Abstract
The targeting of antioxidant systems that allow stem-like cancer cells to avoid the adverse consequences of oxidative stress might be expected to improve the efficacy of cancer treatment. Here, we show that head and neck squamous cell carcinoma (HNSCC) cells that express variant isoforms of CD44 (CD44v) rely on the activity of the cystine transporter subunit xCT for control of their redox status. xCT inhibition selectively induces apoptosis in CD44v-expressing tumor cells without affecting CD44v-negative differentiated cells in the same tumor. In contrast to CD44v-expressing undifferentiated cells, CD44v-negative differentiated cells manifest EGF receptor (EGFR) activation and rely on EGFR activity for their survival. Combined treatment with inhibitors of xCT-dependent cystine transport and of EGFR resulted in a synergistic reduction of EGFR-expressing HNSCC tumor growth. Thus, xCT-targeted therapy may deplete CD44v-expressing undifferentiated HNSCC cells and concurrently sensitize the remaining differentiating cells to available treatments including EGFR-targeted therapy. Cancer Res; 73(6); 1855–66. ©2012 AACR.
Introduction
Despite recent improvement in treatment of head and neck squamous cell carcinoma (HNSCC), the increase in overall survival has been minimal, with treatment failure and disease recurrence continuing to be a problem in many patients (1). Tumor formation, relapse, and metastasis are thought to be driven by a subpopulation of cancer cells. These stem-like cancer cells are generally more resistant to cancer treatments, including chemo- and radiotherapy, compared with non–stem-like cancer cells (2–4). HNSCC comprises a heterogeneous population of cancer cells, with the CD44-expressing subpopulation having been shown to possess the properties of stem-like cancer cells (5, 6). The selective killing of CD44-expressing stem-like cancer cells might therefore be required for effective therapy in patients with HNSCCs.
CD44 is a major marker for stem-like cancer cells in many epithelial tumors (2, 7–9) and is implicated in tumor growth, invasion, and metastasis (10, 11). It exists in numerous variant (v) isoforms generated through alternative mRNA splicing (12). We recently showed that interaction of CD44v with the cystine transporter subunit xCT (SLC7A11) stabilizes the latter protein and thereby potentiates the ability of cancer cells to defend themselves against reactive oxygen species (ROS; refs. 13, 14). Given that cancer cells are exposed to environmental stressors such as oxygen or nutrient deficiency, low pH, inflammatory mediators, and ROS during tumor progression, the ability to avoid the consequences of such exposure is required for cancer cell survival. Stem-like cancer cells in which defense against ROS is enhanced by CD44v are thus thought to drive tumor growth, chemoresistance, and metastasis.
xCT functions together with CD98hc (SLC3A2) as an Na+-independent transporter (system xc−) that mediates exchange of extracellular cystine for intracellular glutamate and thereby promotes synthesis of reduced glutathione (GSH; refs. 15, 16), an important intracellular redox buffer that is associated with cancer cell resistance to anticancer agents (17, 18). Cysteine availability is rate-limiting for GSH synthesis (19), and xCT activity is therefore essential for such resistance (16). Sulfasalazine is a specific inhibitor of xCT-mediated cystine transport and inhibits the growth, invasion, and metastasis of several types of cancer (20–22). We recently showed that sulfasalazine treatment suppressed CD44v-dependent tumor growth and chemoresistance (13) as well as distant metastasis (14). However, whether inhibition of xCT selectively damages stem-like cancer cells, without affecting non–stem-like cancer cells, has remained unclear. We therefore investigated the role of xCT-mediated control of redox status in CD44v-expressing cancer cells and examined whether sulfasalazine selectively kills these stem-like tumor cells in vivo–.
Materials and Methods
Tissue specimens
HNSCC specimens were collected with informed consent from patients who underwent surgery at Keio University Hospital (Tokyo, Japan). The specimens were subjected to immunostaining. Excision samples of HNSCCs (n = 49) were divided into a CD44v-positive group (n = 19) and a CD44v-low or -negative group (n = 30). The CD44v-positive samples were further divided into those from patients who had (n = 12) or had not (n = 7) undergone neoadjuvant chemotherapy. The tumor–node–metastasis (TNM) classification of the specimens ranged from T1 to T4 and N0 to N1 (Supplementary Table S1). The area positive for CD44v or involucrin immunoreactivity was quantified with the use of BZ Analyzer software (Keyence) and with a constant color threshold in 3 fields per slide. This aspect of the study was approved by the Institutional Review Board of Keio University School of Medicine (Tokyo, Japan).
Cell culture
HSC-2 and HSC-3 cells were cultured in Minimum Essential Medium (MEM) supplemented with 10% FBS. HSC-4 and OSC19 cells were cultured in RPMI-1640 and Dulbecco's Modified Eagle's Medium (DMEM), respectively, each supplemented with 10% FBS. The normal keratinocyte cell line HaCaT was cultured in DMEM. All cell lines were maintained under 5% CO2 at 37°C. HSC-2, HSC-3, and HSC-4 cells were obtained from RIKEN Cell Bank and HaCaT cells from Cell Lines Service. These cells were passaged in our laboratory for fewer than 6 months after resuscitation. OSC19 cells were obtained from Kanazawa University (Ishikawa, Japan) and were tested negative for mycoplasma using PCR tests within 6 months before the experiments were carried out. For assay of HNSCC cell differentiation, cells were seeded in 24-well NanoCulture plates (SCIVAX) at a density of 2 × 105 cells/mL.
GSH assay, tumor cell isolation and flow cytometry, and other methods
A GSH assay as well as tumor cell isolation and flow-cytometric analysis were conducted as previously described (13, 14). Other experimental procedures are described in Supplementary Materials and Methods.
Statistical analysis
Data are presented as mean ± SD and were analyzed with the unpaired Student t test. A P value of less than 0.05 was considered statistically significant.
Results
CD44v-positive area is increased in HNSCC tumors of patients who received neoadjuvant chemotherapy
To explore the clinical relevance of CD44v-expressing cells in human HNSCC tumors, we examined CD44v expression in tumor tissue of patients treated with (n = 12) or without (n = 7) neoadjuvant chemotherapy (Supplementary Table S1). The relative area occupied by CD44v-expressing cells was significantly larger in tumors of patients who received neoadjuvant chemotherapy than in those of patients who did not (Fig. 1A and B), suggesting that CD44v-positive HNSCC cells are resistant to chemotherapy compared with CD44v-negative cells. Furthermore, the expression level of the differentiation marker involucrin tended to be reduced in tumor tissue resected after neoadjuvant chemotherapy compared with that obtained from patients not subjected to chemotherapy (Fig. 1B and C). Together, these observations suggested that cytotoxic chemotherapy might preferentially kill CD44v-negative differentiated cells and that the remaining subpopulation of CD44v-positive cells expands after such treatment.
CD44v expression in HNSCC tumors. A, HNSCC tumor sections from patients treated with or without neoadjuvant chemotherapy were subjected to immunofluorescence staining with antibodies to human CD44v9 (red fluorescence) and to involucrin (green fluorescence). Nuclei were also stained with 4′,6-diamidino-2-phenylindole (DAPI; blue fluorescence). Scale bars, 100 μm. B, box-and-whisker plot of the percentage area positive for CD44v or involucrin in HNSCC specimens from patients who did (n = 12) or did not (n = 7) receive neoadjuvant chemotherapy. Data are mean ± SD. **, P < 0.01. C, sections of HNSCC tumors (T2N0M0) and corresponding biopsied normal tissue from patients treated with or without neoadjuvant chemotherapy were subjected to immunofluorescence staining as in A. Boxed regions are shown at higher magnification in the bottom. Scale bars, 100 μm.
CD44v expression in HNSCC tumors. A, HNSCC tumor sections from patients treated with or without neoadjuvant chemotherapy were subjected to immunofluorescence staining with antibodies to human CD44v9 (red fluorescence) and to involucrin (green fluorescence). Nuclei were also stained with 4′,6-diamidino-2-phenylindole (DAPI; blue fluorescence). Scale bars, 100 μm. B, box-and-whisker plot of the percentage area positive for CD44v or involucrin in HNSCC specimens from patients who did (n = 12) or did not (n = 7) receive neoadjuvant chemotherapy. Data are mean ± SD. **, P < 0.01. C, sections of HNSCC tumors (T2N0M0) and corresponding biopsied normal tissue from patients treated with or without neoadjuvant chemotherapy were subjected to immunofluorescence staining as in A. Boxed regions are shown at higher magnification in the bottom. Scale bars, 100 μm.
xCT-mediated cystine transport supports the GSH-dependent antioxidant system in highly CD44v-positive HNSCC cells
The GSH-dependent antioxidant system promotes the resistance of CD44v-expressing gastrointestinal cancer cells to chemotherapy (13). To investigate whether a similar CD44v-dependent mechanism is operative in HNSCC cells, we examined the effects of cisplatin, whose cytotoxicity is mediated, in part, by ROS (23), on the viability of 4 HNSCC cell lines that differ in CD44v expression status (Fig. 2A and Supplementary Fig. S1A). OSC19 cells, in which CD44v is expressed at a high level, were resistant to cisplatin treatment (Fig. 2B). HSC-2 cells, which also express CD44v at a high level, albeit not as high as that in OSC19 cells, also showed resistance to cisplatin, although not to the same extent as did OSC19 cells. In contrast, HSC-3 and HSC-4 cells, both of which express CD44v at a low level, were sensitive to this drug. These results thus suggested that CD44v expression status is related to cisplatin resistance in these cell lines.
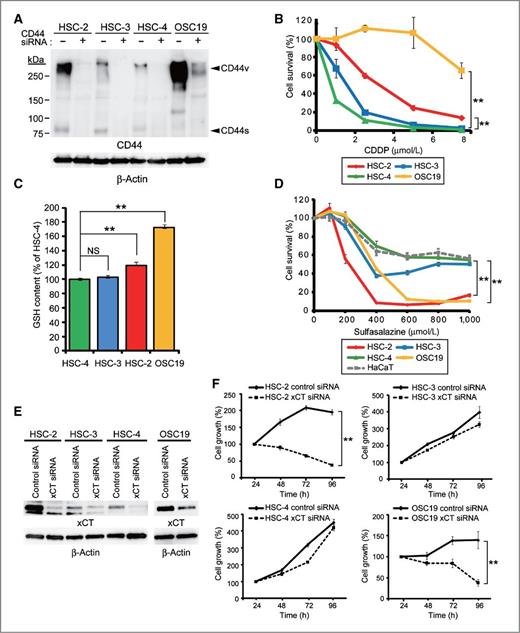
Highly CD44v-expressing HNSCC cells are resistant to cisplatin but sensitive to sulfasalazine. A, HSC-2, HSC-3, HSC-4, and OSC19 cells transfected with control or CD44 siRNAs were subjected to immunoblot analysis with antibodies to CD44 or to β-actin (loading control). The identity of the bands putatively corresponding to variant (CD44v) and standard (CD44s) isoforms of CD44 was confirmed by the effect of the CD44 siRNA. B, viability of cells incubated with various concentrations of cisplatin for 72 hours. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01 for OSC19 or HSC-2 cells versus the corresponding value for HSC-4 cells. C, GSH content of HSC-2, HSC-3, HSC-4, and OSC19 cells. Data are expressed relative to the value for HSC-4 cells and are mean ± SD from 3 independent experiments. **, P < 0.01; NS, not significant. D, viability of HSC-2, HSC-3, HSC-4, and OSC19 cells as well as HaCaT normal keratinocytes incubated with various concentrations of sulfasalazine for 24 hours. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01 for OSC19 or HSC-2 cells versus the corresponding value for HSC-4 cells. E, cells transfected with control or xCT siRNAs were subjected to immunoblot analysis with antibodies to xCT or to β-actin. F, time course of cell proliferation for cell lines transfected with control or xCT siRNAs. Data are mean ± SD from 3 independent experiments. **, P < 0.01.
Highly CD44v-expressing HNSCC cells are resistant to cisplatin but sensitive to sulfasalazine. A, HSC-2, HSC-3, HSC-4, and OSC19 cells transfected with control or CD44 siRNAs were subjected to immunoblot analysis with antibodies to CD44 or to β-actin (loading control). The identity of the bands putatively corresponding to variant (CD44v) and standard (CD44s) isoforms of CD44 was confirmed by the effect of the CD44 siRNA. B, viability of cells incubated with various concentrations of cisplatin for 72 hours. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01 for OSC19 or HSC-2 cells versus the corresponding value for HSC-4 cells. C, GSH content of HSC-2, HSC-3, HSC-4, and OSC19 cells. Data are expressed relative to the value for HSC-4 cells and are mean ± SD from 3 independent experiments. **, P < 0.01; NS, not significant. D, viability of HSC-2, HSC-3, HSC-4, and OSC19 cells as well as HaCaT normal keratinocytes incubated with various concentrations of sulfasalazine for 24 hours. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01 for OSC19 or HSC-2 cells versus the corresponding value for HSC-4 cells. E, cells transfected with control or xCT siRNAs were subjected to immunoblot analysis with antibodies to xCT or to β-actin. F, time course of cell proliferation for cell lines transfected with control or xCT siRNAs. Data are mean ± SD from 3 independent experiments. **, P < 0.01.
Given that the GSH-dependent antioxidant system plays a key role in cisplatin resistance as a result of its ability to scavenge ROS (24), we examined the GSH levels of the 4 cell lines. The abundance of GSH was greater in the highly CD44v-positive OSC19 and HSC-2 cells than in HSC-3 and HSC-4 cells (Fig. 2C). The intracellular GSH level thus also appeared related to cisplatin resistance in HNSCC cells. Given that CD44v stabilizes the cystine transporter subunit xCT and thereby potentiates cystine uptake and ROS defense in cancer cells (13), we next examined the effects of sulfasalazine, a specific inhibitor of xCT-mediated cystine transport (25), on HNSCC cell viability. Sulfasalazine markedly reduced the viability of the highly CD44v-expressing OSC19 and HSC-2 cells, whereas it affected that of HNSCC cells that express CD44v at a low level, including HSC-3 and HSC-4 cells, to a lesser extent (Fig. 2D and Supplementary Fig. S1B), with this pattern of cytotoxicity contrasting with that observed for cisplatin. These data suggested that highly CD44v-positive HNSCC cells rely on xCT-mediated cystine transport and consequent upregulation of the GSH-dependent antioxidant system for cellular redox homeostasis.
Sulfasalazine has been reported to inhibit not only the xCT-mediated cystine transport but also NF-κB signaling (25, 26). Accordingly, to examine further the functional relevance of xCT expression in HNSCC cells, we determined the effects of xCT knockdown on cell growth (Fig. 2E and F). Depletion of xCT resulted in significant inhibition of the proliferation of sulfasalazine-sensitive HSC-2 and OSC19 cells without affecting that of sulfasalazine-resistant HSC-3 and HSC-4 cells. Similar results were obtained by CD44 knockdown in HSC-2 and OSC19 cells (Supplementary Fig. S1C and S1D). Together, these results suggested that sulfasalazine-sensitive HNSCC cells are dependent on the CD44v-xCT system for their survival and proliferation to a greater extent than are sulfasalazine-insensitive cells.
Cell differentiation is associated with the loss of CD44v and xCT dependency in HNSCC cells in vivo
To examine the effect of xCT inhibition on tumor formation by highly CD44v-expressing HNSCC cells, we transplanted HSC-2 and OSC19 cells into nude mice. Sulfasalazine significantly inhibited the growth of OSC19 tumors after 14 days of treatment but did not affect the size of HSC-2 tumors even after treatment for 28 days (Fig. 3A and Supplementary Fig. S2A). Thus, whereas both HSC-2 and OSC19 cells are sensitive to sulfasalazine in vitro, only tumors formed by OSC19 cells are sensitive to this drug in vivo.
xCT inhibition selectively induces apoptosis in CD44v-positive tumor cells in differentiated-type tumors formed by HSC-2 cells. A, volume of tumors formed by OSC19 or HSC-2 cells in nude mice treated daily with sulfasalazine (250 mg/kg) or saline. Data are mean ± SD for 5 mice per group. **, P < 0.01. B, immunofluorescence staining for CD44v and involucrin, together with DAPI staining of nuclei (blue fluorescence), in tumors formed by HSC-2 or OSC19 cells. Asterisks indicate tumor cells positive for involucrin but negative for CD44v; white dashed lines indicate tumor margins. Scale bars, 500 μm. C, weight of tumors formed by HSC-2 cells in nude mice treated daily with retinoic acid (4 mg/kg), sulfasalazine (250 mg/kg), or saline (control) for 4 weeks. Data are mean ± SD for 5 mice per group. **, P < 0.01. D, immunofluorescence staining for CD44v and involucrin, together with DAPI staining of nuclei (blue fluorescence), in tumors formed by HSC-2 cells in nude mice treated as in C. Arrows indicate CD44v-expressing tumor cells, and arrowheads indicate the few remaining such cells after sulfasalazine treatment (left); scale bars, 500 μm. Tumor lysates were also subjected to immunoblot analysis with antibodies to CD44 and to α-tubulin (right). E, flow-cytometric analysis of CD44v expression on lineage marker–negative (Lin−) cells isolated from tumors formed by HSC-2 cells in nude mice treated daily with saline or sulfasalazine (500 mg/kg) for 3 weeks. FSC-H, forward-scatter height. F, immunofluorescence staining for CD44v and cleaved caspase-3, together with DAPI staining of nuclei (blue fluorescence), in tumors formed by HSC-2 cells in nude mice treated with sulfasalazine or saline as in A but for only 7 days (left). Arrows indicate CD44v-expressing tumor cells positive for cleaved caspase-3. Scale bars, 100 μm. The proportion of CD44v-expressing or CD44v-negative tumor cells that were positive for cleaved caspase-3 was also determined (right).
xCT inhibition selectively induces apoptosis in CD44v-positive tumor cells in differentiated-type tumors formed by HSC-2 cells. A, volume of tumors formed by OSC19 or HSC-2 cells in nude mice treated daily with sulfasalazine (250 mg/kg) or saline. Data are mean ± SD for 5 mice per group. **, P < 0.01. B, immunofluorescence staining for CD44v and involucrin, together with DAPI staining of nuclei (blue fluorescence), in tumors formed by HSC-2 or OSC19 cells. Asterisks indicate tumor cells positive for involucrin but negative for CD44v; white dashed lines indicate tumor margins. Scale bars, 500 μm. C, weight of tumors formed by HSC-2 cells in nude mice treated daily with retinoic acid (4 mg/kg), sulfasalazine (250 mg/kg), or saline (control) for 4 weeks. Data are mean ± SD for 5 mice per group. **, P < 0.01. D, immunofluorescence staining for CD44v and involucrin, together with DAPI staining of nuclei (blue fluorescence), in tumors formed by HSC-2 cells in nude mice treated as in C. Arrows indicate CD44v-expressing tumor cells, and arrowheads indicate the few remaining such cells after sulfasalazine treatment (left); scale bars, 500 μm. Tumor lysates were also subjected to immunoblot analysis with antibodies to CD44 and to α-tubulin (right). E, flow-cytometric analysis of CD44v expression on lineage marker–negative (Lin−) cells isolated from tumors formed by HSC-2 cells in nude mice treated daily with saline or sulfasalazine (500 mg/kg) for 3 weeks. FSC-H, forward-scatter height. F, immunofluorescence staining for CD44v and cleaved caspase-3, together with DAPI staining of nuclei (blue fluorescence), in tumors formed by HSC-2 cells in nude mice treated with sulfasalazine or saline as in A but for only 7 days (left). Arrows indicate CD44v-expressing tumor cells positive for cleaved caspase-3. Scale bars, 100 μm. The proportion of CD44v-expressing or CD44v-negative tumor cells that were positive for cleaved caspase-3 was also determined (right).
To explore the mechanism underlying the difference in sulfasalazine sensitivity of HSC-2 cells in vitro versus that in vivo, we focused on histologic differences of tumors formed by HSC-2 and OSC19 cells. Flow cytometry revealed that all HSC-2 and OSC19 cells express CD44v at a high level in vitro (Supplementary Fig. S2B). However, immunofluorescence analysis showed that whereas tumors formed by OSC19 cells consisted mostly of CD44v-positive cells, those formed by HSC-2 cells comprised a heterogeneous population of CD44v-positive and -negative cells (Fig. 3B). Furthermore, CD44v-negative HSC-2 cells in established tumors expressed involucrin (Fig. 3B), a marker of keratinocyte differentiation (27). These results suggested that HSC-2 cells give rise to differentiated tumor cells in vivo, resulting in the formation of heterogeneous tumors, whereas OSC19 cells generate homogeneous undifferentiated tumors.
To examine whether tumor cell differentiation might affect the response to sulfasalazine, we investigated the effects of 9-cis-retinoic acid, which promotes the differentiation of a subset of HNSCC cells including HSC-2 cells (28). In contrast to the lack of effect of sulfasalazine, tumors formed by HSC-2 cells in mice treated with retinoic acid were significantly smaller than those formed in control mice (Fig. 3C), suggesting that the promotion of cell differentiation inhibits the growth of differentiated-type tumors formed by HSC-2 cells. Immunofluorescence and immunoblot analyses showed that tumors formed in sulfasalazine-treated mice contained a reduced number of cells expressing CD44v compared with those formed in mice treated with either saline or retinoic acid (Fig. 3D). Flow cytometry also revealed that sulfasalazine selectively reduced the proportion of highly CD44v-expressing tumor cells and increased that of CD44v-negative cells (Fig. 3E). These findings suggested that sulfasalazine targets highly CD44v-expressing tumor cells.
Given that retinoic acid failed to reduce CD44v expression in HSC-2 tumors (Fig. 3D) but inhibited tumor growth, we examined whether CD44v expression might affect sensitivity to retinoic acid. Retinoic acid failed to reduce CD44 expression and to increase involucrin expression in HSC-2 and OSC19 cells in vitro (Supplementary Fig. S2C). On the other hand, CD44 ablation increased the amount of involucrin mRNA in HSC-2 cells and this effect was enhanced by retinoic acid (Supplementary Fig. S2D), suggesting that a high level of CD44v expression in HNSCC cells might confer resistance to the induction of cell differentiation by retinoic acid. Together, these results suggested that retinoic acid promotes differentiation in tumor cells with a low level of CD44v expression, without affecting that in highly CD44v-expressing cells, and thereby inhibits tumor growth.
To determine whether sulfasalazine actually induces apoptosis in CD44v-expressing tumor cells in vivo, we treated mice bearing HSC-2 cell tumors with sulfasalazine for just 7 days. Immunofluorescence analysis revealed that the proportion of CD44v-expressing tumor cells positive for the cleaved form of caspase-3, a marker of apoptosis, was increased by sulfasalazine treatment (Fig. 3F), suggesting that sulfasalazine selectively killed CD44v-expressing tumor cells. We then examined whether the selective depletion of CD44v-expressing tumor cells by sulfasalazine might enhance the antitumor effect of cisplatin. The weight of HSC-2 tumors was markedly smaller for mice treated with both cisplatin and sulfasalazine than for those treated with saline or cisplatin alone (Supplementary Fig. S2E). The abundance of CD44v in tumor lysates was increased 2.3-fold after cisplatin treatment, whereas it was reduced by 70% after the combined treatment with cisplatin and sulfasalazine (Supplementary Fig. S2E). Together, these results suggested that the selective depletion of CD44v-expressing stem-like tumor cells by sulfasalazine treatment indeed enhances the cisplatin sensitivity of the differentiated-type tumors formed by HSC-2 cells.
Cell differentiation is associated with loss of CD44v and xCT dependency in HNSCC cells in vitro
We further examined whether differentiation reduces the sensitivity of HNSCC cells to sulfasalazine with the use of an adhesion-restricted culture system, which triggers keratinocyte differentiation in vitro (ref. 29; Fig. 4A). Culture under such conditions induced expression of the differentiation markers involucrin and cytokeratin 10 (CK10) in HSC-2, HSC-3, and HSC-4 cells, but not in OSC19 cells, in a time-dependent manner (Fig. 4B). Conversely, the expression level of CD44v was reduced in HSC-2, HSC-3, and HSC-4 cells, but not in OSC19 cells, by adhesion restriction (Fig. 4B). Consistent with our in vivo data, these results thus indicated that HSC-2, HSC-3, and HSC-4 cells are able to undergo differentiation in response to adhesion restriction in vitro whereas OSC19 cells are not.
Differentiation status affects the sensitivity of HNSCC cells to xCT inhibition in vitro. A, microscopic images of HSC-2, HSC-3, HSC-4, and OSC19 cells seeded on low-attachment plates and cultured for 7 days to induce cell differentiation. Scale bars, 100 μm. B, immunoblot analysis of involucrin, CK10, and CD44v isoforms in cells cultured under normal or low-attachment conditions for the indicated times. C, immunoblot analysis of cleaved caspase-3, CD44v, and involucrin in HSC-2 and OSC19 cells cultured on normal or low-attachment plates in the absence or presence of 200 μmol/L (HSC-2) or 100 μmol/L (OSC19) sulfasalazine for 72 hours.
Differentiation status affects the sensitivity of HNSCC cells to xCT inhibition in vitro. A, microscopic images of HSC-2, HSC-3, HSC-4, and OSC19 cells seeded on low-attachment plates and cultured for 7 days to induce cell differentiation. Scale bars, 100 μm. B, immunoblot analysis of involucrin, CK10, and CD44v isoforms in cells cultured under normal or low-attachment conditions for the indicated times. C, immunoblot analysis of cleaved caspase-3, CD44v, and involucrin in HSC-2 and OSC19 cells cultured on normal or low-attachment plates in the absence or presence of 200 μmol/L (HSC-2) or 100 μmol/L (OSC19) sulfasalazine for 72 hours.
We then examined the effects of sulfasalazine on HSC-2 and OSC19 cells cultured under the adhesion-restricted condition. The abundance of the cleaved form of caspase-3 was increased in both HSC-2 and OSC19 cells after treatment with sulfasalazine under normal conditions (Fig. 4C). However, under the adhesion-restricted condition, cleavage of caspase-3 was not detected in sulfasalazine-treated HSC-2 cells whereas it was still apparent in OSC19 cells (Fig. 4C), suggesting that the ability of sulfasalazine to trigger cell death is affected by the differentiation status of HNSCC cells. Together, these observations suggested that sulfasalazine selectively triggers apoptosis in CD44v-expressing undifferentiated stem-like HNSCC cells, without affecting CD44v-negative differentiated cells.
EGF receptor activity is required for survival of differentiating HNSCC cells
Given that the expression level of the EGF receptor (EGFR) is associated with the differentiation capacity of human SCC cells (30) and that EGFR gene amplification has been detected in HSC-2, HSC-3, and HSC-4 cells (31), which we found undergo differentiation in response to adhesion restriction, we investigated EGFR expression in these HNSCC cell lines. The expression and phosphorylation of EGFR were apparent at only low levels in the differentiation-resistant OSC19 cells compared with HSC-2, HSC-3, and HSC-4 cells, whereas expression of the EGFR-related protein HER2 did not differ substantially among the four cell lines (Fig. 5A). Flow cytometry also revealed that the level of EGFR expression at the cell surface was lower in OSC19 cells than in HSC-2 cells (Fig. 5B). These results thus suggested that EGFR signaling is suppressed in the differentiation-resistant OSC19 cells.
EGFR activity is required for survival of CD44-negative differentiating HNSCC cells. A, immunoblot analysis of EGFR, phosphorylated EGFR (phospho-EGFR), and HER2 in HSC-2, HSC-3, HSC-4, and OSC19 cells. B, flow-cytometric analysis of EGFR expression at the surface of HSC-2 and OSC19 cells. C, immunofluorescence staining of phospho-EGFR and CD44v, together with DAPI staining of nuclei (blue fluorescence), in tumors formed in nude mice injected with HSC-2 cells. Arrows indicate CD44v-negative, phospho-EGFR–positive tumor cells, and arrowheads indicate CD44v-postitive, phospho-EGFR–negative tumor cells. Scale bars, 500 μm. D, immunoblot analysis of phospho-EGFR, involucrin, cleaved caspase-3, and CD44v in HSC-2 cells cultured on low-attachment plates for 72 hours in the presence of the indicated concentrations of AG1478 or cetuximab.
EGFR activity is required for survival of CD44-negative differentiating HNSCC cells. A, immunoblot analysis of EGFR, phosphorylated EGFR (phospho-EGFR), and HER2 in HSC-2, HSC-3, HSC-4, and OSC19 cells. B, flow-cytometric analysis of EGFR expression at the surface of HSC-2 and OSC19 cells. C, immunofluorescence staining of phospho-EGFR and CD44v, together with DAPI staining of nuclei (blue fluorescence), in tumors formed in nude mice injected with HSC-2 cells. Arrows indicate CD44v-negative, phospho-EGFR–positive tumor cells, and arrowheads indicate CD44v-postitive, phospho-EGFR–negative tumor cells. Scale bars, 500 μm. D, immunoblot analysis of phospho-EGFR, involucrin, cleaved caspase-3, and CD44v in HSC-2 cells cultured on low-attachment plates for 72 hours in the presence of the indicated concentrations of AG1478 or cetuximab.
In HSC-2 tumors, the abundance of phosphorylated EGFR was high in CD44v-negative differentiated cells (Fig. 5C), suggesting that the activation of EGFR signaling is associated with differentiation of HNSCC cells. To examine this notion further, we treated HSC-2 cells cultured under the adhesion-restricted condition with the EGFR tyrosine kinase inhibitor AG1478 or with the EGFR-targeted antibody cetuximab. Inhibition of EGFR signaling by either agent resulted in downregulation of the differentiation marker involucrin and in the cleavage of caspase-3 (Fig. 5D). These results suggested that activation of EGFR signaling is required for the survival of HNSCC cells that are exposed to a differentiation stimulus.
CD44v expression enhances xCT dependency but reduces EGFR dependency
To examine whether CD44 ablation in HNSCC cells affects the surface expression of xCT, we conducted flow cytometry with a monoclonal antibody that recognizes the surface complex of xCT and CD98hc (13). Ablation of CD44 by transfection of cells with CD44 siRNA reduced the amount of xCT at the surface of HSC-2 cells (Fig. 6A) as well as of OSC19 cells (Supplementary Fig. S3A), suggesting that CD44v promotes the surface expression of the xCT-CD98hc complex in HNSCC cells. Concomitant with the downregulation of xCT expression, the intracellular GSH content was reduced in HSC-2 (Supplementary Fig. S3B and S3D) or OSC19 (Supplementary Fig. S3C and S3D) cells by transfection with CD44 siRNA. Furthermore, we confirmed the interaction of xCT with CD44v in both HSC-2 and OSC19 cells (Supplementary Fig. S3E). Together, our observations suggested that CD44v stabilizes xCT at the cell surface and thereby promotes cystine transport by xCT and consequent GSH synthesis in HNSCC cells.
xCT inhibition selectively reduces the viability of CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy. A, flow-cytometric analysis of xCT and CD44v expression at the surface of HSC-2 cells transfected with control or CD44 siRNAs. Cells were stained with antibodies to CD44v9 and to xCT. B, viability of HSC-2 cells stably expressing control or CD44 shRNAs and incubated for 24 hours with various concentrations of sulfasalazine. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01. C, immunoblot analysis of phospho-EGFR, EGFR, and CD44v in HSC-2 or OSC19 cells transfected with control or CD44 siRNAs. The intensity of the phospho-EGFR band normalized by that of the corresponding EGFR band is shown as fold increase relative to that for control cells. D, viability of HSC-2 cells stably expressing control or CD44 shRNAs and incubated for 24 hours with various concentrations of AG1478. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01. E, weight of tumors formed by HSC-2 cells in nude mice treated daily with sulfasalazine (250 mg/kg) or every 4 days with cetuximab (500 μg), as indicated, for 4 weeks. Control mice received saline. Data are mean ± SD for 5 mice per group. *, P < 0.05; **, P < 0.01. F, effects of xCT-targeted and EGFR-targeted therapies on undifferentiated or differentiated HNSCC cells. CD44v expression promotes xCT-dependent cystine transport and thereby boosts the GSH-dependent antioxidant system in undifferentiated HNSCC cells. Reduced adhesion of cells to the extracellular matrix, a stimulus for terminal differentiation, results in down-regulation of CD44v expression. EGFR is activated in CD44v-negative HNSCC cells and promotes cell survival during differentiation. Combined treatment with inhibitors of xCT-dependent cystine transport and of EGFR may therefore kill HNSCC cells in a cooperative manner.
xCT inhibition selectively reduces the viability of CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy. A, flow-cytometric analysis of xCT and CD44v expression at the surface of HSC-2 cells transfected with control or CD44 siRNAs. Cells were stained with antibodies to CD44v9 and to xCT. B, viability of HSC-2 cells stably expressing control or CD44 shRNAs and incubated for 24 hours with various concentrations of sulfasalazine. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01. C, immunoblot analysis of phospho-EGFR, EGFR, and CD44v in HSC-2 or OSC19 cells transfected with control or CD44 siRNAs. The intensity of the phospho-EGFR band normalized by that of the corresponding EGFR band is shown as fold increase relative to that for control cells. D, viability of HSC-2 cells stably expressing control or CD44 shRNAs and incubated for 24 hours with various concentrations of AG1478. Data are expressed relative to the corresponding value for nontreated cells and are mean ± SD from 3 independent experiments. **, P < 0.01. E, weight of tumors formed by HSC-2 cells in nude mice treated daily with sulfasalazine (250 mg/kg) or every 4 days with cetuximab (500 μg), as indicated, for 4 weeks. Control mice received saline. Data are mean ± SD for 5 mice per group. *, P < 0.05; **, P < 0.01. F, effects of xCT-targeted and EGFR-targeted therapies on undifferentiated or differentiated HNSCC cells. CD44v expression promotes xCT-dependent cystine transport and thereby boosts the GSH-dependent antioxidant system in undifferentiated HNSCC cells. Reduced adhesion of cells to the extracellular matrix, a stimulus for terminal differentiation, results in down-regulation of CD44v expression. EGFR is activated in CD44v-negative HNSCC cells and promotes cell survival during differentiation. Combined treatment with inhibitors of xCT-dependent cystine transport and of EGFR may therefore kill HNSCC cells in a cooperative manner.
We next examined the effect of CD44 expression on sulfasalazine sensitivity in HNSCC cells. The effect of sulfasalazine on the viability of HSC-2 cells expressing CD44 short hairpin RNA (shRNA) was attenuated compared with that apparent for those expressing a control shRNA (Fig. 6B) and was similar to that for HSC-3 and HSC-4 cells (Fig. 2D), which express CD44v at only a low level, suggesting that CD44v expression determines the xCT dependency of HNSCC cells.
We then examined the relation between CD44 expression and EGFR activity. The amount of phosphorylated EGFR was increased 1.6- to 2.0-fold in CD44-depleted HSC-2 cells compared with control cells (Fig. 6C). However, such an effect of CD44 ablation on EGFR phosphorylation was not observed in OSC19 cells, which express EGFR at only a low level (Fig. 5A and B), suggesting that CD44 contributes to the modulation of EGFR activity in EGFR-overexpressing HNSCC cells. Furthermore, the growth-inhibitory effect of the EGFR inhibitor AG1478 was significantly increased by CD44 ablation in HSC-2 cells (Fig. 6D). Together, these results suggested that differentiated HNSCC cells that express CD44v at only a low level rely on the activation of EGFR signaling rather than on xCT activity for their survival.
We investigated the effect of combined treatment with cetuximab and sulfasalazine on tumor growth in the HSC-2 mouse xenograft model. Sulfasalazine significantly increased the antitumor effect of cetuximab (Fig. 6E), suggesting that xCT-targeted therapy might enhance the effect of EGFR-targeted therapy in EGFR-overexpressing HNSCCs.
Finally, we investigated the relevance of sulfasalazine sensitivity to the sensitivity to EGFR-targeted agents in HNSCC cells. We conducted microarray analysis with OSC19 cells, which express CD44v at a high level and are sensitive to sulfasalazine, and with HSC-4 cells, which express CD44v at only a low level and are insensitive to sulfasalazine, and we then conducted gene set enrichment analysis (GSEA; ref. 32). The OSC19/HSC-4 gene set profile was significantly similar to the cetuximab-resistant/-sensitive HNSCC profile (Supplementary Fig. S4 and Supplementary Table S2). Sulfasalazine-sensitive OSC19 cells thus manifest a cetuximab resistance–like gene signature when compared with sulfasalazine-insensitive HSC-4 cells. Collectively, our data suggest that xCT-targeted therapy selectively damages CD44v-expressing tumorigenic HNSCC cells that manifest a high level of intracellluar GSH and resistance to both conventional cancer therapy and EGFR-targeted therapy.
Discussion
A key challenge remaining for anticancer therapy is the selective killing of cancer cells on the basis of cancer-specific features (33, 34). The ability to avoid the consequences of oxidative stress is a feature of stem-like cancer cells (3). We recently showed that the interaction of CD44v with xCT enhances the ability of stem-like cancer cells to defend against ROS and thereby promotes tumor growth (13) and metastasis (14), suggesting that CD44v-expressing stem-like tumor cells rely on xCT-mediated cystine transport for their survival and propagation in vivo. We have now shown that the proliferation and survival of CD44v-expressing HNSCC cells depend on xCT-mediated cystine transport and that inhibition of xCT by sulfasalazine selectively triggers apoptosis in CD44v-expressing undifferentiated tumor cells. Furthermore, ablation of CD44v by RNA interference attenuated xCT dependency but enhanced EGFR dependency in HSC-2 cells. Together, our results suggest that upregulation of the GSH antioxidant system by the CD44v–xCT complex plays a key role in regulation of redox status in CD44v-expressing HNSCC cells.
Like many epithelial tumors, HNSCC is composed of a heterogeneous population of cancer cells corresponding to different stages of differentiation, and such heterogeneity may affect the response to cancer therapy (5, 6). Epidermal homeostasis depends on a balance between stem cell renewal and differentiation and is regulated by extrinsic signals from the extracellular matrix, with human epidermal stem cells having been shown to undergo terminal differentiation under conditions of restricted adhesion (29). HSC-2 cells, but not OSC19 cells, were found to differentiate into CD44-negative, involucrin-positive cells under such conditions. Furthermore, such differentiated HSC-2 cells showed resistance to sulfasalazine treatment, suggesting that differentiated HNSCC cells do not rely on the xCT-dependent antioxidant system for control of their redox status.
CD44-expressing stem-like cells in human HNSCC tumors play a central role in resistance to cancer therapy (5, 6). Indeed, our analysis of clinical HNSCC specimens showed that CD44v-positive tumor cells selectively survive and increase in number after chemotherapy. These observations suggest that definitive treatment should target the highly CD44-expressing cell subpopulation. We found that sulfasalazine selectively damaged the CD44v-expressing cell subpopulation in HSC-2 tumors, which are composed of both CD44v-positive undifferentiated cells and CD44v-negative differentiated cells, as well as enhanced the cytotoxicity of cisplatin. The volume of HSC-2 tumors was little affected by sulfasalazine treatment, however, compared with that of OSC19 tumors, which are composed mostly of CD44v-positive cells. It is thus possible that CD44v–xCT plays a key role in the maintenance of established undifferentiated tumors rather than differentiated-type tumors.
Both EGFR and the extracellular Ca2+ concentration influence keratinocyte differentiation (30, 35). We found that the EGFR-targeting agents AG1478 and cetuximab downregulated the differentiation marker involucrin and induced apoptosis in differentiating HNSCC cells that do not express CD44v, suggesting that CD44v-negative HNSCC cells rely on EGFR activity for survival. Furthermore, ablation of CD44v enhanced EGFR activation as well as EGFR dependency in EGFR-overexpressing HSC-2 cells, without affecting EGFR activation in OSC19 cells, which express EGFR at a low level. A high level of CD44v expression may therefore suppress the clustering of EGFR at the surface of HNSCC cells and thereby negatively regulate EGFR signaling in the absence of a differentiation stimulus. In EGFR-overexpressing HNSCCs, downregulation of CD44v during differentiation may thus increase dependency on EGFR signaling and thereby enhance sensitivity to EGFR-targeted therapy. Together, these findings suggest that reduced expression of CD44v in differentiating HNSCC cells enhances the sensitivity to EGFR-targeted therapy, whereas it diminishes the effect of xCT-targeted therapy (Fig. 6F).
Several types of cancer cells, including prostate, pancreatic, and breast cancer cells, are highly sensitive to sulfasalazine treatment (20, 36, 37). In addition to such cell type specificity, our present data suggest that CD44v expression status might predict the sensitivity of heterogeneous HNSCC tumors to xCT-targeted therapy. Such targeted therapy might deplete CD44v-expressing undifferentiated HNSCC cells and concurrently sensitize the remaining differentiating cells to available treatments including EGFR-targeted therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: H. Saya, O. Nagano
Development of methodology: T. Yae
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M. Yoshikawa, K. Tsuchihashi, T. Yae, T. Masuko, M. Mukai
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M. Yoshikawa, K. Tsuchihashi, T. Yae, T. Masuko, O. Nagano
Writing, review, and/or revision of the manuscript: M. Yoshikawa, O. Nagano
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): T. Yae, T. Motohara, E. Sugihara, N. Onishi, K. Yoshizawa, S. Kawashiri
Study supervision: T. Ishimoto, S. Asoda, H. Kawana, T. Nakagawa, H. Saya, O. Nagano
Acknowledgments
The authors thank I. Ishimatsu, Y. Iwasaki, S. Hayashi, and Y. Imanishi for technical assistance and K. Arai for help with article preparation.
Grant Support
This work was supported by grants (to H. Saya) from, as well as in part by the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct; to O. Nagano) of, the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.