Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3289
Peer-review started: April 8, 2019
First decision: August 1, 2019
Revised: August 22, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 26, 2019
We describe the treatment strategy for a patient who was found to have a partial hydatidiform mole and coexisting fetus (PHMCF) during the second trimester. The patient was a 38-year-old Chinese woman who had become pregnant following in vitro fertilization and embryo transplantation. We wanted to determine the safest therapeutic strategy to terminate the PHMCF during the second trimester.
In this case, we present a patient who was found to have a PHMCF complicated with serious continuous vaginal bleeding and pre-eclampsia during the second trimester. After careful evaluation, the pregnancy was considered to be unsustainable and was terminated via caesarean section (CS). An infant with weak vital signs and a partially cystic placenta measuring 110 mm × 95 mm × 35 mm were delivered by CS. The patient was discharged after 4 d. The serum levels of β-human chorionic gonadotropin decreased to within a normal range 5 wk after the operation, and no evidence of persistent trophoblastic disease or lung metastases was noticed at the 6-mo follow-up.
CS termination of PHMCF during the second trimester may be a relatively safe therapeutic strategy.
Core tip: A partial hydatidiform mole and coexisting fetus (PHMCF), a rare phenomenon in the past, is showing an upward trend in frequency due to the increasing use of assisted reproductive technologies. Our main objective was to find the safest therapeutic strategy to terminate a PHMCF during the second trimester. We determined that termination of PHMCF during the second trimester via caesarean section is a relatively safe therapeutic strategy.
- Citation: Zhang RQ, Zhang JR, Li SD. Termination of a partial hydatidiform mole and coexisting fetus: A case report. World J Clin Cases 2019; 7(20): 3289-3295
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3289.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3289
A partial hydatidiform mole and coexisting fetus (PHMCF), a rare phenomenon of the past, is showing an upward trend in frequency due to the increasing use of assisted reproductive technologies (ART). The reported incidence of complete hydatidiform mole and coexisting fetus, which is essentially a twin pregnancy, is 1 in 22000-100000 pregnancies[1]. However, a PHMCF is a single pregnancy and the reported prevalence is 0.005%-0.01% of all pregnancies[2]. The management of such pregnancies creates a dilemma for both the physician and the parents, particularly when the PHMCF occurs during the second trimester of pregnancy. Empirically, the pregnancy is allowed to proceed provided that severe maternal complications are controlled, and the fetal karyotype and development are normal[3]. A small number of cases of PHMCF have been reported with certain couples choosing to continue the pregnancy until 28 wk of gestation and delivery was achieved via caesarean section (CS) resulting in a number of healthy babies[4,5]. Unfortunately, in most patients, serious complications can occur, posing significant challenges and difficult choices for both the obstetrician and the patients. For these patients, the main problem is to determine whether the pregnancy is sustainable, and if not, the best way to terminate the pregnancy. Herein, we report the case of a PHMCF patient with severe complications during the second trimester, whose pregnancy was considered unsustainable after careful evaluation and eventually terminated by CS. The timeline is shown in Figure 1.
A 38-year-old Chinese woman was found to have blood pressure instability at 21 wk of gestation and was admitted to our hospital for pre-eclampsia.
The patient became pregnant following in vitro fertilization and embryo transplantation (IVF-ET). Three embryos were transferred into the uterus and only one embryo survived. In early pregnancy, the patient had recurrent episodes of fresh vaginal bleeding. On admission, the couple was informed of the high probability for a poor obstetrical outcome with complications; however, they wished to continue the pregnancy.
The patient (gravida 2, para 0) denied having had hypertension, diabetes and coronary heart disease. The patient denied having had hepatitis and tuberculosis. Moreover, the patient denied a history of food and drug allergy.
The patient denied a history of drug abuse, smoking, and drinking. The patient denied a family history of genetic disease and cancer.
The patient’s blood pressure at admission was 160/87 mmHg, and heart rate was 115 bpm. Her temperature and pulse were normal. The fetal heart rate was 140 bpm.
At 13 wk and 3 d, the measurement of serum β-human chorionic gonadotropin (β-HCG) rose to 271600 IU/L, and the result of the Down’s syndrome screening showed a low-risk status. At 21 wk and 4 d, her serum β-HCG level rose to 510427 IU/L, which was abnormally high for the corresponding gestational age.
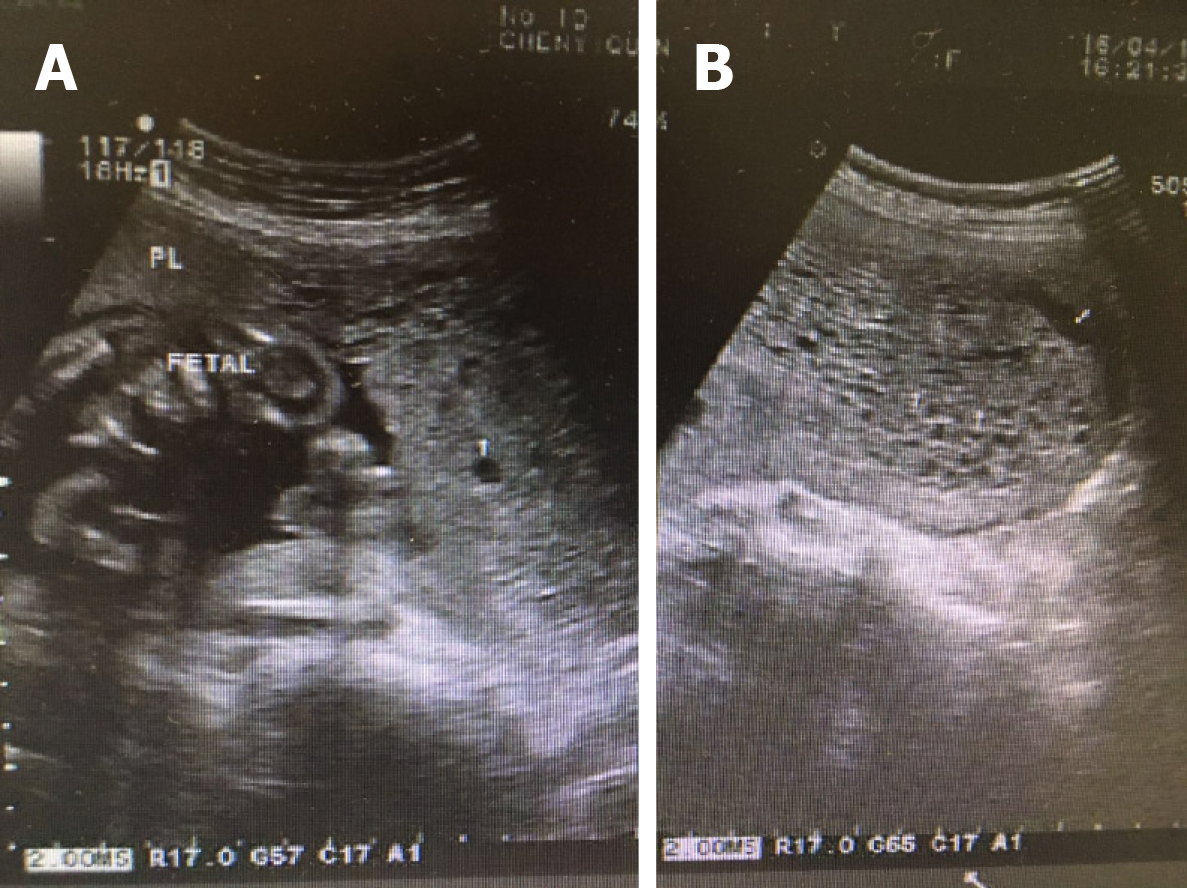
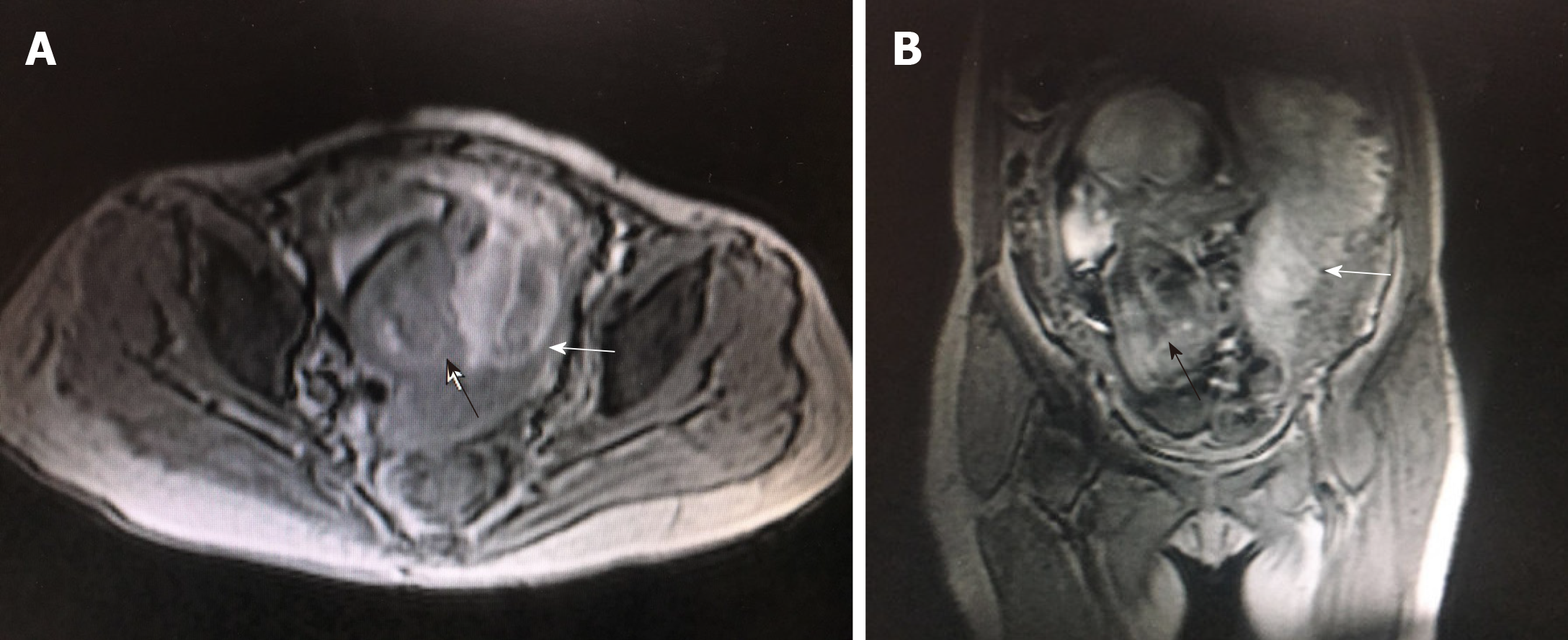
An ultrasound sonographic examination at 55 d of gestational age revealed a single live intrauterine fetus of 7 wk and 4 d, and a blood clot beside it. At 10 wk and 3 d, an ultrasound examination revealed a single live intrauterine fetus of 10 wk and 3 d with a suspected partial hydatidiform mole. However, at 13 wk and 3 d of gestational age, vaginal bleeding disappeared and an ultrasound examination merely revealed a single live intrauterine fetus. At 21 wk and 3 d, a transvaginal sonographic examination revealed a single live intrauterine fetus of 21 wk and 4 d. The left part of the placenta was composed of multiple cystic areas, measuring 126 mm × 42 mm × 114 mm (Figure 2). Two days later, enhanced magnetic resonance imaging revealed a single live intrauterine fetus and abnormal intensified signals at the left part of the placenta with no clear demarcations between the myometrium and placental tissue (Figure 3). The chest computed tomography result was normal.
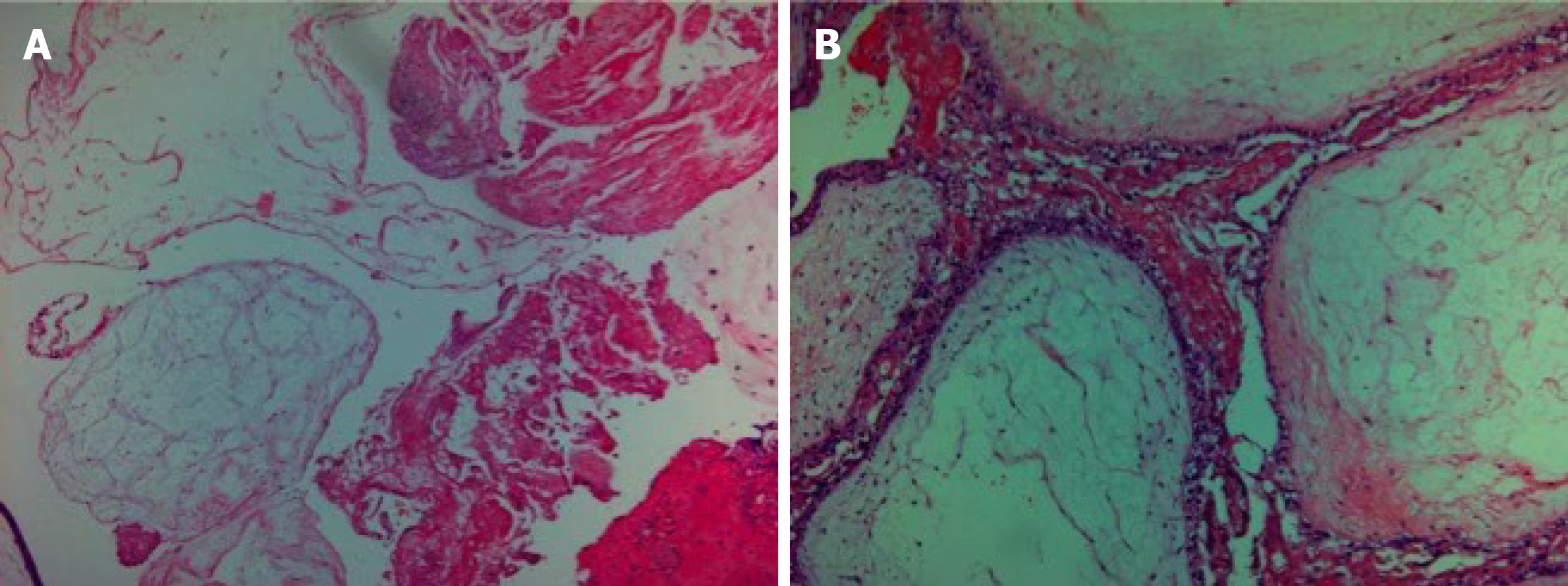
The histopathology was consistent with molar changes (Figure 4).
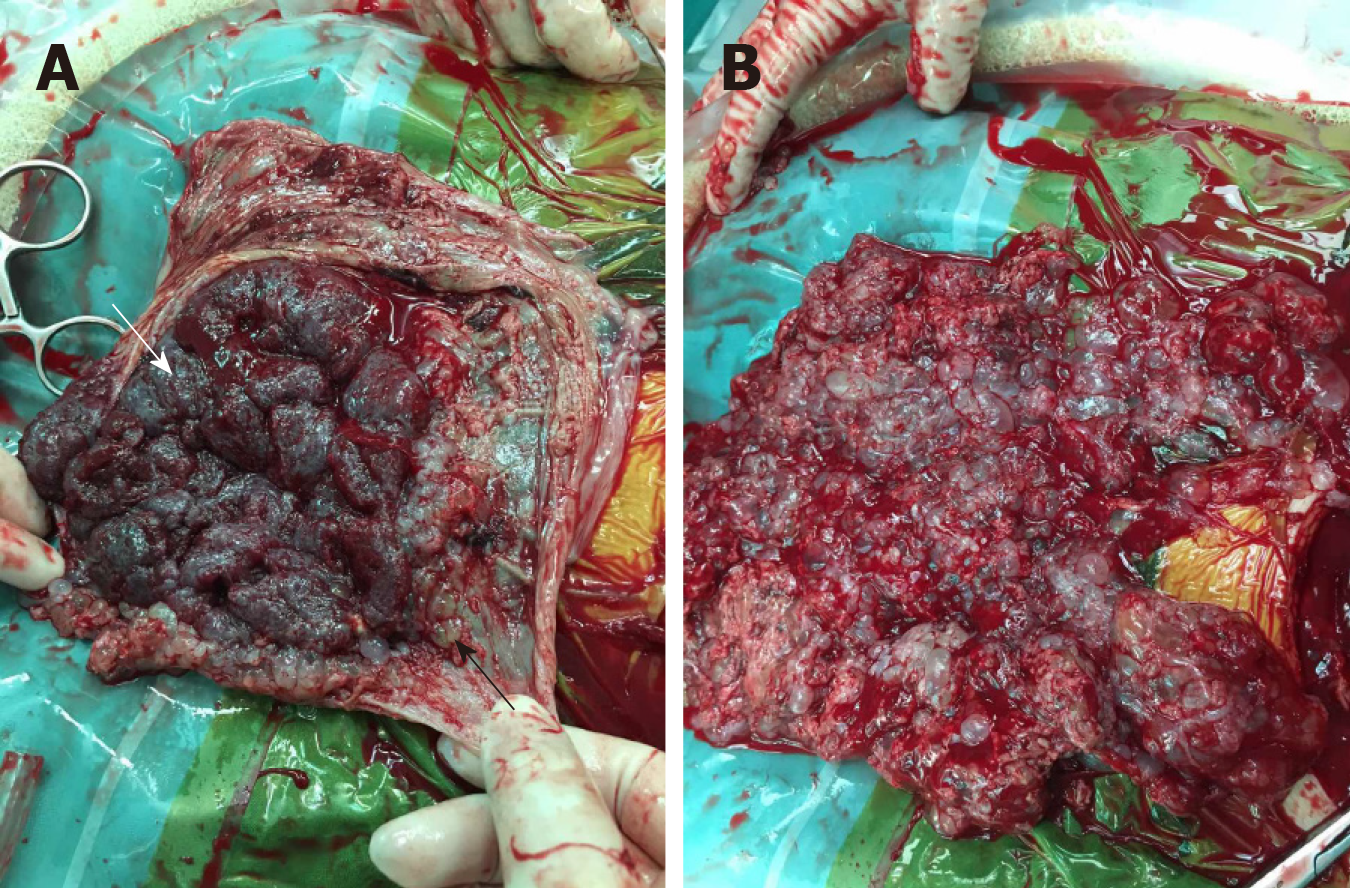
A detailed counseling session was provided to discuss the risks of maternal complications, and the couple decided to terminate the pregnancy following careful consideration. CS was chosen because of the heavy vaginal bleeding and the high probability that a medical induction of labor may lead to an incomplete evacuation of the uterus. An infant with weak vital signs and a partially cystic placenta measuring 110 mm × 95 mm × 35 mm were delivered by CS. Thereafter, vesicular tissue, measuring 180 mm × 160 mm × 45 mm, was extracted and the uterus was completely evacuated (Figure 5).
The serum β-HCG levels of the patient decreased to 5258 IU/L after a week. The patient was discharged and her weekly follow-up of serum β-HCG levels showed an obvious downward trend. After 5 wk, her serum β-HCG levels decreased to the normal range and no evidence of persistent trophoblastic disease (PTD) or lung metastases was noticed at the 6-mo follow-up.
A PHMCF is always associated with complications such as fetal death, vaginal bleeding, early onset pre-eclampsia, hyperthyroidism, and a risk of gestational trophoblastic neoplasia. Consequently, an early diagnosis of PHMCF has been recommended in order to treat this disease. The diagnosis of PHMCF during the first trimester of pregnancy is mainly revealed by ultrasonographic examinations. Unfortunately, a misdiagnosis is not uncommon on the initial ultrasonography due to the reassuring presence of a fetal heartbeat and the misinterpretation of abnormal placental echoes such as those associated with hematomas[2]. Moreover, partial molar pregnancies often present minor cystic changes of the placenta[6], which makes PHMCFs even more difficult to diagnosis. In our case, the ultrasound examination at 55 d revealed a single live intrauterine fetus with a blood clot beside it. At 13 wk of pregnancy, it merely revealed a single live intrauterine fetus. Magnetic resonance imaging can be a useful adjunct to ultrasound for visualizing the normal placental site, the relationship between the fetus and vesicles, and the extension of the disease to the myometrium and parametrium. However, its diagnostic effect in early pregnancy remains unclear[7]. Although several PHMCF cases have been successfully diagnosed during the first trimester[8-11], the overall diagnosis rate is still low. Further investigations are required to find more ideal methods to diagnose PHMCF in the first trimester.
When we failed to diagnose PHMCF early and the pregnancy progressed to the second trimester, we considered two factors to determine whether the pregnancy was sustainable. First, severe maternal complications were controlled, and second, the fetal karyotype and development were normal, as stated above. However, even if the pregnancy is allowed to proceed, the overall live birth rate is very low, as listed in Table 1. In addition, a partial molar pregnancy with an embryo of a normal karyotype during the second trimester, may end up as a fetal demise in the third trimester which may be due to placental mosaicism or placental mesenchymal dysplasia[12,13]. In our case, we could not acquire the fetal karyotype information because the patient refused to allow an amniocentesis since the fetus appeared normal on sonographic examination. Moreover, the onset of severe vaginal bleeding at 22 wk rendered the pregnancy unsustainable.
| Time of pregnancy termination | β-HCG before termination (IU / L) | Methods for pregnancy termination | Live fetus | Persistent trophoblastic disease | Metastasis | IVF-ET | Ref. |
| 17 wk | 449078 | Rivanol and aspiration curettage | No | Yes | Lung metastasis | No | [18] |
| 16 wk | 800842 | Rivanol | No | Yes | Lung metastasis | Yes | [15] |
| 14 wk | 229000 | Misoprostol | No | Yes | Lung metastasis | Yes | [19] |
| 15 wk | 270000 | Suction curettage | No | Yes | No | No | [8] |
| 20 wk | 878000 | Suction curettage | No | No | No | No | [8] |
| 27 wk | Unknown | Spontaneous abortion | No | Yes | Lung metastasis | No | [22] |
| 21 wk | 1100000 | Spontaneous abortion | No | Yes | No | No | [23] |
| 23 wk | 133100 | Spontaneous abortion | No | No | No | No | [24] |
| 15 wk | 600000 | Hysterectomy | No | Yes | No | No | [16] |
| 27 wk | Unknown | Cesarean section | No | No | No | No | [20] |
| 23 wk | 76642 | Cesarean section | No | No | No | No | [21] |
| 23 wk | 510427 | Cesarean section | No | No | No | Yes | Present |
Several methods have been reported to terminate PHMCFs during the second trimester. Suction curettage is a safe way to terminate hydatidiform moles[14]. However, a mid-term pregnancy with a relatively large fetus precludes the use of suction curettage. Medical termination (using rivanol or misoprostol) is not recommended for molar evacuations since these methods increase maternal complications, including blood loss, incomplete evacuation[15], and the development of PTD due to increased intrauterine pressure[16]. Dilatation and evacuation have been recommended by the Evidence-based Practice Guidelines of the Royal College of Obstetricians and Gynecologists as a safe method for termination of pregnancies > 15 wk. However, it should be performed by a skilled specialist, and its use in PHMCFs is still questionable. CS has also been reported for terminating PHMCFs. So far, no PTDs have been reported to develop after this procedure. However, the relatively low survival rate of the neonate and the presence of a uterine scar make the CS procedure a difficult choice for both physicians and patients. Hysterectomy is a viable management choice for women in an emergent situation not wishing to maintain fertility[17]. Bearing in mind that PHMCF patients often are relatively young, this procedure can cause emotional distress and should be considered very carefully. Moreover, PTDs can still develop after a hysterectomy.
To the best of our knowledge, 12 PHMCF cases during the second trimester have previously been reported (Table 1). Three of these cases[15,18,19] of PHMCF were treated with medical termination; unfortunately, all of them resulted in PTDs and metastases. In contrast, two cases[20,21] (and our case) were terminated using CSs and no PTDs or metastases developed. Therefore, these results led to the hypothesis that CS termination may be a safer therapeutic strategy during the second trimester with regard to the risk of developing PTDs.
A plausible relationship between gestational trophoblastic disease (GTD) and ART has been established[15]. This may be because the clinical factors (advanced maternal age, poor oocyte quality and so on) that exist in IVF patients may also predispose them to GTDs. As PHMCF is a rare kind of GTD, it is imaginable that the PHMCF incidence increases along with the increase in the use of ART. Although no statistical analysis can be done due to the small case numbers, our literature review showed that, in addition to our case, three more cases (1/7)[4,15,19] of PHMCF developed following IVF-ET. This highlighted the possible link between IVF-ET and PHMCF.
A PHMCF during the second trimester poses a considerable challenge both for the patients and the doctors. Once the pregnancy is confirmed to be unsustainable, a suitable termination method should be carefully chosen. According to the knowledge gained from the current case and from the literature review, we can conclude that, compared to medical terminations and dilatation and evacuations, CS is a difficult choice, but may be ultimately safer with regard to the risk of developing PTDs or distant metastases.
The CS termination of PHMCFs during the second trimester may be the safest method.
We thank the Department of Shanghai General Hospital, School of Medicine, Shanghai Jiao Tong University and our patient with her family for their extreme cooperation and support.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Raja SG S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Sebire NJ, Foskett M, Paradinas FJ, Fisher RA, Francis RJ, Short D, Newlands ES, Seckl MJ. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet. 2002;359:2165-2166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Albers E, Daneshmand S, Hull A. Placental pathology casebook. Complete hydatidiform mole with coexistent term twin pregnancy. J Perinatol. 2001;21:72-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Chu W, Chapman J, Persons DL, Fan F. Twin pregnancy with partial hydatidiform mole and coexistent fetus. Arch Pathol Lab Med. 2004;128:1305-1306. [PubMed] [Cited in This Article: ] |
| 4. | Sun CJ, Zhao YP, Yu S, Fan L, Wu QQ, Li GH, Zhang WY. Twin pregnancy and partial hydatidiform mole following in vitro fertilization and embryos transfer: a novel case of placental mosaicism. Chin Med J (Engl). 2012;125:4517-4519. [PubMed] [Cited in This Article: ] |
| 5. | Copeland JW, Stanek J. Dizygotic twin pregnancy with a normal fetus and a nodular embryo associated with a partial hydatidiform mole. Pediatr Dev Pathol. 2010;13:476-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Savage JL, Maturen KE, Mowers EL, Pasque KB, Wasnik AP, Dalton VK, Bell JD. Sonographic diagnosis of partial versus complete molar pregnancy: A reappraisal. J Clin Ultrasound. 2017;45:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Herek D, Karabulut N. The role of magnetic resonance imaging in the diagnosis of complete hydatidiform mole in a twin pregnancy. Int J Gynaecol Obstet. 2013;123:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Teng NN, Ballon SC. Partial hydatidiform mole with diploid karyotype: report of three cases. Am J Obstet Gynecol. 1984;150:961-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Gupta K, Venkatesan B, Kumaresan M, Chandra T. Early Detection by Ultrasound of Partial Hydatidiform Mole With a Coexistent Live Fetus. WMJ. 2015;114:208-11; quiz 212. [PubMed] [Cited in This Article: ] |
| 10. | Ingec M, Borekci B, Altas S, Kadanali S. Twin pregnancy with partial hydatidiform mole and coexistent normal fetus. J Obstet Gynaecol. 2006;26:379-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Tay ET. Partial hydatidiform mole and coexisting viable twin pregnancy. Pediatr Emerg Care. 2013;29:1298-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Guven ES, Ozturk N, Deveci S, Hizli D, Kandemir O, Dilbaz S. Partial molar pregnancy and coexisting fetus with diploid karyotype. J Matern Fetal Neonatal Med. 2007;20:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Papoutsis D, Mesogitis S, Antonakou A, Goumalatsos N, Daskalakis G, Papantoniou N, Papaspyrou I, Zirganos N, Antsaklis A. Partial molar pregnancy with a chromosomically and phenotypically normal embryo: presentation of an extremely rare case and review of literature. J Matern Fetal Neonatal Med. 2011;24:1289-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Tidy JA, Gillespie AM, Bright N, Radstone CR, Coleman RE, Hancock BW. Gestational trophoblastic disease: a study of mode of evacuation and subsequent need for treatment with chemotherapy. Gynecol Oncol. 2000;78:309-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Zhou X, Chen Y, Li Y, Duan Z. Partial hydatidiform mole progression into invasive mole with lung metastasis following in vitro fertilization. Oncol Lett. 2012;3:659-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Deaton JL, Hoffman JS, Saal H, Allred C, Koulos JP. Molar pregnancy coexisting with a normal fetus: a case report. Gynecol Oncol. 1989;32:394-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Hurteau JA. Gestational trophoblastic disease: management of hydatidiform mole. Clin Obstet Gynecol. 2003;46:557-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Qian H, Wang J. Medical termination of a partial hydatidiform mole and coexisting fetus during the second trimester: A case report. Oncol Lett. 2015;10:3625-3628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kim CH, Kim YH, Kim JW, Kim KM, Cho MK, Kim SM, Nam JH, Song TB. Triplet pregnancy with partial hydatidiform mole coexisting with two fetuses: a case report. J Obstet Gynaecol Res. 2008;34:641-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Crooij MJ, Van der Harten JJ, Puyenbroek JI, Van Geijn HP, Arts NF. A partial hydatidiform mole, dispersed throughout the placenta, coexisting with a normal living fetus. Case report. Br J Obstet Gynaecol. 1985;92:104-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Lembet A, Zorlu CG, Yalçin HR, Seçkin B, Ekici E. Partial hydatidiform mole with diploid karyotype in a live fetus. Int J Gynaecol Obstet. 2000;69:149-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Cheung NY, Ngan HY, Ghosh A. Persistent gestational trophoblastic disease after a diploid partial hydatidiform mole coexisting with a normal living fetus. Int J Gynaecol Obstet. 1992;38:238-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Sánchez-Ferrer ML, Ferri B, Almansa MT, Carbonel P, López-Expósito I, Minguela A, Abad L, Parrilla JJ. Partial mole with a diploid fetus: case study and literature review. Fetal Diagn Ther. 2009;25:354-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Feinberg RF, Lockwood CJ, Salafia C, Hobbins JC. Sonographic diagnosis of a pregnancy with a diffuse hydatidiform mole and coexistent 46,XX fetus: a case report. Obstet Gynecol. 1988;72:485-488. [PubMed] [Cited in This Article: ] |