Published online Feb 6, 2019. doi: 10.12998/wjcc.v7.i3.357
Peer-review started: October 22, 2018
First decision: November 2, 2018
Revised: November 27, 2018
Accepted: December 21, 2018
Article in press: December 21, 2018
Published online: February 6, 2019
Ghost cell odontogenic carcinoma (GCOC) is a rare malignant odontogenic epithelial tumor with features of benign calcifying odontogenic cysts. Herein, we report two new cases of GCOC and systematically review the previous literature.
In case 1, a 46-year-old man complained of painless swelling of the right maxilla for 3 years, with a 1-mo history of hemorrhinia in the right nasal cavity. In case 2, a 72-year-old man was referred to our hospital with a chief complaint of painful swelling of the right mandible. Initially, the preliminary diagnoses were ameloblastomas. Thus, the two patients underwent resection of the tumor under general anesthesia. Finally, immunohistochemical examination confirmed the diagnosis of GCOC. The patient in case 1 was followed for 2 years, with no evidence of recurrence. However, the patient in case 2 was lost to follow-up.
GCOC is a rare malignant odontogenic epithelial tumor with high recurrence. Local extensive resection is necessary for the definitive treatment of GCOC.
Core tip: Ghost cell odontogenic carcinoma (GCOC) is a rare malignant odontogenic epithelial tumor with features of benign calcifying odontogenic cysts. Herein, we report two cases of GCOC and describe their clinical features, histological characteristics, and treatment. In addition, the tumor affected the tooth, suggesting that the disease may originate from an odontogenic tumor, progressing to malignant GCOC. Moreover, we analyze the reported cases in the English literature and summarize the prognosis and optimal therapy.
- Citation: Jia MQ, Jia J, Wang L, Zou HX. Ghost cell odontogenic carcinoma of the jaws: Report of two cases and a literature review. World J Clin Cases 2019; 7(3): 357-365
- URL: https://www.wjgnet.com/2307-8960/full/v7/i3/357.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i3.357
Ghost cell odontogenic carcinoma (GCOC) is an extremely rare odontogenic epithelial malignant neoplasm within the maxillofacial bones that displays aggressive behavior[1]. It may be derived from de novo or two benign odontogenic neoplasms: Calcifying cystic odontogenic tumors (CCOT) or dentinogenic ghost cell tumors (DGCT)[2,3]. Since Ikemura et al[4] first well documented one case in 1985, about 50 cases have been described thus far. Although histologic diagnostic criteria have been established for CCOT, DGCT, and GCOC, these three tumors manifest diverse nonspecific clinical and radiologic characteristics, making the diagnosis challenging.
The natural history of GCOC is unpredictable, as it may vary from slow progression to rapid destructive growth, with highly local aggressive characteristics, recurrence, and occasional distant metastases. Herein, we report two cases of GCOC and describe their clinical features, histological characteristics, and treatment.
Chief complaints: A 46-year-old man was referred to our hospital for painless swelling of the right maxilla for 3 years. He also complained of a 1-mo history of hemorrhinia in the right nasal cavity.
Personal and family history: His medical and family history did not reveal any relevant information.
Physical examination: No enlarged cervical lymph nodes were palpable. Intra-oral examination revealed asymptomatic swelling that extended from the midline to the maxillary tuberosity, and the overlying mucosa appeared normal. The labial and lingual cortices were expanded, and the swelling was solid in consistency and no fluctuation was elicited when the tumor was pressed. Teeth 11 and 12 demonstrated grade mobility and 13-17 were missing.
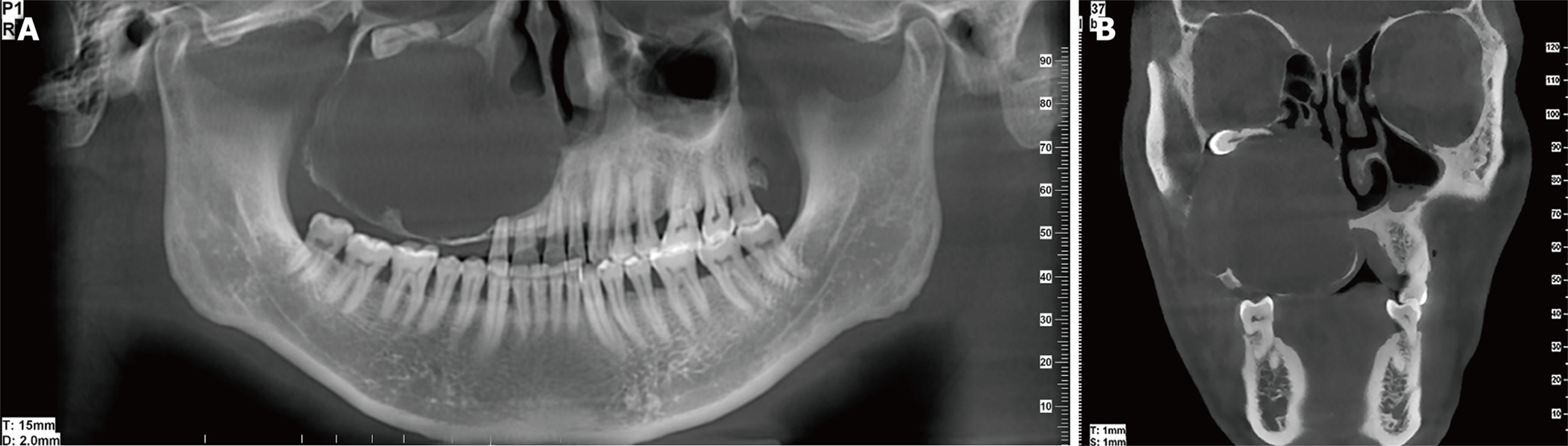
Imaging examinations: Cone-beam computed tomography (CBCT) depicted a round, well-defined unilocular radiolucent lesion filling the right maxillary sinus (Figure 1). The buccal, lateral, and medial bony walls of the right maxilla were destructed, and the tumor extended into the nasal cavity. The roots of teeth 11 and 12 had undergone apical resorption.
Laboratory examinations: Chest radiography showed no evidence of distant metastasis. Complete blood and urine tests were performed with no alterations.
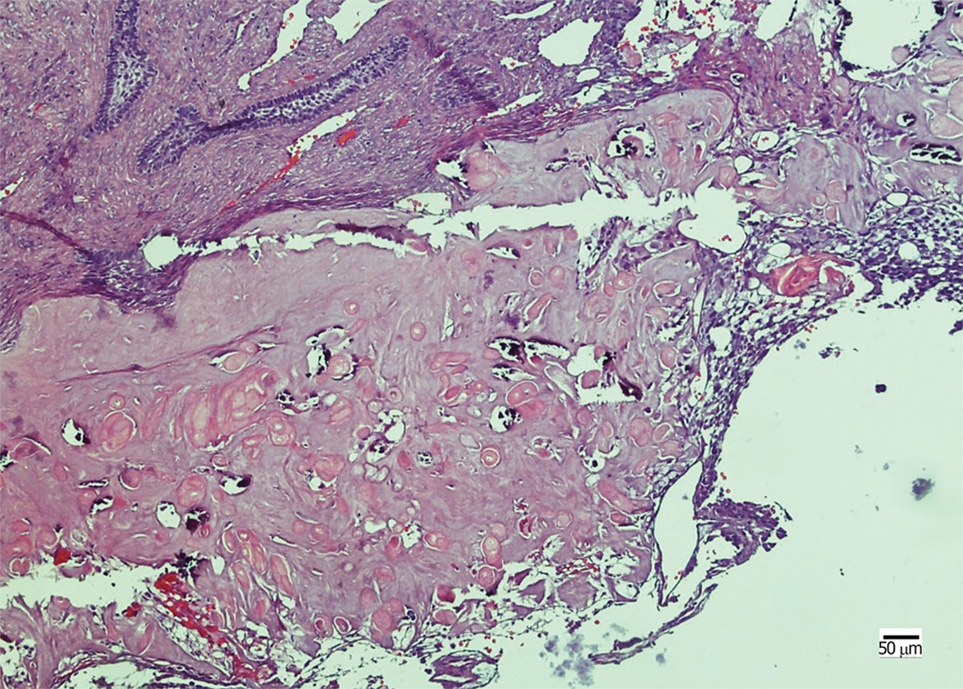
The lesion was punctured preoperatively, and no fluid was found in the lesion. Based on the clinical findings, a provisional diagnosis of ameloblastoma was made. The tumor was resected under general anesthesia. Histopathological examination revealed that the neoplastic nests demonstrated the characteristic features of odontogenic epithelium, showing a well-defined basal layer of columnar cells and cells resembling the stellate reticulum forming an epithelial lining (Figure 2). Masses of ghost cells could be seen in the lining or nests, presenting calcification or accompanying multicellular giant cell reaction. On the basis of the above presentation, a GCOC was suspected. Immunohistochemical staining revealed focal expression of Ki-67, less than 10%. Furthermore, the cells were positive for CK19 and negative for SMA. The patient was followed for 2 years without any sign of recurrence.
Chief complaints: A 72-year-old man was referred to our department with a chief complaint of painful swelling of the right mandible for 3 mo. He tried to mitigate the pain by taking oral antibiotics, but it had no effect.
History of past illness: He had a history of hepatitis B virus infection.
Personal and family history: His family history did not reveal any relevant information.
Physical examination upon admission: Physical examination revealed poor oral hygiene, generalized caries, and periodontal disease. A 5 cm × 3 cm expansive mass was examined in the mandible, and the swelling extended anteroposteriorly from the distal part of the right mandibular first premolar to the anterior border of the ramus of the mandible. The mandibular teeth were not affected, and the overlying mucosa was ulcerated because of the bite of the maxillary teeth. On palpation, the swelling was solid without fluctuation.
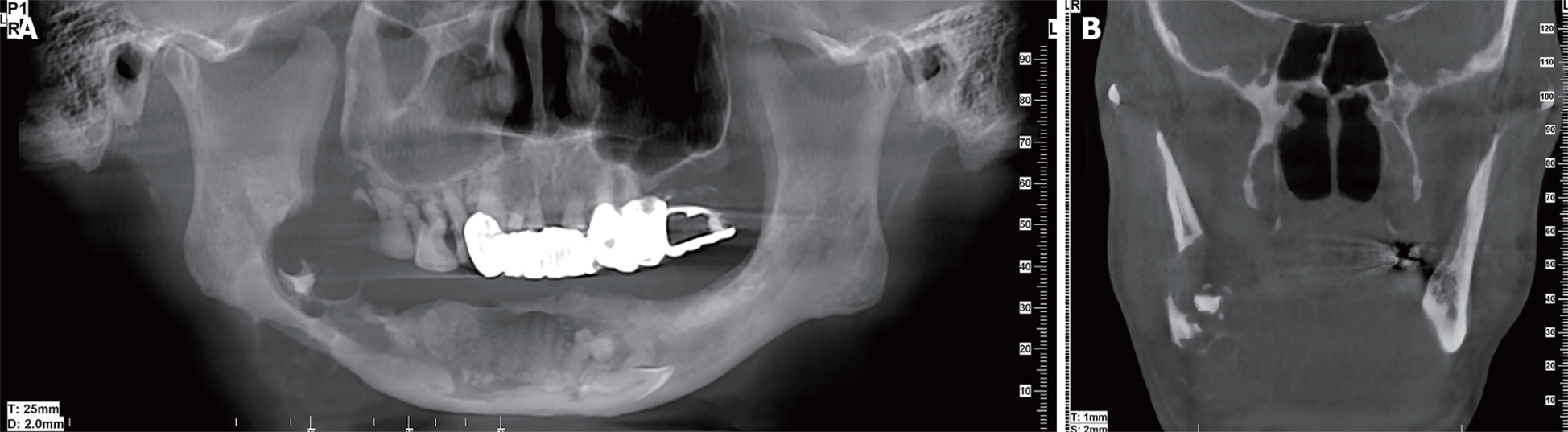
Imaging examinations: CBCT showed a multilocular cyst with ill-defined and extensive bony destruction lesion, with perforation of the buccal and lingual plates of the jaw, and infiltration into the soft tissues (Figure 3).
Laboratory examinations: Changes in routine hematological and urine test results were observed, but within normal limits.
Preliminary diagnosis: Based on the clinical findings, a provisional diagnosis of ameloblastoma was made.
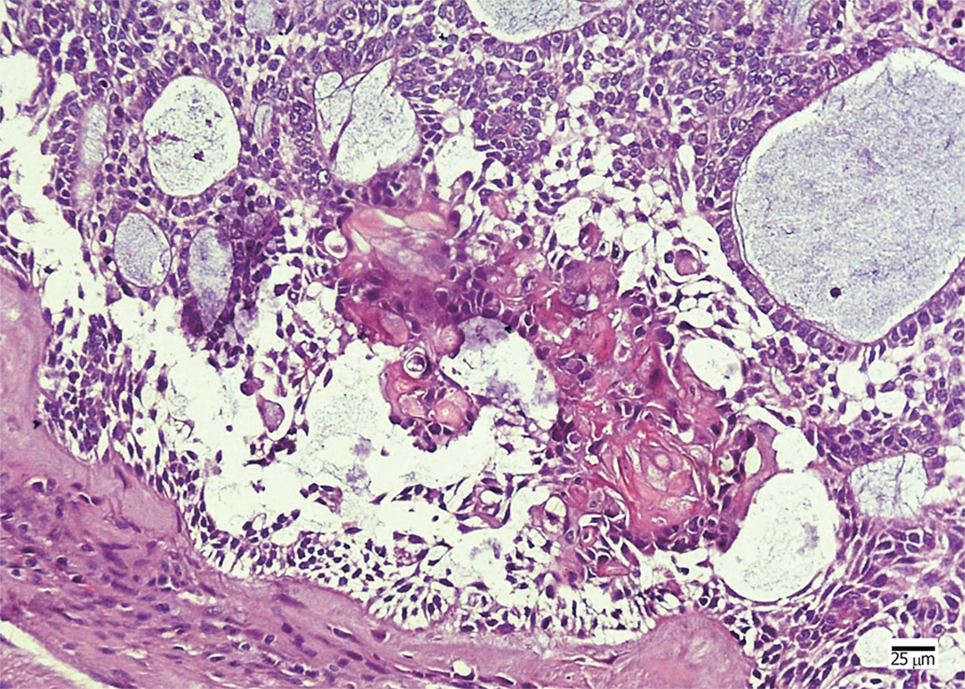
The patient underwent segmental mandibulectomy, owing to his poor economic status, and primary reconstruction could not be performed. Histopathological examination showed that the tumor was a cystic tumor with calcified and necrotic parts, infiltrating the surrounding connective tissue. The neoplastic tumor included an epithelial lining showing a basal layer of columnar cells arranged in a palisading pattern and an overlying layer of stratified cells resembling stellate reticulum, with clusters of ghost cells sporadically or intensively distributed in the tumor nests (Figure 4). The presentation revealed findings consistent with a GCOC. Immunohistochemical staining showed the tumor cells were positive for CK19 and negative for SMA. In addition, the Ki-67 was expressed focally. Unfortunately, the patient was lost to follow-up.
GCOC.
The patient with maxillary involvement underwent maxillectomy. Moreover, another patient with a mandibular lesion underwent segmental resection of the mandible.
The patient in case 1 was followed for 2 years without any sign of recurrence. However, the patient in case 2 was lost to follow-up.
GCOC is an extremely rare tumor with aggressive and destructive behavior. Various terminologies have been used to designate this disease owing to its diverse histopathological characteristics including calcifying GCOC, malignant epithelial odontogenic ghost cell tumor, carcinoma arising from the calcifying odontogenic cysts (COCs), aggressive epithelial ghost cell odontogenic tumor, malignant COC, and malignant calcifying ghost cell odontogenic tumor[5,6]. It is characterized by the presence of ‘‘ghost cells,” which are enlarged polygonal eosinophilic epithelial cells without a nucleus stained with hematoxylin and eosin. The formation of ghost cells is still unclear, and ischemia induced degeneration or metaplastic of epithelial cells may play an important role[7]. Nevertheless, the presence of ghost cells is not a specific feature, as they can also be seen in pilomatricoma, craniopharyngioma, odontoma, and ameloblastic fibro-odontoma[5,8]. These tumors are exclusively seen in an intraosseous location, with an occurrence of approximately 7% in the head and neck regions. Their incidence in the oral cavity is about 0.37% to 2.1% of all odontogenic tumors[5]. They occur most commonly in the maxilla, accounting for 67%[9]. Pathologically, it is reported to arise de novo (55%), followed by malignant transformation of CCOT and DGCT (32.5%), ameloblastoma, and other odontogenic tumors[10]. In the presented cases, both patients had an impacted tooth in the bone lesion, which suggested that the disease may originate from an odontogenic tumor progressing into a malignant GCOC.
Because of the rarity of such disease, less than 50 cases have been reported in the English literature[3-6,8-44] (Table 1). The Asian population appears to be more susceptible to such tumor (20/50), and men are at a higher risk than women with a ratio of 3.2:1. It can occur at any age, ranging from 10 to 89 years, with the peak incidence in the fourth and fifth decades of life. The maxilla is the most commonly involved site, with a ratio of 2.1:1. In addition, the molar area and ramus are the most affected parts of the mandible. Patients are often referred to the hospital with a chief complaint of painful or painless swelling of the bone, and local paresthesia, teeth mobility, and mucosal ulcer can be seen in some patients. Few patients showed enlarged neck lymph nodes, and lymph node metastasis was extremely rare. Pulmonary metastasis was found in two cases, and multiple metastases occurred only in one case. Local recurrence in patients who underwent local surgical resection or curettage was more common, and 56.25% of patients demonstrated recurrence from 3 mo to 7 years of follow-up. Given the rare occurrence of GCOC, most epidemiological information and clinical features are based on case reports; hence, the 5-year survival rate is not yet clear.
| Race | N = 50 |
| Asian | 20 |
| White | 5 |
| Black | 4 |
| Others | 3 |
| N/A | 18 |
| Sex | |
| Male | 38 |
| Female | 12 |
| Age (yr) | |
| 10-20 | 5 |
| 20-30 | 4 |
| 30-40 | 15 |
| 40-50 | 12 |
| 50-60 | 5 |
| 60-70 | 3 |
| 70-80 | 4 |
| ≥ 80 | 2 |
| Location | |
| Maxilla | 34 |
| Mandible | 16 |
| Main complaints | |
| Swelling | 27 |
| Paresthesia | 1 |
| Swelling and paresthesia | 1 |
| Slowly growing mass | 1 |
| Others | 15 |
| N/A | 5 |
| Therapy | |
| Enucleation | 5 |
| Surgery | 30 |
| Enucleation + RT | 2 |
| Surgery + RT | 8 |
| Surgery + CCRT | 2 |
| Surgery + adjuvant carbon ion therapy | 1 |
| N/A | 2 |
| Histological legend | |
| After AB | 2 |
| After COC/CCOT/DGCT | 16 |
| DN | 24 |
| N/A | 8 |
| Follow-up | |
| Recurrence history | 27 |
| No recurrence | 17 |
| N/A | 6 |
| Distant metastasis | 3 |
| Neck metastasis | 4 |
| Death | 10 |
The etiology of GCOC is controversial, and an integrative genomic and transcriptomic analysis of GCOC revealed the expression of multiple oncogenes and changes in tumor suppressor genes. Although the gene expression in the GCOC is unique, no tumor markers have been identified. Because of the different compositions of GCOC tissues, the imaging of GCOC showed that mixed unicameral or multilocular radiolucent-radiopaque lesions with or without clear borders are more common than radioactive lesions[19]. Displacement of tooth roots and various degrees of bony destructions or infiltration of adjacent structures are common; tooth impaction and root resorption can also occur[21,45]. No typical or special presentation is found on CT of such lesions. Although bone destruction is frequently observed, the manifestation of tooth resorption was unusual. Possibly, various compositions of the cancellous bone, cortical bone, and cementum are related to the phenomenon.
Histologically, GCOC shows small, rounded cells with dark nuclei, larger cells with vesicular nuclei, or small cluster or large masses of “ghost cells” regardless of calcification. Mitoses are frequently observed. Immunohistochemical staining with a panel of antibodies demonstrated that neoplastic cells were strongly positive for cytokeratin and negative for vimentin, desmin, SMA, and CD34[43]. The expression of Ki-67 and MMP-9 may be associated with the proliferation, invasion, and prognosis of GCOC[33]. In our two cases, the Ki-67 staining was focally positive, which demonstrated the lower malignancy of GCOC. Moreover, the cytokeratins such as CK19 were present, which showed the nature of odontogenic epithelial tumor. In addition, Rappaport et al[39] reported that mutation of the β-catenin gene was noted at codon 33 in GCOC. Compared to CCOT and DGCT, GCOC demonstrates extensive bone destruction, and tartrate-resistant acid phosphatase and vitronectin receptor were strongly expressed in the ghost cells of GCOC[24]. Further research is required to determine if these biomarkers can be used to determine malignant transformation or high recurrence rate.
The diagnosis of GCOC should be based on clinical symptoms, radiological examination, and histopathology. Accurate diagnosis of GCOC requires extensive sampling of the specimen for the features of malignant epithelial tumors containing benign histology of CCOT or DGCT[42,46].
The recommended treatment for GCOC is wide surgical excision with clean margins[5,42]. No research to date has been able to draw definite conclusions on whether adjunctive radiotherapy with or without chemotherapy has efficient outcome. Adjuvant cetuximab may be an option; however, it has been used in only one case.
To develop a full picture of GCOC treatment, additional studies will be needed to obtain overall information about its pathological and biological features. Taken together, a multidisciplinary team is essential to determine proper therapy and optimal outcome. According to previous case reports, GCOC has a high recurrence rate and shows diverse biological behaviors. Therefore, long-term follow-up is recommended to track and assess clinical changes in such disease. The present cases emphasized the biological behavior of GCOC. Adequate biopsy with meticulous histopathological examination of multiple sections along with adjunctive immunohistochemistry is the key to definitive diagnosis and to differentiating from conventional squamous cell carcinoma. As recurrence and metastasis are very frequent in GCOC, long-term follow-up should be advised. According to some studies, the 5-year survival rate was about 73%[28]. However, the small number of cases made it difficult to determine the exact rate of recurrence, with half of them having a follow-up time shorter than 2 years and with almost half of the patients having a relapse during the follow-up period. Given the short follow-up time and obscure prognosis, it is hard to determine the recurrence rate, disease-free survival, and 5-year survival rate.
In summary, we have introduced another two cases of GCOC that affected the adjacent tooth, which suggested that the disease may originate from an odontogenic tumor progressing into malignant GCOC. During the 2-year follow-up period, no recurrence was reported in case 1. Further investigation is needed to elucidate the etiology and diagnosis, and its treatment remains to be improved.
GCOC is a rare tumor that has been reported mostly in case reports. The literature review as well as the clinical, imaging, and histochemical presentation suggests an odontogenic origin of this tumor. In addition, this carcinoma has high recurrence when treated with enucleation therapy only. It is commonly recognized that extended resection therapy with a negative margin is a comparatively radical cure for GCOC. However, there is no explicit evidence to indicate that adjunctive therapy is effective.
We fully appreciate the help from Professor Jia-Li Zhang in the histopathological diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chen YK, Gorseta K, Muhvić-Urek M, Rattan V, Vieyra JP S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Song H
| 1. | Gorlin RJ, Pindborg JJ, Odont, Clausen FP, Vickers RA. The calcifying odontogenic cyst--a possible analogue of the cutaneous calcifying epithelioma of Malherbe. An analysis of fifteen cases. Oral Surg Oral Med Oral Pathol. 1962;15:1235-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 293] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Ledesma-Montes C, Gorlin RJ, Shear M, Prae Torius F, Mosqueda-Taylor A, Altini M, Unni K, Paes de Almeida O, Carlos-Bregni R, Romero de León E, Phillips V, Delgado-Azañero W, Meneses-García A. International collaborative study on ghost cell odontogenic tumours: calcifying cystic odontogenic tumour, dentinogenic ghost cell tumour and ghost cell odontogenic carcinoma. J Oral Pathol Med. 2008;37:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Arashiyama T, Kodama Y, Kobayashi T, Hoshina H, Takagi R, Hayashi T, Cheng J, Saku T. Ghost cell odontogenic carcinoma arising in the background of a benign calcifying cystic odontogenic tumor of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e35-e40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Ikemura K, Horie A, Tashiro H, Nandate M. Simultaneous occurrence of a calcifying odontogenic cyst and its malignant transformation. Cancer. 1985;56:2861-2864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Goldenberg D, Sciubba J, Tufano RP. Odontogenic ghost cell carcinoma. Head Neck. 2004;26:378-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Sun ZJ, Zhao YF, Zhang L, Li ZB, Chen XM, Zhang WF. Odontogenic ghost cell carcinoma in the maxilla: a case report and literature review. J Oral Maxillofac Surg. 2007;65:1820-1824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Levy BA. Ghost cells and odontomas. Oral Surg Oral Med Oral Pathol. 1973;36:851-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Ellis GL, Shmookler BM. Aggressive (malignant?) epithelial odontogenic ghost cell tumor. Oral Surg Oral Med Oral Pathol. 1986;61:471-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Martos-Fernández M, Alberola-Ferranti M, Hueto-Madrid JA, Bescós-Atín C. Ghost cell odontogenic carcinoma: A rare case report and review of literature. J Clin Exp Dent. 2014;6:e602-e606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Gomes JP, Costa AL, Chone CT, Altemani AM, Altemani JM, Lima CS. Three-dimensional volumetric analysis of ghost cell odontogenic carcinoma using 3-D reconstruction software: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:e170-e175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kamijo R, Miyaoka K, Tachikawa T, Nagumo M. Odontogenic ghost cell carcinoma: report of a case. J Oral Maxillofac Surg. 1999;57:1266-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Castle JT, Arendt DM. Aggressive (malignant) epithelial odontogenic ghost cell tumor. Ann Diagn Pathol. 1999;3:243-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Grodjesk JE, Dolinsky HB, Schneider LC, Dolinsky EH, Doyle JL. Odontogenic ghost cell carcinoma. Oral Surg Oral Med Oral Pathol. 1987;63:576-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Scott J, Wood GD. Aggressive calcifying odontogenic cyst--a possible variant of ameloblastoma. Br J Oral Maxillofac Surg. 1989;27:53-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | McCoy BP, O Carroll MK, Hall JM. Carcinoma arising in a dentinogenic ghost cell tumor. Oral Surg Oral Med Oral Pathol. 1992;74:371-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Dubiel-Bigaj M, Olszewski E, Stachura J. The malignant form of calcifying odontogenic cyst. A case report. Patol Pol. 1993;44:39-41. [PubMed] [Cited in This Article: ] |
| 17. | Siar CH, Ng KH. Aggressive (malignant?) epithelial odontogenic ghost cell tumour of the maxilla. J Laryngol Otol. 1994;108:269-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Alcalde RE, Sasaki A, Misaki M, Matsumura T. Odontogenic ghost cell carcinoma: report of a case and review of the literature. J Oral Maxillofac Surg. 1996;54:108-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kim HJ, Choi SK, Lee CJ, Suh CH. Aggressive epithelial odontogenic ghost cell tumor in the mandible: CT and MR imaging findings. AJNR Am J Neuroradiol. 2001;22:175-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Li TJ, Yu SF. Clinicopathologic spectrum of the so-called calcifying odontogenic cysts: a study of 21 intraosseous cases with reconsideration of the terminology and classification. Am J Surg Pathol. 2003;27:372-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Cheng Y, Long X, Li X, Bian Z, Chen X, Yang X. Clinical and radiological features of odontogenic ghost cell carcinoma: review of the literature and report of four new cases. Dentomaxillofac Radiol. 2004;33:152-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Pindborg JJ, Kramer IRH, Torloni H. WHO International Histological Classification of Tumors: Histological Typing of Odontogenic Tumors, Jaw Cysts and Allied Lesions. Geneva, Switzerland: Springer 1971; 18. [Cited in This Article: ] |
| 23. | Nazaretian SP, Schenberg ME, Simpson I, Slootweg PJ. Ghost cell odontogenic carcinoma. Int J Oral Maxillofac Surg. 2007;36:455-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Roh GS, Jeon BT, Park BW, Kim DR, Hah YS, Kim JH, Byun JH. Ghost cell odontogenic carcinoma of the mandible: a case report demonstrating expression of tartrate-resistant acid phosphatase (TRAP) and vitronectin receptor. J Craniomaxillofac Surg. 2008;36:419-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Li BB, Gao Y. Ghost cell odontogenic carcinoma transformed from a dentinogenic ghost cell tumor of maxilla after multiple recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:691-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Motosugi U, Ogawa I, Yoda T, Abe T, Sugasawa M, Murata S, Yasuda M, Sakurai T, Shimizu Y, Shimizu M. Ghost cell odontogenic carcinoma arising in calcifying odontogenic cyst. Ann Diagn Pathol. 2009;13:394-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Folpe AL, Tsue T, Rogerson L, Weymuller E, Oda D, True LD. Odontogenic ghost cell carcinoma: a case report with immunohistochemical and ultrastructural characterization. J Oral Pathol Med. 1998;27:185-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Lu Y, Mock D, Takata T, Jordan RC. Odontogenic ghost cell carcinoma: report of four new cases and review of the literature. J Oral Pathol Med. 1999;28:323-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Slama A, Boujelbène N, Ben Yacoub L, Trabelsi A, Khochtali H, Sriha B. Ghost cell odontogenic carcinoma of the mandible. Rev Stomatol Chir Maxillofac. 2010;111:158-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Li BH, Cho YA, Kim SM, Kim MJ, Hong SP, Lee JH. Recurrent odontogenic ghost cell carcinoma (OGCC) at a reconstructed fibular flap: a case report with immunohistochemical findings. Med Oral Patol Oral Cir Bucal. 2011;16:e651-e656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Minatani R, Tabuchi K, Nakayama M, Nishimura B, Ashizawa K, Hara A. A Case of Odontogenic Ghost Cell Carcinoma of the Maxilla. Pract Otol. 2012;105:1181-1186. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Zhu ZY, Chu ZG, Chen Y, Zhang WP, Lv D, Geng N, Yang MZ. Ghost cell odontogenic carcinoma arising from calcifying cystic odontogenic tumor: a case report. Korean J Pathol. 2012;46:478-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Gomes da Silva W, Ribeiro Bartholomeu Dos Santos TC, Cabral MG, Azevedo RS, Pires FR. Clinicopathologic analysis and syndecan-1 and Ki-67 expression in calcifying cystic odontogenic tumors, dentinogenic ghost cell tumor, and ghost cell odontogenic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:626-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Ahmed SK, Watanabe M, deMello DE, Daniels TB. Pediatric Metastatic Odontogenic Ghost Cell Carcinoma: A Multimodal Treatment Approach. Rare Tumors. 2015;7:5855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Ali EA, Ali karrar M, El-Siddig AA, Gafer N, Abdel satir A. Ghost cell odontogenic carcinoma of the maxilla: a case report with a literature review. Pan Afr Med J. 2015;21:260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Del Corso G, Tardio ML, Gissi DB, Marchetti C, Montebugnoli L, Tarsitano A. Ki-67 and p53 expression in ghost cell odontogenic carcinoma: a case report and literature review. Oral Maxillofac Surg. 2015;19:85-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Fitzpatrick SG, Hirsch SA, Listinsky CM, Lyu DJ, Baur DA. Ameloblastic carcinoma with features of ghost cell odontogenic carcinoma in a patient with suspected Gardner syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:e241-e245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Ismerim AB, Fernandes AG, Loyola AM, Dos Santos JN. Ghost Cell Odontogenic Carcinoma in the Anterior Mandible: Case Report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e46. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Rappaport MJ, Showell DL, Edenfield WJ. Metastatic Ghost Cell Odontogenic Carcinoma: Description of a Case and Search for Actionable Targets. Rare Tumors. 2015;7:5813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Miwako S, Hiroto I, Takahumi N, Junichi H, Tadahide N, Yoshinori J, Yoshiyuki M. Ghost cell odontogenic carcinoma transformed from dentinogenic ghost cell tumor of the maxilla after recurrences. J Oral Maxillofac Surg Med Pathol. 2017;In press. [Cited in This Article: ] |
| 41. | Namana M, Majumdar S, Uppala D, Avv A, Rao AK. Ghost Cell Odontogenic Carcinoma Arising Denovo with Distant Metastasis: A Case Report and Review of Literature. J Clin Diagn Res. 2017;11:ZD01-ZD03. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Qin Y, Lu Y, Zheng L, Liu H. Ghost cell odontogenic carcinoma with suspected cholesterol granuloma of the maxillary sinus in a patient treated with combined modality therapy: A case report and the review of literature. Medicine (Baltimore). 2018;97:e9816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Remya K, Sudha S, Nair RG, Jyothi H. An unusual presentation of ghost cell odontogenic carcinoma: A case report with review of literature. Indian J Dent Res. 2018;29:238-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Alberola M, Diaz S, Ferrer B, Martos M, Hueto JA, Siurana S, Bescos S, Cajal SRY. Ghost Cell Odontogenic Carcinoma. A Case Report and Review of the Literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:e125. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Kim J, Lee EH, Yook JI, Han JY, Yoon JH, Ellis GL. Odontogenic ghost cell carcinoma: a case report with reference to the relation between apoptosis and ghost cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:630-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Barnes L, Eveson JW, Reichart P, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Head and neck Tumours. Lyon: IARC Press 2005; 283-327, 342-344. [Cited in This Article: ] |