Hong Kong Med J 2019 Dec;25(6):473–82 | Epub 4 Dec 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Assessment and diagnosis of dementia: a review for
primary healthcare professionals
K Lam, FRCP (Edin), FHKAM (Medicine)1;
Windy SY Chan, MClinPharm, DHSc2; James KH Luk, FHKCP, FHKAM
(Medicine)3; Angela YM Leung, PhD, FHKAN (Geron)4
1 Cheshire Home (Shatin), Hospital
Authority, Hong Kong
2 School of Health Sciences, Caritas
Institute of Higher Education, Hong Kong
3 Department of Medicine, Fung Yiu King
Hospital, Hong Kong
4 Centre for Gerontological Nursing, The
Hong Kong Polytechnic University, Hong Kong
Corresponding author: Dr Angela YM Leung (angela.ym.leung@polyu.edu.hk)
Abstract
Dementia is one of the most costly,
disabling diseases associated with ageing, yet it remains underdiagnosed
in primary care. In this article, we present the comprehensive approach
illustrated with a classical case for diagnosing dementia which can be
applied by healthcare professionals in primary care. This diagnostic
approach includes history taking and physical examination, cognitive
testing, informant interviews, neuropsychological testing, neuroimaging,
and the utility of cerebrospinal fluid biomarkers. For the differential
diagnosis of cognitive impairment, the differences and similarities
among normal ageing, mild cognitive impairment, depression, and delirium
are highlighted. As primary care physicians are playing an increasingly
prominent role in the caring of elderly patients in an ageing
population, their role in the diagnosis of dementia should be
strengthened in order to provide a quality care for patients with
dementia.
Introduction
Among people aged >65 years, the global
prevalence of dementia has been estimated as 5%,1
with an overall prevalence of 3.9% in Asia.2
The prevalence increases with ageing, with more than one third of people
aged >85 years having dementia.1
In 2015, it was estimated that 46.8 million people lived with dementia
worldwide, with those numbers expected to almost double every 20 years
until reaching 131.5 million in 2050.3
In China, the burden of dementia is increasing much more rapidly than
previously assumed by the international health community.4 Earlier and more accurate detection of dementia is
critical because it allows patients to plan their future care while they
still have the capacity to make important decisions.5 Only through receiving a diagnosis can a person with
dementia obtain access to cognitive and pharmacological therapies.
However, dementia is underdiagnosed in primary care. Evidence from a
primary care-based screening and diagnosis programme in the United States
revealed that only 19% of patients with a confirmed dementia diagnosis had
been checked for dementia during routine medical care.6
A population-based study showed that approximately
20% of family informants failed to recognise memory problems in elderly
subjects who were found to have dementia on a standardised examination.7 A United Kingdom study showed that
earlier diagnosis may also ease caregiver concerns.8 Planning by families of elderly patients is most
effective when dementia is diagnosed early in the course of the illness.
Accurate diagnosis with subtyping is the prerequisite for providing
optimal therapies specific to different dementia diagnoses. In addition,
reversible causes of cognitive impairment, eg, depression, should be
looked for.9
This article reviews recent approaches in dementia
diagnosis and discusses their applications by healthcare professionals,
particularly those working in the primary care setting.
Definition of dementia
The fifth edition of the Diagnostic and Statistical
Manual of Mental Disorders (DSM-5) provides a common framework for the
diagnosis of neurocognitive disorders, including dementia, which is named
‘major neurocognitive disorder’ in this edition.10
11 It serves as a common
linguistic framework to deal with neurocognitive disorders, thus promoting
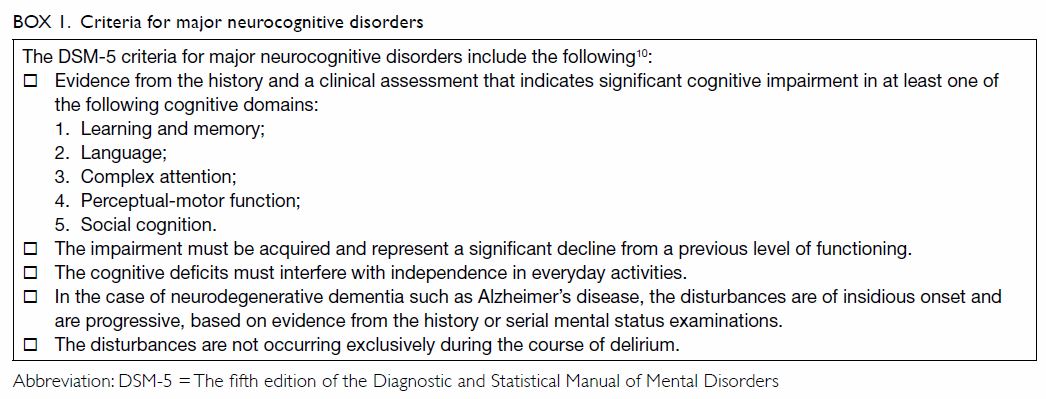
efficient communication among clinicians and researchers.11 The criteria for major neurocognitive disorders are
summarised in Box 1.
The major limitation of the DSM-5 is that it
requires intellectual deficits to be sufficiently severe to impair social
or occupational functioning. Therefore, it necessarily draws an arbitrary
line between dementia and the lack thereof. In clinical settings, patients
usually pass through stages of intellectual decline, including cognitive
deficits thought to occur with normal ageing, mild cognitive impairment
(MCI), and early dementia.12
Differential diagnosis of cognitive impairment
Accurately diagnosing dementia remains a challenge
for healthcare professionals, as there is no definitive diagnostic test to
identify it.
Does Mrs Wong have dementia?
Mrs Wong, aged 83 years, has a history of
hypertension and diabetes mellitus. She lives with her daughter’s family
and her activities of daily living are independent. In the past half year,
her family members have noticed that Mrs Wong sometimes misses
appointments with friends and her favourite Chinese music classes. Mrs
Wong denies physical discomfort but cannot recall the details of her
appointments. Her daughter helps by marking every appointment on a
calendar to remind her. Mrs Wong is unable to handle her own banking and
has become lost on the street when she goes shopping on her own. She
occasionally becomes irritable when she encounters difficulties in
recalling memories.
Not all patients with memory loss complaints have
dementia. There are four common conditions that primary care doctors need
to differentiate from dementia.
Cognitive changes with normal ageing
The normal cognitive decline associated with ageing
consists primarily of mild changes in memory and the rate of information
processing and does not usually affect daily functioning. Normative data
from cross-sectional studies examining neuropsychological performance
demonstrate that the ability to perform new learning or acquisition
declines with age, whereas cued recall remains stable.12 13 14 Agerelated declines are not inevitable, and when they
do occur, careful evaluation for underlying age-related diseases is
warranted.
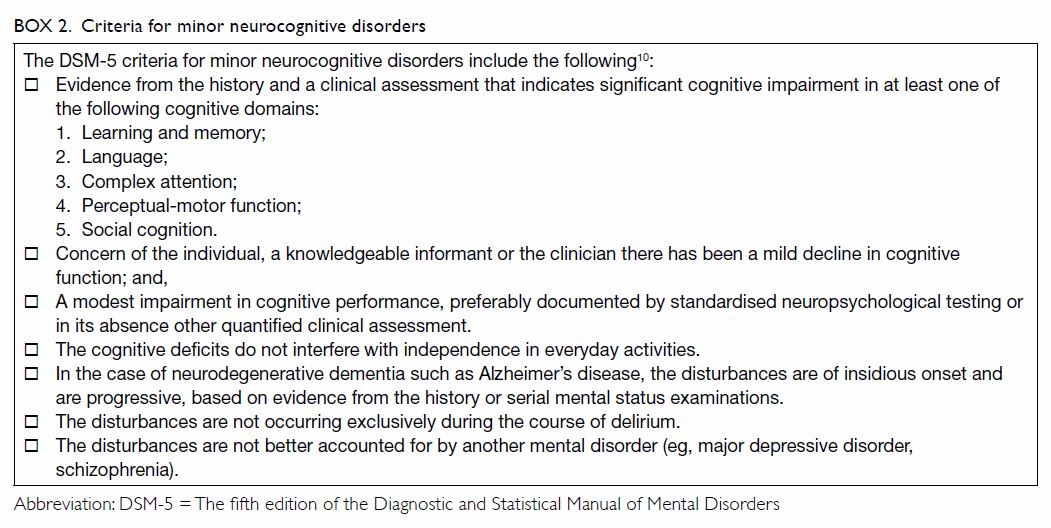
Mild cognitive impairment
Mild cognitive impairment is defined by the
presence of memory difficulty and objective memory impairment but
preserved ability to function in daily life.
While cognitive changes with normal age refer to
normal age-associated memory and cognitive changes in older adults
compared with young normal adults, MCI refers to abnormal changes in
cognitive functioning, and the criteria include a measurable cognitive
deficit in at least one domain.15
16 17
This condition is considered to be a transitional
stage between normal ageing and dementia. There is no pharmacological
treatment for MCI at this time. Studies have shown that subjects with MCI
followed for up to 4 years have an annual conversion rate ranging from 6%
to 25%, but not all of those with MCI evolve to dementia.18 Subjects with MCI may fluctuate between different
trajectories of MCI, including normal cognition, MCI, and dementia.19 Progression from ‘normal to MCI’ or ‘normal to MCI to
dementia’ is not always linear: subjects who develop MCI and later return
to normal may develop dementia later.20
Longitudinal follow-up of MCI subjects is indicated to avoid missing a
diagnosis of dementia conversion.20
The criteria for minor neurocognitive disorders are summarised in Box
2.
Depression
Memory impairment is commonly associated with major
depression in elderly people, and it can cause pseudo-dementia syndrome.
Patients with depression usually present with persistent sadness, loss of
interest in their usual activities, sleep and appetite disturbances, or
feelings of worthlessness or guilt.10
The most commonly used clinical screening tool for depression is the
Geriatric Depression Scale.21
Patients with depression may have signs of psychomotor slowing and apply
little effort to testing, while those with dementia often try hard but
respond with incorrect answers. Antidepressants may improve patients’ mood
and cognitive symptoms. However, dementia can sometimes co-exist with
depression, and treatments may be required for both.
Delirium
Delirium, or acute confusional state, is another
common condition in elderly people. It is usually acute or subacute in
onset and is associated with sensory clouding; patients have fluctuations
in their consciousness level and have difficulty maintaining attention and
concentration.22 Delirium is
associated with a variety of systemic illnesses, infections, and toxic and
metabolic disturbances.
Delirium is uncommon among community-dwelling
elderly people. The Canadian Study of Health and Aging found that the
prevalence of delirium is <0.5% among elderly people living outside of
acute care.23 Nevertheless,
hospitalised elderly patients are more prone to delirium. Studies have
shown that approximately 11% to 25% of hospitalised older patients have
delirium upon admission, and an additional 29% to 31% of older patients
admitted without delirium will develop it.24
25 Elderly patients have a
decreased level of brain reserve, which makes them more prone to
decompensation during acute stress and the development of delirium.
It is important to recognise that patients with
dementia are at increased risk of delirium and that delirium and dementia
may coexist. The most commonly used instrument for screening and
identifying delirium is the Confusion Assessment Method.26 The updated standard diagnostic criteria for delirium
are stipulated in the DSM-5.
Aetiology of dementia
Once a patient is diagnosed with dementia, it is
important to determine the underlying aetiologies. These include various
neurodegenerative diseases as well as metabolic and toxic causes. Among
them, Alzheimer’s disease (AD) is the most common cause of dementia in
elderly individuals, accounting for 60% to 80% of all cases, followed by
vascular dementia (VaD), which accounts for about 20%.4 27 The
distribution of dementia varies across geographical locations and cultural
and socio-economic differences. In the Chinese population, the median
proportions of AD and VaD in all forms of dementia were around 70% and
24%, respectively; other forms constituted 7.5%.4
Dementia with Lewy bodies (DLB) is among the most common forms of
degenerative dementia, accounting for 4.2% of all diagnosed dementia cases
in the community.28 Frontotemporal
dementia (FTD), Parkinson-plus syndromes, alcohol-related dementia,
chronic traumatic encephalopathy, and other central nervous system
illnesses are uncommon causes and are responsible for the majority of the
remaining chronic dementia cases.12
Common subtypes of dementia
Alzheimer’s disease is usually a disease of ageing,
and its incidence increases exponentially with age >65 years. The onset
and progress of AD are insidious, and memory impairment is its most
frequent feature. Deficits in other cognitive domains such as executive
and visuospatial tend to occur relatively early, while language deficits
and behavioural symptoms often manifest later in the course of the
disease.29 30
Vascular dementia is primarily caused by
cerebrovascular disease and/or impaired cerebral blood flow. There are two
main syndromes of VaD: post-stroke dementia and VaD without recent stroke.
Patients with post-stroke dementia experience a
stepwise cognitive decline that may be accompanied by other cortical
stroke signs, including aphasia and apraxia, after a clinically diagnosed
stroke. In contrast, patients with VaD without recent stroke present with
progressive or stepwise cognitive decline with prominent impairment in
executive functioning and processing speed. Brain imaging reveals silent
cerebrovascular disease including infarction or haemorrhage and is a
required element of some of the commonly used diagnostic criteria for VaD,
including the criteria of the NINDS-AIREN (National Institute of
Neurological Disorders and Stroke).31
32
Dementia with Lewy bodies is a form of dementia
caused by abnormal protein structures called Lewy bodies, which co-occur
with symptoms of Parkinsonism such as trembling, stiffness, and slowness.
Other classical features of DLB include rapid eye movement sleep
behavioural disorder and fluctuation of cognition (both are easy to elicit
from history). This disorder often causes vivid and long-lasting visual
hallucinations. Differential diagnosis of DLB includes other degenerative
dementias, especially if complicated by superimposed delirium, medication
toxicity, or seizures. Diagnosis of DLB is made primarily by the revised
criteria for clinical diagnosis.33
Frontotemporal dementia is a heterogeneous
neurodegenerative disorder characterised by frontal and/or temporal lobe
degeneration with early-onset dementia presenting with prominent changes
in social behaviour, personality, or aphasia.34
There are several clinical presentations: behavioural variant FTD, two
forms of primary progressive aphasia (PPA), non-fluent variant PPA, and
semantic variant PPA.34 Among
these, behavioural variant FTD is the most common form, characterised by
progressive personality and behavioural changes including disinhibition,
apathy, loss of empathy, hyperorality, and compulsive behaviours. The
diagnostic criteria for FTD created by an international consortium
synthesise clinical features, neuroimaging, neuropathology, and genetic
testing.35
Behavioural and psychological symptoms of dementia
Behavioural and psychological symptoms of dementia
(BPSD) were defined as ‘symptoms of disturbed perception, thought content,
mood, and behaviour frequently occurring in patients with dementia’ by
consensus among clinicians in 1996.36
37 More than 50% of people with
dementia have BPSD, and these symptoms affect both patients and their
relatives.38 A wide variety of
affective, psychotic, and behavioural symptoms and signs can be signs of
BPSD presentation, including verbal and physical aggression, agitation,
hallucinations, delusions, sleep disturbances, oppositional behaviour, and
wandering.39 Non-pharmacological
interventions have been beneficial and should be offered as the first-line
management.40 Indoor therapeutic
gardening is an effective non-pharmacological intervention to reduce BPSD,
medicine intake, and stress levels among patients with AD.41 Antipsychotic drugs are the common treatment for
BPSD,42 but evidencebased clinical
practice guidelines recommend deprescribing the drugs when the symptoms
have been stabilised or treated for 3 months or more.43 Caregivers’ burden has been associated with patients’
BPSD44; therefore, treating BPSD
brings benefits to both patients and caregivers.
Diagnostic approach
There is no single test to confirm the diagnosis of
dementia. Initial assessment should include careful histories from both
the patient and caregivers, physical examinations, cognitive assessments,
laboratory tests, and neuroimaging.
History and physical examination
Clinical evaluation of patients with suspected
dementia should start with a thorough and detailed history, taken from
both the patient and a relative or close friend.45
It is important for primary care practitioners to rule out other causes of
memory and cognitive impairments and refer appropriate patients for
specialist assessment, especially those with unusual symptoms.46 A review is necessary of the patient’s family history
and medical and psychiatric history, including obstructive sleep apnoea,
cardiovascular disease, remote head trauma, alcohol use, and depression
treatment that may contribute to cognitive decline, as well as the use of
drugs that impair cognition (eg, analgesics, anticholinergics,
psychotropic medications, and sedative-hypnotics).12 47 It is
important to note the age of onset of cognitive impairment and to ask
about family history for patients with cognitive impairment developing at
or before age 65 years. Familial AD has been reported in Hong Kong, and
these patients may need to be referred for genetic counselling and
testing.48 49 This should be followed by a complete physical
examination, including a neurological examination, to identify any
clinical features of obstructive sleep apnoea, focal neurologic signs that
may suggest vascular aetiology, or bradykinesia, rigidity, or tremors that
would suggest a Parkinsonian syndrome.12
Cognitive testing
Cognitive tests provide objective evidence about
cognitive deficits. Nevertheless, a single test will not suffice for all
assessments. Different classes of cognitive tests are better suited to
different tasks—short questionnaires are useful for rapid screening, but
multidomain tests are more useful to support a clinical diagnosis of
dementia.50 In the primary care
setting, abbreviated or brief cognitive instruments such as abbreviated
versions of the Montreal Cognitive Assessment (MoCA) and Mini-Cog are
recommended in persons with symptoms of dementia.46
51
Mini-Mental State Examination
The Mini-Mental State Examination (MMSE) has been
the most commonly used cognitive screening instrument, although patent
protection has led to its decreased use in recent years. It has the
advantages of being brief, easy to administer, and inclusive of multiple
domains, including orientation, recall, attention, calculation, language
manipulation, and constructional praxis.52
53 Published normative data allow
interpretation of MMSE scores according to patient age and education. The
maximum score on the MMSE is 30 points. The Cantonese version of the MMSE
has been locally validated, and its cut-off points are categorised
according to the patient’s education level. For patients with more than 2
years of schooling, the cut-off score is 22.54
55 The pattern of clinical deficit
shown by the MMSE test is also important for the diagnosis of dementia.56 However, the test is not
sensitive to mild dementia, and scores may be influenced by age,
education, and language, motor and visual impairments.12 57 The MMSE
has limited ability to assess progressive cognitive decline in individual
patients over time.58
Although many ‘free’ versions of the MMSE are
available online, the official version is copyrighted.
Montreal Cognitive Assessment
The MoCA has become the more widespread initial
screening test for dementia. In early 2018, news of the President of the
United States having passed the MoCA triggered widespread interest in this
test. The MoCA is a 30-point test designed to detect cognitive impairment
in older adults. Compared with MMSE, it is more sensitive for detection of
MCI and includes items that sample a wider range of cognitive domains,
including memory, language, attention, visuospatial, and executive
functions.59 Cut-offs should be
adjusted based on education level and other appropriate norms.60 The original MoCA takes a longer time (approximately
15 minutes) to complete compared with the MMSE. Free access with
registration is available from the official website at www.mocatest.org.
The MoCA has been validated in different languages, including Chinese. The
Hong Kong–MoCA (HK-MoCA) has been shown to have comparable sensitivity to
the Cantonese version of the MMSE for detection of MCI.61 The optimal cut-off score for HK-MoCA to detect
dementia was 18/19 (sensitivity: 0.923; specificity: 0.918).61 To facilitate screening in busy clinical settings, a
few abbreviated versions of the MoCA have been validated, including the
MoCA 5-min protocol, which is feasible for telephone administration.62
Clinical Dementia Rating
Clinical Dementia Rating (CDR) was designed to
assess the severity of AD in longitudinal studies and clinical trials. In
a semi-structured interview with the patient and caregiver, impairments in
six domains (memory, orientation, judgement and problem solving, community
affairs, home and hobbies, and personal care) are assessed.63 A caregiver who knows the patient well should be
present for an accurate and valid CDR assessment. The global CDR score is
assigned based on performance in each domain. It is time-consuming, but
the test has established validity and inter-rater reliability and is
useful for following disease progression over time.64
Mini-Cog test
The Mini-Cog test consists of two components: a
three-item recall test for memory and a simply scored clock drawing test.
The assessment and instructions can be accessed at www.mocatest.org.
The results of the clock drawing test are considered normal if all numbers
are present in the correct sequence and the hands display the correct time
in a readable way.65 The
advantages of the Mini-Cog include high sensitivity for predicting
dementia status, short testing time relative to the MMSE, ease of
administration, and diagnostic value not limited by the subject’s
education or language. Nevertheless, these tests are not appropriate when
assessing patients with aphasic or anomic disorders, and more prospective
data are required for further validation of this test.66
Informant interview
The AD8 Dementia Screening Interview is a brief,
eight-item questionnaire for informants to detect dementia and cognitive
impairment. Informants are asked whether the patient has exhibited any
increase in eight deficits or behaviours. A positive response to two or
more questions had a sensitivity of 93% and a specificity of 46%.67 The AD8 is readily administered by nurses and is
useful as a screening tool by primary healthcare doctors.68 The interview questions and scoring guidelines can be
accessed from Washington University in St Louis at http://alzheimer.wustl.edu/cdr/ad8.htm.
Neuropsychological testing
Neuropsychological testing usually involves an
extensive evaluation of multiple cognitive domains (eg, attention,
orientation, executive function, verbal memory, spatial memory, language,
calculations, mental flexibility, and conceptualisation) and may be
necessary when the bedside assessment fails to differentiate between the
changes associated with normal ageing and early dementia. Moreover,
neuropsychological testing can identify patterns of deficits that suggest
a particular cause of dementia and assist in narrowing the differential
diagnosis of dementia syndrome. However, these scores can also be
influenced by education and age.18
69
Diagnosing Mrs Wong’s dementia
Mrs Wong scored 18 on the HK-MoCA assessment, which
was the cut-off score for dementia adjusted by her age and education
level.
Mrs Wong presented with a memory complaint
evidenced by the collateral history from her family members. In her daily
life, she was noted to have impaired executive functioning (inability to
handle banking) and topographic disorientation (getting lost while
shopping alone). Her HK-MoCA score also confirms the presence of memory
impairment. Moreover, her impairment has reached the point of interfering
with her social functioning. Therefore, Mrs Wong meets the clinical
definition of dementia.
Neuroimaging
Although the literature regarding indications for
neuroimaging in evaluating dementia remains inconclusive, most dementia
specialists suggest that a structural brain image be obtained for newly
diagnosed patients to assess any cerebrovascular lesions, neoplasms,
subdural haematomas, or hydrocephalus. Imaging analysis techniques that
quantify the volume of brain structures or lesions may be useful in the
future for diagnosing AD. Fluorodeoxyglucose positron emission tomography
(FDG-PET) and other functional neuroimaging techniques are usually used by
specialists to assess complex or unclear cases of neurodegenerative
dementia.18 70 Occasionally, PET imaging with different tracers for
biomarkers (eg, amyloid tracers with 18F-florbetapir) in
dementia may be indicated for the differential diagnosis of dementing
disorders, especially in the presence of overlapping clinical features.71 Scanning with 18F-florbetapir
is not available in Hong Kong, but imaging with Pittsburgh Compound B and
18F-flutametamol are available in Hong Kong. A local study has
shown that 18FDG-PET with or without 11C-PIB brain
imaging improved the accuracy of diagnosis of dementia subtype in 36% of a
case series of Chinese dementia patients.72
Volumetric analysis of magnetic resonance imaging
(MRI) may identify patterns of regional atrophy that are more specific in
certain subtypes of dementia. In some studies, volumetric MRI has shown
that patients with AD had a greater degree of hippocampal atrophy than
patients with DLB had, and patients with DLB had more pronounced cortical
atrophy than patients with Parkinson disease dementia.73 74
Although basic structural brain imaging (computed
tomography or MRI) can be obtained in the primary care setting, highly
specialised imaging is recommended for specialist settings only, as most
studies using specialised modalities have focused on subtyping rather than
the existence versus absence of dementia.46
Laboratory tests
Laboratory tests should be performed to identify
infectious, metabolic, toxic, and inflammatory disorders that can cause
neuropsychological impairment. The American Academy of Neurology
recommends screening for vitamin B12 deficiency and hypothyroidism in
patients with dementia. Screening for neurosyphilis is not recommended
unless there is a high clinical suspicion. Because of the potential for
both false positives and false negatives, genetic testing is not currently
recommended unless a specific characteristic family history is present.
Lumbar puncture, electroencephalography, and/or serologic tests may be
useful in patients with dementia who are aged <55 years or in those
with rapid progression, unusual dementia, or immunosuppression.12 18 75
Cerebrospinal fluid biomarkers (total-tau and p-tau)
Cerebrospinal fluid biomarkers (including Aβ42
protein, total tau, and phospho-tau) are widely investigated biomarkers of
AD and can be supportive of a diagnosis of AD but are not yet recommended
for routine diagnostic purposes.30
76 None of these tests are valid
as a standalone diagnostic test. These cerebrospinal fluid biomarkers can
be measured only in private laboratory settings on a self-paid basis. Such
tests are not well accepted in Hong Kong because of the
invasiveness of collecting cerebrospinal fluid.
Determining the underlying cause(s) of Mrs Wong’s
dementia
Delirium or depression, which may mimic symptoms of
dementia, should first be excluded. Medication history is checked to rule
out any drugs with potential anticholinergic properties, which may also
cause cognitive impairment.
With vascular risk factors (a history of
hypertension and diabetes mellitus), there is a potential contributing
cerebrovascular component to Mrs Wong’s dementia. Mrs Wong did not have a
history of clinical stroke and her dementia is of a slow progressive
course, not one of the stroke-like episodes of stepwise decline, as might
be the case in VaD.
On physical examination, Mrs Wong was not found to
have any Parkinsonism features or abnormal neurological signs that would
be suggestive of dementia related to Parkinsonism. There were no features
suggestive of DLB. Screening for other reversible causes of dementia,
including thyroid disorder and vitamin B12 deficiency, were negative. Mrs
Wong underwent a computed tomography scan of the brain, which showed mild
cerebral atrophy and some periventricular ischaemia. On the basis of these
findings, Mrs Wong met the clinical diagnostic criteria for AD.
Role of primary healthcare professionals
The World Alzheimer Report 2016 indicated that
with the rising prevalence of dementia, the usual specialist-led approach
may not expand rapidly enough to keep up with the increased need for care.
Thus, primary and community care should have a more prominent role in
improving the coverage of diagnosis and continuing dementia care.77
Screening for dementia among people at risk in
primary care practices was found to significantly promote the recognition
of dementia, but because of the risk of receiving a false-positive
diagnosis, additional diagnostic assessment should be mandatory.78 Other perspectives exist on systematic consideration
of the respective roles of primary and specialty care in long-range
dementia care.79 Following recent
research on the causes of and treatments for cognitive impairment, changes
in clinical practice have occurred, and there is increased awareness of
cognitive impairment detection during routine health check-ups in the
primary care setting. The objectives of primary care physicians (PCPs) are
to identify AD and other types of dementia, arrive at early diagnoses, and
prevent and treat AD complications such as falls and malnutrition. It is
also important for PCPs to develop close interactions with specialists,
including geriatricians, psychogeriatricians, and neurologists.80 For complex case diagnosis and management, a prompt
referral to a specialist is required.
In one study, the collaborative care delivered by
an interdisciplinary team resulted in a significant improvement in both
the quality of care and BPSD among both primary care patients and their
caregivers.81 Structured
partnerships among primary healthcare professionals may serve as bridges
between primary care, specialty care, and community-based services.82 To facilitate such management, it is advised that
PCPs include a relevant history in the referral letter to facilitate
subtyping of the patient’s dementia (online supplementary Appendix).
Because of primary care providers’ growing role,
there is a need for better professional education and training in both
diagnosis and management of dementia. In the United Kingdom, general
practitioners work one session per week in their local Memory Clinics,
where they can learn directly from experts and establish working
relationships with secondary dementia caregivers. It is highly recommended
that we adopt the same educational programme in Hong Kong. In addition,
primary doctors can refer to the Hong Kong Reference Framework for
Preventive Care for Older Adults in Primary Care Settings–Module for
Cognitive Impairment (2017) written by the Primary Care Office, Department
of Health.83
Conclusion
It is important that dementia is recognised at the
earliest stage and that timely evaluations are carried out to initiate
appropriate therapy, with patients able to participate in management
decisions. The initial step in evaluation of a patient with suspected
dementia should be the taking of a focused history of cognitive and
behavioural changes, followed by a complete physical examination.
Delirium, depression, and MCI should be considered in the differential
diagnosis of memory impairment. Many useful screening tools are now
available for dementia. Once a diagnosis of dementia has been made,
neuroimaging (eg, brain computed tomography or MRI scan) and laboratory
tests should be performed to rule out any reversible causes of dementia.
Advanced imaging, such as FDG-PET imaging or amyloid PET scanning with
special tracers, may be indicated for specific cases to reach a definitive
diagnosis. In the future, primary care will play a more prominent role in
dementia management. Close interactions between PCPs and specialists are
needed for complex case management, especially those requiring
pharmacological management.
Author contributions
All authors have made substantial contributions to
the concept or design of the study, acquisition of data, analysis or
interpretation of data, drafting of the article, and critical revision for
important intellectual content. All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Acknowledgement
The authors would like to thank Mr David CH Lau,
who offered professional advice on cognitive tests.
Conflicts of interest
As an editor of the journal, JKH Luk was not
involved in the peer review process of the article. Other authors have
declared no conflicts of interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
References
1. Qiu C, Kivipelto M, von Strauss E.
Epidemiology of Alzheimer’s disease: occurrence, determinants, and
strategies toward intervention. Dialogues Clin Neurosci 2009;11:111-28.
2. Catindig JA, Venketasubramanian N, Ikram
MK, Chen C. Epidemiology of dementia in Asia: insights on prevalence,
trends and novel risk factors. J Neurol Sci 2012;321:11-6. Crossref
3. Prince M, Wimo A, Guerchet M, et al.
World Alzheimer Report 2015—The Global Impact of Dementia. London:
Alzheimer’s Disease International; 2015.
4. Chan KY, Wang W, Wu JJ, et al.
Epidemiology of Alzheimer’s disease and other forms of dementia in China,
1990-2010: a systematic review and analysis. Lancet 2013;381:2016-23. Crossref
5. Prince M, Bryce R, Ferri C. World
Alzheimer report 2011: The benefits of early diagnosis and intervention.
London: Alzheimer’s Disease International; 2011.
6. Boustani M, Callahan CM, Unverzagt FW,
et al. Implementing a screening and diagnosis program for dementia in
primary care. J Gen Intern Med 2005;20:572-7. Crossref
7. Ross GW, Abbott RD, Petrovitch H, et al.
Frequency and characteristics of silent dementia among elderly
Japanese-American men. The Honolulu-Asia Aging Study. JAMA 1997;277:800-5.
Crossref
8. Fahy M, Wald C, Walker Z, Livingston G.
Secrets and lies: the dilemma of disclosing the diagnosis to an adult with
dementia. Age Ageing 2003;32:439-41. Crossref
9. Tripathi M, Vibha D. Reversible
dementias. Indian J Psychiatry 2009;51 Suppl 1:S52-5.
10. American Psychiatric Association.
Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed.
Arlington, VA: Arlington; 2013. Crossref
11. Sachdev PS, Blacker D, Blazer DG, et
al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev
Neurol 2014;10:634-42. Crossref
12. Ross GW, Bowen JD. The diagnosis and
differential diagnosis of dementia. Med Clin North Am 2002;86:455-76. Crossref
13. Howieson DB, Holm LA, Kaye JA, Oken
BS, Howieson J. Neurologic function in the optimally healthy oldest old.
Neuropsychological evaluation. Neurology 1993;43:1882-6. Crossref
14. Small SA, Stern Y, Tang M, Mayeux R.
Selective decline in memory function among healthy elderly. Neurology
1999;52:1392-6. Crossref
15. Crook T, Bartus RT, Ferris SH,
Whitehouse P, Cohen GD, Gershon S. Age-associated memory impairment:
proposed diagnostic criteria and measures of clinical change—report of a
national institute of mental health work group. Dev Neuropsychol
1986;2:261-76. Crossref
16. Levy R. Aging-associated cognitive
decline. Working Party of the International Psychogeriatric Association in
collaboration with the World Health Organization. Int Psychogeriatr
1994;6:63-8. Crossref
17. Petersen RC, Smith GE, Waring SC,
Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical
characterization and outcome. Arch Neurol 1999;56:303-8. Crossref
18. Petersen RC, Stevens JC, Ganguli M,
Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection
of dementia: mild cognitive impairment (an evidence-based review). Report
of the Quality Standards Subcommittee of the American Academy of
Neurology. Neurology 2001;56:1133-42. Crossref
19. Pandya SY, Clem MA, Silva LM, Woon FL.
Does mild cognitive impairment always lead to dementia? A review. J Neurol
Sci 2016;369:57-62. Crossref
20. Lopez OL, Becker JT, Chang YF, et al.
Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular
Health Study–Cognition Study. Neurology 2012;79:1599-606. Crossref
21. Yesavage JA, Brink TL, Rose TL, et al.
Development and validation of a geriatric depression screening scale: a
preliminary report. J Psychiatr Res 1982-1983;17:37-49. Crossref
22. Jorm AF, Fratiglioni L, Winblad B.
Differential diagnosis in dementia. Principal components analysis of
clinical data from a population survey. Arch Neurol 1993;50:72-7. Crossref
23. Andrew MK, Freter SH, Rockwood K.
Prevalence and outcomes of delirium in community and non-acute care
settings in people without dementia: a report from the Canadian Study of
Health and Aging. BMC Med 2006;4:15. Crossref
24. Levkoff S, Cleary P, Liptzin B, Evans
DA. Epidemiology of delirium: an overview of research issues and findings.
Int Psychogeriatr 1991;3:149-67. Crossref
25. Vasilevskis EE, Han JH, Hughes CG,
Wesley Ely E. Epidemiology and risk factors for delirium across hospital
settings. Best Pact Res Clin Anaesthesiol 2012;26:277-87. Crossref
26. Leung JL, Leung VC, Leung CM, Pan PC.
Clinical utility and validation of two instruments (the Confusion
Assessment Method Algorithm and the Chinese version of Nursing Delirium
Screening Scale) to detect delirium in geriatric inpatients. Gen Hosp
Psychiatry 2008;30:171-6. Crossref
27. Rizzi L, Rosset I, Roriz-Cruz M.
Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed
Res Int 2014;2014:908915. Crossref
28. Vann Jones SA, O’Brien JT. The
prevalence and incidence of dementia with Lewy bodies: a systematic review
of population and clinical studies. Psychol Med 2014;44:673- 83. Crossref
29. Ballard C, Gauthier S, Corbett A,
Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet
2011;377:1019-31. Crossref
30. McKhann GM, Knopman DS, Chertkow H, et
al. The diagnosis of dementia due to Alzheimer’s disease: recommendations
from the National Institute on Aging-Alzheimer’s Association workgroups on
diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement
2011;7:263-9.Crossref
31. Hachinski V, Iadecola C, Petersen RC,
et al. National Institute of Neurological Disorders and Stroke–Canadian
Stroke Network vascular cognitive impairment harmonization standards.
Stroke 2006;37:2220-41. Crossref
32. Román GC, Tatemichi TK, Erkinjuntti T,
et al. Vascular dementia: diagnostic criteria for research studies. Report
of the NINDS-AIREN International Workshop. Neurology 1993;43:250-60. Crossref
33. McKeith IG, Boeve BF, Dickson DW, et
al. Diagnosis and management of dementia with Lewy bodies: fourth
consensus report of the DLB Consortium. Neurology 2017;89:88-100. Crossref
34. Olney NT, Spina S, Miller BL.
Frontotemporal dementia. Neurol Clin 2017;35:339-74. Crossref
35. Rascovsky K, Hodges JR, Knopman D, et
al. Sensitivity of revised diagnostic criteria for the behavioural variant
of frontotemporal dementia. Brain 2011;134(Pt 9):2456-77.
36. Finkel SI, Silva JC, Cohen GD, Miller
S, Sartorius N. Behavioral and psychological symptoms of dementia: a
consensus statement on current knowledge and implications for research and
treatment. Am J Geriatr Psychiatry 1998;6:97-100. Crossref
37. International Psychogeriatric
Association. The IPA complete guides to behavioral and psychological
symptoms of dementia. Milwaukee, WI; 2010.
38. Hersch EC, Falzgraf S. Management of
the behavioral and psychological symptoms of dementia. Clin Interv Aging
2007;2:611-21. Crossref
39. Tible OP, Riese F, Savaskan E, von
Gunten A. Best practice in the management of behavioural and psychological
symptoms of dementia. Ther Adv Neurol Disord 2017;10:297-309. Crossref
40. Bessey LJ, Walaszek A. Management of
behavioral and psychological symptoms of dementia. Curr Psychiatry Rep
2019;21:66. Crossref
41. Pedrinolla A, Tamburin S, Brasioli A,
et al. An indoor therapeutic garden for behavioral symptoms in Alzheimer’s
disease: a randomized controlled trial. J Alzheimers Dis 2019;71:813-23. Crossref
42. Luk JH. Pharmacological management of
behavioural and psychological symptoms of dementia. Asian J Gerontol
Geriatr 2017;12:65-8.
43. Bjerre LM, Farrell B, Hogel M, et al.
Deprescribing antipsychotics for behavioural and psychological symptoms of
dementia and insomnia: evidence-based clinical practice guideline. Can Fam
Physician 2018;64:17-27.
44. Huang SS, Lee MC, Liao YC, Wang WF,
Lai TJ. Caregiver burden associated with behavioral and psychological
symptoms of dementia (BPSD) in Taiwanese elderly. Arch Gerontol Geriatr
2012;55:55-9. Crossref
45. Knopman DS, Boeve BF, Petersen RC.
Essentials of the proper diagnoses of mild cognitive impairment, dementia,
and major subtypes of dementia. Mayo Clin Proc 2003;78:1290-308. Crossref
46. Pink J, O’Brien J, Robinson L, Longson
D; Guideline Committee. Dementia: assessment, management and support:
summary of updated NICE guidance. BMJ 2018;361:k2438. Crossref
47. Campbell NL, Boustani MA, Lane KA, et
al. Use of anticholinergics and the risk of cognitive impairment in an
African American population. Neurology 2010;75:152-9. Crossref
48. Shea YF, Chu LW, Chan AO, Ha J, Li Y,
Song YQ. A systematic review of familial Alzheimer’s disease: differences
in presentation of clinical features among three mutated genes and
potential ethnic differences. J Formos Med Assoc 2016;115:67-75. Crossref
49. Shea YF, Chu LW, Lee SC, Chan AO. The
first case series of Chinese patients in Hong Kong with familial
Alzheimer’s disease compared with those with biomarker-confirmed sporadic
late-onset Alzheimer’s disease. Hong Kong Med J 2017;23:579-85. Crossref
50. Brown J. The use and misuse of short
cognitive tests in the diagnosis of dementia. J Neurol Neurosurg
Psychiatry 2015;86:680-5. Crossref
51. Creavin S, Wisniewski S, Noel-Storr A,
Cullum S; MMSE review team. Cognitive tests to help diagnose dementia in
symptomatic people in primary care and the community. Br J Gen Pract
2018;68:149-50. Crossref
52. Folstein MF, Folstein SE, McHugh PR.
“Mini-mental state”. A practical method for grading the cognitive state of
patients for the clinician. J Psychiatr Res 1975;12:189-98. Crossref
53. Tangalos EG, Smith GE, Ivnik RJ, et
al. The Mini-Mental State Examination in general medical practice:
clinical utility and acceptance. Mayo Clin Proc 1996;71:829-37. Crossref
54. Chiu HF, Lee HC, Chung WS, Kwong PK.
Reliability and validity of the Cantonese version of Mini-Mental State
Examination—a preliminary study. JHKC Psych 1994;4:25-8.
55. Chiu HF, Lam C, Chi I, et al.
Prevalence of dementia in Chinese elderly in Hong Kong. Neurology
1998;50:1002-9. Crossref
56. Anthony JC, LeResche L, Niaz U, von
Korff MR, Folstein MF. Limits of the ‘Mini-Mental State’ as a screening
test for dementia and delirium among hospital patients. Psychol Med
1982;12:397-408. Crossref
57. Crum RM, Anthony JC, Bassett SS,
Folstein MF. Population-based norms for the Mini-Mental State Examination
by age and educational level. JAMA 1993;269:2386-91. Crossref
58. Creavin ST, Wisniewski S, Noel-Storr
AH, et al. Mini-Mental State Examination (MMSE) for the detection of
dementia in clinically unevaluated people aged 65 and over in community
and primary care populations. Cochrane Database Syst Rev
2016;(1):CD011145.
59. Nasreddine ZS, Phillips NA, Bédirian
V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool
for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. Crossref
60. Rossetti HC, Lacritz LH, Cullum CM,
Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in
a population-based sample. Neurology 2011;77:1272-5. Crossref
61. Yeung PY, Wong LL, Chan CC, Leung JL,
Yung CY. A validation study of the Hong Kong version of Montreal Cognitive
Assessment (HK-MoCA) in Chinese older adults in Hong Kong. Hong Kong Med J
2014;20:504-10. Crossref
62. Wong A, Nyenhuis D, Black SE, et al.
Montreal Cognitive Assessment 5-minute protocol is a brief, valid,
reliable, and feasible cognitive screen for telephone administration.
Stroke 2015;46:1059-64. Crossref
63. Washington University in St Louis.
CDR® Dementia Staging Instrument. Available from: https://knightadrc.
wustl.edu/cdr/cdr.htm. Accessed 30 Aug 2019.
64. Morris JC. The Clinical Dementia
Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-4.
Crossref
65. Mini-Cog©–Screening for cognitive
impairment in older adults. Available from: https://mini-cog.com/.
Accessed 30 Aug 2019.
66. Borson S, Scanlan J, Brush M,
Vitaliano P, Dokmak A. The Mini-Cog: a cognitive ‘vital signs’ measure for
dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry
2000;15:1021-7. Crossref
67. Galvin JE, Roe CM, Xiong C, Morris JC.
Validity and reliability of the AD8 informant interview in dementia.
Neurology 2006;67:1942-8. Crossref
68. Shaik MA, Khoo CH, Thiagarajah AG, et
al. Pilot evaluation of a dementia case finding clinical service using the
informant AD8 for at-risk older adults in primary health care: a brief
report. J Am Med Dir Assoc 2016;17:673.e5-8. Crossref
69. Cahn DA, Salmon DP, Butters N, et al.
Detection of dementia of the Alzheimer type in a population-based sample:
neuropsychological test performance. J Int Neuropsychol Soc 1995;1:252-60.
Crossref
70. Corey-Bloom J, Thal LJ, Galasko D, et
al. Diagnosis and evaluation of dementia. Neurology 1995;45:211-8. Crossref
71. Berti V, Pupi A, Mosconi L. PET/CT in
diagnosis of dementia. Ann N Y Acad Sci 2011;12281:81-92. Crossref
72. Shea YF, Ha J, Lee SC, Chu LW. Impact
of 18FDG PET and 11C-PIB PET brain imaging on the diagnosis of Alzheimer’s
disease and other dementias in a regional memory clinic in Hong Kong. Hong
Kong Med J 2016;22:327-33. Crossref
73. Barber R, Ballard C, McKeith IG,
Gholkar A, O’Brien JT. MRI volumetric study of dementia with Lewy bodies;
a comparison with AD and vascular dementia. Neurology 2000;54:1304-9. Crossref
74. Beyer MK, Larsen JP, Aarsland D. Gray
matter atrophy in Parkinson disease with dementia and dementia with Lewy
bodies. Neurology 2007;69:747-54. Crossref
75. Geschwind MD, Shu H, Haman A, Sejvar
JJ, Miller BL. Rapidly progressive dementia. Ann Neurol 2008;64:97-108. Crossref
76. Shea YF, Chu LW, Zhou L, et al.
Cerebrospinal fluid biomarkers of Alzheimer’s disease in Chinese patients:
a pilot study. Am J Alzheimers Dis Other Demen 2013;28:769-75. Crossref
77. Prince M, Comas-Herrera A, Knapp M,
Guerchet M, Karagiannidou M. World Alzheimer Report 2016—improving
healthcare for people living with dementia: coverage, quality and costs
now and in the future. London: Alzheimer’s Disease International; 2016.
78. Eichler T, Thyrian JR, Hertel J, et
al. Rates of formal diagnosis of dementia in primary care: the effect of
screening. Alzheimers Dement (Amst) 2015;1:87-93. Crossref
79. Borson S, Frank L, Bayley PJ, et al.
Improving dementia care: the role of screening and detection of cognitive
impairment. Alzheimers Dement 2013;9:151-9. Crossref
80. Cohen CA, Pringle D, LeDuc L. Dementia
caregiving: the role of the primary care physician. Can J Neurol Sci
2001;28 Suppl 1:S72-6. Crossref
81. Callahan CM, Boustani MA, Unverzagt
FW, et al. Effectiveness of collaborative care for older adults with
Alzheimer disease in primary care: a randomized controlled trial. JAMA
2006;295:2148-57. Crossref
82. Reuben DB, Roth CP, Frank JC, et al.
Assessing care of vulnerable elders—Alzheimer’s disease: a pilot study of
a practice redesign intervention to improve the quality of dementia care.
J Am Geriatr Soc 2010;58:324-9. Crossref
83. Hong Kong Reference Framework for
Preventive Care for Older Adults in Primary Care Settings. Module on
Cognitive Impairment. Department of Health, Hong Kong SAR Government;
2017.