Coronary slow flow (CSF) phenomenon, also known as cardiac syndrome Y, is characterised by angiographically normal or near-normal coronary arteries with delayed opacification of the distal vasculature.1 Far from being a simply angiographic finding, it has clinical implications, such as angina, acute coronary syndrome and sudden death. The prevalence ranges from 1–5% of coronary angiograms and it is more frequent in young male smokers.2
Researchers have been trying to characterise this phenomenon and understand its pathophysiological mechanisms. Myocardial biopsy studies have demonstrated the presence of microvascular disease and an increased resting coronary vasomotor tone.3,4
Different therapeutic options have been used and oral calcium channel blockers (CCBs) have proven to be the most effective as they attenuate the associated microvascular dysfunction.2 There is still a great deal that is unknown about its nature and prognosis, which we cover in this review.
How is Coronary Slow Flow Defined?
CSF was first described in 1972 by Tambe et al. after they identified it in six patients.5 It is defined as the delayed opacification of the coronary vasculature at the distal level in the absence of significant obstructive artery disease as a consequence of a primary coronary microvascular disorder.6 It has been considered to be an early stage of atherosclerotic disease according to some studies.7 An increased resting coronary vasomotor tone in coronary resistance vessels has been reported and Sezgin et al. revealed that endothelium-dependent flow-mediated dilatation is impaired in these patients secondary to elevated homocysteine levels.8 Lower nitric oxide (NO) and elevated NO synthase levels with impaired endothelial function have also been demonstrated in several studies.8–11 The three coronary vessels can be involved. Multivessel involvement with CSF represents a more severe and diffuse condition with a worse prognosis.12
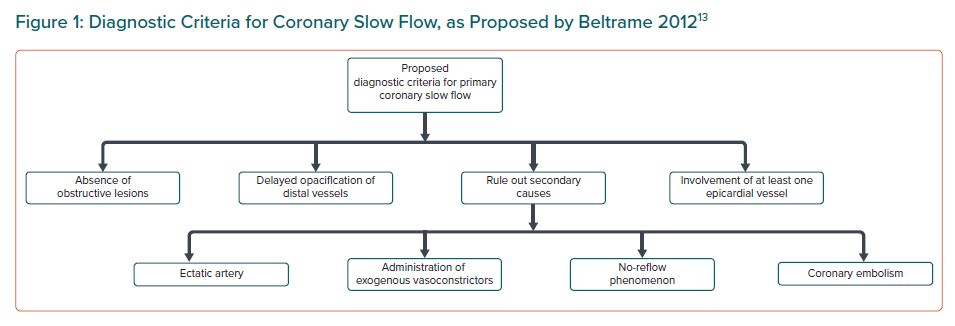
In the absence of coronary obstructive lesions, delayed contrast opacification is considered a marker of increased microvascular resistance and characteristically unequivocal of the phenomenon of CSF, which may cause angina secondary to myocardial ischaemia and lead to acute coronary syndrome.1 Beltrame proposed a definition of CSF based on the following angiographic criteria:13
- No evidence of obstructive epicardial coronary artery disease.
- Delayed distal vessel contrast opacification, as evidenced by either: thrombolysis in MI-2 (TIMI-2) flow (requiring ≥3 beats to opacify the vessel) or corrected TIMI frame count >27 frames.
- The delayed distal vessel opacification is in at least one epicardial vessel.
A definitive diagnosis can only be made once secondary causes have been ruled out such as no-reflow phenomenon, coronary embolism, ectatic artery or administration of exogenous vasoconstrictors.13 CSF is a specific pathological entity that should be considered as a clinical syndrome rather than simply an angiographic phenomenon.14,15 Figure 1 shows the diagnostic criteria for CSF.
The Pathophysiology of Coronary Slow Flow
Angina is the term used to define symptoms secondary to myocardial ischaemia. Atherosclerotic coronary disease continues to be the main cause; however, it does not always correlate with angiographic evidence of obstructive coronary lesions and coronary arteries can be normal or near normal but with a slow progression of contrast material. There are several hypotheses and theories about the substrate and mechanism for CSF, but microvascular and endothelial dysfunction are highly suspected. The normal coronary vasculature consists of epicardial vessels with little resistance to blood flow; and the microvasculature is the main source for regulation of myocardial flow. Coronary vascular tone is regulated via the endothelium and reflects the balance of opposite factors such as endothelin, the most potent vasconstrictor agent, and vasodilators agents such as NO, which seems to be the most important contributor to acute regulation of vascular tone.16
Beltrame et al. reported the presence of an increased resting coronary vasomotor tone in coronary resistance vessels in patients with CSF, suggesting the presence of microvascular spasm.9 Otherwise on the molecular level, endothelin-1 and neuropeptide Y (hence ‘syndrome Y’) have been implicated as possible mediators of the microvascular constriction response.17 Sezgin et al. revealed that endothelium-dependent flow-mediated dilatation is impaired in patients with CSF; and Camsari et al. demonstrated a decreased level of NO in these patients supporting the theory of endothelial dysfunction.8,18 Other authors have theorised that high homocysteine levels are responsible for endothelial dysfunction in CSF.19,20
Pekdemir et al. demonstrated that the decreased fractional flow reserve in patients with CSF was attributed to increased resistance in the epicardial coronary arteries due to diffuse atherosclerotic disease.21 This supports the theory that CSF caused by the deterioration of endothelial function could be considered a precursor of atherosclerosis. Similarly, the study by Yilmaz et al. showed that patients with CSF have a high incidence of metabolic syndrome responsible for microvascular dysfunction.7
However, other theories have emerged that challenge the hypothesis of endothelial dysfunction in the pathogenesis of CSF. Kopetz et al. published the first study that assessed endothelial function and biomarkers of endothelial activation in a large group of CSF patients compared to a healthy control cohort.22 They found that endothelial function was not impaired in patients with CSF and concluded that abnormalities in endothelial function and the endothelial NO pathway do not play a major role in the pathogenesis of CSF suggesting that further investigations are needed to elucidate the importance of other biological factors, such as platelet activation or the role of endothelin.
Otherwise, regarding small vessel involvement in CSF, the study by Mangieri et al., which used an endomyocardial biopsy and histopathological investigation, revealed not only the thickening of the vessels with reduction of their lumen, but also mitochondrial abnormalities and reduction in glycogen content; the histopathological abnormalities are suggestive of small vessel disease which could contribute to increased flow resistance.3 In the same way, Beltrame et al. indicated that CSF was associated with a chronically elevated resting coronary microvascular tone characterised by a low oxygen saturation in the coronary sinus.23
Besides endothelial dysfunction, other authors have proposed that inflammatory phenomena and conditions associated with changes in platelet properties and changes in blood rheological properties could also be associated with CSF.24 Li et al. examined inflammatory mechanisms and showed that the plasma concentration of high-sensitivity C-reactive protein and interleukin-6 was increased in CSF patients.25 Additionally, higher levels of plasma soluble adhesion molecules, such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin have been described in these patients, as well as other inflammatory markers such as serum acid uric levels and increased red cell distribution width.26,27
Anatomical factors have also been implicated in the pathogenesis of CSF. Studies with multidetector CT coronary angiography have demonstrated that the angulations of the main coronary arteries from the aorta were smaller in these patients. Wang et al. have also shown a higher tortuosity and more distal branches in coronary arteries in this cohort.6,28
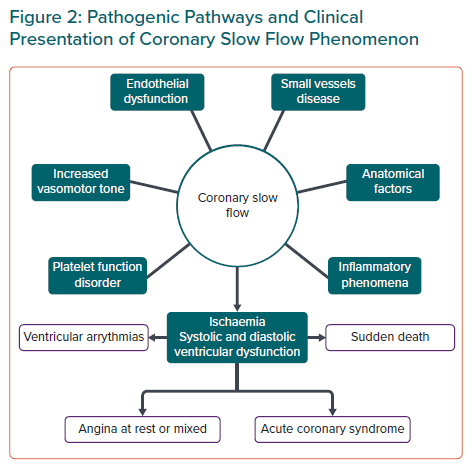
As we have seen and, in conclusion, various and heterogeneous theories have tried to explain the pathogenesis of CSF, with questions still pending that will require longer series and new studies if they are to be resolved. (Figure 2).
Clinical Aspects of Coronary Slow Flow
CSF is usually associated with recurrent angina at rest or mixed rather than with angina on exertion.13 Other clinical forms of presentation are acute coronary syndrome, ST-segment elevation MI, ventricular arrhythmias and even sudden cardiac death probably due to increased QTc dispersion (QTcd) in these patients.29,30 In most cases it occurs in young men who are smokers and have a predisposition to develop metabolic syndrome, postulating endothelial dysfunction as a common pathophysiological mechanism between both.6,7,13
In the study by Hawkins et al., male sex and obesity predicted the presence of CSF as independent risk factors and patients with a diagnosis of CSF had significantly lower levels of HDL.12 Mosseri et al. studied six patients with CSF and found that the clinical presentation was varied, although the symptoms improved with sublingual nitrates in all of them. In four of them, the left ventricular (LV) ejection fraction was preserved and end-diastolic pressure was at the high limit of normality, which reflects a reduced compliance in the left ventricle. Supraventricular arrhythmias and conduction disorders were identified in four of the patients, probably in relation to the associated small vessel disease. Only one patient experienced electrical changes during the episode of pain, probably due to the balanced ischaemia that involves the simultaneous microvascular involvement.31–33
A question of special interest is the development of ventricular arrhythmias and sudden death in these patients attributed to increased QTc dispersion. The study by Atak et al. found that QTc dispersion (defined as the difference between the maximum and minimum QTc interval in a 12-lead ECG) was increased in patients with CSF and it was mainly due to a shortened minimum QTc interval. This increased LV repolarisation heterogeneity can be attributed to myocardial ischaemia caused by microvascular dysfunction or CSF.30
According to the review by Wang et al., metabolic syndrome was more frequent in patients with CSF in the presence of higher levels of total cholesterol, low-density lipoprotein and fasting blood glucose and a higher BMI.6 In addition, a relationship between this phenomenon and increased insulin resistance and glucose intolerance has been described.34,35 Everything points to a common pathophysiology of endothelial dysfunction between metabolic syndrome and CSF. In a more recent study carried out by Sanghvi et al., hypertension, dyslipidaemia and smoking were identified as independent predictors of CSF, with acute coronary syndrome being the most frequent presentation.36
With respect to the detection of ischaemia, Ciavolella et al. reported that 69% of their patients with CSF had functional and perfusion abnormalities that matched the coronary territories that demonstrated the delayed contrast dye run-off.37 In the same way, Alvarez et al. also reported a high percentage of patients with CSF and abnormal ischaemic evaluation.2 These studies confirm that CSF is a cause of ischaemia detected by non-invasive testing.
Whereas all three coronary arteries can be involved, several case series have shown that the left anterior descending artery was most frequently affected.24 Ventricular dysfunction has also been described related to CSF, these patients can present with impaired LV systolic and diastolic function which has an impact on exercise capacity. 38,39 Several studies have correlated these findings. Wang et al. performed a study to evaluate LV and right ventricular (RV) systolic and diastolic function using 2D longitudinal strain and strain rate. They found a deterioration of ventricular function in patients with CSF with similar findings to previous published research, such as Baykan et al. using tissue Doppler imaging.40,41 These findings reveal the need to develop therapeutic interventions in patients with CSF, not only to alleviate symptoms but also to improve LV and RV function.
Prevalence and Predictors of Coronary Slow Flow
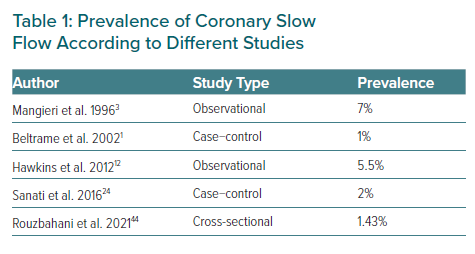
The CSF phenomenon is not a very common finding in routine coronary angiography. Since Tambe et al. first reported this condition as a new angiographic finding, its real incidence is not well known. In 1996, Mangieri et al. reported an incidence in their institution of about 7% of patients with chest pain, normal epicardial coronary arteries and abnormal coronary progression of dye.3,5 In other series, an incidence of 1–5% of patients undergoing coronary angiography has been found.
A case-control study conducted by Beltrame et al. obtained a prevalence of 65 of CSF cases among a total of 6,000 coronary angiograms. They also observed that this phenomenon probably relates to small vessel dysfunction and is associated with significant morbidity and possibly mortality, finding smoking as the most commonly associated factor.1
In 2016, Sanati et al. carried out a cross-sectional study to investigate the demographic and clinical findings of the CSF phenomenon at a tertiary centre in Iran. They found a prevalence of 2% similar to other previous reports. The most frequently affected coronary artery was the left anterior descending. As a conclusion of their research, hypertension and a low HDL-c level could be independent predictors of CSF.24
In India, Mukhopadhyay et al. conducted the first case-control study to analyse clinical presentation, angiographic profile and risk factors associated with CSF. Eighty patients were analysed with classic cardiovascular risk factors (age, sex, BMI, diabetes, hypertension, dyslipidaemia and smoking) as well as haematological and biochemical parameters (haematocrit, platelet count, uric acid, homocysteine, fibrinogen, high sensitivity C-reactive protein and HbA1c) were assessed. BMI was the only independent factor associated with CSF.42
Another case-control study carried out in Japan by Li et al. among 124 patients, showed that the adenosine phosphate-induced platelet aggregation rate was an independent predictor of CSF. LV global and regional diastolic function was impaired in CSF patients and the number of coronary arteries involved determined the severity of LV dysfunction, underlining the need for follow-up in CSF patients with three involved coronary arteries.43
More recently, Rouzbahani et al. performed a cross-sectional study to investigate the prevalence and predictors of CSF, finding a prevalence in their population similar to previous studies (1.43%) with BMI and hypertension being independent predictors of the presence of CSF as reported by other authors.44 The prevalence of CSF according to different studies is summarised in Table 1.
Treatment of Coronary Slow Flow
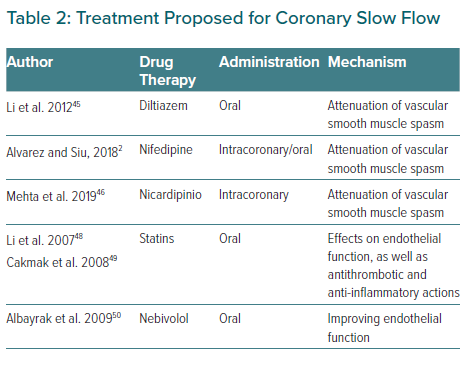
CSF remains under-recognised and the specific standard of care for treatment has not yet been established. It has been suggested that the possible pathophysiology of this entity underlies the microcirculation. Taking into account the demonstrated role of calcium antagonists in patients with microvascular angina, vasospastic angina and CSF, which share a common pathophysiology, Li et al. carried out a clinical trial between 2004 and 2009 in which CSF patients were randomly assigned to a treatment group for diltiazem sustained release capsules 90 mg twice daily (n=40) or a placebo control group. This study aimed to observe the chronic effects of oral diltiazem in patients with CSF. They concluded that patients treated with diltiazem showed a significant improvement in relation to coronary flow, exercise test tolerance and angina, possibly through the attenuation of vascular smooth muscle.44
Once it had been demonstrated that oral CCBs could attenuate the microvascular effects associated with CSF, Alvarez et al. conducted a study evaluating the subsequent use of oral CCBs in patients whose angiographic slow flow had been resolved with intracoronary CCBs.2 They analysed 15 patients diagnosed with CSF, none of whom had been previously treated with CCBs or nitrates. They observed an immediate angiographic improvement of the phenomenon after administration of intracoronary nifedipine. Subsequently, they administered CCBs orally with a follow-up of 13.6 months and observed that all subjects improved symptomatically from their chest pain. As previously mentioned, although the specific standard of care for treatment has not yet been established, symptomatic improvement with CCBs has been shown. Similarly, Mehta et al. confirmed in 30 patients that intracoronary nicardipine was highly effective in reversing CSF implicating a microvascular spasm in the pathogenesis of CSF. Previous studies with mibefradil (a calcium T-channel blocker) also reduced angina frequency and improved physical quality of life, supporting the microspastic pathogenesis of CSF.45–47
Li et al. also hypothesised about the possible effect of statins on CSF. Increasing evidence suggests that the reduction of cardiovascular events conferred by statins is related not only to cholesterol reduction but also to direct effects on endothelial function, as well as antithrombotic and anti-inflammatory actions. Therefore, these authors hypothesise that these pleiotropic effects of statins may be beneficial for these patients due to their pharmacological basis.48 In another study with statins, Cakmak et al. also concluded that 40 mg simvastatin improved myocardial perfusion in patients with CSF.49
Nebivolol has also been shown to be useful for the treatment of CSF. Albayrak et al. investigated the efficacy of nebivolol in patients with SCF by monitoring its effects on endothelial function and different markers of inflammation. They confirmed that nebivolol was effective at improving endothelial function in patients with CSF, controlling chest pain, and having a favourable effect on brachial artery dilation in patients with CSF.50 It may also be beneficial to improve oxidative stress parameters and reduces QTc interval in patients with CSF in which, as we have previously mentioned, QT interval dispersion is increased, reducing the risk of ventricular arrhythmias.51,52 The efficacy of nitrates is controversial – in some studies CSF was not reversed with intracoronary nitroglycerin administration while others have shown increase in the coronary flow.3 Several studies have compared its effects with other drugs and Ozdogru et al. compared the effects of intracoronary nitrate and diltiazem in patients with CSF during coronary angiography showing that although both therapies improved the TIMI frame count, diltiazem was superior to nitroglycerine.53 In the same way, Sani et al. compared the efficacy of nicorandil versus nitroglycerin for alleviation of angina symptoms in CSF in a randomised trial.54 Patients reported greater reductions in pain intensity with nicorandil versus nitroglycerin. These results were similar to those previously reported by Sadamatsu et al.55
Otherwise, in the acute setting, different therapies can be used; dyridamole have been shown to have beneficial effects with different routes of administration, such as intracoronary, IV or oral.3 Other intracoronary drugs, such as verapamil, adenosine or nitroprusside, have also been used for the management of acute patients.56 Also in the acute presentation, Chalikias et al. have recently published the use of intracoronary atropine, with an anticholinergic effect, as an appropriate agent to quick reversal of CSF.57
In conclusion, different agents and therapies have been studied for CSF and previous reports have shown the benefit of using dipyridamole by decreasing the microvascular tone, and others have proved the effects of angiotensin-converting enzyme inhibitors by modulating coronary microvascular tone but until now there is no clear treatment for this phenomenon.58,59 Evidence for the management of these patients is weak as there are no large randomised control trials but CCBs are the most promising treatment. Further studies are needed in the acute setting and for outpatients. A summary of treatment for CSF is shown in Table 2.
Prognosis of Coronary Slow Flow
CSF could be considered within the atherosclerosis spectrum caused by microvascular dysfunction or even a systemic phenomenon. Few studies have analysed the prognosis of CSF and some authors considered this entity as a syndrome different to any kind of coronary heart disease. Due to its unknown pathogenesis many questions still remain and prognosis of CSF is not clearly determined. Years ago it was considered a benign entity with ‘normal’ coronary arteries and many patients were discharged. However, as previously mentioned, these patients can present with various asymptomatic clinical conditions including sudden cardiac death due to ischaemia and ventricular arrhythmias. Otherwise, chronic and recurrent angina pectoris, without a definitive treatment, negatively affects quality of life.14 The recent study published by Candemir et al. showed that ischaemic myocardial scar in MRI may be present in patients with CSF; this finding should be investigated in further and longer studies and could help to clarify not only the pathogenesis but also the prognosis of CSF.60
Final Questions About Coronary Slow Flow
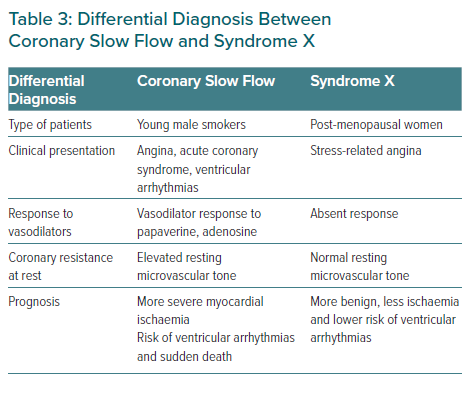
Since its first description, many questions have been raised about CSF. Previous authors considered it to be an angiographic subgroup of syndrome X.61 However, given its peculiarities, some authors have considered this entity to be a syndrome, as proposed by Li et al., differentiating it from syndrome X and even naming it syndrome Y, given the proposed role of neuropeptide Y.4,14,62 The former occurs more frequently in postmenopausal women, while the latter, as previously mentioned, is more frequent in male smokers and characterised by delayed coronary opacification. Otherwise, the CSF prognosis is not as benign as syndrome X. CSF should also be distinguished from the delay of contrast in the context of reperfusion therapy or other secondary causes such as coronary ectasia, coronary spasm or coronary embolisms.4 Differential diagnosis between CSF and syndrome X is reflected in Table 3.
Another questionable aspect is whether we are dealing with a localised coronary condition or a systemic disease. As reflected by Wang et al. reduced endothelial function is implicated in CSF as measured by flow-mediated dilatation of the brachial artery, suggesting that endothelial dysfunction appears to be a generalised process affecting both coronary and peripheral vasculature. Previous studies demonstrated an increased carotid intima-media thickness (IMT) in patients with CSF with a significant correlation between coronary IMT and carotid IMT, all these findings support the hypothesis of a systemic vascular involvement in this group of patients.6,63
Anxiety and depression have been associated with CSF. The pathogenesis of CSF involves mechanisms similar to those linked to anxiety/depression (inflammation, microvascular abnormalities, endothelial dysfunction and anatomical factors of epicardial arteries) which could support the hypothesis that it is a systemic disease.64
There are many questions to be resolved about CSF pathogenesis, treatment and prognosis. Further and longer case series will help us to understand this partially unknown phenomenon.
Conclusion
Over the years, there have been many publications about CSF phenomenon or syndrome. Far from being a simple finding in coronary angiography, it is an entity defined with diagnostic criteria, responsible for symptoms ranging from angina to ventricular arrhythmias, with ischaemia demonstrated. Its pathogenesis has not been completely clarified and the creation of national and international registries would help to clarify and spread knowledge about this phenomenon.