Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5112
Peer-review started: February 25, 2021
First decision: April 18, 2021
Revised: April 28, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: July 6, 2021
Obstructive sleep apnea (OSA) has been suggested as an independent risk factor for nonalcoholic fatty liver disease (NAFLD), and continuous positive airway pressure (CPAP) is the first-line therapy for OSA.
To clarify the efficacy of effective CPAP therapy on NAFLD of OSA patients by serum markers and transient elastography (TE) using FibroScan® (Echosens, Paris, France).
We prospectively enrolled 123 consecutive patients with OSA who met the indications for CPAP. Liver fibrosis and steatosis were assessed using TE. Before and after 6 mo of CPAP therapy, serum markers and TE were assessed for all patients. The mean usage rate of CPAP therapy for 6 mo was arbitrarily calculated in each patient and expressed as “mean compliance index” (m-CI).
In 50 OSA patients with NAFLD, both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were significantly decreased after 6 mo of CPAP therapy. Univariate analysis showed that decreased body weight (BW), decreased body mass index (BMI), decreased AST level, decreased hemoglobin A1c, and high m-CI were significantly related with improved ALT level. In multivariate regression model adjusted for quantities of BW change during 6 mo of CPAP therapy, high m-CI tended to improve ALT level (P = 0.051). All 17 OSA patients with NAFLD, high m-CI and no BMI changes showed significant improvements in AST and ALT levels. Meanwhile, no significant changes in TE data or serum fibrosis markers were seen.
Some NAFLD could be associated with chronic intermittent hypoxia due to OSA independent of BW changes. In those cases, adequate reoxygenation from effective CPAP therapy may improve NAFLD.
Core Tip: Obstructive sleep apnea (OSA) is a condition of chronic intermittent hypoxia (CIH) during sleep. We evaluated the efficacy of reoxygenation by adequate effective continuous positive airway pressure (CPAP) therapy for liver injury in nonalcoholic fatty liver disease (NAFLD) with OSA patients. The results indicate that effective CPAP therapy for 6 mo in OSA patients with NAFLD could significantly improve serum transaminase activities independent of body weight (BW) changes. Some NAFLD patients could be associated with CIH due to OSA independent of BW changes. We propose that all NAFLD patients be analyzed by polysomnography for the diagnosis of OSA if any symptoms related to OSA become apparent.
- Citation: Hirono H, Watanabe K, Hasegawa K, Kohno M, Terai S, Ohkoshi S. Impact of continuous positive airway pressure therapy for nonalcoholic fatty liver disease in patients with obstructive sleep apnea. World J Clin Cases 2021; 9(19): 5112-5125
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5112.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5112
Nonalcoholic fatty liver disease (NAFLD) seems to be becoming the most common liver disease worldwide as virus-related liver diseases are being successfully addressed. NAFLD affects 20%-40% of the general population in Western countries[1,2], and 9%-30% of the Japanese population[3,4]. Overnutrition and/or lack of exercise leads to metabolic syndrome, including visceral obesity, impaired glucose tolerance, dyslipidemia, and hypertension. NAFLD is also considered as the hepatic manifestation of metabolic syndrome. However, whether NAFLD represents a risk factor for cardiovascular disease and cerebral vessel disease remains unclear[5-8]. NAFLD encompasses a large spectrum of histological liver alterations, ranging from simple steatosis to steatosis accompanied by hepatocellular injury, inflammation, and fibrosis, termed nonalcoholic steatohepatitis (NASH). NASH has the potential to progress to cirrhosis, which is an established risk factor for hepatocellular carcinoma. In this setting, NAFLD is considered a major public issue all over the world, and improved treatment is imperative. However, the pathogenesis is still poorly understood, and effective pharmacotherapy as an adjunct to lifestyle modifications has yet to be established.
Obesity is the highest risk factor for NAFLD and is known to be frequently complicated by obstructive sleep apnea (OSA). OSA is a common medical condition characterized by repetitive partial or complete obstruction of the upper airway, resulting in increased negative intrathoracic pressure, sleep fragmentation, and chronic intermittent hypoxia (CIH) during sleep. Several experimental studies have demonstrated that CIH from OSA may be relevant to the presence and severity of NAFLD[9-11]. Moreover, OSA is independently associated with an increased prevalence of metabolic syndrome[12] and also leads to chronic systemic inflammations, insulin resistance, and liver injury. Repetitive hypoxia/reoxygenation during the transient cessation of breathing in OSA resembles ischemia/reperfusion injury and is known to promote production of reactive oxygen species, leading to oxidative stress[13]. Savransky et al[10] demonstrated that CIH in lean mice led to lipid peroxidation of liver tissue and increased levels of the active proinflammatory transcription factor nuclear factor kappa βin the nuclear fraction of hepatocytes, suggesting that CIH induces oxidative stress in the liver[10].
Continuous positive airway pressure (CPAP) therapy can treat upper airway collapse during sleep, and is now accepted as the standard and first-line therapy in the current management of OSA. The benefits of CPAP therapy have been demonstrated and established in terms of decreasing hypoxic events and daytime sleepiness, and of ameliorating metabolic and cardiovascular diseases[14-16]. Whether CPAP therapy improves the liver injury of NAFLD with OSA has been controversial[17-24]. However, several recent studies have suggested that adequate oxygenation by CPAP therapy during sleep for longer than 3 mo is associated with improvements in liver enzymes[25-31], steatosis[20], and fibrosis[28]. Adherence to CPAP therapy could also be a key point in the effectiveness against liver injury in NAFLD with OSA [25,28,30,31].
Liver biopsy is the gold-standard method for diagnosing and assessing severity of hepatic steatosis and for staging fibrosis in NAFLD. However, this method is invasive and associated with pain and a small risk of serious complications. Instead, liver stiffness measurement (LSM) using transient elastography (TE) is a non-invasive, simple-to-perform, totally innocuous, and reproducible method to assess liver fibrosis, and has been demonstrated to be effective in NAFLD. On the other hand, a non-invasive method for assessing hepatic steatosis has also been needed. As fat affects ultrasound propagation, a novel attenuation parameter has been developed to detect and quantify steatosis. This parameter, controlled attenuation parameter (CAP), is based on the ultrasonic properties of the reflected radiofrequency signals acquired by TE using the FibroScan® M probe (3.5 MHz; Echosens, Paris, France)[32]. Although many reports have demonstrated the efficacy of CPAP therapy for liver enzyme, hepatic steatosis, and fibrosis in NAFLD with OSA, evaluations by LSM and CAP using the FibroScan® are not well known. We therefore undertook the present study to evaluate the efficacy of adequate effective CPAP therapy for liver injury in NAFLD with OSA by LSM and CAP using FibroScan® along with serological parameters.
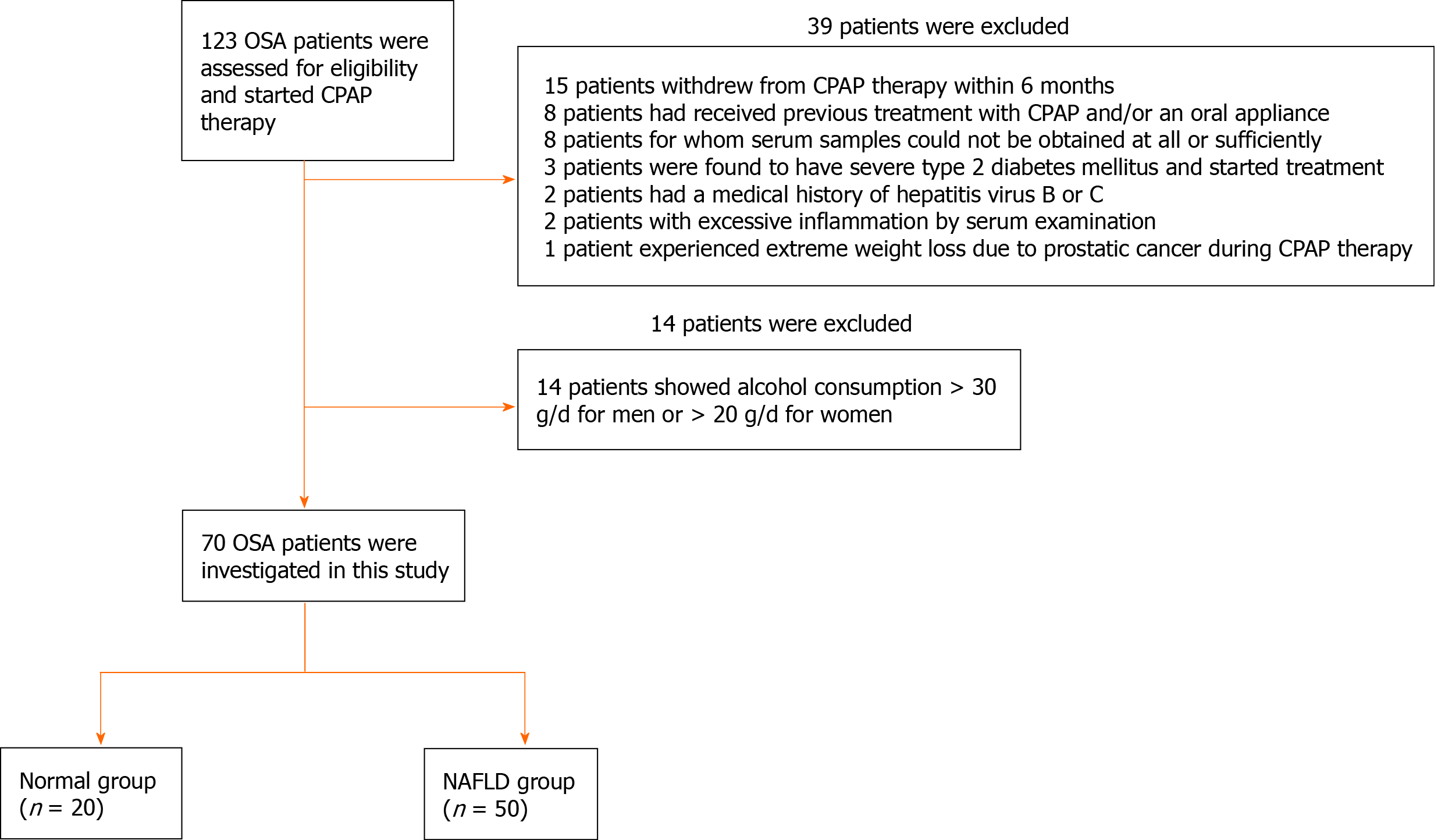
In a prospective cohort study, we identified patients from October 2016 to September 2018 who presented to our sleep laboratory with symptoms of snoring, with or without witnessed episodes of sleep apnea or daytime sleepiness. Patients were analyzed by polysomnography (PSG) for the diagnosis of OSA in an overnight hospitalization, and initially, 123 patients who were diagnosed with moderate to severe OSA [apnea-hypopnea index (AHI) > 15 events/h] and started CPAP therapy were consecutively enrolled. Of all the OSA patients, 15 patients who withdrew from CPAP therapy because of distress or transfer to a different hospital within 6 mo, 8 patients who had received previous treatment with CPAP and/or an oral appliance, 8 patients for whom serum samples could not be obtained at all or sufficiently, 3 patients who were found to have severe type 2 diabetes mellitus and started treatment, 2 patients who had a medical history of hepatitis virus B or C, 2 patients with excessive inflammation by serum examination, and 1 patient who experienced extreme weight loss due to prostatic cancer during CPAP therapy were excluded. In addition, 14 patients showing alcohol consumption > 30 g/d for men or > 20 g/d for women were also excluded. Finally, 70 patients were investigated in this study (Figure 1).
Informed consent was obtained from each patient prior to enrolment in this study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board of The Nippon Dental University School of Life Dentistry at Niigata, No. ECNG-H-247.
All 70 OSA patients were classified into following two groups according to the presence of fatty liver evaluated by ultrasonography (US): A Normal group and an NAFLD group (Figure 1). The NAFLD group was defined as the group in which patients were diagnosed with the presence of fatty liver by high-resolution US (Xario SSA-660A; Toshiba, Tokyo, Japan) evaluated by four trained and experienced gastroenterologists regardless of the presence or absence of elevated serum liver enzymes. The Normal group was defined as the remaining patients without fatty liver. The definition of fatty liver by US was the decisive presence of bright hepatic echogenicity compared with the right kidney and posterior attenuation of the echo beam. All subjects with moderate to severe OSA were treated by auto-CPAP under a set pressure range of 4–16 hPa for 6 mo.
Clinical and laboratory assessments including TE measurements for the two groups were performed at the diagnosis of OSA. To investigate the influences of CPAP therapy on NAFLD with OSA, patients from the NAFLD group were re-evaluated after 6 mo of CPAP therapy in a similar manner. In terms of compliance with CPAP therapy for 6 mo for all patients in the NAFLD group, those meeting the following conditions were re-selected and used to assess the impact of CPAP therapy for NAFLD: (1) alanine aminotransferase (ALT) ≥ 30 U/L at diagnosis of OSA; (2) good compliance with CPAP therapy for 6 mo, as defined below (Compliance with CPAP therapy for 6 mo); and (3) change in body mass index (BMI) during the 6-month CPAP period was < ± 1 kg/m2.
Patients were investigated for clinical data (age, sex, weight, and height) and completed a questionnaire regarding underlying pathologies (type 2 diabetes, hypertension, hyperlipidemia, or hyperuricemia). Body weight and height were measured using digital scales, and BMI was calculated by dividing weight (in kilograms) by the square of height (in meters). Venous blood samples were obtained in the morning following overnight fasting for 12 h. Laboratory evaluations in all patients included aspartate aminotransferase (AST), ALT, γ-glutamyl transpeptidase (GGT), albumin, uric acid, triglycerides (TG), low-density lipoprotein cholesterol, high-density lipoprotein cholesterol (HDL-C), high-sensitivity C-reactive protein (hs-CRP), hemoglobin (Hb)A1c, Mac-2-binding protein glycan isomer (M2BPGi), type Ⅳ collagen 7 s, and platelet count. Insulin resistance was also evaluated using the homeostasis model assessment-insulin resistance (HOMA-IR) method as follows: HOMA-IR = fasting glucose (mg/dL) × fasting plasma insulin (μIU/mL)/405[33].
LSM and CAP measurements were investigated by FibroScan® performed by the aforementioned four trained gastroenterologists, who were blinded to the clinical data of the patients. All patients were measured using a 3.5-MHz standard M probe. After the patients had fasted overnight, FibroScan® was performed on the right lobe through the intercostal spaces with the patient in a dorsal decubitus position and the right arm in maximal abduction[34]. For LSM, the examination was considered reliable if 10 valid measurements were obtained and interquartile range/median LSM was less than 30%.
In this study, compliance with CPAP therapy for 6 mo in each patient was determined from the mean usage rate of CPAP therapy for 6 mo, arbitrarily defined as “mean compliance index” (m-CI). This value was calculated in each patient as: m-CI = [sum of the following calculations for each of the 6 mo of treatment: [(CPAP usage rate of days per calendar month) × (ratio of days of CPAP use for > 4 h/d to total days of CPAP use in the same calendar month)]/6 mo. Compliance with CPAP therapy for 6 mo was defined as “good” for m-CI > 0.5.
Results are expressed as mean and standard deviation (SD) for quantitative variables and as frequencies with percentages for categorical variables. The Shapiro-Wilk normality test was used to ascertain the normality of data distributions in a population. Comparisons between two groups were analyzed using Student's t-test for equal variances or Welch's t-test for unequal variances if data were normally distributed, and the Mann-Whitney U test if data were not normally distributed. Within-group comparisons were examined by a paired t-test for normally distributed data or the Wilcoxon signed-rank test for non-normally distributed data. Categorical variables were compared using chi-square test or Fisher’s exact test. Values of P < 0.05 were considered statistically significant. All statistical analyses were performed using JMP® version 13.2 software (SAS Institute, Cary, NC, United States).
We prospectively enrolled 123 consecutive patients (74.8% men; age, 56.3 ± 13.6 years; BMI, 26.5 ± 4.7 kg/m2) with moderate to severe OSA who met the indications for treatment with CPAP by PSG (data not shown). A total of 70 patients (47 men, 23 women) were finally included in this study, with 20 patients in the Normal group and 50 patients in the NAFLD group. Clinical and laboratory evaluations of the two groups are shown in Table 1. Clinical features of the two groups were similar in terms of sex ratio and underlying diagnosis. However, BMI was significantly higher in the NAFLD group (27.57 ± 0.6 kg/m2) than in the Normal group (23.05 ± 0.97 kg/m2; P < 0.001). Blood samples from all patients of the two groups were available for analysis before CPAP therapy. AST, ALT, GGT, uric acid, TG, hs-CRP, and HOMA-IR were all significantly higher in the NAFLD group than in the Normal group (P < 0.05 each). On the other hand, HDL-C was significantly lower in the NAFLD group than in the Normal group (P = 0.0017). Regarding liver fibrosis, no significant difference was found between groups in M2BPGi, type IV collagen 7 s, or platelet count. Assessments of liver fibrosis (LSM) and steatosis (CAP measurement) by FibroScan® before CPAP therapy were also available for all patients. Both LSM and CAP were significantly higher in the NAFLD group than in the Normal group (P = 0.0101 and P < 0.001, respectively).
| Normal group (n = 20) | NAFLD group (n = 50) | P value | |
| Demographics | |||
| Age (yr) | 61.5 ± 10.3 | 54.7 ± 14.1 | 0.0301 |
| Male sex, n (%) | 12 (60) | 35 (70) | 0.421 |
| BMI (kg/m2) | 23.05 ± 0.97 | 27.57 ± 0.6 | < 0.001 |
| History, n (%) | |||
| Type 2 diabetes | 0 (0) | 6 (12) | 0.1052 |
| Hypertension | 10 (50) | 20 (40) | 0.4450 |
| Hyperlipidemia | 2 (10) | 16 (32) | 0.0571 |
| Hyperuricemia | 1 (5) | 2 (4) | 0.852 |
| Biochemical parameter | |||
| AST (U/L) | 21.2 ± 4.2 | 28.2 ± 10.9 | 0.0035 |
| ALT (U/L) | 18.7 ± 5.6 | 37.6 ± 19.1 | < 0.001 |
| GGT (U/L) | 23.7 ± 15.3 | 45.6 ± 40.0 | 0.0008 |
| Albumin (g/dL) | 4.57 ± 0.07 | 4.57 ± 0.35 | 0.9545 |
| Uric acid (mg/dL) | 5.18 ± 1.02 | 5.81 ± 1.17 | 0.048 |
| TG (mg/dL) | 91.0 ± 58.6 | 138.3 ± 66.9 | 0.0015 |
| LDL-C (mg/dL) | 125.9 ± 29.4 | 133.9 ± 35.9 | 0.3754 |
| HDL-C (mg/dL) | 68.5 ± 17.7 | 55.9 ± 12.9 | 0.0017 |
| hs-CRP (mg/dL) | 0.0396 ± 0.0413 | 0.1545 ± 0.2423 | 0.0028 |
| HbA1c (%) | 5.82 ± 0.35 | 6.16 ± 0.78 | 0.1835 |
| HOMA-IR | 1.14 ± 1.14 | 2.31 ± 1.70 | 0.0002 |
| Serum fibrosis markers | |||
| M2BPGi | 0.460 ± 0.206 | 0.507 ± 0.226 | 0.4201 |
| Type IV collagen 7s (ng/mL) | 3.09 ± 0.54 | 3.07 ± 0.69 | 0.6767 |
| Platelet count (× 104/μL) | 24.00 ± 5.47 | 25.28 ± 6.49 | 0.4197 |
| Transient elastography measurements | |||
| LSM (kPa) | 3.72 ± 0.88 | 4.80 ± 2.21 | 0.0101 |
| CAP (dB/m) | 225.7 ± 41.3 | 304.2 ± 51.7 | < 0.001 |
Influences of CPAP therapy on the liver were evaluated in a total of 50 OSA patients from the NAFLD group. Data were compared between before and after 6 mo of CPAP therapy (Table 2). AST and ALT levels after 6 mo of CPAP therapy were significantly lower than those before CPAP therapy in all 50 patients (P = 0.005 and P = 0.021, respectively), and body weight (BW) tended to be decreased (P = 0.076). However, no significant difference in CAP measurement was evident between before and after 6 mo of CPAP therapy (P = 0.987), and LSM (P = 0.617) and all other serum fibrosis markers showed the same result. In OSA patients of the Normal group, no significant differences in data between before and after CPAP therapy were seen for any measured values (data not shown).
| n | Before CPAP | After 6 mo of CPAP | P value | |
| Demographics | ||||
| BW (kg) | 50 | 75.6 ± 13.4 | 74.8 ± 13.0 | 0.076 |
| BMI (kg/m2) | 50 | 27.6 ± 4.4 | 27.4 ± 4.4 | 0.153 |
| Biochemical parameter | ||||
| AST (U/L) | 50 | 28.2 ± 10.9 | 24.7 ± 7.5 | 0.005 |
| ALT (U/L) | 50 | 37.6 ± 19.1 | 33.1 ± 22.4 | 0.021 |
| GGT (U/L) | 50 | 45.6 ± 40.0 | 42.5 ± 40.1 | 0.299 |
| Albumin (g/dL) | 50 | 4.57 ± 0.35 | 4.54 ± 0.37 | 0.445 |
| Uric acid (mg/dL)) | 50 | 5.81 ± 1.17 | 5.79 ± 1.29 | 0.836 |
| TG (mg/dL) | 47 | 139.5 ± 67.8 | 126.0 ± 54.8 | 0.105 |
| LDL-C (mg/dL) | 50 | 133.9 ± 35.9 | 134.2 ± 45.7 | 0.949 |
| HDL-C (mg/dL) | 50 | 55.9 ± 12.9 | 56.1 ± 11.4 | 0.866 |
| hs-CRP (mg/dL) | 49 | 0.154 ± 0.242 | 0.152 ± 0.205 | 0.953 |
| HbA1c (%) | 50 | 6.16 ± 0.78 | 6.06 ± 0.55 | 0.120 |
| HOMA-IR | 47 | 2.28 ± 1.73 | 2.46 ± 1.63 | 0.369 |
| Serum fibrosis markers | ||||
| M2BPGi | 50 | 0.507 ± 0.226 | 0.539 ± 0.314 | 0.365 |
| Type IV collagen 7s (ng/mL) | 50 | 3.07 ± 0.69 | 3.09 ± 0.60 | 0.684 |
| Platelet count (× 104/μL) | 49 | 25.28 ± 6.49 | 24.78 ± 6.43 | 0.257 |
| Transient elastography measurements | ||||
| LSM (kPa) | 48 | 4.61 ± 1.78 | 4.70 ± 1.94 | 0.617 |
| CAP (dB/m) | 48 | 304.0 ± 52.2 | 303.9 ± 44.1 | 0.987 |
In 50 OSA patients from the NAFLD group, relationships between improvement of ALT level and other factors for 6 mo of CPAP therapy were evaluated (Table 3). In univariate analyses, the factors of decreased BW, BMI, AST level and HbA1c and the factor of high m-CI after 6 mo of CPAP therapy were significantly related with improvement of ALT level (P < 0.05 each). In the multivariate regression model adjusted for quantities of BW change, the factor of high m-CI tended to be associated with improved ALT level (β = -0.254, P = 0.051).
| Factor | n | Partial regression coefficient | Standard error | β | P value |
| Univariate model | |||||
| Decreased factors for 6 mo | |||||
| BW | 50 | 2.051 | 0.600 | 0.443 | 0.001 |
| BMI | 50 | 5.578 | 1.664 | 0.436 | 0.002 |
| AST | 50 | 1.318 | 0.134 | 0.818 | < 0.001 |
| TG | 47 | 0.030 | 0.036 | 0.124 | 0.407 |
| LDL-C | 50 | -0.027 | 0.063 | -0.061 | 0.674 |
| HbA1c | 50 | 11.076 | 4.064 | 0.366 | 0.009 |
| HOMA-IR | 47 | 0.521 | 1.496 | 0.052 | 0.729 |
| M2BPGi | 50 | 4.126 | 7.657 | 0.078 | 0.593 |
| LSM | 48 | 1.871 | 1.656 | 0.164 | 0.264 |
| CAP | 48 | 0.020 | 0.047 | 0.064 | 0.667 |
| m-CI for 6 mo of CPAP therapy | 50 | -11.878 | 5.354 | -0.305 | 0.031 |
| Mutivariate regression model | |||||
| m-CI for 6 mo of CPAP therapy | 50 | -9.890 | 4.928 | -0.254 | 0.051 |
Of the 50 OSA patients in the NAFLD group, 17 patients fulfilled the following three conditions: (1) BMI change < ± 1.0 for 6 mo of CPAP therapy; (2) ALT level before CPAP therapy ≥ 30 U/L; and (3) m-CI for 6 mo of CPAP therapy > 0.5. After excluding the factor of BMI change, both AST and ALT levels were significantly decreased after 6 mo of good CPAP therapy in OSA patients with NALFD. However, no significant improvement in CAP measurement including other factors was evident between before and after 6 mo of CPAP therapy (Table 4).
| n | Before CPAP | After 6 mo of CPAP | P value | |||
| Demographics | ||||||
| Age (yr) | 17 | 57.1 ± 12.2 | - | - | ||
| Male sex, number (%) | 17 | 13 (76.4) | - | - | ||
| BW (kg) | 17 | 74.36 ± 13.44 | 74.23 ± 13.27 | 0.7700 | ||
| BMI (kg/m2) | 17 | 27.02 ± 4.36 | 27.03 ± 4.37 | 0.9691 | ||
| Biochemical parameter | ||||||
| AST (U/L) | 17 | 33.3 ± 9.8 | 28.5 ± 7.5 | 0.0167 | ||
| ALT (U/L) | 17 | 48.6 ± 18.4 | 40.9 ± 19.1 | 0.0177 | ||
| GGT (U/L) | 17 | 45.2 ± 24.1 | 45.6 ± 29.9 | 0.8904 | ||
| Albumin (g/dL) | 17 | 4.51 ± 0.36 | 4.50 ± 0.35 | 0.9071 | ||
| Uric acid (mg/dL) | 17 | 5.59 ± 0.86 | 5.45 ± 1.14 | 0.3776 | ||
| TG (mg/dL) | 16 | 123.4 ± 33.8 | 139.4 ± 48.8 | 0.0819 | ||
| LDL-C (mg/dL) | 17 | 145.8 ± 34.0 | 139.8 ± 34.5 | 0.3002 | ||
| HDL-C (mg/dL) | 17 | 58.3 ± 12.8 | 57.1 ± 13.9 | 0.5143 | ||
| hs-CRP (mg/dL) | 16 | 0.1087 ± 0.1202 | 0.0881 ± 0.0671 | 0.3740 | ||
| HbA1c (%) | 17 | 6.28 ± 0.92 | 6.16 ± 0.62 | 0.2636 | ||
| HOMA-IR | 16 | 2.38 ± 1.36 | 2.87 ± 2.18 | 0.1436 | ||
| Serum fibrosis markers | ||||||
| M2BPGi | 17 | 0.519 ± 0.215 | 0.556 ± 0.148 | 0.2151 | ||
| Type IV collagen 7s (ng/mL) | 17 | 3.35 ± 0.70 | 3.40 ± 0.53 | 0.7018 | ||
| Platelet count (× 104/μL) | 17 | 25.44 ± 7.30 | 24.65 ± 5 .70 | 0.3100 | ||
| Transient elastography measurements | ||||||
| LSM (kPa) | 17 | 4.59 ± 0.97 | 4.79 ± 1.42 | 0.3243 | ||
| CAP (dB/m) | 17 | 306.1 ± 58.2 | 319.8 ± 43.3 | 0.2105 | ||
To the best of our knowledge, this is the first prospective cohort study to investigate the effects of reoxygenation by effective CPAP therapy for OSA patients with NAFLD using TE by FibroScan®. This study demonstrated that effective CPAP therapy for 6 mo in OSA patients with NAFLD could significantly improve serum transaminase activities independent of BW changes. However, no changes were seen in liver stiffness or steatosis from TE or serum fibrosis markers, or in insulin resistance, hs-CRP, or other biochemical parameters.
We first compared anthropometric data, serum biochemical parameters, serum fibrosis markers, and TE measurements between OSA patients of the NAFLD and Normal groups before CPAP therapy. In the analysis of HOMA-IR, NAFLD with OSA represented high insulin resistance as well as NALFD without OSA[35]. TE measurement showed that LSM and CAP measurements in the NAFLD group were significantly higher than those in the Normal group. We also examined hs-CRP in OSA patients of the two groups before CPAP therapy. The concentration of hs-CRP is known to offer a marker of systemic inflammation, and a low-level elevation of hs-CRP level is known to be seen in metabolic syndrome, cardiovascular disease, type 2 diabetes, hypertension[36], some cancers[37], and NAFLD[38]. In this study, hs-CRP was significantly higher in OSA patients with NAFLD than in OSA patients without NAFLD, presumably reflecting the systemic inflammation associated with NAFLD.
In 50 OSA patients with NAFLD, we compared data between before and after 6 mo of CPAP therapy, and both AST and ALT levels were significantly decreased after therapy compared to before. However, LSM and CAP measurement, and other serum biochemical parameters including serum fibrosis markers, were unchanged after therapy. On the other hand, BW tended to decrease after CPAP therapy (P = 0.076). BW loss is known as the most important factor in improving abnormal transaminases for NAFLD[39]. We then considered that improvement of abnormal transaminases in OSA patients with NAFLD may depend more on BW loss than on the reoxygenation achieved by effective CPAP therapy for 6 mo. We therefore next performed uni- and multivariate regression analyses of factors associated with improved ALT level, particularly for the factor of compliance with CPAP therapy.
Compliance with CPAP therapy is one of the most influential factors for the effectiveness of the therapy and is an indispensable factor in this study. Good compliance leads to adequate reoxygenation, but several factors can exert deleterious influences on compliance. The most common reason for poor compliance cited by patients is a troublesome experience using CPAP every night[40]. Although compliance with CPAP therapy is important, standardized evaluations remain lacking. Several studies have used the term “adherence” as a definition of good compliance with CPAP therapy. Kribbs et al[41] and Salepci et al[42] defined adherence as > 4 h per night for > 70% of monitored days, and this definition has been used in many studies. Many prior studies have also expressed adherence to CPAP therapy as mean ± SD for hours per night[18,21,22]. However, detail of the calculations used for this parameter have not been well described. This study therefore originally quantified and expressed compliance with CPAP therapy as a mean usage rate of therapy over 6 mo using a new value, m-CI. In the multivariate regression model, the factor of m-CI for 6 mo of CPAP therapy tended to be associated with improvements in ALT level (P = 0.051), indicating a high probability that compliance with CPAP therapy was associated with improved ALT level independent of BW changes during therapy. The number of participants in this study was so small that the only adjusted factor in multivariate analysis was change in BW.
In addition, to confirm the effectiveness of adequate CPAP therapy for NAFLD with OSA independent of BW changes, we conducted a comparison of biochemical parameters and FibroScan® data between before and after 6 mo of CPAP therapy in 17 OSA patients with NAFLD who met the following three criteria: No BMI change (BMI < ± 1 kg/m2) in 6 mo; high ALT level (ALT ≥ 30 U/L) before therapy; and good CPAP therapy for 6 mo (m-CI > 0.5). Both AST and ALT levels were significantly decreased after 6 mo of CPAP therapy. However, LSM and CAP measurements including serum fibrosis markers and other biochemical parameters were unchanged. This suggests that reoxygenation by effective CPAP therapy for OSA patients with NAFLD first might improve hepatitis, but does not first improve liver steatosis or fibrosis. Moreover, hs-CRP representing systemic inflammation was also unchanged between before and after CPAP therapy. As levels of hs-CRP were too low, there might be no significant difference in this study. Other systemic inflammatory markers should be considered.
Many studies have investigated the impacts of CPAP therapy on NAFLD with OSA. Kohler et al[18] and Sivam et al[21] investigated the influences on liver enzymes of NAFLD by CPAP therapies for relatively short durations in randomized controlled trials (RCTs). However, no significant improvements in AST or ALT levels were seen compared with controls in either study. On the other hand, several cohort studies have demonstrated improvements in transaminase levels among NAFLD patients with OSA following CPAP therapy for longer than 3 mo[17,19,26-28]. Likewise, a recent meta-analysis indicated that CPAP therapy was associated with significant decreases in both AST and ALT levels among OSA patients, and was more effective in OSA patients receiving treatment for > 3 mo[25]. Similarly, the present study demonstrated that 6 mo of CPAP therapy improved both AST and ALT levels. An RCT to investigate the impacts on NAFLD in OSA patients on CPAP therapy for > 3 mo is warranted.
The influences on liver steatosis and fibrosis of CPAP therapy remain controversial in patients with NAFLD and OSA. Many techniques can be used to evaluate liver steatosis. Sivam et al[21] compared the percentage of intrahepatic lipid by liver volume using magnetic resonance spectroscopy between 2 mo of therapeutic CPAP therapy and 2 mo of sham therapy, finding no significant difference[21]. Moreover, short-term (< 6 mo) CPAP therapy in other studies did not decrease liver fat as evaluated by liver attenuation values on computed tomography (CT)[43,44]. Shpirer et al[19] also evaluated liver steatosis by liver attenuation index from CT, but demonstrated a significant improvement of liver steatosis with long-term (2-3 years) CPAP therapy[19]. In the present study, we evaluated liver steatosis by CAP measurements of TE using FibroScan®, finding that 6 mo of CPAP therapy did not significantly improve liver steatosis. Improvement of liver steatosis from NAFLD in OSA patients may require over 2 years of effective CPAP therapy. As for liver fibrosis, evaluations can include serum markers, liver fibrosis indexes, and imaging. Buttacavoli et al[20] investigated liver fibrosis by LSM from TE using FibroScan®, the same method as used in our study, and compared values between before and after 6–12 mo of CPAP therapy in 11 OSA patients[20]. However, no significant differences were identified, supporting our results for 6 mo of CPAP. On the other hand, a few reports have demonstrated significant improvement of liver fibrosis from NAFLD by CPAP therapy. Mesarwi et al[45] evaluated liver fibrosis by serum lysyl oxidase (LOX), an enzyme that cross-links collagen and can serve as a biomarker of hepatic fibrosis[46], and demonstrated significant reductions in serum LOX among 8 OSA patients after 3 mo of CPAP therapy[45]. In addition, Wai et al[47] used AST-to-platelet ratio index (APRI)[47] to evaluate liver fibrosis, and assessed adequate CPAP adherence using a cutoff of > 4 h/night > 70% of treatment nights[28]. Significant improvements in AST, ALT, and APRI were seen with 3 mo CPAP therapy (P < 0.01 each)[28]. Moreover, a dose-response relationship seemed to be present, with patients showing good adherence to CPAP therapy also displaying significantly larger decreases in AST and ALT levels than patients with poor adherence (P < 0.01). Multivariable logistic regression analysis identified CPAP therapy with good adherence (odds ratio 3.93, 95% confidence interval 1.29–11.94) as an independent predictor of NAFLD regression after adjusting for obesity class and severity of OSA[28]. Effective CPAP therapy for an adequate duration was associated with significant biochemical improvement and reductions in CIH-related liver fibrosis, suggesting that reoxygenation over the long term could reduce liver fibrosis from NAFLD only in terms of serum markers. However, to the best our knowledge, no studies have demonstrated patent improvement of liver fibrosis on either visual images or pathological specimens. Further studies are needed to clarify the impacts of reoxygenation on liver fibrosis of NAFLD with OSA.
Several limitations to this study should be considered. The first and biggest limitation of this prospective cohort study was the small sample size, particularly for OSA patients with NAFLD. Only 50 OSA patients in the NAFLD group were investigated by multivariate analysis regarding improvement of ALT levels. Only BW change could be adjusted for due to this small sample size. Second, this study did not include untreated samples and could not compare variations in ALT levels between OSA patients with NAFLD showing good compliance with CPAP therapy and patients with no therapy. Setting patients with moderate to severe OSA to receive no therapy as negative controls would be difficult and ethically questionable. Third, NAFLD in this study was not defined histopathologically using the gold standard of liver biopsy, and the definition was somewhat obscure and determined by the existence of fatty liver images on US and by alcohol consumption. Fourth, our investigation could only be conducted for 6 mo. This was because if OSA patients in our sleep laboratory showed improvements in general condition (e.g., decreased BW and fat around the neck) and AHI after 6 mo of CPAP therapy, therapy would often be changed to combination therapies with an oral appliance. Combination therapy could not be evaluated for adequate reoxygenation and was thus not included in this study.
In conclusion, our findings suggest that transaminase activity in NAFLD patients with OSA could be associated with CIH due to OSA, independent of BW changes, and adequate reoxygenation by effective CPAP therapy for an extended duration of at least 6 mo may thus improve activity of NAFLD. A period of years might be required to improve liver steatosis and fibrosis following adequate reoxygenation. The CIH of OSA might just be part of the cause of NAFLD. However, what kind of NAFLD patients with OSA are indicated for effective CPAP therapy to improve activity of NAFLD has yet to be clarified. Progression from NAFLD to NASH could be preventable if patients with OSA and NAFLD can be appropriately selected and receive effective CPAP therapy. More detailed analyses including experimental research are required. We propose that all NAFLD patients should be analyzed by PSG for the diagnosis of OSA if any symptoms of OSA are apparent.
Several experimental studies have demonstrated that chronic intermittent hypoxia (CIH) from obstructive sleep apnea (OSA) may be relevant to the presence and severity of nonalcoholic fatty liver disease (NAFLD). Continuous positive airway pressure (CPAP) therapy is the first-line therapy for OSA, and good compliance with CPAP therapy leads to adequate reoxygenation.
It has been controversial whether reoxygenation by CPAP therapy improves the liver injury of NAFLD with OSA.
We evaluated the efficacy of reoxygenation by adequate effective CPAP therapy for liver injury in NAFLD with OSA patients.
We prospectively enrolled 123 consecutive patients with OSA who met the indications for CPAP. After excluding for their underlying disease or excessive alcohol consumption, 70 OSA patients were finally included in this study. They were classified into following two groups according to the presence of fatty liver evaluated by ultrasonography: A Normal group and an NAFLD group. Liver fibrosis and steatosis were assessed by transient elastography (TE) using FibroScan® (Echosens, Paris, France). Before and after 6 mo of CPAP therapy, serum markers and TE were assessed for OSA patients in the NAFLD group. The mean usage rate of CPAP therapy for 6 mo was arbitrarily calculated in each patient and expressed as “mean compliance index” (m-CI).
Influences of CPAP therapy on the liver were evaluated in 50 OSA patients from the NAFLD group. In 50 OSA patients with NAFLD, both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were significantly decreased after 6 mo of CPAP therapy. In multivariate regression model adjusted for quantities of body weight (BW) change during 6 mo of CPAP therapy, high m-CI tended to improve ALT level (P = 0.051). All 17 OSA patients with NAFLD, high m-CI and no BMI changes showed significant improvements in AST and ALT levels. Meanwhile, no significant changes in TE data or serum fibrosis markers were seen.
Some NAFLD patients could be associated with CIH due to OSA independent of BW changes. We propose that all NAFLD patients be analyzed by polysomnography for the diagnosis of OSA if any symptoms related to OSA become apparent.
This prospective study demonstrated the efficacy of reoxygenation by adequate effective CPAP therapy for liver injury in NAFLD with OSA patients. An randomized controlled trials to investigate the impacts on NAFLD in OSA patients on CPAP therapy for longer than 3 mo is warranted. Progression from NAFLD to nonalcoholic steatohepatitis could be preventable if patients with OSA and NAFLD can be appropriately selected and receive effective CPAP therapy.
The authors wish to thank the members of examination laboratory at The Nippon Dental University Medical Hospital for their excellent technical support.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gassler N S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1522] [Cited by in F6Publishing: 1515] [Article Influence: 116.5] [Reference Citation Analysis (1)] |
| 2. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 3. | Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46 Suppl 1:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T; JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 361] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 5. | Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Avillach P, Egger P, Dhalwani NN, Kendrick S, Celis-Morales C, Waterworth DM, Alazawi W, Sattar N. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 6. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 886] [Cited by in F6Publishing: 854] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 7. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 736] [Cited by in F6Publishing: 823] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 8. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1326] [Cited by in F6Publishing: 1361] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 9. | Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871-G877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Takayama F, Egashira T, Kawasaki H, Mankura M, Nakamoto K, Okada S, Mori A. A Novel Animal Model of Nonalcoholic Steatohepatitis (NASH): Hypoxemia Enhances the Development of NASH. J Clin Biochem Nutr. 2009;45:335-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 443] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 13. | Atkeson A, Jelic S. Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc Health Risk Manag. 2008;4:1327-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1677] [Cited by in F6Publishing: 2112] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 15. | Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, Jagia P, Kumar A. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277-2286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 16. | Aggarwal S, Nadeem R, Loomba RS, Nida M, Vieira D. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep-disordered breathing and heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol. 2014;37:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Chin K, Nakamura T, Takahashi K, Sumi K, Ogawa Y, Masuzaki H, Muro S, Hattori N, Matsumoto H, Niimi A, Chiba T, Nakao K, Mishima M, Ohi M. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med. 2003;114:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Kohler M, Pepperell JC, Davies RJ, Stradling JR. Continuous positive airway pressure and liver enzymes in obstructive sleep apnoea: data from a randomized controlled trial. Respiration. 2009;78:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Shpirer I, Copel L, Broide E, Elizur A. Continuous positive airway pressure improves sleep apnea associated fatty liver. Lung. 2010;188:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Buttacavoli M, Gruttad'Auria CI, Olivo M, Virdone R, Castrogiovanni A, Mazzuca E, Marotta AM, Marrone O, Madonia S, Bonsignore MR. Liver Steatosis and Fibrosis in OSA patients After Long-term CPAP Treatment: A Preliminary Ultrasound Study. Ultrasound Med Biol. 2016;42:104-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Sivam S, Phillips CL, Trenell MI, Yee BJ, Liu PY, Wong KK, Grunstein RR. Effects of 8 wk of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012;40:913-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Jullian-Desayes I, Tamisier R, Zarski JP, Aron-Wisnewsky J, Launois-Rollinat SH, Trocme C, Levy P, Joyeux-Faure M, Pepin JL. Impact of effective vs sham continuous positive airway pressure on liver injury in obstructive sleep apnoea: Data from randomized trials. Respirology. 2016;21:378-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Hang LW, Chen CF, Wang CB, Wu TN, Liang WM, Chou TC. The association between continuous positive airway pressure therapy and liver disease development in obstructive sleep apnea/hypopnea syndrome patients: a nationwide population-based cohort study in Taiwan. Sleep Breath. 2017;21:461-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Labarca G, Cruz R, Jorquera J. Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Non-Alcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. J Clin Sleep Med. 2018;14:133-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Chen LD, Lin L, Zhang LJ, Zeng HX, Wu QY, Hu MF, Xie JJ, Liu JN. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: A meta-analysis. Clin Respir J. 2018;12:373-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Chen LD, Zhang LJ, Lin XJ, Qi JC, Li H, Wu Z, Xu QZ, Huang YP, Lin L. Association between continuous positive airway pressure and serum aminotransferases in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2018;275:587-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Sundaram SS, Halbower AC, Klawitter J, Pan Z, Robbins K, Capocelli KE, Sokol RJ. Treating Obstructive Sleep Apnea and Chronic Intermittent Hypoxia Improves the Severity of Nonalcoholic Fatty Liver Disease in Children. J Pediatr 2018; 198: 67-75. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Kim D, Ahmed A, Kushida C. Continuous Positive Airway Pressure Therapy on Nonalcoholic Fatty Liver Disease in Patients With Obstructive Sleep Apnea. J Clin Sleep Med. 2018;14:1315-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Bajantri B, Lvovsky D. A Case of Concomitant Obstructive Sleep Apnea and Non-Alcoholic Steatohepatitis Treated With CPAP Therapy. Gastroenterology Res. 2018;11:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Parikh MP, Gupta NM, McCullough AJ. Obstructive Sleep Apnea and the Liver. Clin Liver Dis. 2019;23:363-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Liu X, Miao Y, Wu F, Du T, Zhang Q. Effect of CPAP therapy on liver disease in patients with OSA: a review. Sleep Breath. 2018;22:963-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 555] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 33. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22373] [Cited by in F6Publishing: 23297] [Article Influence: 597.4] [Reference Citation Analysis (0)] |
| 34. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1967] [Cited by in F6Publishing: 1813] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 35. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1074] [Cited by in F6Publishing: 1051] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 36. | Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, Mirhafez SR, Moohebati M, Esmaily H, Ghazavi H, Saberi Karimian M, Parizadeh SM, Mohammadi M, Mohaddes Ardabili H, Ferns GA, Ghayour-Mobarhan M. Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J Clin Lab Anal. 2016;30:672-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Lee S, Choe JW, Kim HK, Sung J. High-sensitivity C-reactive protein and cancer. J Epidemiol. 2011;21:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Nigam P, Bhatt SP, Misra A, Vaidya M, Dasgupta J, Chadha DS. Non-alcoholic fatty liver disease is closely associated with sub-clinical inflammation: a case-control study on Asian Indians in North India. PLoS One. 2013;8:e49286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Eslamparast T, Tandon P, Raman M. Dietary Composition Independent of Weight Loss in the Management of Non-Alcoholic Fatty Liver Disease. Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Gao W, Sun M, Chen B. Adherence to CPAP in patients with obstructive sleep apnea in a Chinese population. Respir Care. 2012;57:238-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 831] [Cited by in F6Publishing: 810] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 42. | Salepci B, Caglayan B, Kiral N, Parmaksiz ET, Comert SS, Sarac G, Fidan A, Gungor GA. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58:1467-1473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67:1081-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 44. | Kritikou I, Basta M, Tappouni R, Pejovic S, Fernandez-Mendoza J, Nazir R, Shaffer ML, Liao D, Bixler EO, Chrousos GP, Vgontzas AN. Sleep apnoea and visceral adiposity in middle-aged male and female subjects. Eur Respir J. 2013;41:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 45. | Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, Torbenson MS, Pedrosa RP, Lorenzi-Filho G, Steele KE, Schweitzer MA, Magnuson TH, Lidor AO, Schwartz AR, Polotsky VY. Lysyl Oxidase as a Serum Biomarker of Liver Fibrosis in Patients with Severe Obesity and Obstructive Sleep Apnea. Sleep. 2015;38:1583-1591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Kagan HM. Lysyl oxidase: mechanism, regulation and relationship to liver fibrosis. Pathol Res Pract. 1994;190:910-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2976] [Article Influence: 141.7] [Reference Citation Analysis (0)] |