Synthesis of Nano-Ag particles using sodium borohydride
Ferdos Parsa Mehr, Masoumeh Khanjani*, Parya Vatani
Department of Chemical Engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran. Correspondence Author Email: s.khanjani@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310367
Article Received on :
Article Accepted on :
Article Published : 22 Aug 2015
These particles were synthesized by the chemical reduction method of AgNO3 using NaBH4. The borohydride anions were adsorbed onto silver nanoparticles and addition of PVP prevented the aggregation of particles. UV–vis absorption spectra, dynamic light scattering (DLS) and transmission electron microscopy (TEM) have been used to trace the growth process and elucidate the structure of the silver nanoparticles. A solution in its UV–Vis absorption spectrum showed surface plasmon resonance absorption bands about 420 nm. We obtain silver colloids of average size 47 nm with a standard deviation of 15 nm after separation and washing procedures.
KEYWORDS:chemical synthesis; Ag nanoparticloes; Sodium Borohydride
Download this article as:| Copy the following to cite this article: Mehr F. P, Khanjani M, Vatani P. Synthesis of Nano-Ag particles using sodium borohydride. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Mehr F. P, Khanjani M, Vatani P. Synthesis of Nano-Ag particles using sodium borohydride. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10436 |
Introduction
Nanotechnology deals with processes that take place on the nanometer scale, that is, from approximately 1 to 100 nm. Properties of metal nanoparticles are different from those of bulk materials made from the same atoms. A bulk material should have constant physical properties regardless of its size, but at the nanoscale, size-dependent properties are often observed. Thus, the properties of the materials change as their size approaches the nanoscale and the percentage of atoms at the surface of a material becomes significant. In the previous studies, many works have mainly focused on the synthesis and study of the antibacterial activity of silver nanoparticles in a general manner with respect to the antibacterial activity through the interaction of spherical silver nanoparticles with bacterial cell [1]. Silvert et al. obtained fine sized Ag particles whose size distribution was between 15 and 36 nm by polyol reduction. Despite these efforts, it remains difficult to prepare silver particles of less than 20 nm and with a narrow distribution from an aqueous solution. For the polyol process, the reaction temperature must consistently remain at 120 °C or higher, which adds to the cost of production. Much finer particles can be fabricated using micelles and the microemulsion method, but this also requires the use of organic solvents [2]. Further, the initial concentration of silver precursor in this method is still limited to less than 0.05 M for size control purposes [3, 4]. Dong et al. reported Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Silver nanoparticles with different sizes and shapes were synthesized by solution phase routes, and their interactions with Escherichia coli were studied. [5]. Thus silver powder of modified surfaces with controlled morphology can be prepared by several methods including the reduction of silver nitrate by reducing agents [6, 7, 8].
We herein describe an easy synthetic route for silver nanoparticles by sodium borohydride as reducing agent. The formation of silver nanoparticles can be observed by a change in colour since they form brown colour on synthesis. The nanoparticles were characterized by different methods.
Materials and Methods
All the chemicals and reagents used were of analytical grade. An aqueous solution of sodium borohydride was added to an aqueous solution of silver nitrate (0.05 M). The drop-wise addition under continuous stirring at room temperature afforded a precipitate. After the complete addition of sodium borohydride, stirring was continued for an additional 10 min. The reduction of the Ag+ ions by sodium borohydride in the solutions was monitored by sampling the aqueous component and measuring the UV–Vis spectrum of solutions. Particle-size distributions of the samples were also obtained using dynamic light scattering (DLS). Furthermore, the silver nanoparticles were characterized by transmission electron microscopy (TEM).
Results and Discussions
It is well known that silver can be reduced from Ag+ to Ag0 by various reducing agents same sodium borohydride. The absorption band at about 420 nm is known to be due to surface plasmon resonance in nano-silver solutions. In fact, the energy of absorption would depend on the degree of plasmon resonance i.e. it may shift either side of this value depending on the ratio of silver ions and zero-valent silver. The UV–Visible spectrum (Fig. 1) of the solution showed a well-defined surface plasmon resonance at ∼420 nm. The technique outlined above has proven to be very useful for analyzing nanoparticles [9].
Dynamic light scattering is a used method for the determination of nanoparticle size. The silver particles’ size histograms show that the nanoparticles size is 47± 15 nm (Fig. 2). TEM micrograph of silver colloidal particles is shown in Fig. 3. It can be noticed that the graphs show that the particles are spherical in shape.
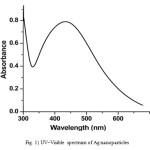 |
Figure 1: UV–Visible spectrum of Ag nanoparticles Click here to View figure |
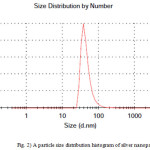 |
Figure 2: A particle size distribution histogram of silver nanoparticles |
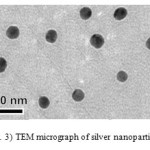 |
Figure 3: TEM micrograph of silver nanoparticles Click here to View figure |
Conclusions
Colloidal silver nanoparticles were synthesized by sodium borohydride. The size of the silver nanoparticles was 47± 15 nm. UV–Vis spectroscopy confirmed silver nanoparticles. Also, nanoparticles were characterized by DLS and TEM. From a technological point of view, these obtained silver nanoparticles have potential applications in the various fields and this simple procedure has several advantages such as cost effectiveness and scale commercial production.
References
- Afreen, R.V., Ranganath, E. Int. J. Environ. Sci 2011, 1(7), 1582.
- Silvert, P. Y.; Urbina, R. H.; Elhsissen, K. T. J. Mater. Chem., 1997, 7, 293.
- Egorova, E. M.; Revina, A. A. Colloids Surf. A: Physicochem. Eng. Asp., 2006,168, 87.
- Xie, Y.; R. Ye; Liu, H. Colloids Surf. A: Physicochem. Eng. Asp., 2006, 279, 175.
- Dong, P.V.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Int. Nano Lett., 2012, 2, 9.
- Ghorbani, H.R. Orient J chem., 2015, 31(1), 303.
- Maleknia, L.,; Rashidi, A. S. Orient J chem., 2015, 31(1), 257.
- Sahni, G.; Panwar, A.; Kaur, B. Int. Nano Lett., 2015, 5(2), 93.
- Ghorbani, H.R.; Soltani, S. Orient J chem., 2015, 31(1), 303.

This work is licensed under a Creative Commons Attribution 4.0 International License.









