Technological Improvement Lithium Recovery Methods from Primary Resources
Gaukhar Bishimbayeva1,2, Dinara Zhumabayeva1, Nurlan Zhandayev2, Arailym Nalibayeva1 , Konstantin Shestakov3, Igor Levanevsky3 and Asem Zhanabayeva1
, Konstantin Shestakov3, Igor Levanevsky3 and Asem Zhanabayeva1
1Institute of Organic Catalysis and Electrochemistry named after D.V. Sokolsky, 142, D. Kunaev str., 050010, Almaty, Kazakhstan.
2LLP "Institute of High Technologies" of JSC "NAC "Kazatomprom", 168 Bogenbay batyr str., 050012, Almaty, Kazakhstan.
3JSC "Ulba Metallurgical Plant", 102, Abay Avenue, 070005, Ust-Kamenogorsk, Kazakhstan.
Corresponding Author E-mail: aray77@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/340611
Article Received on : 12-10-2018
Article Accepted on : 9-12-2018
Article Published : 07 Dec 2018
This article reviews lithium recovery methods from primary resources and lithium raw materials processing methods. Lithium world reserves as well as deposits by countries are given. Summary of global market for and marketing of lithium raw materials are provided. The main lithium recovery methods from minerals (sulphate, lime, sulphuric) are shown. The data about obtaining in Kazakhstan an experimental sample of lithium carbonate from spodumene ore is provided.
KEYWORDS:Global Market; Lithium-Ion Battery; Lithium; Processing; Spodumene; Technology
Download this article as:| Copy the following to cite this article: Bishimbayeva G, Zhumabayeva D, Zhandayev N, Nalibayeva A, Shestakov K, Levanevsky I, Zhanabayeva A. Technological Improvement Lithium Recovery Methods from Primary Resources. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Bishimbayeva G, Zhumabayeva D, Zhandayev N, Nalibayeva A, Shestakov K, Levanevsky I, Zhanabayeva A. Technological Improvement Lithium Recovery Methods from Primary Resources. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=53641 |
Introduction
Today, lithium is becoming increasingly popular in high-tech industries such as aircraft manufacturing, space industry, automotive, nuclear energy, as well as in energy storage systems. In connection with the sharp increase in the production of electric vehicles in the world, lithium has come to be called “future gasoline” and “white oil”. In recent years, there has been a steady increase in the consumption of lithium in the field of high technologies. In 2015, global demand for lithium compounds exceeded 150 thousand tons in terms of lithium carbonate. By 2020, it is expected to grow up to 300-320 thousand tons, and by 2025 – up to 550 thousand tons. Mass introduction of electric vehicles and hybrids will be the main reason for this growth. In light of this, new lithium recovery methods will be of high value.
At the same time, the distribution of lithium reserves in the world is uneven. Access to its stock plays a significant role and affects technological development.
Currently, lithium minerals are mined mainly from pegmatite. There are reserves of mineral raw materials containing mainly spodumene and petalite, which are intensively explored and mined in Canada, Finland and other countries. Spodumene is the main commercial lithium mineral and contains about 8% lithium (in terms of Li2O oxide). About 50% of spodumene is mined in Australia and processed into lithium carbonate in China.
Another type of lithium deposits is the brines of some very salty lakes. Chile and Argentina produce the largest part of the world’s lithium from salt lakes, in aggregate, approximately 46% of total lithium production (FMC, Rockwood and S.Q.M.).
New lithium producers, both traditional and novices are likely to focus on the lithium carbonate market for LIB and will receive steady profits in the upcoming few years. In general, a relatively uniform price increase is expected. It should be noted that general trend of a surge in demand for lithium in the long-term period is envisioned by the most analysts. The lithium industry is divided into the extractive sector and sectors associated with the processing of raw materials and manufacturing.1
World production of lithium is divided into two groups in terms of raw materials used. The first group (Australia, Canada, Brazil, and China) recovers this metal from solid minerals, and the second group (Chile and Argentina) – from bittern (a strong brine solution of mineral lakes located in arid regions). World lithium reserves – the part of total resources that can be recovered via existing technologies – are estimated by the US Geological Survey (USGS) at the amount of more than 14 million tons. Estimates of total resources vary from 40 to 60 million tons.
At the same time, 76% of the world’s reserves are accounted for by bittern of salt lakes. The largest of them are concentrated in South America – the so-called “South American triangle” – the frontier of Chile, Argentina and Bolivia. In Bolivia, there are salt lakes “salars” with an area of up to 10 thousand km2 and which have content of Li2O in bittern from 0.02% to 0.3%, and some zones – up to 0.9%. In Chile, “salars”, similar to the Salar deposit de Atacama deposit, where streams that were flowing for millions of years from the adjacent mountains, evaporated over time and formed fields of mineral salts, characterized by exceptionally high average content Li2O- up to 0.3% and maximum – up to 2.1%.
The USA. US Salt Lakes, located in the western states, have an average LiO2 content in bittern from 0.0075 up to 0.015%. Albemarle Rockwood Lithium produces lithium carbonate from bittern at company Silver Peak in Nevada state. The total lithium reserves in the US are estimated by the USGS at 38 thousand tons.
Argentina. Since 1998, American FMC Corp. manages the Salar field de Hombre Muerto deposit (lithium content is 220-1000 ppm, total reserves are estimated at 800 thousand tons), the output is 12 thousand tons of lithium carbonate per year and 5-7 thousand tons of lithium chloride per year. In Argentina, the total lithium reserves are estimated by the USGS at the amount of 2 million tons.
China. Company Tibet Lithium New Technology Development Co. produces 5,000 tons of lithium carbonate per year from bittern of Zabayu Salt Lake’s deposit in East Tibet. CITIC Guoan Lithium Science & Technology Co. produces 35 thousand tons of lithium carbonate per year from the Taijinaier deposit Salt Lake’s deposit (reserves are estimated at 940 thousand tons of Lithium) in Qinghai Province. It is the largest lithium production plant in China.
Lithium recovery from minerals. Australia. The largest supplier of lithium minerals is the Australian company Talison Lithium Ltd., which is developing the Greenbushes spodumene deposit, at the same time known as the world’s largest deposit of tantalite. Supplies from Greenbushes fill 60% of the global demand for spodumene concentrate. Factors that augment Talison Lithium’s leading position among the producers of spodumene concentrate are cheap production and geographic location – the company delivers most of its products to the rapidly growing Chinese market of lithium. The total lithium reserves in Australia are estimated by the USGS at 1.5 million tons.
Canada. Canadian company Tantalum Mining Corp. (TANCO) produces spodumene concentrate (7.25% Li2O) at its mine, which is located in the province of Manitoba. Company Avalan Ventures is working within the framework of the lithium project at pegmatite deposit Big Whopper (Ontario province). Total reserves of lithium in Canada are estimated by the USGS at 360 thousand tons.
Zimbabwe. The petalite concentrate is produced by Bikita Mining company, which develops the Bikita pegmatite deposit (Lithium content is 1.4%, the amount of total resources of the deposit is not known precisely and sums up to 56.7 thousand tons by some estimates). The recoverable lithium reserves in Zimbabwe are estimated conservatively by the USGS at 23 thousand tons.
Brazil. Minas Gerais and Ceara pegmatite deposits. The amount of total resources of the deposits is not known precisely and according to some estimates, they amount to 85 thousand tons of lithium. The recoverable lithium reserves of Brazil are conservatively estimated by the USGS at 48 thousand tons.
China. Company Xinjuang Nonferrous Metal Corp. produces lithium carbonate from local deposits in Jiangsu Province. Total reserves of China are estimated at 3.2 million tons.1
Russia. At present, lithium is not recovered from its own deposits in Russia.
Lithium reserves in Russia. The state balance of the Russian Federation takes into account lithium reserves in 15 deposits, one of them is classified as off-balance reserves. Mainly lithium reserves (63%) are concentrated in Eastern Siberia (including 31% in the Irkutsk region, 19% in the Republic of Tuva, 13% in the Trans-Baikal territory), in the Murmansk region (35%) and the Primorye Territory (about 2%).2
The Russian raw materials base is structurally and qualitatively different from the foreign one. In Russia, there are no balance reserves of lithium in terms of mineralized waters and brines, whereas in the far abroad they are its main carriers.
Presumably, Russia’s demand for lithium compounds in the military and nuclear industries will remain at the current level, however, export deliveries of lithium-7 hydroxide for foreign nuclear power plants will increase due to increased consumption of this product.
Kazakhstani deposits Bakkennoye, Verkhne-Baymurzinskoye and Akhmetkino all have promising reserves of remaining ores that contain lithium oxide. The results of previous geological prospecting and exploration as well as and mining operations in these areas indicate that the prospects for identifying new industrial facilities and increasing the resource base of already known deposits are not exhausted.
Lithium Raw Materials Processing Methods
The processing of lithium raw materials includes the stages of comminution and beneficiation of raw materials, production of lithium compounds and obtaining metallic lithium. The ores beneficiation is carried out using various processes such as thermal concentration, optical sorting, magnetic methods, flotation and heavy media separation to produce concentrates containing 4-6% Li2O.3-6 The processing products of lithium concentrates are lithium salts (carbonate, sulfate, chloride) and hydroxide. Purification of the obtained lithium compounds is carried out by such methods as precipitation, ion exchange, liquid-liquid extraction, membranes. The most common final product – lithium carbonate used as the starting material for the preparation of other compounds, in particular lithium chloride, from which produce lithium metal.5, 7
The combination of industrial methods of processing lithium raw materials and obtaining the final chemical products includes:
Sulfate –ratio method – sintering of various types of lithium raw materials (ores and concentrates) with K2SO4.
Li2O·Al2O3·4SiO2 + К2SO4 → Li2SO4 + К2O·Al2O3·4SiO2
Next, lithium sulfate is dissolved in water and lithium carbonate is precipitated from its solution with soda:
Li2SO4 + Na2CO3 → Li2 CO3 + Na2SO4
High cost led to the replacement of this once traditional method by others, more accessible and effective.8
Lime method – sintering of lithium mineral concentrates with lime or limestone at a temperature of 1200-1250°C, followed by decomposition of the cake with water and production of lithium hydroxide from a solution of its multiple evaporation. This method requires the use of rich raw materials, since when sintering with lime, dilution occurs. Industrial production, based on the application of this method, was created in the USA (a plant in San Antonio, Texas) for the processing of spodumene concentrates Kings-Mountain and lepidolite concentrates from African countries. 9, 10
The key point of this method of processing is sintering at a temperature of 900-950°C for lepidolite and 1150-1200°C for spodumene with chalk or lime, followed by leaching the obtained sinter with water, and separating LiOH·H20 from lithium hydroxide monohydrate from the resulting solution during evaporation. The main reactions of the process can be represented by the following equations:
Li2O·Al2O3·4SiO2 + 8CaO → Li2O·Al2O3 + 4(2CaO·SiO2)
With an excess of CaO during leaching, lithium aluminate is obtained, which reacts with Ca(OH)2 to form lithium hydroxide:
Li2O·Al2O3 + Ca(OH)2 → 2LiOH + CaO·Al2O3
If potassium or sodium is present in the minerals included in the concentrate, then during leaching, potassium and sodium interact with Ca(OH)2 to form sodium and potassium hydroxides. To obtain pure lithium hydroxide, the solution is evaporated in several stages.8
The lime process is a fairly simple method, but it has several significant drawbacks: this method is applicable to lithium-rich concentrates, and lithium extraction for such concentrates does not exceed 70%, due to the limited solubility of lithium hydroxide (9.83% by mass at 0°C; 11.1% at 25°C; 11.52% at 50°C; 14.96% at 100°C) in water.11
Sulfuric acid method – includes decrepitating of spodumene raw material at a temperature of 1100°C, which ensures the transfer of spodumene to the β-modification, and subsequent treatment of this middlings with sulfuric acid at a temperature of 250-300°C to obtain lithium sulfate.8,9,12
α-Li(Na)Al[Si2O6] → β-Li(Na)[AlSi2O6]
Li[AlSi2O6] + H2SO4 → Li2SO4 + H[AlSi2O6]
Treatment of the latter with a solution of calcined soda makes it possible to obtain lithium carbonate as the final product.10,13 The application of this method requires a considerable flow of H2SO4 – 250 kg per ton of concentrate. Industry-wide sulfuric acid technology was used in the USA for the processing of Canadian spodumene concentrates with 3-5% Li2O (plant in Minneapolis, Minnesota).14 This technology has been used for direct hydrometallurgical processing of lithium ores without prior flotation enrichment, characterized by significant loss of lithium. Sulfuric acid processing of lithium raw material provides recovery of 80% lithium and surpasses all other technological processes in terms of efficiency. It is used in the largest US enterprise (Bessemer-City plant) for the processing of local spodumene ore and Canadian spodumene.1 Sulfuric acid decomposition is used for lithium concentrates of all types: spodumene, lepidolite and amblygonitic.15-17 As a result of the decomposition of the concentrate with concentrated sulfuric acid at 250-400°C and the subsequent leaching of the product with water, solutions containing lithium sulfate are obtained, from which lithium carbonate precipitates.
Lime-chloride method is the least common. Depending on the mineral composition, the ore is burned in the temperature range of 880-1100°C in the presence of chlorine gas or HCl. In the process of chlorination, when the ore is sintered with NH4Cl and CaCl2 in a furnace at 750°C, ~ 98% of the lithium contained in the spodumene is converted to its chloride, which can be leached with water.8,11,12
The production process of lithium chloride from the collective concentrate of amblygonite and lepidolite includes the step of obtaining lithium sulfate by the impact of sulfur trioxide on the concentrate at a temperature of 600°C, followed by leaching of Li2SO4 with water, and then the step of treating the solution with barium chloride, evaporating it and recovery of chloride lithium by amyl alcohol. Metallic lithium is obtained by electrolysis of lithium chloride melt and metallothermic methods: from chloride – by reduction by calcium, from hydroxide – by magnesium. Of particular interest is the development of lithium production directly from spodumene batch, using ferrosilicon or aluminum as reducing agents.
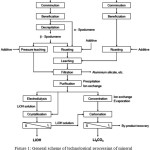 |
Figure 1: General scheme of technological processing of mineral lithium-containing raw materials17 |
Extraction of lithium from the lithium containing clays such as such as montmorillonite, kaolinite, hectorite compared with the extraction of minerals and brines is more cost-consuming and costly. The total cost of the process is related to the cost of such processes as micronizing, high temperature calcination, electrolysis and evaporation. But as a result produces a better product as clay richer in lithium than saline. Production costs are fully compensated high price quality of the final product.18,19
Crocker L. and co-workers extensive and successful research has been conducted to extract lithium from the montmorillonite-type clays of the Mc Dermitt Caldera. Limestone-gypsum roasting and selective chlorination proved most successful for extracting lithium from the clays; 80-pct li recovery was achieved using either technique. With both processes, lithium silicate in the clay was converted to a water- soluble compound–either lithium sulfate or lithium chloride. The lithium was then recovered as a carbonate by water leaching the calcine, concentrating the leach solution by evaporation, and precipitating the lithium with soda ash.20 Mohammad R Barzegari et al. used an efficient method extracting the maximum amount of lithium from clay deposits while minimizing the extraction of magnesium and calcium ions. For research, limestone-gypsum calcination with water leaching was selected. As a result of the research, it turned out that the highest lithium extraction of 75.65% was obtained at a furnace temperature of 1100°C, firing time of 5 hours, the ratio of calcium carbonate to feed 1:5 and the ratio of calcium sulfate in a ratio of 1: 5. In both cases, magnesium was completely removed, and the maximum calcium content (in optimal technical and economic conditions) in the leach solution was 0.1%.21 The recovery of 88% lithium from boron clay containing 0.2% Li was obtained by the authors using a gypsum sediment process. The clay mixture with these additives was sintered at 915°C for 110 min, and then leaching was calcined with water at room temperature.22,23
Improvement of the Sulfuric Acid Method of Processing Lithium Raw Materials
Purpose of this work is the preparation of an experimental industrial sample of lithium carbonate with a lithium carbonate content not less than 99.5%. The technology of obtaining lithium carbonate from domestic spodumene concentrate was develop and tested in the plant laboratory of the “Ulba Metallurgical Plant” JSC. The results of the work are expected to be used to create a new production in the vertically integrated lithium industrial cluster, and will also be used as part of the state program for the development of the rare metal industry to create lithium batteries for alternative energy industry.
Currently, the need for high-purity lithium carbonate for the production of batteries is constantly increasing, and the technologies used today for processing spodumenes are based mainly on the production of technical grade lithium carbonate with a basic substance content not exceeding 99% by weight. To obtain lithium carbonate with a basic substance content of more than 99%, additional cleaning operations are performed on a technical grade lithium carbonate. At the same time, it is advisable to create a technology for the production of high-purity lithium carbonate directly during the processing of spodumene concentrate.
The main impurities present in technical lithium carbonate, which require purification, are sodium, potassium, calcium and sulfate-ion. There are a number of techniques to reduce the content of silk metals in lithium carbonate. This is the replacement of soda with carbonate or ammonium bicarbonate during the precipitation of lithium carbonate, as well as the conversion of lithium carbonate to soluble bicarbonate by treatment with carbon dioxide and subsequent decarbonization, in which lithium carbonate precipitates.
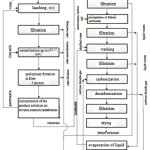 |
Figure 2: Flow sheet for the production of lithium carbonate from spodumene concentrate. |
Since the decrepitating process has been studied as a method of selective grinding and calcination of the concentrate, in this calculation, firing is used instead of this operation. The firing temperature 1100°C. NC JSC “UMP” indicates that for the treatment of β-spodumene by concentrated acid temperature recommended for beginning to form lithium sulfate is 250-300°C. The process of opening the products with water is carried out at 80°C for 1 hour. The excess acidity of the solution of lithium sulfate was 20-30 g/dm3. After filtration, the solution of lithium sulphate is neutralized up to pH=7 by lime. To remove the sulfate ion from the resulting lithium carbonate, the dissolved lithium sulfate was converted to lithium hydroxide by the causticization method. Lithium hydroxide was obtained by interaction of lithium sulphate with barium hydroxide. Lithium carbonate precipitation is carried out using an ammonium carbonate mixture (ACM). Purification of precipitated lithium carbonate is carried out by carbonization-decarbonization methods.
The objective of the proposed technology improvement is the development of a method for obtaining high-grade lithium carbonate directly from spodumene, bypassing the stage of obtaining a technical grade product, simplifying the process of cleaning a lithium-containing solution from impurities.
The technical result is to obtain high-grade lithium carbonate in a single technological process of processing spodumene, reducing the number of technological operations of cleaning from impurities, eliminating the expensive operation of concentrating a solution of lithium sulfate by the method of evaporation.
The technical result is achieved by a method of obtaining high-grade lithium carbonate from spodumene concentrate, including spodumen calcining, sulfation, water leaching, purification of lithium sulfate from impurities, lithium concentration in solution, reagent lithium carbonate precipitation, washing of lithium carbonate, characterized in that lithium concentration is carried out by a membrane method reverse osmosis, cleaning of metal and anions from impurities is carried out by causification of lithium sulfate, lithium carbonate is deposited by coal mmoniynoy salt at a temperature not more than 40°C, followed by heating to 90°C. Moreover, the concentration of lithium by the method of reverse osmosis is carried out with the return of a portion of the flow of retentate to membrane filtration, and the proportion of circulating retentate is calculated so that the total salt content of the lithium sulfate solution supplied to membrane filtration does not exceed 35-40 g /l.
An essential feature of the method is the use of reverse osmosis membranes for the concentration of a solution of lithium sulfate, which makes it possible to exclude from the technology a very laborious and expensive method of evaporation of a solution of lithium sulfate. Conducting the concentration with the return of part of the flow of retentate on membrane filtration leads to an increase in the degree of concentration of lithium in the retentate. Determination of the share of circulating retentate according to the salt content allows to ensure optimal filtration regimes using the reverse osmosis method. An increase in the salt content of the lithium sulfate solution supplied to the filtration above 40 g /l is not advisable, since it will lead to a decrease in the performance of the membrane.
The proposed method is also distinguished by the fact that the purification from metal impurities, forming water-insoluble hydroxides, and sulfate ion is carried out in one stage in the process of causticization – the conversion of lithium sulfate into lithium hydroxide. This technique allows you to further carry out the precipitation of lithium carbonate with a relatively cheap reagent, ammonium carbon, and exclude the additional introduction of sodium ions into the process.
A distinctive feature of the method is to conduct the precipitation of lithium carbonate with an ammonium salt at a temperature of no more than 400°C with further heating to 900°C to increase the degree of precipitation of lithium from the solution by lowering the solubility of lithium carbonate with increasing temperature.
The advantage of the method is due to the use of the proposed complex of technological operations in combination with the modes of their conduct.
Technical and economic evaluations confirm the competitiveness of the lithium – containing ore development project in the Eastern Kazakhstan and comply with general situation in the global market for the production of lithium carbonate for power storage (lithium batteries). Based on the results in the most promising spodumene deposit “Ahmetkino”, it contains about 3 million tons of ore with an average lithium content of 0.77% Li2O. For comparison, in the Trans-Baikal region of the Russian Federation, on the basis of Pervomaisky MCC, it is planned to organize the processing of spodumene ores of the Zavitinsky deposit with a content of 0,3-0,6% Li2O.
The feasibility study of the production cost shows that the proposed approach has the potential to reduce the cost. According to the data, using modern enrichment technologies, the cost of spodumene concentrate will be about 280 USD/ton. In addition, it is possible to reduce the cost of producing lithium carbonate by partially replacing barium hydroxide with calcium hydroxide (“lime milk”).
Conclusion
Lithium is one of the rare metals with many applications, and the demand for lithium is expected to increase with increasing use of electric and electronic devices / hybrid electric vehicles. According to geochemical characteristics, lithium belongs to the group of rare and scattered elements and practically does not form rich deposits and has few of its own minerals.
Thus, the growing global interest in lithium salts is due to the expansion of its consumption, in particular, the increased production of lithium-ion batteries. Lithium is a key element in the production of batteries, the demand for which is constantly growing. The analysis shows that the current global consumption of lithium is currently favorable for the emergence of a new manufacturer of lithium compounds, and Kazakhstan has every chance to become one of them, since it has studied the large reserves of lithium-containing ores in the Eastern region. In addition, due to new technological developments in the enrichment of lithium-containing ores, pegmatites can compete with the extraction of lithium from salars. Taking into account the forecast of the intensive development of the lithium economy, these lithium reserves are of practical interest for development.
Organization of processing of lithium concentrates in the production of lithium metals and lithium salts is one of the priorities of the Republic of Kazakhstan. This is largely due to the following factors: the presence of its own lithium resource base in the form of spodumene ore deposits, developed industrial infrastructure of the Eastern region of Kazakhstan, production facilities, scientific potential and experience in the development of related technologies.
Acknowledgements
This work was conducted under research grant project of AP05135814 “Development of full cycle technology for preparation of innovative electrode materials for lithium batteries from domestic raw materials”. The authors would like to place on record their sincere gratitude to the Ministry of Science and Education of the Republic of Kazakhstan for financial support.
References
- Global and China Lithium Battery Electrolyte Industry Report, 2017-2021, 2017.
- http://rareearth.ru/en/pub/20161026/02870.html
- Banks, M.K.; McDaniel W.T.; Sales P.N. Min. Eng. Trans. AIME, 1953, 181-190.
- Siame E.; Pascoe D. Min. Eng., 2011, 24, 595-602.
- Brand F.; Haus R. Min. Eng., 2010, 23, 659 – 671.
CrossRef - http://www.galaxyresources.com.au/documents/gxy_ar_31_dec_2011.pdf
- Swain, B. J. Chem. Technol. Biotechnol., 2016, 91, 10, 2549–2562.
CrossRef - Garrett, D. E. Elsevier Science Publishing Co Inc, Academic Press Inc, 2004.
- Romare, M.; Olofsson, Y. Dep. Energy Environ., Appl. Physics, Chalmers Univ. Technol., Gothenberg, Sweden, 2013.
- Abe, Y. Rare JOGMEC Mineral Resources Report, 2010.
- Chagnes, A. Lithium Process Chemistry, 2015, 41–80.
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J Ore Geol. Rev., 2012, 48, 55–69.
CrossRef - http://minerals.usgs.gov/minerals/pubs/commodity/lithium/myb1-2015-lithi.pdf
- Meshram, P.; Pandey, B.D.; Mankhand T.R., Hydrometallurgy, 2014.
- Averill, W.A.; Olson D.L. Energy, 1978, 3, 305–313.
CrossRef - Choubey, P.K.; Kim, M.; Srivastava, R.R.; Lee, J.-C.; Lee, J.-Y. Miner. Eng., 2016, 89, 119–137.
CrossRef - Barbosa, L.I.; González, J.A.; Ruiz, M.C. Thermochim. Acta, 2015, 605, 63–67.
CrossRef - Zhang, M.; Yan, Y. Science Press, Beijing (In Chinese). 2015
- Mohr, S.; Mudd, G.; Giurco, D. Lithium Resources and Production: A Critical Global, 2010.
- Angerer, G., Marcheider-Weidemann F., Wendl M., Wietschel M. Fraunhofer Fraunhofer ISI: Karlsruhe, Germany, 2009.
- Jandová, J.; Dvořák, P.; Vu, H.N. Hydrometallurgy, 2010, 103, 12–18.
CrossRef - Tran, T.; Van, T. Luong. Lithium Process Chemistry Resources. Extraction, Batteries and Recycling. Elsevier, 2015, 81-124.
- Buyukburc, A.; Koksa, G. Clays Clay Miner., 2005, 53(3), 301–309.
CrossRef - May, J.T.; Witsowsky, D.S.; Siedel, D.C. Extracting lithium from clays by roast-leach treatment. US Bureau of Mines Report of Investigations No. 8432. 1980.
- Crocker L.; Lien R.H.; May J.T.; Witkowsky D.S.; Seidel D.C. Lithium and its recovery from low-grade Nevada clays. Bureau of Mines, US Department of the Interior, Bulletin 691, 1988.
- Barzegari, M.R.; Ghorbankarimi, G.; Saadati, H.; Torshizian, H. International Academic Journal of Science and Engineering, 2016, 3(4), 70-79.
- Buyukburc, A.; Maraslıoglu, D.; Bilici, M.S. U.; Koksal, G. Miner. Eng., 2006, 19, 515–517.
CrossRef - Crowson P. Minerals Handbook, Stockton Press, New York, NY, USA, 1980, 1987–1992.

This work is licensed under a Creative Commons Attribution 4.0 International License.









