Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.10840
Peer-review started: April 30, 2022
First decision: May 29, 2022
Revised: June 13, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: October 26, 2022
The growing worldwide burden of insulin resistance (IR) emphasizes the importance of early identification for improved management. Obesity, particularly visceral obesity, has been a key contributing factor in the development of IR. The obesity-associated chronic inflammatory state contributes to the deve
Core Tip: Visfatin is an adipocytokine that is produced by visceral fat and other sources. It has been shown to influence glucose and lipid metabolism, as well as enhance the chronic inflammatory state linked to obesity. The findings on the relationship between visfatin and IR in obese patients are controversial. This review aims to better understand how visfatin contributes to the emergence of IR and to assess the possibility of utilizing visfatin levels as a biomarker for the early detection of IR and IR-related diseases, including cardiovascular and renal diseases.
- Citation: Abdalla MMI. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases 2022; 10(30): 10840-10851
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/10840.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.10840
Accumulation of adipose tissue, particularly visceral adiposity, is the most common contributory factor in the development of insulin resistance (IR). Adipose tissue is an endocrine tissue that secretes many peptides that are adipocytokines including leptin, adiponectin, resistin and visfatin. Adipokines are reported to affect glucose and lipid metabolism as well as food intake[1]. Alterations in the levels of adipokines and genetic polymorphism such as adiponectin SNP + 45 are related to the emergence of IR and its related comorbidities[2]. The serum and salivary levels of these adipokines were extensively assessed as biomarkers for early diagnosis of IR and its consequences for cardiovascular and renal diseases. Visfatin is an adipokine that has attracted the attention of researchers for its role in the pathogenesis of IR and the possibility of using its levels as a biomarker for IR detection[3-7].
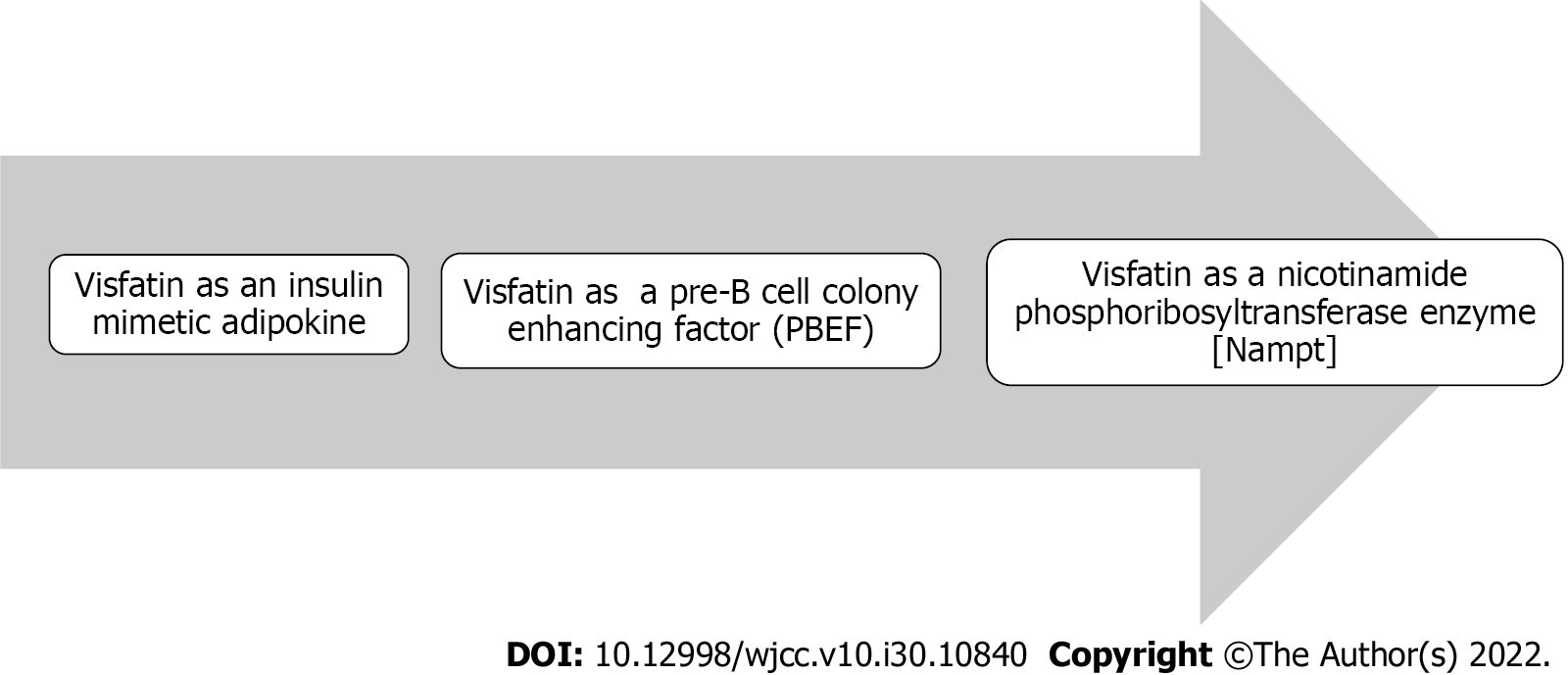
Visfatin is a 52-kDa molecule, first described in 2004 by Fukuhara et al[7] as an insulin-mimetic adipokine. It was named visfatin because it was thought to be produced exclusively from visceral adipose tissue[6,7], in addition to other sources including subcutaneous adipose tissue[8], skeletal muscle[9], liver[10], immune cells[11], cardiomyocytes[12], brain cells[13] and renal glomeruli[14].
Visfatin’s insulin-like actions were demonstrated by a reduction in plasma glucose concentration 30 min after intravenous infusion of recombinant visfatin into c57BL/6J mice and insulin-resistant obese mice[7]; an outcome that the authors hypothesized was independent of insulin. The insulin-mimetic action of visfatin might be mediated by an increase in peripheral tissue glucose uptake, a decrease in both gluconeogenesis and glucose release, and stimulation of the insulin signaling cascade[7]. The glucose-lowering effect of visfatin attracted researchers ‘ curiosity about the prospect of visfatin becoming a novel diabetic medication. Extensive research has been conducted to investigate the relationship between visfatin levels, type 2 diabetes mellitus (T2DM), and IR. However, the findings are inconsistent, ranging from a positive correlation between plasma visfatin level and T2DM[15,16] to the existence of an inverse relationship between plasma visfatin level and type 1 diabetes[17].
Visfatin is structurally identical to the pre-B cell colony enhancing factor (PBEF)[18], a cytokine that was isolated in 1994 from a human lymphocyte and was known to accelerate the maturation of B cell precursors[19]. PBFE (visfatin) is regarded as a proinflammatory cytokine that has been shown to have multiple physiological functions related to cell metabolism[20,21], immunomodulation[20,22] and inflammation[23,24]. It has been found in various immune cells other than B cells and has been shown to inhibit apoptosis of macrophages[20]. Extracellular PBEF promotes proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-16, transforming growth factor (TGF)-β1, as well as the chemokine receptor CCR3. PBEF also boosts IL-6, TNF-α and IL-1 production in CD14+ monocytes, macrophages, and dendritic cells, improves T cell function, and is required for the development of B and T lymphocytes[20].
In 2001, Martin et al[24] discovered a significant homology between the mammalian PBEF gene and nicotinamide phosphoribosyltransferase (NAMPT); the enzyme that is required for production of NAD, and subsequently, is capable of modulating its intracellular levels[25-27]. NAD is a crucial coenzyme involved in a variety of cellular activities, including the generation of ATP[28] and the synthesis of regulatory proteins such as silent information regulator 2 (Sir2), which has seven types of deacetylase proteins known as sirtuins: SIRT1–SIRT7[29]. Sirtuins are involved in various metabolic functions[30-32]. SIRT1 deacetylases have been demonstrated to regulate many transcription factors required for cell survival, apoptosis and proliferation, including tumor suppressor p53[33-35], a protein forkhead box class O protein[36-38], and peroxisome proliferator-activated receptor-γ coactivator-1α[39,40]. SIRT1 also inhibits the signaling of nuclear factor-κB and suppresses inflammation[41-43]. Visfatin may thus alter metabolic processes involved in glucose and lipid metabolism via its NAMPT enzyme-like activity[3,44,45]. Figure 1 depicts the discovery of visfatin as a structurally similar hormone to the cytokine PBEF, whose gene is homologous to the NAMPT enzyme. As a result, PBEF and NAMPT are synonyms for visfatin.
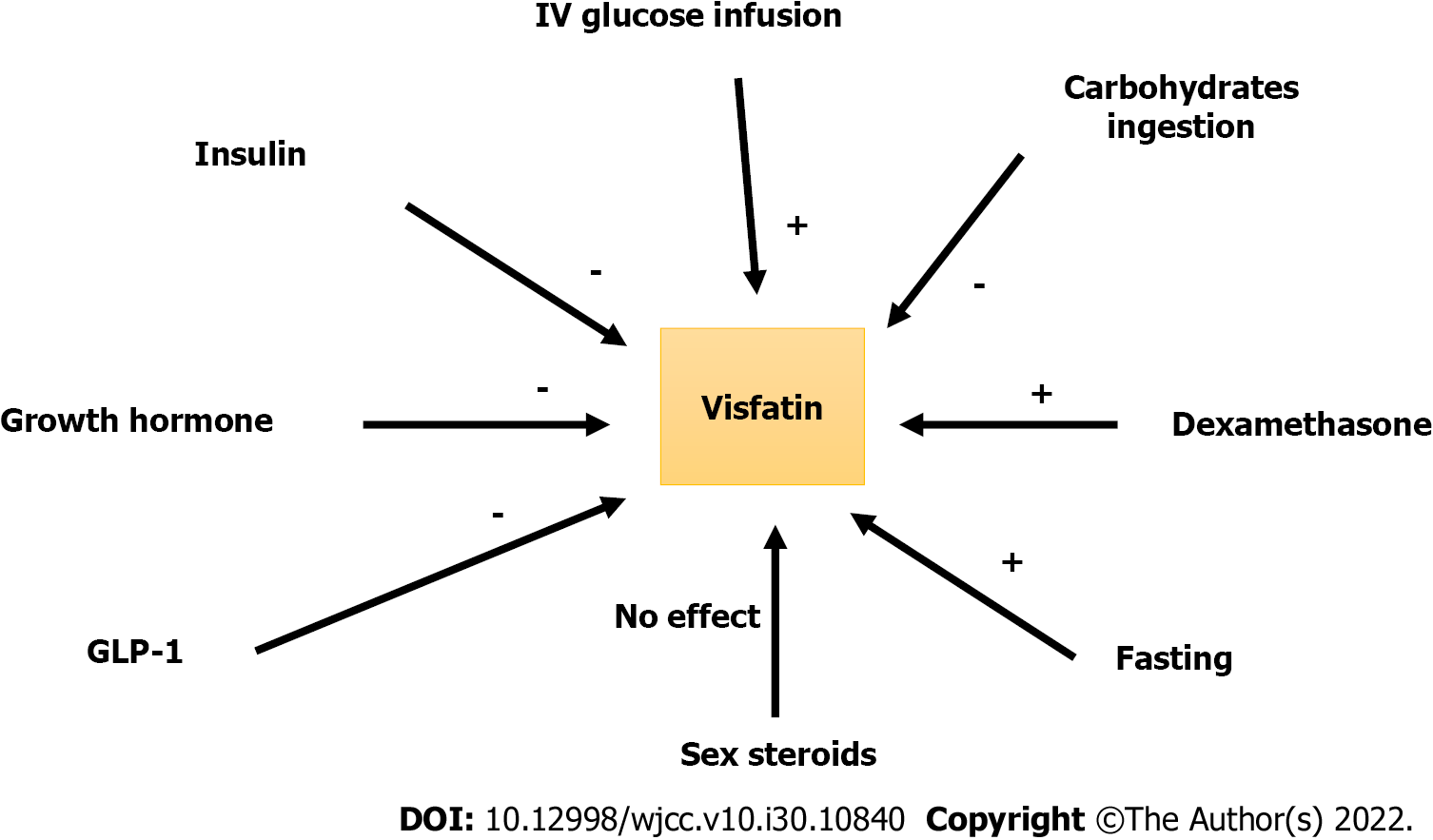
Despite several research published since the discovery of visfatin, the regulation of visfatin (PBEF/NAMPT) appears to be complex. The majority of published research is inconsistent and provides conflicting results. Dexamethasone raised visfatin mRNA expression in 3T3-L1 adipocytes while growth hormone and TNF-α decreased it[18,46,47]. Glucose infusion elevated plasma visfatin levels in healthy participants during clamp testing[48]. However, another study published in 2011 by Bala et al[49] found that 75 g carbohydrate intake following a 2-h oral glucose tolerance test (OGTT) significantly reduced plasma visfatin levels primarily in overweight and female participants, who were more affected than normal healthy glucose-tolerant individuals. The significant inhibitory effect of insulin and glucagon-like peptide (GLP)-1 on visfatin production following oral glucose ingestion might explain the effects of intravenous versus oral glucose consumption[49]. Insulin and GLP-1 dramatically decreased visfatin secretion in vitro and in animal experiments. According to Bala et al[49], insulin infusion dramatically reduced visfatin release from 3T3-L1 adipocytes by nearly 50%, and GLP-1 lowered visfatin production from 3T3-L1 adipocytes by roughly 50%. According to the same study, insulin and GLP-1 under an oral glucose load, reduce visfatin levels among those who are healthy, insulin-sensitive and have normal glucose tolerance[49]. The authors concluded that the inhibitory effect of GLP-1 on visfatin production from 3T3-L1 adipocytes during OGTT may indicate the emergence of a novel incretin-like action described by a GLP-1/visfatin/axis; the significance of which remains unknown. In another investigation, researchers discovered that intravenous glucose, mannitol, and sex hormones (estradiol and testosterone) had no effect on visfatin release[50]. Figure 2 summarizes the control of visfatin secretion in normal, healthy and glucose-tolerant individuals.
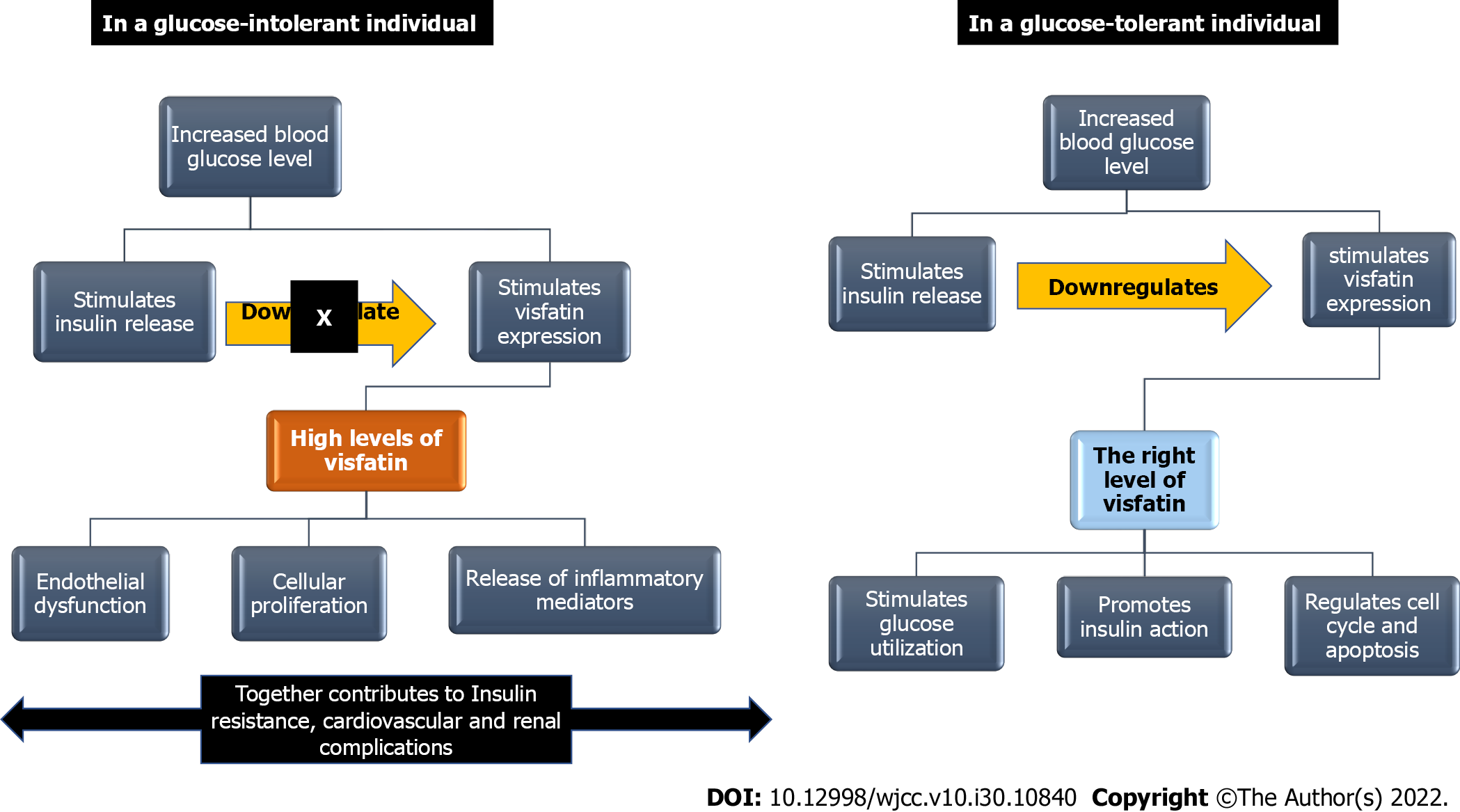
Visfatin (NAMPT), with its two isoforms, the extracellular (eNAMPT) and intracellular (iNAMPT) forms, is crucial for NAD biosynthesis, with the higher activity of the extracellular form. NAD is required for various processes, including metabolic processes, glucose-stimulated insulin secretion, cell survival, cell cycle control, and apoptosis[51]. However, elevated levels of visfatin have been linked to increased levels of inflammatory markers such as IL-6, IL-8, C-reactive protein, and monocyte chemotactic protein-1[52,53], endothelial dysfunction[54], and increase in oxidative stress[55-58]. These findings point to the existence of an average physiological level of visfatin at which it is properly controlled and fulfils its physiological functions, as well as a threshold level at which its pathological consequences occur. Studies have shown that an increased level of visfatin is associated with IR and T2DM[15,59], metabolic syndrome (MetS)[60], polycystic ovary syndrome[61], and T2DM-associated complications such as cardiovascular[62,63], cerebrovascular[64,65], and renal[66-68] diseases. The significance of visfatin in the pathogenesis of IR and its associated consequences reflects the potential utility of visfatin as an early biomarker for IR in high-risk patients, especially obese adults, and as a biomarker for T2DM sequelae.
The impairment of glucose metabolism and the development of multiorgan IR following the adipose-tissue-specific deletion of visfatin[69] has demonstrated that visfatin is a major target in obesity, diabetes, and dyslipidemia[70]. Visfatin interacts with insulin receptors at locations other than insulin-binding sites, lowering blood glucose levels via increased muscle and liver cell glucose utilization[5]. It also prompts the action of insulin by inducing phosphorylation of insulin receptors 1 and 2. It also prompts the activation of phosphatidylinositol-3 kinase (PI3K), protein kinase B, and mitogen-activated protein kinase[71]. Visfatin is thought to have endocrine, paracrine and autocrine functions. Visfatin’s autocrine effects in the liver may be important in modifying insulin sensitivity, as indicated by a decrease in glucose-stimulated insulin release linked with decreased visfatin expression and NAD production in the Fao rat hepatocytes[72].
Central visfatin improved glucose homeostasis in diabetic rats by boosting insulin release and sensitivity via the hypothalamus, demonstrating that visfatin is a positive modulator of glucose homeostasis via transmitting hypothalamic signals to the periphery[73]. The increased level of visfatin has been reported in obese patients with T2DM compared with obese patients without T2DM. The high visfatin level in these patients was correlated with an increase in inflammatory markers[59]. The high levels of visfatin found in patients who have uncontrolled glucose levels may represent a regulatory response in an attempt to maintain glucose homeostasis; however, as the insulin secretion/insulin actions are impaired in those patients, there is an imbalance in the regulation of visfatin, with a subsequent marked rise in visfatin level, exceeding the threshold. The increase in visfatin levels promotes the release of inflammatory mediators, as evidenced by the significant association between high visfatin levels with the high levels of inflammatory mediators regardless of the presence of T2DM and MetS[53,54,74] suggesting a role of visfatin in the development of IR and T2DM in a dose-dependent manner. Figure 3 summarizes the role of visfatin in glucose homeostasis and its potential role in the development of IR and IR-related complications.
Increased visfatin levels are associated with endothelial dysfunction, smooth muscle cell proliferation in the arterial wall, the formation of atherosclerotic plaques, and acute coronary syndrome[21,54,75]. The proliferative, proinflammatory and proangiogenic effects of visfatin are mediated via stimulation of PI3K, nuclear factor (NF)-B, signal transducer and activator of transcription (STAT), extracellular signal-regulated kinases (ERKs) and Toll-like receptor 4[54,76,77]. The visfatin-induced cell proliferation is dose-dependent, and it is mediated by increasing the release of vascular endothelial growth factor, fibroblast growth factor-2 (FGF-2), MCP-1, IL-6, and thromboxane A2[77-80]. The increased levels of visfatin in patients with atherosclerosis and acute coronary syndrome are caused by the expression of visfatin by the foam cells and smooth muscle cells of the atherosclerotic plaques, in addition to the increased expression of visfatin by the epicardial and perivascular adipose tissue, in which it is suggested to function in a paracrine fashion on the blood vessels[11,81]. Studies have shown that visfatin stimulates the release of inducible nitric oxide synthase (iNOS), a proinflammatory enzyme associated with vascular complications in diabetic patients[82-84]. The changes in visfatin levels in ST-elevation myocardial infarction (STEMI) followed the same pattern as the troponin levels, suggesting the usefulness of visfatin as a biomarker for STEMI[85]. Visfatin levels are also associated with cardiac fibrosis, as visfatin induces the proliferation of fibroblasts and increases the release of type I and III collagen[86].
Stroke is one of the cardiovascular diseases escalating in both developed and developing countries[87]. Diabetes is an established risk factor for stroke because of the cerebrovascular changes induced by hyperglycemia[88]. Recent research has found an increase in visfatin levels in stroke patients, indicating that a high visfatin level may be a risk factor for strokes[89,90]. The association observed between high visfatin levels and cerebrovascular strokes can be explained by the endothelial dysfunction induced by high visfatin levels[54,90].
The global prevalence of chronic kidney disease (CKD) among diabetic patients is steadily increasing[91]. An increased visfatin level is associated with the progression of CKD in diabetic and nondiabetic patients[92,93]; an observation that can be explained by endothelial dysfunction associated with high visfatin levels[93], IR[3], inflammation[53,74,94], oxidative stress[57,58], synthesis of profibrotic molecules in the mesangial cells, and increased expression of renin, angiotensinogen, and angiotensin receptors, as well as angiotensin II[95,96], thus increasing the activity of NADPH oxidase. This proinflammatory enzyme causes an increase in glomerular permeability[97]. A decrease in serum visfatin level was noted upon improving endothelial dysfunction following renal transplantation[98]. Overall, these data imply that visfatin has a role in mediating the CKD associated with T2DM. However, more studies are required to elucidate the relative contribution of visfatin compared with other inflammatory biomarkers, and to indicate the threshold level of visfatin at which those complications appear.
To assess the evidence on the relationship between visfatin levels and IR in obesity, PubMed and Google Scholar were searched for articles published between 2015 and March 2022. The search was limited to English-language articles. The references in the discovered texts were further analyzed for other relevant articles. After the search, 15 papers were chosen for evaluation and they are discussed in ascending sequence.
The first research chosen was by Nourbakhsh et al[99] in 2015. The study was conducted among 73 children in Iran; 42 obese patients versus 31 controls. The study involved the measurement of serum visfatin and insulin using ELISA, and IR was assessed using the homeostatic model assessment for IR (HOMA-IR). Fasting plasma glucose (FPG) and lipid profile were also tested, and MetS was defined using the International Diabetes Federation (IDF) criteria. The study revealed that obese children had significantly greater serum visfatin than nonobese children without MetS or IR. In obese individuals, visfatin levels correlated with FPG, insulin, and HOMA-IR[99].
A positive correlation between serum visfatin levels and IR among obese children was further investigated by Salama et al[100] in 2015. The study was conducted among 33 Egyptian children (22 obese and 11 control). Anthropometric measurements such as body mass index (BMI), waist circumference (WC), hip circumference (HC), and waist-to-hip ratio (WHR) were measured using standardized methods. The serum levels of insulin and visfatin were measured using ELISA. IR was calculated using HOMA-IR. The lipid profile and FPG were also measured. The study revealed significantly higher levels of serum visfatin in obese children (9.18 ± 3.04) compared with controls (4.33 ± 3.01). In obese children, Spearman’s correlation analysis revealed a positive correlation between serum visfatin with height, body weight, BMI, WC, HC and HOMA-IR[100].
The association between serum visfatin and IR was further evaluated in a study conducted in Turkey, Bursa, in 2015 by Gul et al[101]. The study evaluated the plasma levels of visfatin, insulin, and HOMA-IR in 18 obese versus 19 nonobese premenopausal women with polycystic ovary syndrome (PCOS). The study revealed a significantly higher HOMA-IR among obese patients with PCOS than nonobese PCOS patients and controls with no significant difference between the groups in serum visfatin levels. The study also revealed an absence of correlation between serum visfatin levels and HOMA-IR in the studied groups[101]. The difference between this result and the previous studies’ results can be explained by the difference in the demographics of the studied groups: Age, gender, and associated conditions.
In Poland, Liang and co-researchers evaluated the correlation between serum visfatin with glucose and lipid metabolism among pregnant women with gestational diabetes mellitus (GDM) in a prospective study conducted between 2012 and 2013. The BMI, FPG, serum visfatin, HOMA-IR and lipid profile were assessed. The study revealed significantly higher levels of serum visfatin, FPG, hemoglobin A1c, and HOMA-IR in women with GDM than the control group. Correlation analysis showed a negative correlation between serum visfatin with FBG and HOMA-IR among the control group. In contrast, a positive correlation is reported between serum visfatin levels with HOMA-IR, weight gain during pregnancy, and BMI at childbirth in women with GDM. The data of this study were first published in 2015[102]. The increase in serum visfatin levels associated with increased FPG in pregnant women may represent a regulatory response to control blood glucose levels; however, any further increase in visfatin levels may have contributed to IR and GDM.
The association between serum visfatin and GDM was further studied by Tsiotra et al[103] in Athens, Greece in 2018. Tsiotra et al[103] evaluated the expression of visfatin by the visceral and subcutaneous adipose tissues and placenta in 15 obese and nonobese women with GDM, compared to a control group that consisted of 23 obese and nonobese women with standard glucose tolerance. The study revealed a lower circulating visfatin level in obese women with GDM than in nonobese women with normal glucose tolerance. The study reported comparable visfatin mRNA expression in all tissues.
In Egypt in 2018, the association between serum visfatin with IR and proinflammatory cytokines in patients with T2DM was studied by Hetta and co-researchers[104]. The case–control study involved the assessment of anthropometric measurements, blood pressure, and serum levels of visfatin, CRP, IL-6, TNF-α and HOMA-IR in 80 people with diabetes in comparison to a control group of 40 healthy participants. The study reported significantly higher levels of visfatin, CRP, IL-6 and TNF- in the diabetic group compared with the healthy control group. Serum visfatin was also shown to be positively correlated with BMI, WC, HOMA-IR and proinflammatory markers[104]. The study results provided evidence of the potential role of serum levels of visfatin in the pathogenesis of IR, an effect that the activation of the proinflammatory cytokines could mediate.
In addition to assessing the association between serum levels of visfatin and IR, the interest in the use of saliva as an alternative tool to serum for the measurement of biomarkers has been developed. The use of salivary levels of visfatin as a biomarker for T2DM was investigated in a study conducted by Srinivasan et al[105] in the USA and published in 2018. The study involved measuring levels of visfatin, TNF-α, IL-6, resistin and ghrelin in unstimulated fasting saliva samples collected from 40 subjects (20 diabetics and 20 healthy controls). It revealed significantly higher salivary levels of visfatin in patients with T2DM than in the control group, supporting the potential use of salivary visfatin as a biomarker for T2DM[105].
Compared with blood sampling, saliva sampling is simple, safe and noninvasive. The use of saliva as a diagnostic tool is evolving not only due to the rapid advances that are being made in the fields of nanotechnology and molecular diagnostics but also because saliva contains biomarkers that are ideal for early detection and monitoring of oral as well as systemic diseases including biomarkers for IR such as resistin, TNF-α, leptin, visfatin, adiponectin, IL-6 and CRP[106].
The association between serum levels of visfatin and GDM was further investigated in a study conducted by Souvannavong-Vilivong et al[107] in Bangkok, Thailand, and published in 2019. The study involved the measurement of serum visfatin among a sample of pregnant women with GDM class A1 (n = 37) compared with a control group with normal pregnancy (n = 37). The results reported significantly higher levels of serum visfatin and plasma glucose levels in women with GDM class A1. This was associated with a negative correlation between serum levels of visfatin with neonatal weight and length, thus supporting the use of serum visfatin level as a biomarker for GDB and prediction of pregnancy outcomes in patients with GDM[107].
The usefulness of serum visfatin as a biomarker for GDM was further investigated by Bawah et al[108] in Ghana and published in 2019. The study examined the alterations of serum levels of visfatin with GDM in a case–control study that included 140 women in their first trimester (70 with GDM and 70 without GDM). In addition to BMI measurement, serum levels of visfatin, resistin and leptin, and lipid profile were assessed. The study reported significantly higher serum visfatin levels in patients with GDM compared with the controls. It also showed a significant positive correlation between serum levels of visfatin with age, total cholesterol, triglycerides, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, and leptin, and a negative correlation with high-density lipoprotein cholesterol[108].
Additional evidence of the increased serum visfatin in women with GDM was provided in a study conducted by Manoharan et al[109] in India and published in 2019. The study involved measuring cord plasma levels of visfatin, insulin, HOMA-IR, insulin sensitivity, and beta-cell function. The pregnancy outcomes such as birth weight were also assessed. Forty pregnant women were recruited for the study (n = 20 with GDM and n = 20 with normal pregnancy). The results revealed significantly higher cord plasma insulin, visfatin, and HOMA-IR levels in women with GDM than in the control group[109].
The association between serum visfatin and IR among obese children was evaluated in a study conducted by Yin et al[110] in China and published in 2019. A total of 244 children (160 obese and 84 lean) were recruited. The study involved assessing serum levels of visfatin, high-sensitivity CRP (hs-CRP), TNF-α, IL-6, angiotensin-2, vascular cellular adhesion molecule (VCAM)-1, E-selectin levels, anthropometric measurements, insulin, glucose, and lipid profile. The study reported significantly higher levels of visfatin in obese children compared with controls. There was a positive correlation between serum visfatin with each of the following; BMI, WC, hs-CRP, TNF-α, IL-6, angiotensin-2, VCAM-1, and E-selectin levels, thus supporting the role of elevated serum visfatin in prompting inflammation and IR in obese children[110].
A more recent study on the association between serum visfatin and IR in patients with Alzheimer’s disease (AD) was conducted by Sharifipour et al[111] in Iran and published in 2020. The study involved the measurement of serum visfatin, blood glucose levels, HOMA-IR, and BMI among 60 subjects divided into two groups: 34 subjects with AD and 26 normal subjects. The study reported a significant increase in HOMA-IR and decrease in serum visfatin levels in patients with AD compared with the control group, with no significant change in BMI or serum insulin levels among the two groups. The results reported a negative correlation between serum visfatin and HOMA-IR, providing evidence of systemic IR and lower serum visfatin in nonobese, non-overweight AD patients[111].
The effect of obesity on serum levels of visfatin in patients with PCOS was further evaluated by Abdul-Maksoud et al[112] in a recent study conducted in Egypt and published in 2020. The study involved measuring serum levels of visfatin, insulin and HOMA-IR in 210 women (70 healthy women: 35 obese, and 35 nonobese + 140 with PCOS: 70 nonobese and 70 obese). There was upregulation of visfatin expression in PCOS, thus supporting the use of serum visfatin as a biomarker for PCOS[112].
A recent cross-sectional study was published in 2020 on the correlation between serum visfatin levels and IR in obese women. The study was conducted by Alnowihi et al[113] in Saudi Arabia, where 83 women were recruited for the study between January 2014 and 2016, divided into three groups according to their BMI (35 obese, 15 overweight and 33 lean). The study involved the evaluation of anthropometric measurements, serum levels of visfatin, insulin, lipid profile, and HOMA-IR. The study reported significantly higher levels of visfatin, insulin, IR, and glucose in the obese group when compared with the lean and overweight groups. The study also reported a positive correlation between serum visfatin levels and BMI, WC, HC, insulin, and HOMA-IR[113].
A study on the use of salivary visfatin as a biomarker for diabetes was published in 2021. This was a case–control study conducted by Eroglu Içli and Bildaci in Turkey. It involved the collection of saliva samples from 91 pregnant women between 24 and 28 weeks’ gestation. Salivary levels of visfatin were measured using ELISA. Classification of the patients was based on their OGTT results into two groups: the GDM group, which consisted of 18 patients, and a control group comprising 73 patients with normal OGTT. The study reported a positive correlation between salivary visfatin levels and 1-h glucose levels in the GDM group, a cut-off value of 13.5 ng/mL for the expected levels of salivary visfatin, a sensitivity of 72% and a specificity of 63% of saliva visfatin as a screening test for GDM[114].
Visfatin is important in glucose homeostasis because of its insulin-like actions mediated by NAD biosynthesis. Increased visfatin levels in obesity may indicate a regulatory response to keep blood glucose levels stable. However, exceeding a threshold level appears to be associated with an increase in inflammation, which may contribute to the development of IR, T2DM, and their associated complications, such as cardiovascular and renal diseases. The available evidence supports the potential use of visfatin serum levels as a biomarker for T2DM, including GDM. Salivary visfatin levels appear to be a potential biomarker for IR. However, the number of studies assessing visfatin in saliva in relation to diabetes and related complications is still limited.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shuang WB, China; Srinivasan AR, India; Xia YK, China S-Editor: Chen YL L-Editor: Kerr C P-Editor: Chen YL
| 1. | Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, Ryu MJ, Ko YB, Lee MA, Lee J, Ku BJ, Shong M, Lee KH, Kim HJ. The Roles of Adipokines, Proinflammatory Cytokines, and Adipose Tissue Macrophages in Obesity-Associated Insulin Resistance in Modest Obesity and Early Metabolic Dysfunction. PLoS One. 2016;11:e0154003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Dong Y, Huang G, Wang X, Chu Z, Miao J, Zhou H. Meta-analysis of the association between adiponectin SNP 45, SNP 276, and type 2 diabetes mellitus. PLoS One. 2020;15:e0241078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Zhang Y, Huo Y, He W, Liu S, Li H, Li L. Visfatin is regulated by interleukin6 and affected by the PPARγ pathway in BeWo cells. Mol Med Rep. 2019;19:400-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 621] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 5. | Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15:1851-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 113] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 6. | Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol Med. 2005;11:344-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1269] [Cited by in F6Publishing: 1247] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 8. | Frydelund-Larsen L, Akerstrom T, Nielsen S, Keller P, Keller C, Pedersen BK. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise. Am J Physiol Endocrinol Metab. 2007;292:E24-E31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298:E117-E126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Kukla M, Ciupińska-Kajor M, Kajor M, Wyleżoł M, Żwirska-Korczala K, Hartleb M, Berdowska A, Mazur W. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Pol J Pathol. 2010;61:147-153. [PubMed] [Cited in This Article: ] |
| 11. | Dahl TB, Yndestad A, Skjelland M, Øie E, Dahl A, Michelsen A, Damås JK, Tunheim SH, Ueland T, Smith C, Bendz B, Tonstad S, Gullestad L, Frøland SS, Krohg-Sørensen K, Russell D, Aukrust P, Halvorsen B. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 343] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 12. | Yang R, Chang L, Wang M, Zhang H, Liu J, Wang Y, Jin X, Xu L, Li Y. MAPK pathway mediates the induction of visfatin in neonatal SD rat cardiomyocytes pretreated with glucose. Biomed Rep. 2014;2:282-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Xiao H, Cheng M, Zhang LJ, Liu K. Visfatin expression and genetic polymorphism in patients with traumatic brain injury. Int J Clin Exp Med. 2015;8:9799-9804. [PubMed] [Cited in This Article: ] |
| 14. | Kang YS, Lee MH, Song HK, Kim JE, Ghee JY, Cha JJ, Lee JE, Kim HW, Han JY, Cha DR. Chronic Administration of Visfatin Ameliorated Diabetic Nephropathy in Type 2 Diabetic Mice. Kidney Blood Press Res. 2016;41:311-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 453] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 16. | Dogru T, Sonmez A, Tasci I, Bozoglu E, Yilmaz MI, Genc H, Erdem G, Gok M, Bingol N, Kilic S, Ozgurtas T, Bingol S. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Toruner F, Altinova AE, Bukan N, Arslan E, Akbay E, Ersoy R, Arslan M. Plasma visfatin concentrations in subjects with type 1 diabetes mellitus. Horm Res. 2009;72:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Sommer G, Garten A, Petzold S, Beck-Sickinger AG, Blüher M, Stumvoll M, Fasshauer M. Visfatin/PBEF/Nampt: structure, regulation and potential function of a novel adipokine. Clin Sci (Lond). 2008;115:13-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 310] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Sun Z, Lei H, Zhang Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013;24:433-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | van der Veer E, Nong Z, O'Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Ognjanovic S, Ku TL, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am J Obstet Gynecol. 2005;193:273-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Martin PR, Shea RJ, Mulks MH. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J Bacteriol. 2001;183:1168-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Kim MK, Lee JH, Kim H, Park SJ, Kim SH, Kang GB, Lee YS, Kim JB, Kim KK, Suh SW, Eom SH. Crystal structure of visfatin/pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866. J Mol Biol. 2006;362:66-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754-50763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 737] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 28. | Cuenoud B, Ipek Ö, Shevlyakova M, Beaumont M, Cunnane SC, Gruetter R, Xin L. Brain NAD Is Associated With ATP Energy Production and Membrane Phospholipid Turnover in Humans. Front Aging Neurosci. 2020;12:609517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2558] [Cited by in F6Publishing: 2571] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 30. | Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 31. | de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP, Avogaro A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59:1006-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 32. | Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2318] [Cited by in F6Publishing: 2410] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 33. | Herbert KJ, Cook AL, Snow ET. SIRT1 inhibition restores apoptotic sensitivity in p53-mutated human keratinocytes. Toxicol Appl Pharmacol. 2014;277:288-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Sasca D, Hähnel PS, Szybinski J, Khawaja K, Kriege O, Pante SV, Bullinger L, Strand S, Strand D, Theobald M, Kindler T. SIRT1 prevents genotoxic stress-induced p53 activation in acute myeloid leukemia. Blood. 2014;124:121-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010;1804:1684-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 36. | Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1039] [Cited by in F6Publishing: 1058] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 37. | Pektas SD, Dogan G, Edgunlu TG, Karakas-Celik S, Ermis E, Tekin NS. The Role of Forkhead Box Class O3A and SIRT1 Gene Variants in Early-Onset Psoriasis. Indian J Dermatol. 2018;63:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 38. | Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene. 2012;31:1546-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 39. | Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem. 2010;285:21590-21599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 40. | Panes JD, Godoy PA, Silva-Grecchi T, Celis MT, Ramirez-Molina O, Gavilan J, Muñoz-Montecino C, Castro PA, Moraga-Cid G, Yévenes GE, Guzmán L, Salisbury JL, Trushina E, Fuentealba J. Changes in PGC-1α/SIRT1 Signaling Impact on Mitochondrial Homeostasis in Amyloid-Beta Peptide Toxicity Model. Front Pharmacol. 2020;11:709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | de Gregorio E, Colell A, Morales A, Marí M. Relevance of SIRT1-NF-κB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 42. | Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, Considine T, Riera T, Suri V, White B, Ellis JL, Vlasuk GP, Loh C. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One. 2012;7:e46364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 43. | Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939-1948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 544] [Cited by in F6Publishing: 582] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 44. | Dakroub A, A Nasser S, Younis N, Bhagani H, Al-Dhaheri Y, Pintus G, Eid AA, El-Yazbi AF, Eid AH. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Filippatos TD, Derdemezis CS, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma levels of visfatin/pre-B cell colony-enhancing factor in obese and overweight patients with metabolic syndrome. J Endocrinol Invest. 2007;30:323-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J Endocrinol. 2005;185:R1-R8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | MacLaren R, Cui W, Cianflone K. Visfatin expression is hormonally regulated by metabolic and sex hormones in 3T3-L1 pre-adipocytes and adipocytes. Diabetes Obes Metab. 2007;9:490-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 49. | Bala M, Martin J, Kopp A, Hanses F, Buechler C, Schäffler A. In vivo suppression of visfatin by oral glucose uptake: evidence for a novel incretin-like effect by glucagon-like peptide-1 (GLP-1). J Clin Endocrinol Metab. 2011;96:2493-2501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Marcinkowska M, Lewandowski KC, Lewiński A, Bieńkiewicz M, Basińska-Lewandowska M, Salata I, Randeva HS. Visfatin levels do not change after the oral glucose tolerance test and after a dexamethasone-induced increase in insulin resistance in humans. Endokrynol Pol. 2007;58:188-194. [PubMed] [Cited in This Article: ] |
| 51. | Amjad S, Nisar S, Bhat AA, Shah AR, Frenneaux MP, Fakhro K, Haris M, Reddy R, Patay Z, Baur J, Bagga P. Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol Metab. 2021;49:101195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 52. | Oki K, Yamane K, Kamei N, Nojima H, Kohno N. Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin Endocrinol (Oxf). 2007;67:796-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Liu SW, Qiao SB, Yuan JS, Liu DQ. Association of plasma visfatin levels with inflammation, atherosclerosis and acute coronary syndromes (ACS) in humans. Clin Endocrinol (Oxf). 2009;71:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Romacho T, Valencia I, Ramos-González M, Vallejo S, López-Esteban M, Lorenzo O, Cannata P, Romero A, San Hipólito-Luengo A, Gómez-Cerezo JF, Peiró C, Sánchez-Ferrer CF. Visfatin/eNampt induces endothelial dysfunction in vivo: a role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci Rep. 2020;10:5386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 55. | Buyukaydin B, Guler EM, Karaaslan T, Olgac A, Zorlu M, Kiskac M, Kocyigit A. Relationship between diabetic polyneuropathy, serum visfatin, and oxidative stress biomarkers. World J Diabetes. 2020;11:309-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (2)] |
| 56. | Marseglia L, D'Angelo G, Manti M, Aversa S, Fiamingo C, Arrigo T, Barberi I, Mamì C, Gitto E. Visfatin: New marker of oxidative stress in preterm newborns. Int J Immunopathol Pharmacol. 2016;29:23-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Oita RC, Ferdinando D, Wilson S, Bunce C, Mazzatti DJ. Visfatin induces oxidative stress in differentiated C2C12 myotubes in an Akt- and MAPK-independent, NFkB-dependent manner. Pflugers Arch. 2010;459:619-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Cheleschi S, Tenti S, Mondanelli N, Corallo C, Barbarino M, Giannotti S, Gallo I, Giordano A, Fioravanti A. MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-κB Pathway in Human Osteoarthritic Chondrocytes. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Laudes M, Oberhauser F, Schulte DM, Freude S, Bilkovski R, Mauer J, Rappl G, Abken H, Hahn M, Schulz O, Krone W. Visfatin/PBEF/Nampt and resistin expressions in circulating blood monocytes are differentially related to obesity and type 2 diabetes in humans. Horm Metab Res. 2010;42:268-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur J Clin Invest. 2008;38:71-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Sun Y, Wu Z, Wei L, Liu C, Zhu S, Tang S. High-visfatin levels in women with polycystic ovary syndrome: evidence from a meta-analysis. Gynecol Endocrinol. 2015;31:808-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Duman H, Özyıldız AG, Bahçeci İ, Duman H, Uslu A, Ergül E. Serum visfatin level is associated with complexity of coronary artery disease in patients with stable angina pectoris. Ther Adv Cardiovasc Dis. 2019;13:1753944719880448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 64. | Yu D, Huang B, Wu B, Xiao J. Association of serum vaspin, apelin, and visfatin levels and stroke risk in a Chinese case-control study. Medicine (Baltimore). 2021;100:e25184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Yu PL, Wang C, Li W, Zhang FX. Visfatin Level and The Risk of Hypertension and Cerebrovascular Accident: A Systematic Review and Meta-Analysis. Horm Metab Res. 2019;51:220-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Mahmood N, Junejo AM, Jamal Q, Awan R. Association of visfatin with chronic kidney disease in a cohort of patients with and without diabetes. J Pak Med Assoc. 2010;60:922-926. [PubMed] [Cited in This Article: ] |
| 67. | Malyszko J, Malyszko JS, Pawlak K, Mysliwiec M. Visfatin and apelin, new adipocytokines, and their relation to endothelial function in patients with chronic renal failure. Adv Med Sci. 2008;53:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Kang YS, Song HK, Lee MH, Ko GJ, Han JY, Han SY, Han KH, Kim HK, Cha DR. Visfatin is upregulated in type-2 diabetic rats and targets renal cells. Kidney Int. 2010;78:170-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Shaker O, El-Shehaby A, Zakaria A, Mostafa N, Talaat S, Katsiki N, Mikhailidis DP. Plasma visfatin and retinol binding protein-4 levels in patients with type 2 diabetes mellitus and their relationship to adiposity and fatty liver. Clin Biochem. 2011;44:1457-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, Qi N, Imai S, Yoshino J. NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell Rep. 2016;16:1851-1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 71. | Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 471] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 72. | Skop V, Kontrová K, Zídek V, Pravenec M, Kazdová L, Mikulík K, Sajdok J, Zídková J. Autocrine effects of visfatin on hepatocyte sensitivity to insulin action. Physiol Res. 2010;59:615-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Kim DS, Kang S, Moon NR, Park S. Central visfatin potentiates glucose-stimulated insulin secretion and β-cell mass without increasing serum visfatin levels in diabetic rats. Cytokine. 2014;65:159-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Sawicka K, Michalska-Jakubus M, Potembska E, Kowal M, Pietrzak A, Krasowska D. Visfatin and chemerin levels correspond with inflammation and might reflect the bridge between metabolism, inflammation and fibrosis in patients with systemic sclerosis. Postepy Dermatol Alergol. 2019;36:551-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Zheng LY, Xu X, Wan RH, Xia S, Lu J, Huang Q. Association between serum visfatin levels and atherosclerotic plaque in patients with type 2 diabetes. Diabetol Metab Syndr. 2019;11:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Moulton KS. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr Opin Lipidol. 2006;17:548-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Erten M. Visfatin as a Promising Marker of Cardiometabolic Risk. Acta Cardiol Sin. 2021;37:464-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 78. | Lee WJ, Wu CS, Lin H, Lee IT, Wu CM, Tseng JJ, Chou MM, Sheu WH. Visfatin-induced expression of inflammatory mediators in human endothelial cells through the NF-kappaB pathway. Int J Obes (Lond). 2009;33:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Bae YH, Bae MK, Kim SR, Lee JH, Wee HJ, Bae SK. Upregulation of fibroblast growth factor-2 by visfatin that promotes endothelial angiogenesis. Biochem Biophys Res Commun. 2009;379:206-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Adya R, Tan BK, Chen J, Randeva HS. Pre-B cell colony enhancing factor (PBEF)/visfatin induces secretion of MCP-1 in human endothelial cells: role in visfatin-induced angiogenesis. Atherosclerosis. 2009;205:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 82. | Kang YS, Kang YG, Park HJ, Wee HJ, Jang HO, Bae MK, Bae SK. Melatonin inhibits visfatin-induced inducible nitric oxide synthase expression and nitric oxide production in macrophages. J Pineal Res. 2013;55:294-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Pacher P, Obrosova IG, Mabley JG, Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 84. | Romacho T, Azcutia V, Vázquez-Bella M, Matesanz N, Cercas E, Nevado J, Carraro R, Rodríguez-Mañas L, Sánchez-Ferrer CF, Peiró C. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia. 2009;52:2455-2463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Zheng M, Lu N, Ren M, Chen H. Visfatin associated with major adverse cardiovascular events in patients with acute myocardial infarction. BMC Cardiovasc Disord. 2020;20:271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Yu XY, Qiao SB, Guan HS, Liu SW, Meng XM. Effects of visfatin on proliferation and collagen synthesis in rat cardiac fibroblasts. Horm Metab Res. 2010;42:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Chen R, Ovbiagele B, Feng W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am J Med Sci. 2016;351:380-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 88. | Janghorbani M, Hu FB, Willett WC, Li TY, Manson JE, Logroscino G, Rexrode KM. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses' Health Study. Diabetes Care. 2007;30:1730-1735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 89. | Ilhan N, Susam S, Canpolat O, Belhan O. The emerging role of leptin, Adiponectin and Visfatin in Ischemic/Hemorrhagic stroke. Br J Neurosurg. 2019;33:504-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus. Metabolism. 2007;56:451-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 91. | Jitraknatee J, Ruengorn C, Nochaiwong S. Prevalence and Risk Factors of Chronic Kidney Disease among Type 2 Diabetes Patients: A Cross-Sectional Study in Primary Care Practice. Sci Rep. 2020;10:6205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 92. | Hsu CY, Huang PH, Chen TH, Chiang CH, Leu HB, Huang CC, Chen JW, Lin SJ. Increased Circulating Visfatin Is Associated With Progression of Kidney Disease in Non-Diabetic Hypertensive Patients. Am J Hypertens. 2016;29:528-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Bessa SS, Hamdy SM, El-Sheikh RG. Serum visfatin as a non-traditional biomarker of endothelial dysfunction in chronic kidney disease: an Egyptian study. Eur J Intern Med. 2010;21:530-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Heo YJ, Choi SE, Jeon JY, Han SJ, Kim DJ, Kang Y, Lee KW, Kim HJ. Visfatin Induces Inflammation and Insulin Resistance via the NF-κB and STAT3 Signaling Pathways in Hepatocytes. J Diabetes Res. 2019;2019:4021623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 95. | Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol. 2004;286:F1039-F1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 96. | Huang Q, Guo Y, Zeng H, Xie W, Yan H, Ding H. Visfatin stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Endocr Res. 2011;36:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Boini KM, Zhang C, Xia M, Han WQ, Brimson C, Poklis JL, Li PL. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim Biophys Acta. 2010;1801:1294-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Yilmaz MI, Saglam M, Carrero JJ, Qureshi AR, Caglar K, Eyileten T, Sonmez A, Oguz Y, Aslan I, Vural A, Yenicesu M, Stenvinkel P, Lindholm B, Axelsson J. Normalization of endothelial dysfunction following renal transplantation is accompanied by a reduction of circulating visfatin/NAMPT. A novel marker of endothelial damage? Clin Transplant. 2009;23:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Nourbakhsh M, Nourbakhsh M, Gholinejad Z, Razzaghy-Azar M. Visfatin in obese children and adolescents and its association with insulin resistance and metabolic syndrome. Scand J Clin Lab Invest. 2015;75:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 100. | Salama HM, Galal A, Motawie AA, Kamel AF, Ibrahim DM, Aly AA, Hassan EA. Adipokines Vaspin and Visfatin in Obese Children. Open Access Maced J Med Sci. 2015;3:563-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Gul OO, Cander S, Gul B, Açıkgoz E, Sarandol E, Ersoy C. Evaluation of insulin resistance and plasma levels for visfatin and resistin in obese and non-obese patients with polycystic ovary syndrome. Eur Cytokine Netw. 2015;26:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Liang Z, Wu Y, Xu J, Fang Q, Chen D. Correlations of serum visfatin and metabolisms of glucose and lipid in women with gestational diabetes mellitus. J Diabetes Investig. 2016;7:247-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 103. | Tsiotra PC, Halvatsiotis P, Patsouras K, Maratou E, Salamalekis G, Raptis SA, Dimitriadis G, Boutati E. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides. 2018;101:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 104. | Hetta HF, Ez-Eldeen ME, Mohamed GA, Gaber MA, ElBadre HM, Ahmed EA, Abdellatief RB, Abd-ElBaky RM, Elkady A, Nafee AM, Zahran AM, Ahmad M. Visfatin Serum Levels in Obese Type 2 Diabetic Patients: Relation to Proinflammatory Cytokines and Insulin Resistance. Egypt J Immunol. 2018;25:141-151. [PubMed] [Cited in This Article: ] |
| 105. | Srinivasan M, Meadows ML, Maxwell L. Assessment of Salivary Adipokines Resistin, Visfatin, and Ghrelin as Type 2 Diabetes Mellitus Biomarkers. Biochem Res Int. 2018;2018:7463796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 106. | Desai P, Donovan L, Janowitz E, Kim JY. The Clinical Utility of Salivary Biomarkers in the Identification of Type 2 Diabetes Risk and Metabolic Syndrome. Diabetes Metab Syndr Obes. 2020;13:3587-3599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Souvannavong-Vilivong X, Sitticharoon C, Klinjampa R, Keadkraichaiwat I, Sripong C, Chatree S, Sririwichitchai R, Lertbunnaphong T. Placental expressions and serum levels of adiponectin, visfatin, and omentin in GDM. Acta Diabetol. 2019;56:1121-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Bawah AT, Seini MM, Abaka-Yawason A, Alidu H, Nanga S. Leptin, resistin and visfatin as useful predictors of gestational diabetes mellitus. Lipids Health Dis. 2019;18:221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Manoharan B, Bobby Z, Dorairajan G, Vinayagam V, Packirisamy RM. Adipokine levels and their association with insulin resistance and fetal outcomes among the newborns of Indian gestational diabetic mothers. Saudi Med J. 2019;40:353-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 110. | Yin C, Hu W, Wang M, Xiao Y. The role of the adipocytokines vaspin and visfatin in vascular endothelial function and insulin resistance in obese children. BMC Endocr Disord. 2019;19:127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 111. | Sharifipour E, Sharifimoghadam S, Hassanzadeh N, Ghasemian Mojarad N, Ghoreishi A, Hejazi SA, Rohampour K. Altered plasma visfatin levels and insulin resistance in patients with Alzheimer's disease. Acta Neurol Belg. 2020;120:901-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Abdul-Maksoud RS, Zidan HE, Saleh HS, Amer SA. Visfatin and SREBP-1c mRNA Expressions and Serum Levels Among Egyptian Women with Polycystic Ovary Syndrome. Genet Test Mol Biomarkers. 2020;24:409-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 113. | Alnowihi SM, Al Doghaither HA, Osman NN. Serum visfatin concentration and its relationship with sex hormones in obese Saudi women. Int J Health Sci (Qassim). 2020;14:9-13. [PubMed] [Cited in This Article: ] |
| 114. | Eroglu Içli H, Bildaci TB. Measuring visfatin levels in saliva: an alternative approach to gestational diabetes screening. Arch Endocrinol Metab. 2021;65:747-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |