Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2520
Peer-review started: December 30, 2019
First decision: April 1, 2020
Revised: April 24, 2020
Accepted: May 20, 2020
Article in press: May 20, 2020
Published online: June 26, 2020
Recent innovations in intensive care have improved the prognosis of patients with severe brain injuries and brought more patients with disorders of consciousness (DoC). Data are lacking regarding the long-term outcomes of those patients in China. It is necessary to study the long-term outcomes of patients with prolonged DoC in light of many factors likely to influence crucial decisions about their care and their life.
To present the preliminary results of a DoC cohort.
This was a two-center prospective cohort study of inpatients with vegetative state (VS)/unresponsive wakefulness syndrome (UWS). The study outcomes were the recovery from VS/UWS to minimally conscious state (MCS) and the long-term status of patients with prolonged DoC considered in VS/UWS or MCS for up to 6 years. The patients were evaluated using the Glasgow coma scale, coma recovery scale-revised, and Glasgow outcome scale. The endpoint of follow-up was recovery of full consciousness or death. The changes in the primary clinical outcome improvement in clinical diagnosis were evaluated at 12 mo compared with baseline.
The study population included 93 patients (62 VS/UWS and 31 MCS). The post-injury interval range was 28-634 d. Median follow-up was 20 mo (interquartile range, 12-37 mo). At the endpoint, 33 transitioned to an emergence from MCS or full consciousness, eight had a locked-in syndrome, and there were 35 patients remaining in a VS/UWS and 11 in an MCS. Seven (including one locked-in syndrome) patients (7.5%) died within 12 mo of injury. Compared with the unresponsive group (n = 52) at 12 mo, the responsive group (n = 41) had a higher proportion of males (87.8% vs 63.5%, P = 0.008), shorter time from injury (median, 40.0 d vs 65.5 d, P = 0.006), higher frequency of vascular etiology (68.3% vs 38.5%, P = 0.007), higher Glasgow coma scale score at admission (median, 9 vs 6, P < 0.001), higher coma recovery scale-revised score at admission (median, 9 vs 2.5, P < 0.001), at 1 mo (median, 14 vs 5, P < 0.001), and at 3 mo (median, 20 vs 6, P < 0.001), lower frequency of VS/UWS (36.6% vs 90.0%, P < 0.001), and more favorable Glasgow outcome scale outcome (P < 0.001).
Patients with severe DoC, despite having strong predictors of poor prognosis, might recover consciousness after a prolonged time of rehabilitation. An accurate initial diagnosis of patients with DoC is critical for predicting outcome and a long-term regular follow-up is also important.
Core tip: Data are lacking regarding the long-term outcomes of patients with disorders of consciousness (DoC) in China. This was a two-center prospective cohort study of inpatients with prolonged DoC for up to 6 years, included 93 patients (62 vegetative state/unresponsive wakefulness syndrome and 31 minimally conscious state). The results showed that patients with severe DoC, despite having strong predictors of poor prognosis, might recover consciousness after a prolonged time of rehabilitation.
- Citation: Chen WG, Li R, Zhang Y, Hao JH, Du JB, Guo AS, Song WQ. Recovery from prolonged disorders of consciousness: A dual-center prospective cohort study in China. World J Clin Cases 2020; 8(12): 2520-2529
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2520.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2520
Recent innovations in intensive care has improved the prognosis of patients with severe brain injuries, which are usually due to traumatic brain injuries (TBI), hypoxic ischemic encephalopathy (HIE), or stroke[1]. Patients may progress from coma to disorders of consciousness (DoC), which include the “vegetative state” (VS) (recently termed “unresponsive wakefulness syndrome”, UWS) and the “minimally conscious state” (MCS)[1-3]. Sometimes these clinical conditions are temporary, but they may also be the stabilized state of the acute brain event and the patients will never recover consciousness; they then enter the stage of prolonged DoC[1-3].
Reliable epidemiologic data on DoC and emergence from prolonged DoC are scarce. When using a proxy definition, the prevalence is estimated at 5000-42000 for VS/UWS and 112000-280000 for MCS[4]. In Europe, the prevalence is 0.2-6.1 cases per 100000 population[5]. The most important concern for DoC patients and their family is prognosis because it is central to the decisions regarding medical treatment and eventual rehabilitation therapy, and profound ethical issues are involved[6,7]. Indeed, there is a lack of evidence in the literature regarding late improvements in DoC patients and an even more deplorable lack of accurate epidemiological data about the long-term outcomes of DoC in China[8,9]. Lifetime care for a patient with prolonged DoC can exceed $1000000[1-3]. In China, the accessibility to proper management services for DoC patients is a major issue, especially for the areas far from large cities[9]. It is therefore necessary to study the long-term outcomes of patients in prolonged DoC in light of many factors likely to influence crucial decisions about their care and their life.
Because of this lack of knowledge on the long-term outcome of patients with DoC, we implemented a two-center prospective study that aimed to collect data about the patients with prolonged DoC admitted to two specialized units in Beijing and Nantong (China). The present real-world prospective cohort study aimed to assess the recovery rate of the inpatients with a VS/UWS to MCS and to analyze and compare the long-term outcomes of patients with prolonged DoC considered in VS/UWS or MCS 1 year after the standard follow-up in the prognostic study, then yearly afterwards up to 6 years, hoping to investigate the factors associated with a higher likelihood of transition to MCS at rehabilitation facilities in China and further our understanding of the rehabilitation potential of the most severely affected patients with DoC. We also hypothesized that there was potential for some level of recovery despite the presence of strong unfavorable prognostic markers. Here we present the preliminary results six years after the start of the study.
Between October 2012 and September 2018, we recruited all consecutive comatose patients with severe brain damage admitted to the department of rehabilitation medicine of the Affiliated Hospital of Nantong University, Nantong and Xuan Wu Hospital, Capital Medical University, Beijing, China. The study was approved by the ethics committee of Xuan Wu hospital according to the guidelines of the Helsinki declaration (1964 and 2000). Written informed consent was obtained from all patients’ legal guardians for the use of their clinical data for research purposes.
The inclusion criteria for patient enrolment were: (1) Native Chinese speaker; (2) Diagnosis of prolonged DoC (VS/UWS or MCS, at least 28 d) after brain injury of traumatic or non-traumatic (e.g., anoxic, vascular, and encephalitic) causes; (3) Normal vision and auditory function; (4) Normal cognitive interpersonal functions prior to brain injury; (5) No history of vital organ injury or failure, stroke, cancer or brain tumor, neuropathy, brain surgery, or familial psychosis; and (6) The patient and his/her family members cooperated with treatment. The exclusion criteria were: (1) Artificial therapeutic coma; or (2) History of brain injury prior to the event leading to the prolonged DoC considered for enrollment in the present study.
All patients enrolled in the study were assessed using the Glasgow coma scale (GCS)[10] and coma recovery scale-revised (CRS-R)[11] at least three times within 1 wk of admission. For patients with major medical complications, the GCS and CRS-R evaluations were performed when the medical condition had stabilized. One month after starting the study, all data were evaluated to ensure their uniformity and accuracy.
In order to examine the outcomes in this study and obtain a significant prognostic value, follow-up was conducted at 1, 3, 6, and 12 mo after admission. Most patients were regularly managed in a single regional rehabilitation center or long-stay hospital dedicated to patients in prolonged DoC. Phone call follow-up was performed if the patients were discharged from or transferred to other hospitals during the 12-mo follow-up. In the case where the patient had already died, the physician had to provide the last cognitive and medical state of the patient. The investigators determining outcomes were blinded to test results.
All patients received rehabilitation wherever possible and appropriate. The rehabilitation program was based on passive joint movements and placing the patients into an upright sitting position using a tilt table.
The endpoint of follow-up was recovery of full consciousness or death. The state of consciousness and emergence from MCS (EMCS) was assessed by the CRS-R and the functional disability according to the Glasgow outcome scale (GOS) outcome[12].
The changes in the primary clinical outcome were evaluated at 12 mo compared with baseline. Patients who progressed to EMCS or regained full consciousness [including the “locked-in syndrome” (LIS)] were classified as “awareness” (good outcome); those whose clinical diagnosis remained in VS/UWS or MCS (including MCS+/-) were classified as “unawareness” (poor outcome). Patients who recovered awareness and then died because of new etiologic events and those who died remaining unawareness were included in this category.
Statistical analyses were conducted using SPSS 22.0 (IBM, Armonk, NY, United States). Descriptive results are summarized as proportions (percentages), means ± SD, or medians (interquartile ranges). Categorical variables were tested using the χ2 test or Fisher exact test. Continuous variables were compared with the Student’s t test or the Mann-Whitney U test. The cumulative awareness rates were calculated using the Kaplan-Meier method, and the differences between groups were assessed by the log-rank test. P values < 0.05 were considered statistically significant.
The study population was composed of 93 patients (62 VS/UWS and 31 MCS, 43 from Beijing and 50 from Nantong). Median follow-up was 20 mo (interquartile range, 12-37 mo). Demographic data of the patients are shown in Supplementary Table 1. Over the 6-year period, for the 93 patients initial GCS score was ≤ 8 at onset. The post-injury interval range was 28-634 d. The CRS-R total score range was 0-17. There were 69 males (74.2%) and 24 females (25.8%). Mean age was 49.8 ± 16.9 years (range, 7-85 years). Ten had HIE (10.8%), 35 had TBI (37.6%), and the remaining 48 had stroke (51.6%). There were 62 (66.7%) patients in VS/UWS (age range: 7-80 years; time since injury range: 28-496 d; CRS-R score range: 0-8) and 31 in MCS (age range: 9-85 years; time since injury range: 28-634 d; CRS-R score range: 6-17). All of the patients were put on a specialized program for slow-to-recover brain injury. The two diagnostic groups had no significant differences in age, duration, or etiology, but there was a significant difference in sex (P = 0.04) (Table 1).
| Characteristic | All (n = 93) | Group | P value | |
| VS/UWS (n = 62) | MCS (n = 31) | |||
| Age, yr, mean ± SD | 49.8 ± 16.9 | 49.7 ± 16.4 | 50.1 ± 18.0 | 0.897 |
| Maximum | 85 | 80 | 85 | |
| Minimum | 7 | 7 | 9 | |
| Sex, male, n (%) | 69 (74.2) | 42 (67.7) | 27 (87.1) | 0.044 |
| Post injury days, median (Q25, Q75) | 60 (35, 98) | 59 (37, 95.5) | 61 (32, 126) | 0.446 |
| Maximum | 634 | 496 | 634 | |
| Minimum | 28 | 28 | 28 | |
| Etiology, n (%) | ||||
| Traumatic | 35 (37.6) | 25 (40.3) | 10 (32.3) | 0.368 |
| Vascular | 48 (51.6) | 29 (46.8) | 19 (61.3) | |
| Anoxic | 10 (10.8) | 8 (2.9) | 2 (6.5) | |
| GCS score at admission, median (Q25, Q75) | 8 (5.5, 9) | 6 (4, 8) | 10 (9, 11) | < 0.001 |
| CRS-R score at admission, median (Q25, Q75) | 5 (2, 9) | 3 (1, 5) | 10.5 (9, 13) | < 0.001 |
| Outcome, n (%) | ||||
| VS/UWS | 35 (37.6) | 35 (56.5) | 0 (0.0) | < 0.001 |
| MCS- | 10 (10.8) | 6 (9.7) | 4 (12.9) | |
| MCS+ | 1 (1.1) | 1 (1.6) | 0 (0.0) | |
| EMCS | 4 (4.3) | 2 (3.2) | 2 (6.5) | |
| CS | 29 (31.2) | 11 (17.7) | 18 (58.1) | |
| LIS | 7 (7.5) | 1 (1.6) | 6 (19.4) | |
| Death | 7 (7.5) | 6 (9.7) | 1 (3.2) | |
| GOS outcome, n (%) | ||||
| Death (1) | 7 (7.5) | 6 (9.7) | 1 (3.2) | < 0.001 |
| VS/UWS (2) | 35 (37.6) | 35 (56.5) | 0 (0.0) | |
| Severe disability (3) | 24 (25.8) | 11 (17.7) | 13 (41.9) | |
| Moderate disability (4) | 16 (17.2) | 5 (8.1) | 11 (35.5) | |
| Good (5) | 11 (11.8) | 5 (8.1) | 6 (19.4) | |
Six years after study start, 88/93 patients (61 in VS/UWS and 27 in MCS) had available 12-mo follow-up data; five patients (one in VS/UWS and four with MCS) had not reached the 12-mo time point. We present the preliminary results of the patients with 12 mo of follow-up at the end of the 6-year observation period. At that time point, of the 93 patients, 33 transitioned to an EMCS or full consciousness, eight had an LIS, and there were 35 patients remaining in a VS/UWS and 11 in an MCS. Seven (including one LIS) patients (7.5%) died within 12 mo of injury: Two from infectious diseases, one from respiratory complications, one from heart failure, one from intracranial hemorrhage relapse, and two from an unrecorded/unknown cause. It should be noted that no patient became vegetative during the follow-up period.
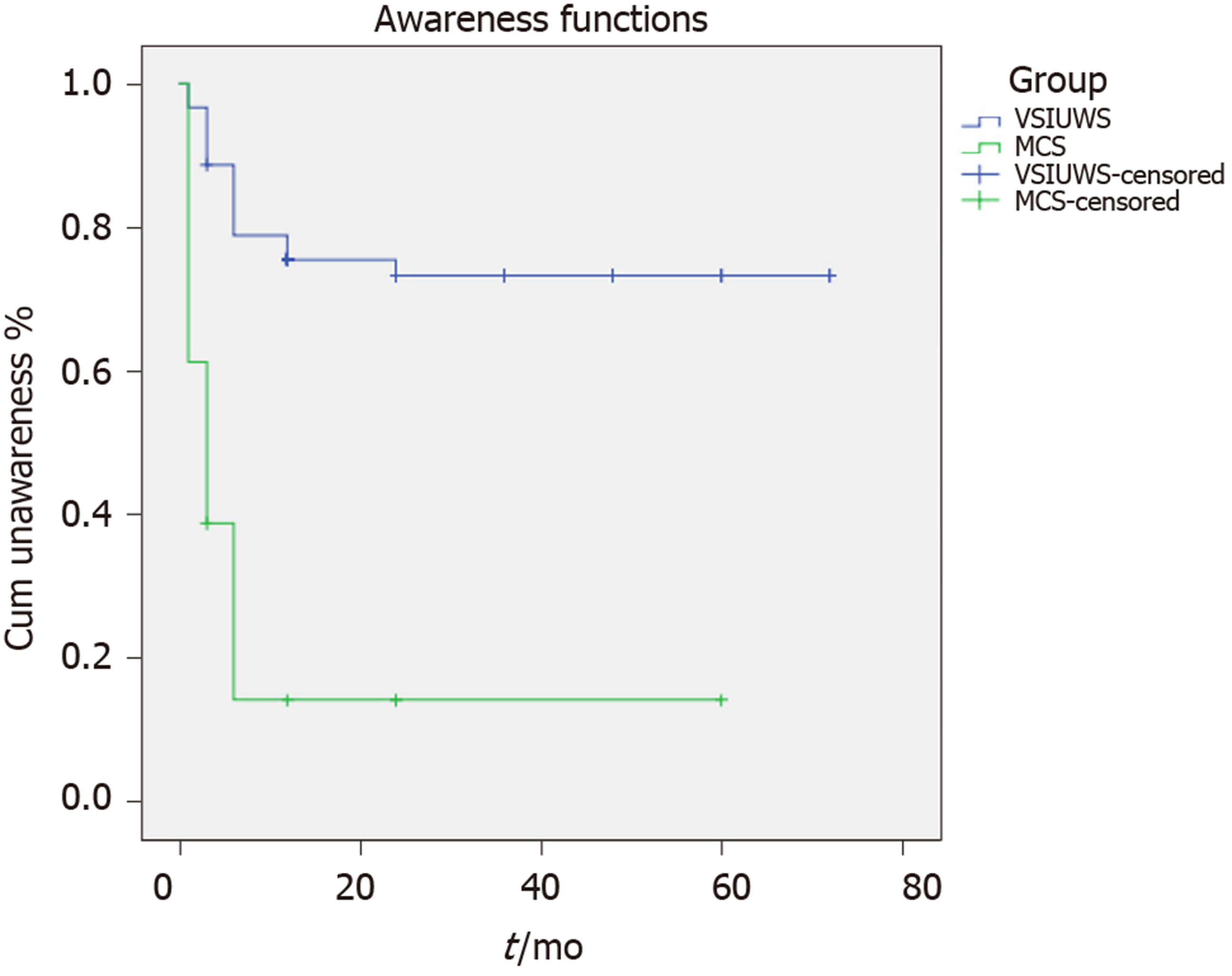
The 62 patients who were in a VS/UWS had a mean age of 49.7 years (range: 7-80) and a median number of days post-injury of 59 (range: 28-496). The median CRS-R total score at admission was 3 (range: 0-8). Of the 62 patients in the VS/UWS group at 1 mo, two patients were corrected as LIS (one died at 12-mo follow-up), and 16 transitioned to MCS (13 in MCS- and three in MCS+), while 12 patients recovered consciousness (including two in EMCS) (19.4%), three died, and one was in MCS- at 12 mo of follow-up. Of the remaining 44 patients at the end of follow-up, only two recovered full consciousness (one recovered full consciousness at 24 mo), five transitioned to MCS-, and two died, while 35 were still in a VS/UWS (56.5%). At that time, of the 31 patients with MCS (22 in MCS- and nine in MCS+) at admission, except six who showed an LIS and one who died, MCS+ in all other five patients and MCS- in 15 patients transitioned to EMCS or full conscious (64.5%), and MCS- in the other four patients did not change (12.9%). The Kaplan-Meier plot of time from injury to awareness is shown in Figure 1.
Table 2 presents the factors associated with improved responsiveness. Compared with the unresponsive group at 12 mo, the responsive group had a higher proportion of males (87.8% vs 63.5%, P = 0.008), a shorter time from injury (median, 40 d vs 65 d, P = 0.006), higher frequency of vascular etiology and lower frequency of traumatic etiology (P = 0.007), higher GCS score at admission (median, 9 vs 6, P < 0.001), higher CRS-R score at admission (median, 9 vs 2.5, P < 0.001), higher CRS-R scores at 1 mo (median, 14 vs 5, P < 0.001) and 3 mo (median, 20 vs 6, P < 0.001), lower frequency of VS/UWS diagnosis (36.6% vs 90.4%, P < 0.001), and more favorable GOS outcome (P < 0.001). The functional outcomes of the 41 awareness patients were: 13 with severe disabilities (31.7%), 16 with moderate disabilities (39.0%), 11 with good outcome (26.8%), and one death (2.4%).
| Characteristic | Outcome | P value | |
| Unawareness (n = 52) | Awareness (n = 41) | ||
| Age, yr, mean ± SD | 49.4 ± 16.2 | 50.3 ± 17.8 | 0.797 |
| Maximum | 79 | 85 | |
| Minimum | 7 | 13 | |
| Sex, male, n (%) | 33 (63.5) | 36 (87.8) | 0.008 |
| Days post injury, median (Q25, Q75) | 65.5 (38.5, 136.5) | 40.0 (30.0, 87.0) | 0.006 |
| Maximum | 496 | 634 | |
| Minimum | 29 | 28 | |
| Etiology, n (%) | |||
| Traumatic | 23 (44.2) | 12 (29.3) | 0.007 |
| Vascular | 20 (38.5) | 28 (68.3) | |
| Anoxic | 9 (17.3) | 1 (2.4) | |
| GCS score at admission, median (Q25, Q75) | 6 (4, 8) | 9 (7.5, 10) | < 0.001 |
| CRS-R score at admission, median (Q25, Q75) | 2.5 (1, 5) | 9 (6, 12) | < 0.001 |
| CRS-R score change at 1 mo, median (Q25, Q75) | 5 (3, 7) | 14 (10, 19) | < 0.001 |
| CRS-R score change at 3 mo, median (Q25, Q75) | 6 (4, 8) | 20 (17, 22) | < 0.001 |
| Diagnosis, n (%) | |||
| VS/UWS | 47 (90.4) | 15 (36.6) | < 0.001 |
| MCS- | 5 (9.6) | 17 (41.5) | |
| MCS+ | 0 | 9 (22.0) | |
| GOS outcome, n (%) | |||
| Death (1) | 6 (11.5) | 1 (2.4) | < 0.001 |
| VS/UWS (2) | 35 (67.3) | 0 | |
| Severe disability (3) | 11 (21.2) | 13 (31.7) | |
| Moderate disability (4) | 0 | 16 (39.0) | |
| Good (5) | 0 | 11 (26.8) | |
Table 3 presents the univariable and multivariable analyses for recovery of awareness as the outcome. In the univariable analysis, VS/UWS vs MCS (OR = 16.29, 95%CI: 5.32-49.93, P < 0.001), etiology of stroke vs TBI (OR = 2.68, 95%CI: 1.09-6.62, P = 0.03), GCS score at admission (OR = 1.77, 95%CI: 1.37-2.28, P < 0.001), and CRS-R score at admission (OR = 1.59, 95%CI: 1.32-1.91, P < 0.001) were associated with recovered awareness. In the multivariable analysis, days post injury (OR = 0.99, 95%CI: 0.974-0.996, P = 0.006), GCS score at admission (OR = 0.31, 95%CI: 0.12-0.82, P = 0.02), and CRS-R score at admission (OR = 4.82, 95%CI: 1.94-11.95, P = 0.001) were independently associated with recovered awareness.
| Univariable analysis | Multivariable analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age | 1.003 (0.979, 1.028) | 0.794 | 1.004 (0.962, 1.048) | 0.860 |
| Sex | 0.241 (0.081, 0.719) | 0.011 | 0.949 (0.196, 4.600) | 0.948 |
| Days post injury | 0.997 (0.992, 1.001) | 0.172 | 0.985 (0.974, 0.996) | 0.006 |
| Group (VS/UWS vs MCS) | 16.293 (5.317, 49.925) | < 0.001 | 0.083 (0.004, 1.701) | 0.106 |
| Etiology | ||||
| Stroke vs TBI | 2.683 (1.087, 6.623) | 0.032 | 1.824 (0.410, 8.126) | 0.430 |
| HIE vs TBI | 0.213 (0.024, 1.885) | 0.165 | 0.006 (0.000, 4.798) | 0.134 |
| GCS score at admission | 1.766 (1.370, 2.277) | < 0.001 | 0.312 (0.119, 0.823) | 0.019 |
| CRS-R score at admission | 1.590 (1.322, 1.913) | < 0.001 | 4.822 (1.946, 11.949) | 0.001 |
The results suggest that patients with severe DoC, without distinction for age, etiology, duration, or extent of DoC, may recover consciousness, despite having strong predictors of poor prognosis. The present preliminary analysis provides information on consecutive patients with severe brain injury and DoC. The number of patients with VS/UWS was higher than that of patients with MCS, and the frequency of vascular etiology was higher than that of traumatic and anoxic etiologies. These findings are supported by an Italian multicenter rehabilitation registry[13], but not by a German multicenter rehabilitation registry[14], where the frequency of anoxic etiology was higher than that of the vascular or traumatic etiology, and time since injury was shorter (i.e., mean 28 d). Therefore, the discrepancies among registries could likely be related to the differences in the eligibility criteria.
The first aim of this present longitudinal study was to present the process of consciousness recovery on clinical evolution after 12-mo of follow-up. Patients with severe brain injury, irrespective of the cause, often develop VS/UWS after an initial coma and subsequently develop MCS, but not always reaching full consciousness or self-awareness. Upon study entry, the patients with VS/UWS and those with MCS showed different clinical scale scores, which is consistent with the diagnoses of the patients, but the two groups had similar age, time post-injury, and etiology, which are different from the findings of previous studies[15,16]. So far, 26/31 MCS patients, but only 16/62 VS/UWS patients, in our cohort recovered consciousness or corrected to an LIS, and thus the clinical outcomes were significantly different between the two diagnostic groups. This finding is supported by previous studies of patients with MCS showing a better short-[17-19] and long-term outcome (> 12 mo post-injury)[17,20]. Nevertheless, the patient populations remain heterogeneous among the studies and within the studies in terms of time post-injury, frequency of traumatic or non-traumatic etiology, level of consciousness, and sample size.
The secondary aim of this longitudinal study was to examine the factors affecting prognosis. Anoxic DoC has a worse prognosis than vascular or traumatic DoC, and longer VS/UWS durations are associated with a lower likelihood of regaining awareness. Unfortunately, only one patient with HIE emerged from MCS, while all the other patients with HIE remained VS/UWS or MCS- after up to 4-year follow-up. Thus, our observations are in line with the generally very poor functional prognosis of HIE. It has been suggested that VS/UWS be considered permanent when lasting > 12 mo after TBI or 3 mo after the original anoxic/vascular condition, but late recovery of consciousness has been reported[21,22]. In any case, emergence can occur months or even years after DoC, and long time to emergence always leads to severe or extremely severe functional disability and poor functioning. Moreover, our study included DoC patients who entered the rehabilitation centers and were followed for 12 mo. Therefore, compared with previous studies, the patients’ duration of onset was longer, which was more likely to reflect the late prognosis of DoC. Lammi et al[23] found that recovery after a prolonged MCS is highly variable among patients. MCS duration does not seem to influence the psychosocial condition or functional recovery of the patients. Patients in VS/UWS with a transition to MCS within 8 wk of DoC onset are likely to recover to higher functional levels, with many of them able to go home and resume daily activities[24], supporting the present study. Since our cohort had enrolled more MCS patients with vascular etiology, some of whom were corrected to LIS, more patients with vascular etiology recovered from DoC. It has to be stressed that the data presented here are preliminary, but they appear to confirm that patients with anoxic DoC have worse outcomes than patients with traumatic or vascular DoC, as supported by the literature[17,20,24-26].
In the present study, although the preliminary data cannot provide definitive diagnostic and prognostic information, they strongly indicate that patients in prolonged DoC should not be pooled for prognostic purposes at admission. First, in all 93 patients, we screened out eight cases of LIS, who should not be classified as prolonged DOC strictly. Second, the analysis showed that the potential for unfavorable outcome was significantly greater in VS/UWS than in MCS. Thirty-one patients in the MCS group had a median duration of 61 d, slightly longer than those reported in previous studies. However, the results suggested that six patients were corrected to an LIS, and that 80% of the remaining 25 patients converted to EMCS within 6 mo, while 18 patients regained full consciousness. Of the 62 patients with VS/UWS, two were corrected to an LIS, 13 entered to EMCS, 11 of whom recovered complete consciousness, and 10 of these 11 patients had been converted to MCS at 1-mo follow-up. Another patient achieved EMCS only one year after follow-up, and eventually recovered full consciousness at 2-year follow-up. We found that, contrary to VS/UWS, the permanence of MCS cannot be established 1 year after coma onset. Between 3 and 6 mo after coma onset, most patients in MCS emerged from this condition. At this stage, patients in MCS seem to have an important potential for recovery given that, at 1-year follow-up, approximately 50% of them recovered at least partial independence for locomotion and activities of daily living. After 6 mo, that potential seems to drop given that, among four patients who remained in MCS for more than 6 mo, none regained full consciousness. After 6 mo in the MCS state, the prognosis becomes even worse, which is the same as in VS/UWS. Among 39 patients who remained in VS/UWS for 3 mo of follow-up, only one regained full ambulation and 34 remained in this condition at the endpoint. To our knowledge, only one study has reported the outcome at 1 year after patients were considered in MCS after 6 mo of follow-up and this outcome was poor because none of the four patients could live with family; all needed total assistance for activities of daily living and had to be institutionalized[20]. Unsurprisingly, our data report very poor functional outcomes, because 47 patients with DoC remained in the same condition or died during follow-up. Nevertheless, EMCS after 1 year was not exceptional and also occurred mainly during the second year of coma. Long MCS duration is associated with a lower probability of recovery, in a similar way to that for VS/UWS. Altogether, these observations tend to confirm the difference in outcomes already suggested between MCS and VS/UWS. This difference lends further support to the need for a clear distinction between MCS and VS/UWS and, thus, for better ways to differentiate the two conditions. Although the design of our study precluded a detailed description of the clinical and functional status of patients who emerged from MCS, most patients remained severely disabled on the motor and cognitive points of view.
Our findings emphasize the clinical importance to follow patients with DoC. VS/UWS can be considered permanent in TBI patients after 12 mo and non-TBI patients after 3 mo. This view has been challenged. Avesani et al[27] described two people diagnosed with VS/UWS who, at respectively 6 and 12 mo after their original trauma, had achieved a moderate level of functional independence following a significant motor and cognitive recovery after 5 years and suggested that it is important to conduct regular follow-ups to better evaluate changes and, if it is necessary, to re-adjust the rehabilitation accordingly. Also, our results have shown that patients admitted to rehabilitation in an unresponsive state can show considerable recovery even after a prolonged time. Although slow regeneration of axons in patients with brain injury could be an intriguing hypothesis as a biological mechanism of delayed recovery, no neurological interpretation of late recovery from VS/UWS has been advanced and early predictors might not apply[8]. Our previous study has supported the use of ERP in clinical practice to predict the likelihood of recovery from DoC[28]. An accurate initial diagnosis of patients with DoC is critical for predicting outcome. Misdiagnosis may lead to a worse prognosis for patients, which may restrict their access to recovery. The adoption of homogeneous assessment procedures will provide valuable and reliable data for investigating clinical issues regarding the diagnosis and prognosis of DoC, as well as the effectiveness of treatment strategies for long-term clinical progression. At the same time, a decision to conduct or withhold specialized neurorehabilitation in traumatic or non-traumatic DoC survivors should be considered comprehensively.
A strength of this study is that it included all patients with brain injury admitted to two specialized centers. Of course, enrolling patients at rehabilitation facilities will bias the data and show more positive outcomes than what could be observed in non-specialized centers. The present study only included a small number of patients from only two study centers, limiting generalizability. The third limitation of this study stems from the inclusion of a disproportionate number of patients in VS/UWS as a result of stroke (compared to other published studies). Finally, the present study examined prolonged DoC and the long-term complications were not taken into consideration.
In conclusion, we present a novel prospective real-world cohort study on the 12-mo outcome of patients with a severe condition at two specialized units in Beijing and Nantong with different etiologies. The results suggest that patients with severe DoC, despite having strong predictors of poor prognosis, might recover consciousness after a prolonged time of rehabilitation. An accurate initial diagnosis of patients with DoC is critical for predicting outcome and a long-term regular follow-up is also important. This preliminary study indicates that establishing a rehabilitation-based registry for patients with severe DoC after brain injury is feasible and probably relevant to improve patient management.
Recent innovations in intensive care have improved the prognosis of patients with severe brain injuries and brought more patients with disorders of consciousness (DoC). The most important concern for DoC patients and their family is prognosis because it is central to the decisions regarding medical treatment and eventual rehabilitation therapy, and profound ethical issues are involved. In China, the accessibility to proper management services for DoC patients is a major issue.
Data are lacking regarding the long-term outcomes of DoC patients in China. It is necessary to study the long-term outcomes of patients in prolonged DoC in light of many factors likely to influence crucial decisions about their care and their life.
The study aimed to assess the recovery rate of the inpatients with prolonged DoC after the standard follow-up, hoping to investigate the factors associated with a higher likelihood to recover consciousness at rehabilitation facilities in China and further our understanding of the rehabilitation potential of the most severely affected patients with DoC.
This was a two-center prospective cohort study of inpatients with vegetative state (VS)/unresponsive wakefulness syndrome (UWS). The study outcomes were the recovery from VS/UWS to minimally conscious state (MCS) and the long-term status of patients with prolonged DoC considered in VS/UWS or MCS for up to 6 years. The patients were evaluated using the Glasgow coma scale (GCS), coma recovery scale-revised, and Glasgow outcome scale. The endpoint of follow-up was recovery of full consciousness or death. The changes in the primary clinical outcome improvement in clinical diagnosis were evaluated at 12 mo compared with baseline.
The study population included 93 patients (62 VS/UWS and 31 MCS). At the endpoint, 33 transitioned to an emergence from MCS or full consciousness, eight had a locked-in syndrome, and there were 35 patients remaining in a VS/UWS and 11 in an MCS. Compared with the unresponsive group, the responsive group had a higher proportion of males, shorter time from injury, higher frequency of vascular etiology, higher Glasgow coma scale score and coma recovery scale-revised score at admission, lower frequency of VS/UWS, and more favorable Glasgow outcome scale outcome.
Patients with severe DoC, despite having strong predictors of poor prognosis, might recover consciousness after a prolonged time of rehabilitation. An accurate initial diagnosis of patients with DoC is critical for predicting outcome and a long-term regular follow-up is also important.
This preliminary study indicates that establishing a rehabilitation-based registry for patients with severe DoC after brain injury is feasible and probably relevant to improve patient management.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lanza G S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017;26:1118-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 2. | Monti MM, Laureys S, Owen AM. The vegetative state. BMJ. 2010;341:c3765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Georgiopoulos M, Katsakiori P, Kefalopoulou Z, Ellul J, Chroni E, Constantoyannis C. Vegetative state and minimally conscious state: a review of the therapeutic interventions. Stereotact Funct Neurosurg. 2010;88:199-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | van Erp WS, Lavrijsen JC, Vos PE, Bor H, Laureys S, Koopmans RT. The vegetative state: prevalence, misdiagnosis, and treatment limitations. J Am Med Dir Assoc. 2015;16:85.e9-85.e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | van Erp WS, Lavrijsen JC, van de Laar FA, Vos PE, Laureys S, Koopmans RT. The vegetative state/unresponsive wakefulness syndrome: a systematic review of prevalence studies. Eur J Neurol. 2014;21:1361-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, Barbano R, Hammond FM, Laureys S, Ling GSF, Nakase-Richardson R, Seel RT, Yablon S, Getchius TSD, Gronseth GS, Armstrong MJ. Comprehensive systematic review update summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91:461-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, Barbano R, Hammond FM, Laureys S, Ling GSF, Nakase-Richardson R, Seel RT, Yablon S, Getchius TSD, Gronseth GS, Armstrong MJ. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91:450-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 336] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 8. | Baricich A, de Sire A, Antoniono E, Gozzerino F, Lamberti G, Cisari C, Invernizzi M. Recovery from vegetative state of patients with a severe brain injury: a 4-year real-practice prospective cohort study. Funct Neurol. 2017;32:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Zhao J. Disorders of Consciousness in China. Neurosci Bull. 2018;34:605-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8080] [Cited by in F6Publishing: 7715] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 11. | Kalmar K, Giacino JT. The JFK Coma Recovery Scale--Revised. Neuropsychol Rehabil. 2005;15:454-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5148] [Cited by in F6Publishing: 5313] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 13. | Pascarella A, Fiorenza S, Masotta O, Tibollo V, Vella D, Nardone AM, Rossi M, Volanti P, Madonia F, Cstronovo G, De Cicco D, Guarnaschelli C, Achilli MP, Chiapparino C, Angelillo MT, Tommasi MA, Pisano F, Grioni G, Vezzadini G, Ferriero G, Salvaderi S, Bellazzi R, Estraneo A. Multicentre registry of brain-injured patients with disorder of consciousness: rationale and preliminary data. Funct Neurol. 2018;33:19-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Grill E, Klein AM, Howell K, Arndt M, Bodrozic L, Herzog J, Jox R, Koenig E, Mansmann U, Müller F, Müller T, Nowak D, Schaupp M, Straube A, Bender A. Rationale and design of the prospective German registry of outcome in patients with severe disorders of consciousness after acute brain injury. Arch Phys Med Rehabil. 2013;94:1870-1876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Perrin F, Schnakers C, Schabus M, Degueldre C, Goldman S, Brédart S, Faymonville ME, Lamy M, Moonen G, Luxen A, Maquet P, Laureys S. Brain response to one's own name in vegetative state, minimally conscious state, and locked-in syndrome. Arch Neurol. 2006;63:562-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 284] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Estraneo A, Loreto V, Guarino I, Boemia V, Paone G, Moretta P, Trojano L. Standard EEG in diagnostic process of prolonged disorders of consciousness. Clin Neurophysiol. 2016;127:2379-2385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Bruno MA, Majerus S, Boly M, Vanhaudenhuyse A, Schnakers C, Gosseries O, Boveroux P, Kirsch M, Demertzi A, Bernard C, Hustinx R, Moonen G, Laureys S. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol. 2012;259:1087-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Seel RT, Douglas J, Dennison AC, Heaner S, Farris K, Rogers C. Specialized early treatment for persons with disorders of consciousness: program components and outcomes. Arch Phys Med Rehabil. 2013;94:1908-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Steppacher I, Kaps M, Kissler J. Will time heal? A long-term follow-up of severe disorders of consciousness. Ann Clin Transl Neurol. 2014;1:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Luauté J, Maucort-Boulch D, Tell L, Quelard F, Sarraf T, Iwaz J, Boisson D, Fischer C. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology. 2010;75:246-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 21. | Higashi K, Hatano M, Abiko S, Ihara K, Katayama S, Wakuta Y, Okamura T, Yamashita T. Five-year follow-up study of patients with persistent vegetative state. J Neurol Neurosurg Psychiatry. 1981;44:552-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Levin HS, Saydjari C, Eisenberg HM, Foulkes M, Marshall LF, Ruff RM, Jane JA, Marmarou A. Vegetative state after closed-head injury. A Traumatic Coma Data Bank Report. Arch Neurol. 1991;48:580-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: a follow-up study 2 to 5 years after traumatic brain injury. Arch Phys Med Rehabil. 2005;86:746-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1-4 year follow-up. Prog Brain Res. 2009;177:73-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Estraneo A, Moretta P, Loreto V, Lanzillo B, Cozzolino A, Saltalamacchia A, Lullo F, Santoro L, Trojano L. Predictors of recovery of responsiveness in prolonged anoxic vegetative state. Neurology. 2013;80:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Avesani R, Gambini MG, Albertini G. The vegetative state: a report of two cases with a long-term follow-up. Brain Inj. 2006;20:333-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Li R, Du J, Huo S, Hao J, Song W. Coherence in P300 as a predictor for the recovery from disorders of consciousness. Neurosci Lett. 2017;653:332-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |