Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5952
Peer-review started: August 28, 2020
First decision: September 29, 2020
Revised: October 3, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: December 6, 2020
The coronavirus disease 2019 (COVID-19) outbreak has brought great challenges to public health. Aggravation of COVID-19 is closely related to the secondary systemic inflammatory response. Glucocorticoids are used to control severe diseases caused by the cytokine storm, owing to their anti-inflammatory effects. However, glucocorticoids are a double-edged sword, as the use of large doses has the potential risk of secondary infection and long-term serious complications, and may prolong virus clearance time. Nonetheless, the risks and benefits of glucocorticoid adjuvant therapy for COVID-19 are inconclusive.
To determine the effect of methylprednisolone in severe and critically ill patients with COVID-19.
This single-center retrospective study included 102 adult COVID-19 patients admitted to a ward of a designated hospital in Wuhan, Hubei Province from January to March 2020. All patients received general symptomatic treatment and organ function support, and were given different respiratory support measures according to their conditions. In case of deterioration, considering the hyperinflammatory state of the patients, methylprednisolone was intravenously administered at 0.75-1.5 mg/kg/d, usually for less than 14 d. Patient vital signs and oxygenation were closely monitored, in combination with imaging and routine blood tests such as C-reactive protein, biochemical indicators (liver and kidney function, myocardial enzymes, electrolytes, etc.), and coagulation function. Patient clinical outcomes were discharge or death.
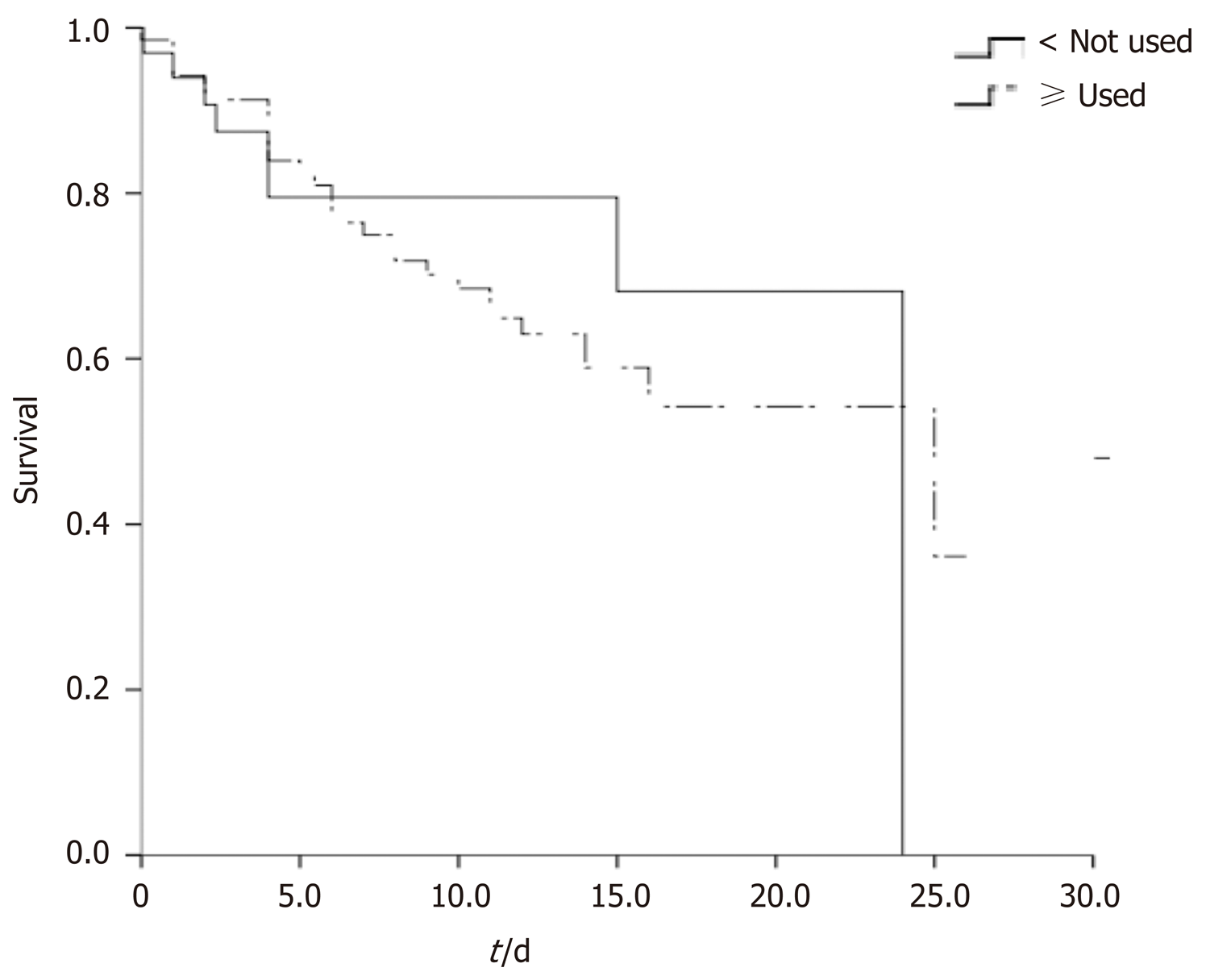
A total of 102 severe and critically ill COVID-19 patients were included in this study. They were divided into treatment (69, 67.6%) and control groups (33, 32.4%) according to methylprednisolone use. Comparison of baseline data between the two groups showed that the treatment group patients had higher aspartic acid aminotransferase, globulin, hydroxybutyrate dehydrogenase, and lactate dehydrogenase. There was no significant difference in other baseline data between the two groups. With regard to prognosis, 29 (78.4%) patients in the treatment group died as opposed to 40 (61.5%) in the control group. The mortality was higher in the treatment group than in the control group; however, according to the log-rank test and the Kaplan–Meier survival curve, the difference in mortality between both groups was insignificant (P = 0.655). The COX regression equation was used to correct the variables with differences, and the results showed that methylprednisolone treatment did not improve prognosis.
Methylprednisolone treatment does not improve prognosis in severe and critical COVID-19 patients.
Core Tip: Glucocorticoids were used in the treatment of severe acute respiratory syndrome, influenza A, and coronavirus disease 2019 (COVID-19) in the past. Many studies believe that glucocorticoids can effectively reduce inflammation caused by viruses. In this study, 102 patients with severe and critical COVID-19 were studied and divided into either a treatment group or a control group according to methylprednisolone use. We found that the difference in mortality between both groups was insignificant (P = 0.655), and the results showed that methylprednisolone treatment did not improve prognosis.
- Citation: Zhu HM, Li Y, Li BY, Yang S, Peng D, Yang X, Sun XL, Zhang M. Effect of methylprednisolone in severe and critical COVID-19: Analysis of 102 cases. World J Clin Cases 2020; 8(23): 5952-5961
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5952.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5952
The coronavirus disease 2019 (COVID-19) outbreak has brought great challenges to public health and governance in various countries. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which uses angiotensin converting enzyme 2 (ACE2) as a receptor to invade cells and cause lung injury. Aggravation of COVID-19 is closely related to the secondary systemic inflammatory response[1]. A previous study found that compared with non-intensive care unit (ICU) patients, COVID-19 patients admitted to the ICU have higher plasma levels of inflammatory factors such as IL-2, IL-7, IL-10, GSCF, IP-10, MCP1, MIP1A, and TNF-α. This indicates obvious inflammatory reactions in severe and critical patients[2]. Severe and critically ill COVID-19 patients reportedly account for more than 20% of all COVID-19 patients[3,4]. Some patients get worse in 7-10 d due to the rapid progression of the disease. It gradually develops into acute respiratory distress syndrome (ARDS), septic shock, and even death via multi-organ failure[5]. At present, there is no effective SARS-CoV-2-specific antiviral therapy, resulting in great difficulty in clinical treatment.
Glucocorticoids are used to control severe diseases caused by the cytokine storm, owing to their rapid, powerful, and nonspecific anti-inflammatory effects. They are often used as an adjunct treatment for viral pneumonia[6,7]. Domestic guidelines, literature, and the first-line treatment guidelines for critical COVID-19 patients all recommend short courses, medium courses, and small doses of glucocorticoids for inhibiting excessive immune injury, which are the applied measures for critical COVID-19 patients[8]. It was previously thought that proper and reasonable corticosteroid use could reduce the excessive inflammatory reaction of severe pneumonia and help severe patients survive respiratory failure and inflammatory exudation. However, glucocorticoids are a double-edged sword, as the use of large doses has the potential risk of secondary infection and long-term serious complications, and may prolong virus clearance time. Nonetheless, the risks and benefits of glucocorticoid adjuvant therapy for COVID-19 are inconclusive. The WHO’s 2019-nCoV-related severe infection clinical guidelines[9] and a review article in Lancet[10] do not recommend the use of glucocorticoids in the treatment of patients with COVID-19, but Chinese experts and scholars believe that the early application of hormones is really helpful for alleviating the condition of patients[11].
This study summarized the clinical treatment strategies for critical COVID-19 patients during the epidemic. The clinical data and treatment options of 102 severe and critical patients in a designated hospital in Wuhan, Hubei Province from January to March 2020 were analyzed. The clinical effect and prognosis of critical COVID-19 patients treated with low doses and short courses of glucocorticoids, were also analyzed, to provide a basis for clinical glucocorticoid use.
This single-center retrospective study included adult COVID-19 patients admitted to a ward of a designated hospital in Wuhan, Hubei Province from January to March 2020.
The inclusion criteria were: (1) Age ≥ 18 years; and (2) The diagnosis criteria[12] were in accordance with the COVID-19 diagnosis and treatment protocol (trial version 7) formulated by the National Health Commission of China, and included epidemiological history, clinical COVID-19 expression, and the presence of one of the etiological or serological evidence (positivity for novel coronavirus nucleic acid or serum-specific antibody). The study patients were all severe or critically ill. Severely ill patients presented with: (1) Shortness of breath, RR ≥ 30 times/min; (2) Resting oxygen saturation ≤ 93%; or (3) PaO2/FiO2 ≤ 300 mmHg, and critically patients presented with: (1) Respiratory failure and mechanical ventilation need; (2) Shock; or (3) Organ failure requiring ICU monitoring.
The exclusion criteria were: (1) Combined with basic lung diseases (such as COPD and pulmonary fibrosis); (2) Complicated with a malignant tumor; and (3) There were contraindications to the use of methylprednisolone.
According to the treatment principles formulated by the above diagnosis and treatment plan, all patients received general symptomatic treatment and organ function support, and were given different respiratory support measures according to their conditions, including nasal catheter oxygen inhalation, mask oxygen inhalation, high-flow nasal catheter oxygen inhalation, noninvasive mechanical ventilation, and invasive mechanical ventilation. Treatment involved conventional antiviral treatment in combination with secondary infection and complication prevention and treatment, and basic disease treatment. During treatment, patient oxygenation was closely monitored. In case of deterioration, considering the hyperinflammatory state of the patients, methylprednisolone was intravenously administered at 0.75-1.5 mg/kg/d, usually for less than 14 d[12].
All subjects enrolled in our study had a case report form, and data were collected within 12 h after hospital admission, including C-reactive protein, biochemical indicators (liver and kidney function, myocardial enzymes, electrolytes, etc.), and coagulation function. Patient vital signs and oxygenation were closely monitored. Patient clinical outcomes were discharge or death.
This survey was a retrospective study collecting only the clinical data of patients. Since it did not bring risks to patients’ physiology and did not interfere with patients’ treatment plan, and researchers protected patients’ information from disclosure, Xuanwu Hospital of Capital Medical University agreed to exempt this study from ethical review.
All data were analyzed using SPSS version 22.0 for Windows. Continuous data with a normal distribution are presented as the mean ± SD. Non-normally distributed variables are presented as the median with interquartile ranges (IQRs) and were analyzed using a non-parametric test. Categorical data were analyzed using the Chi-square test. Survival rates were estimated using the Kaplan–Meier method. The log-rank test was used to compare the unadjusted survival curves. Cox regression models were used to estimate the hazard ratios associated with patient mortality risk, incorporating baseline differences. All tests were two-sided, and a P value of < 0.05 was considered statistically significant.
A total of 102 severe and critically ill COVID-19 patients were enrolled in this study, including 18 critically ill and 84 severe patients. They were divided into treatment (69, 67.6%) and control groups (33, 32.4%) according to methylprednisolone use. Comparison of baseline data between the two groups showed that the treatment group patients had the following characteristics: Higher aspartic acid aminotransferase (P < 0.05), globulin (P < 0.05), hydroxybutyrate dehydrogenase (P < 0.05), and lactate dehydrogenase (P < 0.05) (Table 1). There was no significant difference in other baseline data between the two groups.
| Control group | Treatment group | P value | |
| Male | 19 (57.6%) | 34 (49.3%) | 0.120 |
| Critically ill | 3 (9.1%) | 15 (21.7%) | 0.117 |
| Hypertension | 15 (45.5%) | 31 (44.9%) | 0.170 |
| Diabetes | 16 (48.5%) | 12 (17.4%) | 0.145 |
| Renal insufficiency | 5 (15.2%) | 7 (10.1%) | 0.387 |
| CHD | 2 (6.1%) | 10 (14.5%) | 0.091 |
| Aspartic acid aminotransferase (U/L) | 27 (20, 40) | 35 (26, 54) | 0.043 |
| Alanine aminotransferase (U/L) | 26 (19, 44) | 36 (20, 57) | 0.164 |
| Alkaline phosphatase (U/L) | 47 (40, 66) | 55 (43, 74) | 0.157 |
| Lactate dehydrogenase (U/L) | 253 (183, 379) | 295 (213, 534) | 0.025 |
| Glutamyl transpeptidase (U/L) | 25 (18, 32) | 33 (21, 56) | 0.072 |
| Total bilirubin (μmol/L) | 9.6 (6.7, 13.2) | 11.1 (7.7, 16.3) | 0.110 |
| Albumin (g/L) | 32.2 (29.7, 35.2) | 29.8 (27.9, 33.8) | 0.146 |
| Globulin (g/L) | 28.9 (26, 32.1) | 31.4 (28.2, 35.9) | 0.010 |
| Prealbumin (mg/L) | 142.6 (113.7, 175.8) | 136.6 (85.5, 209.8) | 0.709 |
| Total bile acid (μmol/L) | 2.2 (1.7, 3.8) | 2.4 (1.6, 4.4) | 0.722 |
| Urea nitrogen (mmol/L) | 4.25 (3.3, 6.35) | 4.84 (3.72, 7.75) | 0.244 |
| Creatinine (μmol/L) | 73.5 (62.2, 90.9) | 69.55 (58.2, 89.55) | 0.419 |
| Uric acid (μmol/L) | 226 (175.1, 287.6) | 233.2 (177.3, 329.2) | 0.622 |
| Calcium (mmol/L) | 1.95 (1.87, 2) | 1.91 (1.81, 2) | 0.122 |
| Creatine kinase (U/L) | 104.5 (45.5, 196) | 85.5 (54, 197) | 0.747 |
| CKMB (U/L) | 13 (9.5, 18.5) | 13 (10, 19) | 0.699 |
| Alpha hydroxybutyrate dehydrogenase (U/L) | 205 (149, 310.5) | 248 (180, 449) | 0.020 |
| Total carbon dioxide (mmol/L) | 22.6 (20.9, 27.9) | 24 (21.4, 26.7) | 0.589 |
| C-reactive protein (mg/L) | 20.98 (4, 40.95) | 34.08 (8.47, 81.4) | 0.140 |
| Cystatin (mg/L) | 0.78 (0.66, 0.92) | 0.88 (0.75, 1.03) | 0.098 |
| Red blood cells (T/L) | 4.05 (3.57, 4.59) | 4.1 (3.79, 4.55) | 0.335 |
| Hemoglobin (g/L) | 122 (105, 138) | 129.5 (120, 140) | 0.242 |
| Platelets (g/L) | 198 (143, 291) | 197 (139, 258) | 0.667 |
| Lymphocytes (g/L) | 0.92 (0.75, 1.3) | 0.87 (0.61, 1.3) | 0.658 |
| Monocytes (g/L) | 0.38 (0.33, 0.48) | 0.36 (0.25, 0.5) | 0.562 |
| Erythrocyte distribution width | 12.5 (11.9, 12.9) | 12.5 (12.1, 13.1) | 0.660 |
With regard to prognosis, 29 (78.4%) patients in the treatment group died as opposed to 40 (61.5%) in the control group. The mortality was higher in the treatment group than in the control group; however, according to the log-rank test and the Kaplan–Meier survival curve, the difference in mortality between the two groups was insignificant (log-rank 0.199, P = 0.655; Figure 1). Considering the effect of baseline difference on patient prognosis in the two groups, the COX regression equation was used to correct the variables with differences, and the results showed that methylprednisolone treatment did not improve prognosis (Table 2).
| B | SE | Wald | P value | 95%CI | |
| Aspartic acid aminotransferase | 0.010 | 0.007 | 1.930 | 0.165 | 0.996, 1.024 |
| Lactate dehydrogenase | 0.006 | 0.005 | 1.291 | 0.256 | 0.996, 1.015 |
| Globulin | -0.007 | 0.031 | 0.050 | 0.823 | 0.935, 1.055 |
| Alpha hydroxybutyrate dehydrogenase | -0.001 | 0.006 | 0.024 | 0.877 | 0.988, 1.010 |
| Methylprednisolone use | -0.579 | 0.437 | 1.754 | 0.185 | 0.238, 1.320 |
COVID-19 is caused by SARS-CoV-2, which currently has no vaccine nor specific antiviral drug. Therefore, the treatment of severe and critical COVID-19 patients remains difficult, with a high mortality rate. Similar to severe pneumonia caused by SARS, Middle East respiratory syndrome coronavirus (MERS-CoV), and other viruses, severe COVID-19 disease develops rapidly. The main reason for this is the hyperactivation of the immune system (cytokine storm) by the disease, causing serious damage to the lungs and other organs. The cytokine storm theory was first proposed by Ferrara et al[13] in 1993. It refers to the rapid and large-scale production of many cytokines, including TNF-α, IL-1, IL-6, IL-12, IFN-α, IFN-β, IFN-γ, MCP-1, and IL-8, after an infection, resulting in multi-organ failure and possibly death. It is a primary cause of ARDS and multiple organ failure[14]. In the defense against pathogens, immune cells secrete a large number of cytokines, which in turn stimulate immune cells. Usually, this positive feedback response is regulated to some extent. However, in some cases, the regulatory mechanism fails, leading to large-scale immune cell activation, and thus further cytokine secretion, referred to as the cytokine storm[15]. SARS, MERS, the influenza A (H1N1) virus, and the avian influenza virus can cause a cytokine storm. The serum levels of cytokines, including IL-17, IP-10, IL-6, KC, G-CSF, GM-CSF, MCP-1, and MIG, in patients with severe infection are reportedly significantly increased[16,17]. Huang et al[2] found that the concentrations of IL-1 β, IL-1ra, IL-7, IL-8, IL-9, IL-10, FGF, GCSF, IFN-γ, IP10, MCP1, MIP1a, MIP1b, PDGF, TNF-α, and VEGF in the initial plasma of COVID-19 patients were higher than those in the control group. They also found that the inflammatory factors in the plasma of ICU patients were higher than those of non-ICU patients, and that the cytokine storm was more obvious. The lung is the key target of the cytokine storm caused by SARS-CoV-2. Hypercytokinemia is the basis of a hyperinflammatory symptomatic state that leads to injury of alveolar epithelial cells and vascular endothelial cells as well as lung infiltration supported by neutrophils and macrophages[18].
Glucocorticoids can play an effective anti-inflammatory role by rapidly inducing the synthesis of anti-inflammatory factors and are used for the treatment of inflammatory diseases of various etiologies. In a retrospective study of 401 SARS patients, it was found that the correct use of glucocorticoids can reduce severe SARS patient mortality and shorten the length of hospitalization, without secondary infection and other complications[6]. A study of 2141 patients with pneumonia caused by the H1N1 pdm09 virus, from 407 hospitals in mainland China, found that low to moderate corticosteroid doses (25-150 mg/d of methylprednisolone or equivalent dose) could reduce the mortality in PaO2/FiO2 < 300 mmHg H1N1 influenza virus pneumonia[19]. Many similar studies have shown that the rational use of glucocorticoids in the treatment of SARS can reduce mortality, improve symptoms, and slow disease progression[6,20-22]. However, a 2004 randomized controlled trial study found that early treatment with glucocorticoids (within 7 d of onset) was related to a subsequent high plasma viral load, which delays the SARS-CoV clearance. On the contrary, it aggravates the disease, possibly related to its simultaneous inhibition of positive cytokines such as IFN-α and IL-6[23]. Xu et al[24] reported that glucocorticoid-treated COVID-19 patients were more likely to have complications such as ARDS, shock, acute kidney injury, and secondary infection, compared to control group patients. Previous studies on the treatment of pneumonia caused by SARS, MERS, and the H1N1 virus with glucocorticoids remain controversial, with some studies reporting that it does not provide survival benefits and may cause adverse effects such as diabetes[25], femoral head necrosis[26], osteoporosis, secondary infection, mental symptoms, gastrointestinal bleeding, increased viral clearance time, and other serious long-term complications[23,27-30].
Some previous SARS consensus and guidelines have suggested the use of glucocorticoid therapy according to the condition, but the specific course and usage are not clear, and there is no recommended level[31,32]. The COVID-19 treatment plan issued by China proposed that patients with progressively deteriorating oxygenation indexes and rapid disease progression can use small and medium doses of glucocorticoids such as methylprednisolone, as appropriate and for a short time[12]. The 2019 novel coronavirus guidelines of the WHO suggested that unless special reasons are denied, regular systemic glucocorticoid therapy should not be administered[9]. This suggests the lack of a consensus or definitive guidelines on the effects of glucocorticoid treatment, thereby indicating that caution should be exercised while determining the dosage and treatment course.
Considering the advantages and disadvantages of the above-mentioned glucocorticoid treatment, and referring to the relevant consensus and guidelines, the present study adopted low-dose hormone treatment for severe and critical COVID-19 patients with rapid disease progression. The results of this retrospective study showed that there was no significant difference in the effect of low-dose methylprednisolone on the mortality of severe and critical COVID-19 patients. At present, glucocorticoid therapy for COVID-19 is still controversial, and high-quality clinical evidence is lacking. Some clinical meta-analysis research and review articles report that glucocorticoid treatment of COVID-19 patients will not bring about survival benefits and may result in a longer virus clearance time, and should therefore be used cautiously[33-35]. On the other hand, the administration of glucocorticoid therapy to severe COVID-19 patients can alleviate clinical symptoms, shorten mechanical ventilation time, and reduce mortality. However, its prolongation of the virus clearance time remains controversial[36-39]. Some scholars have stated that the inconclusive clinical evidence should not be the reason for giving up glucocorticoid use in COVID-19 treatment. They rather recommend to advocate for rational glucocorticoid use, and careful formulation of a short-term and low-dose glucocorticoid treatment plan.
This was a single-center retrospective study with a limited research method and sample size, failing to elucidate the impact of hormone therapy on survival. Obesity is one of the most critical risk factors which aggravates the mortality of COVID-19, and metformin has been reported to reduce COVID-19 mortality in elderly, obese, and diabetic patients through weight loss[40]. Most of the patients in this study were severe and critically ill, and their body weight could not be measured at admission. The loss of body weight in baseline data may affect the clinical effect of methylprednisolone. Therefore, further high-quality research is required to explore the evidence, drug selection, dosage, course of treatment, complications, and withdrawal methods of glucocorticoid therapy.
The results of the present study do not elucidate the survival benefit of methylprednisolone treatment, suggesting caution in glucocorticoid treatment of COVID-19 patients. Each patient’s situation and the advantages and disadvantages of glucocorticoid treatment should be fully considered, and long-term and high-dose glucocorticoid use should be avoided.
Coronavirus disease 2019 (COVID-19) has spread to many countries and regions all over the world and has become a worldwide public health event. COVID-19 is an acute infectious disease caused by a new coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)], which is clinically categorized into mild, moderate, severe, and critical illness. Severe and critically ill patients progress rapidly with dyspnea, hypoxemia, and even life-threatening complications such as multiple organ dysfunction syndrome, sepsis, and shock. At present, there is no significant and effective drug for severe and critical patients with COVID-19. Glucocorticoids have been used in the treatment of SARS, Middle East respiratory syndrome, influenza A, and other infectious respiratory diseases worldwide, but their efficacy is still controversial.
In clinical practice, some severe and critical patients with COVID-19 benefit from the application of glucocorticoids, but some patients have various adverse effects. Therefore, whether glucocorticoids should be used in patients with COVID-19 and how to use them are a problem worthy of discussion.
The main objective of this study was to determine the effect of methylprednisolone in severe and critical patients with COVID-19.
One hundred and two severe and critically ill patients with COVID-19 were divided into treatment (69, 67.6%) and control groups (33, 32.4%). In the treatment group, methylprednisolone was intravenously administered at 0.75-1.5 mg/kg/d, usually for less than 14 d. We compared the general information, underlying diseases, laboratory examination indexes, and mortality of the two groups. The log-rank test and the Kaplan–Meier survival curve were used to explore the difference in mortality between the two groups, and the COX regression equation was used to correct the variables with differences.
The treatment group patients had higher aspartic acid aminotransferase (P < 0.01), globulin (P < 0.01), hydroxybutyrate dehydrogenase (P < 0.01), and lactate dehydrogenase (P < 0.01). Twenty-nine (78.4%) of patients in the treatment group died as opposed to 40 (61.5%) in the control group. The mortality was higher than that of the control group. And the results showed that methylprednisolone treatment did not improve prognosis.
Methylprednisolone treatment does not improve prognosis in severe and critical COVID-19 patients.
Methylprednisolone treatment in severe and critically ill patients with COVID-19 should be comprehensively evaluated and used with caution.
The authors gratefully acknowledge the useful suggestions given by Dr. Zhang M.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Arabey AA S-Editor: Huang P L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Zhou YG, Fu BQ, Zheng XH, Wang DS, Zhao CC, Qi YJ, Sun R, Tian ZG, Xu XL, Wei HM. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. NSR. 2020. [DOI] [Cited in This Article: ] [Cited by in Crossref: 727] [Cited by in F6Publishing: 652] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 2. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28479] [Article Influence: 7119.8] [Reference Citation Analysis (3)] |
| 3. | Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1186] [Cited by in F6Publishing: 1351] [Article Influence: 337.8] [Reference Citation Analysis (0)] |
| 4. | CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1413] [Cited by in F6Publishing: 1448] [Article Influence: 362.0] [Reference Citation Analysis (0)] |
| 5. | Kofi Ayittey F, Dzuvor C, Kormla Ayittey M, Bennita Chiwero N, Habib A. Updates on Wuhan 2019 novel coronavirus epidemic. J Med Virol. 2020;92:403-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Siemieniuk RA, Meade MO, Alonso-Coello P, Briel M, Evaniew N, Prasad M, Alexander PE, Fei Y, Vandvik PO, Loeb M, Guyatt GH. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:519-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 8. | Tomazini BM. Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP; COALITION COVID-19 Brazil III Investigators. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 921] [Cited by in F6Publishing: 820] [Article Influence: 205.0] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. 2020; Available from: https://apps.who.int/iris/handle/10665/330893. [Cited in This Article: ] |
| 10. | Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1415] [Cited by in F6Publishing: 1400] [Article Influence: 350.0] [Reference Citation Analysis (0)] |
| 11. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14113] [Cited by in F6Publishing: 14137] [Article Influence: 3534.3] [Reference Citation Analysis (0)] |
| 12. | National Health Commission; National Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin Med J (Engl). 2020;133. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Ferrara JL. Cytokine dysregulation as a mechanism of graft versus host disease. Curr Opin Immunol. 1993;5:794-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 172] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Shao MM, Zhang FX. Progress in immunopathological mechanism of influenza virus mediated lung injury. Zhongyao Xinyao Yu Linchuang Yaoli. 2014;25:236-240. [Cited in This Article: ] |
| 15. | Darwish I, Mubareka S, Liles WC. Immunomodulatory therapy for severe influenza. Expert Rev Anti Infect Ther. 2011;9:807-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75:185-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 562] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 17. | Arisan ED, Dart A, Grant GH, Arisan S, Cuhadaroglu S, Lange S, Uysal-Onganer P. The Prediction of miRNAs in SARS-CoV-2 Genomes: hsa-miR Databases Identify 7 Key miRs Linked to Host Responses and Virus Pathogenicity-Related KEGG Pathways Significant for Comorbidities. Viruses. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 18. | Pelaia C, Tinello C, Vatrella A, De Sarro G, Pelaia G. Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther Adv Respir Dis. 2020;14:1753466620933508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 19. | Li H, Yang SG, Gu L, Zhang Y, Yan XX, Liang ZA, Zhang W, Jia HY, Chen W, Liu M, Yu KJ, Xue CX, Hu K, Zou Q, Li LJ, Cao B, Wang C; National Influenza A(H1N1)pdm09 Clinical Investigation Group of China. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir Viruses. 2017;11:345-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Sung JJ, Wu A, Joynt GM, Yuen KY, Lee N, Chan PK, Cockram CS, Ahuja AT, Yu LM, Wong VW, Hui DS. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Yam LY, Lau AC, Lai FY, Shung E, Chan J, Wong V; Hong Kong Hospital Authority SARS Collaborative Group (HASCOG). Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54:28-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, Yin Z, Huang S, Deng Z, Wei M, Xiong J, Hawkey PM. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52:715-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 23. | Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 434] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 24. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5228] [Cited by in F6Publishing: 5526] [Article Influence: 1381.5] [Reference Citation Analysis (2)] |
| 25. | Xiao JZ, Ma L, Gao J, Yang ZJ, Xing XY, Zhao HC, Jiao JS, Li GW. [Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy]. Zhonghua Nei Ke Za zhi. 2004;43:179-182. [PubMed] [Cited in This Article: ] |
| 26. | Guo KJ, Zhao FC, Guo Y, Li FL, Zhu L, Zheng W. The influence of age, gender and treatment with steroids on the incidence of osteonecrosis of the femoral head during the management of severe acute respiratory syndrome: a retrospective study. Bone Joint J. 2014;96-B:259-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA; Saudi Critical Care Trial Group. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197:757-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 720] [Cited by in F6Publishing: 762] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 28. | Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Ruan SY, Lin HH, Huang CT, Kuo PH, Wu HD, Yu CJ. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2014;18:R63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Auyeung TW, Lee JS, Lai WK, Choi CH, Lee HK, Lee JS, Li PC, Lok KH, Ng YY, Wong WM, Yeung YM. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Chinese Thoracic Society. Expert consensus on clinical diagnosis and treatment standard of severe acute respiratory syndrome. Zhonghua Jiehe He Huxi Zazhi. 2003;26:323-324. [Cited in This Article: ] |
| 32. | Lim WS, Anderson SR, Read RC; SARS Guidelines Committee of the British Thoracic Society; British Infection Society; Health Protection Agency. Hospital management of adults with severe acute respiratory syndrome (SARS) if SARS re-emerges--updated 10 February 2004. J Infect. 2004;49:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Zha L, Li S, Pan L, Tefsen B, Li Y, French N, Chen L, Yang G, Villanueva EV. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust. 2020;212:416-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 232] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 34. | Lu X, Chen T, Wang Y, Wang J, Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24:241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 35. | Li H, Chen C, Hu F, Wang J, Zhao Q, Gale RP, Liang Y. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34:1503-1511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 36. | Fang X, Mei Q, Yang T, Li L, Wang Y, Tong F, Geng S, Pan A. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect. 2020;81:147-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 37. | Liu J, Zheng X, Huang Y, Shan H, Huang J. Successful use of methylprednisolone for treating severe COVID-19. J Allergy Clin Immunol. 2020;146:325-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | Gong Y, Guan L, Jin Z, Chen S, Xiang G, Gao B. Effects of methylprednisolone use on viral genomic nucleic acid negative conversion and CT imaging lesion absorption in COVID-19 patients under 50 years old. J Med Virol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12365] [Article Influence: 3091.3] [Reference Citation Analysis (1)] |
| 40. | El-Arabey AA, Abdalla M. Metformin and COVID-19: A novel deal of an old drug. J Med Virol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |