A review of the massive Chilean palm Jubaea chilensis
Una revisión de la enorme palma chilena Jubaea chilensis
DOI:
https://doi.org/10.15446/caldasia.v39n2.68728Palabras clave:
Arecaceae, Butia, mediterranean ecosystem, palm honey, Parajubaea (en)Arecaceae, Butia, ecosistema mediterráneo, miel de palma, Parajubaea (es)
Descargas
Diverse information about the Chilean palm Jubaea chilensis, an endemic species to the Chilean Mediterranean ecosystem, has been generated along the two last centuries. The objective of this review is to bring together in a single document all the information to date on this species. Diverse sources of information were considered, from where the information gathered was systematized and analyzed to be presented in five broad themes. J. chilensis belongs to the Arecaceae family, and can reach 33 m height and two m in diameter, making it one of the most massive palms. Is the southernmost palm in the Americas, and because of its massiveness, it can resist prolonged periods of drought and low temperatures. Therefore, the species is used ornamentally in many countries. Its original population has decreased dramatically in recent centuries due to land use conversion and harvesting. Although it is now a protected species, the Chilean palm is classified as having vulnerable preservation status. Its principal threats, as indiscriminate fruit harvesting and the decreased of vegetal cover, have led to the aging of the palm population without proper regeneration. More research at the species level is highly recommended. Three broad thematic areas should be the focus for deepening the knowledge about the species: biology, specifically in terms of ecophysiology and reproduction; determine its environmental requirements; and, resulting from the application of the knowledge of the first two themes, the reconstruction of its natural populations.
Diversa información sobre la palma chilena Jubaea chilensis, una especie endémica de los ecosistemas mediterráneos chilenos, ha sido generada a lo largo de los dos últimos siglos. El objetivo de esta revisión es reunir en un documento toda la información a la fecha sobre esta especie. Variadas fuentes de información fueron consideradas, desde donde la información reunida fue sistematizada y analizada para ser presentada en cinco temas generales. J. chilensis pertenece a la familia Arecaceae, y puede alcanzar 33 m de altura y dos metros de diámetro, convirtiéndola en una de las más masivas del mundo. Es la palmera más austral de América, y debido a su masividad, puede resistir periodos prolongados de sequía y bajas temperaturas. Es por esto que esta especie es utilizada como ornamental en muchos países. Su población original ha disminuido drásticamente en los últimos siglos debido al cambio de uso de suelo y las cosechas. A pesar de que ahora es una especie protegida, la palma chilena presenta un estado de conservación vulnerable. Sus principales amenazas, como la cosecha indiscriminada de sus frutos y la disminución de la cobertura vegetal, han permitido que las poblaciones de palmas envejezcan sin una regeneración apropiada. Más investigación al nivel de especie es altamente recomendada. Tres áreas temáticas deberían ser el foco para profundizar el conocimiento sobre la especie: su biología, específicamente en términos de ecofisiología y reproducción; determinar sus requerimientos ambientales; y, como resultado de la aplicación del conocimiento de las primeras dos temáticas, la reconstrucción de sus poblaciones naturales.
Recibido: 25 de agosto de 2016; Aceptado: 26 de mayo de 2017
ABSTRACT
Diverse information about the Chilean palm Jubaea chilensis, an endemic species to the Chilean Mediterranean ecosystem, has been generated along the two last centuries. The objective of this review is to bring together in a single document all the information to date on this species. Diverse sources of information were considered, from where the information gathered was systematized and analyzed to be presented in five broad themes. J. chilensis belongs to the Arecaceae family, and can reach 33 m height and two m in diameter, making it one of the most massive palms. Is the southernmost palm in the Americas, and because of its massiveness, it can resist prolonged periods of drought and low temperatures. Therefore, the species is used ornamentally in many countries. Its original population has decreased dramatically in recent centuries due to land use conversion and harvesting. Although it is now a protected species, the Chilean palm is classified as having vulnerable preservation status. Its principal threats, as indiscriminate fruit harvesting and the decreased of vegetal cover, have led to the aging of the palm population without proper regeneration. More research at the species level is highly recommended. Three broad thematic areas should be the focus for deepening the knowledge about the species: biology, specifically in terms of ecophysiology and reproduction; determine its environmental requirements; and, resulting from the application of the knowledge of the first two themes, the reconstruction of its natural populations.
Key words:
Arecaceae, Butia, mediterranean ecosystem, palm honey, Parajubaea.RESUMEN
Diversa información sobre la palma chilena Jubaea chilensis, una especie endémica de los ecosistemas mediterráneos chilenos, ha sido generada a lo largo de los dos últimos siglos. El objetivo de esta revisión es reunir en un documento toda la información a la fecha sobre esta especie. Variadas fuentes de información fueron consideradas, desde donde la información reunida fue sistematizada y analizada para ser presentada en cinco temas generales. J. chilensis pertenece a la familia Arecaceae, y puede alcanzar 33 m de altura y dos metros de diámetro, convirtiéndola en una de las más masivas del mundo. Es la palmera más austral de América, y debido a su masividad, puede resistir periodos prolongados de sequía y bajas temperaturas. Es por esto que esta especie es utilizada como ornamental en muchos países. Su población original ha disminuido drásticamente en los últimos siglos debido al cambio de uso de suelo y las cosechas. A pesar de que ahora es una especie protegida, la palma chilena presenta un estado de conservación vulnerable. Sus principales amenazas, como la cosecha indiscriminada de sus frutos y la disminución de la cobertura vegetal, han permitido que las poblaciones de palmas envejezcan sin una regeneración apropiada. Más investigación al nivel de especie es altamente recomendada. Tres áreas temáticas deberían ser el foco para profundizar el conocimiento sobre la especie: su biología, específicamente en términos de ecofisiología y reproducción; determinar sus requerimientos ambientales; y, como resultado de la aplicación del conocimiento de las primeras dos temáticas, la reconstrucción de sus poblaciones naturales.
Palabras clave:
Arecaceae, Butia, ecosistema mediterráneo, miel de palma, Parajubaea.INTRODUCTION
Arecaceae, the palm tree family, has 181 genera, classified in five sub-families, with more than 2,600 species (Baker and Dransfield 2016). Although the Arecaceae family has a wide distribution and a large number of species, the endemic Chilean palm, Jubaea chilensis (Mol.) Baill., is the only palm among continental native Chilean flora and therefore is considered a singular and important element of Chilean Mediterranean ecosystems (González et al. 2009). Despite these characteristics, the Chilean palm population has been decreasing steadily. The current population is estimated to be only 2.5% of the population at the beginning of the 19th century (González et al. 2009). In 1853 the famous French naturalist Claude Gay (1800 - 1873) pointed out that the Chilean palms formed rather dense stands that unfortunately were rapidly diminishing as a result of their indiscriminate harvesting for the extraction of palm honey or sap. Diverse information about the Chilean palm has been generated in the last 160 years, which it is important to systematize and analyze. The objective of this review is to bring together in a single document all the information to date on the species J. chilensis, describing it in relation to its taxonomic family, its natural context, and its productive and ornamental uses. A base text has been developed, which for the first time highlights the unique position of this species among palm species. The exhaustive analysis of existing information has allowed for generating a more complete and critical view of the species. Finally, after systematizing and analyzing the information, we seek to identify the priority areas for further research to improve decision-making in public policies and planning for management and conservation of the species.
MATERIALS AND METHODS
Diverse sources of information were considered for the review, including old texts, university theses, articles in popular magazines and mainstream scientific journals, government and university websites and botanical gardens in different parts of the world, as well as websites of Chilean and international greenhouses that market the palm species. The information gathered was systematized and analyzed to be presented in five broad themes that are presented below with their respective methodologies.
The first theme is the characterization of J. chilensis, which is divided into (a) taxonomy and phylogenetic relationships; (b) the size of the species compared to its peers; and (c) a morphological and anatomical description. Information about the species was reviewed exhaustively for the three sub-themes. The size of J. chilensis was compared to that of other palm species based on Balslev et al. (2011), who described it as part of the large tall-trunked palm group (LT-Tp), characterized by trunks 20-35 m in height and diameters of 0.2-1 m. Based on Balslev et al. (2011) and diverse bibliographical sources, a database was constructed with the heights and diameters reported for American palm species. The results are presented as histograms of frequency for each size group. This analysis seeks to provide statistical support for otherwise unsupported statements in the literature claiming J. chilensis is the largest palm.
The second theme is the natural distribution and ecology of the species in which, based on diverse texts and sources, the natural conditions under palms of the species J. chilensis develop and the plant communities it is associated. To confirm that J. chilensis is, in fact, the southernmost palm species in the world, as several authors have stated, we compared its natural distribution with those of other species in the Americas and worldwide. We also compare the original distribution of the species to current populations, which makes evident the magnitude of the decline of the species. To determine the climatic ranges in which J. chilensis currently grows, we gathered and analyzed climatic data from meteorological stations closest to current populations of the species. We also sought to provide statistical support to statements in the literature regarding the resistance of the species to cold and drought.
The third aspect reviewed is the propagation and management of the Chilean palm, under which more up-to-date information was gathered about propagation and management of the species, mainly university thesis and local studies.
The fourth theme is the ornamental uses of the Chilean palm and the commercial harvest of palm honey. The main sources of information on the uses of the species were synthesized and analyzed, with emphasis on extraction of palm honey and the ornamental use of the Chilean palm. It should be noted that to determine the presence of the Chilean palm worldwide required an extensive review of official sources like mainstream magazines and unofficial sources like the lists of collections of botanical gardens and the webpages of institutions dedicated to the study of plants that mention J. chilensis in their respective geographic areas. For the first time, a map was generated with the confirmed presence of this species in different parts of the world.
Finally, we discuss the main threats to this species based on the results of several publications. We also consider existing and proposed legislation for the conservation of this species.
RESULTS
Characterization of Jubaea chilensis
Taxonomy and phylogenetic relations
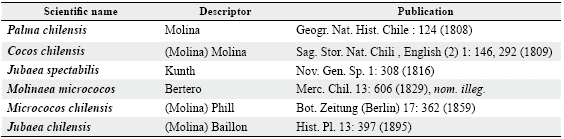
The species J. chilensis belongs to the subtribe Attaleinae, and with another ten genera to the tribe Cocoseae, subfamily Arecoideae, family Arecaceae and order Arecales (Baker and Dransfield 2016). Jubaea is a monotypic genus and the only genus endemic to the Pacific coast of South America (Pintaud and Ludeña 2008). It is named after King Juba II, who ruled Numidia between 85 and 46 BC, because of his great interest in botany (Muñoz-Schick et al. 2012). Table 1 shows the names given to this species and the authors that have described it, to the last accepted name: J. chilensis by Henri Ernest Baillon in 1895. There was a native palm species in Easter Island, now extinct, that botanists named Paschalococos disperta (Dransfield et al. 1984), which other authors added later to the Jubaea genus (Mieth and Bork 2010).
Table 1: Synonymy of J. chilensis and its describers.
The monotypic characteristic of Jubaea genus is probably due to the prolonged isolation of Chilean forests from those of the rest of the continent, especially from tropical forests. This isolation is associated with the development of the "Arid Diagonal" (Villagrán and Armesto 2005) that describes a strip of territory with low rainfall crossing South America from northern Peru to the Atlantic Patagonian coastal range (Bruniard 1982). This phenomenon disconnected J. chilensis from other species of the family in the Tropical regions, including Tropical Andes, specifically from its related genera Parajubaea (Bolivia), Syagrus, and Butia (Brazil and Uruguay) (Henderson et al. 1995), all in tropical areas with dry winters.
Based on morphological and molecular evidence of all the genera belonging to the Attaleinae subtribe, Butia is considered the closest to Jubaea (Gunn 2004). Butia and Parajubaea (Seubert 1998a) are very close to Jubaea in their morphologies, particularly their root anatomies and the presence of lateral pores in the endocarp (Gunn 2004). However, there are some general differences among the three species (Fig. 1). In detail, while J. chilensis has smooth petioles and pedunculated male flowers with 18 stamens, Butia has conspicuously toothed petioles and only six stamens and Parajubaea has between 13-18 stamens (Moraes and Henderson 1990, Seubert 1998b, Dransfield et al. 2008). Jubaea chilensis lacks raphide bundles and raphide containing idioblasts in the staminate petals, as observed in Butia (Martel et al. 2013).
Figure 1: a.
Jubaea chilensis, b. Butia capitata
(Photo: Ramón Vallejos) and c.
Parajubaea torallyi
(Photo: Janine Gray).
An analysis of maximum parsimony (Gunn 2004) and a molecular phylogeny of Bayesian approach (Meerow et al. 2009) place Butia and Jubaea in their own clade (100/100% BP) and suggest that Butia capitata, B. eriosphata and J. chilensis are closely related. The three genera, Jubaea, Parajubaea and Butia, have 32 chromosomes (2n), as do most of the species of the Attaleinae subtribe (77%) (Dransfield et al. 2008, Meerow et al. 2009).
Size of the species compared to its peers
Jubaea chilensis has been described as a giant herb, a giant grass or an arborescent monocotyledon species (Senerman 1970, Tomlinson 2006). According to the classification of growth forms of palm trees (Balslev et al. 2011), the growth habits of J. chilensis corresponds to "large tall-trunked palms", LT-Tp, which species characterized by trunks 20-35 m in height and trunk diameters of 0.2-1 m.
The LT-Tp category represents approximately 4% of all palm species in the world, but if we consider only the 789 species in the Americas, this rises to 13%. Considering the maximum heights for the species of the LT-Tp category (Fig. 2), J. chilensis is in the height class of 35 m which represents the percentile 98 (Fig. 2a) and in the diameter class of 2 m or percentile 100 (Fig. 2b). This gives the species the record for being the largest and most massive American palm, also confirming the description by Dransfield et al. (2008) as one of the most massive palm species in the world.
Figure 2: Frequency distribution of a. maximum heights (n = 82) and b. maximum diameters (n = 78) of the American palm species classified in the group of "large tall-stemmed palms". Data source: Edwards (1903), Parish (1907), Bernal and Henderson (1986), Henderson (1990), Henderson et al. (1995), Joyal (1996), Moraes (1996), Zona (1996, 2000, 2002), Borschsenius et al. (1998), Evans (2001), La Torre-Cuadros and Islebe (2003), Gentil and Ferreira (2005), Del Cañizo (2011), Sanin and Galeano (2011).
Hurtado (2013) developed a volume function for a group of 52-year-old palms at the National Botanical Garden in Viña del Mar:
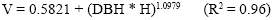
Where V is the volume in m3; DBH is diameter at breast height (1.3 m) in m; and H is the total height in m. According to this function, a palm tree 2 m in diameter and 33 m high has a stem volume of 57.9 m3.
Morphological and anatomical description
The Chilean palm has a massive and smooth trunk up to 33 m in height (Edwards 1903), and a diameter of between 0.43 to 2 m (Rubinstein 1969, Muñoz 1971). The trunk has a distinctive cylindrical shape with irregular swelling at or near the base and generally tapered toward the apex; unramified, dark gray, with a thinner part in the upper portion of the trunk in mature palms. Leaf scars can be distinguished for many years (Figs. 3a-c) (Rodríguez et al. 1983, Henderson et al. 1995). The thinnest part in mature individuals (Fig. 3d) is explained as a physiological variation produced after the first flowering and fruiting, which generates a loss of vegetative vigor and a reduction in meristematic activity in the apex (Rubinstein 1969, Senerman 1970). Consequently, the height at which the thinnest portion of the trunk begins indicates when the first flowering occurred, when the plant changed from the mature vegetative to mature reproductive stage. This happens when the plant is 10 to 13 m high and circa 40 years old (Rubinstein 1969).
Figure 3: Details of a J. chilensis stem. a. complete cross section (diameter = 0.6 m), b. close-up of the cut, c. leaf scars on the trunk surface (Photo: Ramón Vallejos), d. stem thickening in and adult specimen (Photo: Eduardo Guzmán).
The only shoot apical meristem is in the upper part and is protected by leaves. The trunk is composed internally of base tissue with many randomly distributed vascular bundles, covered by a combustion-resistant bark (Senerman 1970). The internal structure of the trunk is compact, with a high air content that avoids heat propagation harming internal tissues and offering protection from fire (Urban 1934, Senerman 1970). In its first 20 years, the Chilean palm grows mainly in diameter and after that increases in height (Álvarez de Araya and Matte 1964).
In the early years of the chilean palm, the leaves are simple, smooth, 15 to 20 cm long and approximately 1 cm wide (Fig. 4a). With time the leaves grow longer and wider. The first pinnate leaves appear after five years, and from then onwards new leaves are all pinnate and steadily larger in size (Angulo 1985). The leaves are perennial (Angulo 1985), feathery and grouped in the upper extreme of the trunk forming a dense crown (Muñoz 1971, Henderson et al. 1995). They are 2 to 3 m long and in some cases can reach 5 m, they can be 50 to 60 cm wide (Rubinstein 1969, Muñoz 1971) (Figs. 4b-c). The rachis has a triangular section in its distal portion and is rounded in the underside (Dransfield et al. 2008) with 110 to 120 stiff leaflets forming a plane with their margins folded in the direction of the base. The leaflets vary in length, with the shortest ones in the leaf apex (Rodríguez et al. 1983, Angulo 1985). The hook-shaped leaflets are tightly packed together, but irregularly grouped (Dransfield et al. 2008). Unlike species of other genera of the subtribe, the leaf of J. chilensis is anatomically distinct because of its 3-to-4-cell layers-deep adaxial hypodermis, larger vascular bundles attached to both leaf surfaces by fiber girders, adaxial girders and often massive and non-vascular fiber bundles (Tomlinson et al. 2011).
Figure 4: Leaf and root morphology of J. chilensis.
a. seedling single-leaf, b. adult leaf, c upper side view of a juvenile palm apex, d. six-year old palm root, e. adult palm root (Photo: Eduardo Guzmán).
The first roots of J. chilensis in its early growth stage are replaced by secondary roots in the entire trunk base (Urban 1934) that are 1 cm in diameter and 2-3 m long (Senerman 1970) (Figs. 4d-e). The deep roots fasten the plant firmly to the ground, allowing it to resist extreme wind conditions (Urban 1934). The roots of the Chilean palm exhibit in their outer section a solid cork that replaces the rhizodermis, exodermis structures, and the outside bark area, while its inner bark area consists of thick homogeneously distributed fibers and a solid hollow cylinder formed by brachysclereids (Seubert 1998b). Finally, the endodermis has U-shaped cells with inner walls that are slightly thicker than the outer walls (Seubert 1998b).
Inflorescences, which are produced 3-12 times per year in the Chilean palm, emerge from the leaf base. Its flowers are short (2.5- 3 cm), actinomorphic, unisexual, and diclino-monoecious. Staminate flowers (Fig. 5a), located towards the apex, have three petals and 18 erect stamens in two series and anthers with two linear cells that are opened by longitudinal grooves (Muñoz 1971). The pollen is asymmetrically ellipsoidal, with a distal opening groove and a longer axis of 46 - 54 μm. Pistillate flowers are globular and slightly larger than the male flowers and have three sepals, three rounded and overlapping petals, as well as a triloculated and triovulated gynoecium (Dransfield et al. 2008). The development of inflorescences consists of two bracts (Figs. 5b - d), first, a fibrous bract that falls away and then a ligneous bract 1 - 1.2 m in length that remains until the fruit ripens (Urban 1934, Rubinstein 1969, Muñoz 1971). Within the bracts there is a racemose inflorescence 1.1 m in length, with an average of 142 pins distributed helically (Manríquez et al. 1992 cited in Cabello 2006).
Figure 5: Chilean palm flower, fruits and seeds. a. staminate flower, b. racemes of fruit (Photo: Paulina Fernández), c. racemes of fruit protected by bracts, d. bract varieties, e. fruit, f. halved fruit showing the endocarp inside, g endocarp with pores, h. halved endocarp and internal seed, i. germinated seed, j. germination boxes from Oasis la Campana (Photo: Eduardo Guzmán). Scale bar = 1cm.
The fruits are drupes with fibrous and smooth 2.2-cm-long spherical exocarpe and a green-yellow skin towards yelloworange (Figs. 5c - f) (Urban 1934, Muñoz 1971, Camilo 2008). The mesocarpe is fleshy and eatable. Inside the fruit there is a smooth spherical seed 2 cm in diameter and weighing approximately 2.6 g (Rodríguez et al. 1983, Camilo 2008). The seed (Figs. 5g -h) is closely attached to a woody endocarpe composed of three pores on its widest part. The seed has a homogeneous endosperm and an embryo in front of one pore (Urban 1934, Gunn 2004). Two of the pores are elliptically shaped, while the third one is circular (Urban 1934). It is in the latter where germination occurs (Figs. 5i- j). The woody endocarp with the seed inside is called a "coquito" (little coconut in Spanish). The seed can be eaten after breaking open the endocarp. An individual produces 10,000 fruits in a good year (Urban 1934).
The seed of the Chilean palm has a very high fatty acid content (68.6%) predominately saturated fatty acids (85%) (Masson et al. 2008). Also notable is the significant linoleic acid content (2.15%), which is essential for human wellbeing (Masson et al. 2008). Moreover, the fatty lauric and mistiric acids present in the seeds have nematicidic effects on Caenorhabditis elegans Maupas, 1900 (Gu et al. 2005).
Natural distribution and ecology
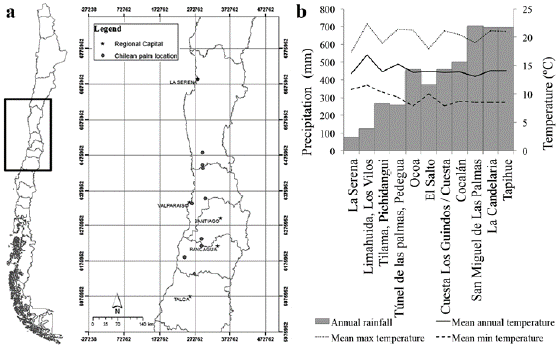
The Chilean palm is currently found from La Serena city, in the Coquimbo Region, (29° 54' S, 71° 15' W), to Curicó city in the Maule Region (35° 15' S, 71° 47' W) (Urban 1934, Rodríguez et al. 1983, Henderson et al. 1995) (Fig. 6a). Although several authors have described J. chilensis as the southernmost palm species in the continent (Urban 1934, Parsons 2007, Pintaud and Ludeña 2008), this is not true, which is in fact Rhopalostylis sapida H.Wendl and Drude, 1878, located 43° - 44° S on the Chatham Islands, New Zealand (Dugand 1965, Parsons 2007, Dransfield et al. 2008).
Figure 6: Natural distribution of Chilean palm populations. a. Chile territory and Chilean palm main locations, b. temperature and rainfall conditions of the natural distribution of the Chilean palm. Data sources: Santibáñez et al. 1990, 1993, Rioseco and Tesser c2014.
Jubaea chilensis grows in dry areas, mainly with a Mediterranean climate with low-to-medium rainfall (Rodríguez et al. 1983, Henderson et al. 1995). The species is found in valleys, close to streams and hillsides from the Chilean coastal mountain range (Urban 1934) to 1,600 m above sea level (Rundel and Weisser 1975). Moreover, it is among the few pinnate-leafed palm species that can survive temperatures from -10 to -12°C, withstanding temperatures as low as -22°C, like in Perpignan, France, in the year 1956 (Del Cañizo 2011).
Its natural distribution, including areas with extreme temperatures, indicates that J. chilensis is tolerant to cold, a marked difference from many tropical palm species. This feature has enabled the Chilean palm to grow successfully in some places quite far south in Chile like Frutillar (Region of Lagos, 41° 7' S, 73° 3' W), with a mean temperature of 7.5 °C in the coldest month and a minimum extreme temperature of 4.1 °C (CLIMATE-DATA.ORG 2015) and other places in the world as described in the section "Uses around the world". Within its natural range, the species withstands a mean temperature range of 8.8 °C to 20.2 °C (Santibáñez et al. 1990, 1993) (Fig. 6b). Rainfall can be as low as 78.5 mm y-1 on average with extreme values like 8.5 mm y-1 that occurred in 1969 in the northern part of its range (Rioseco and Tesser c2014) to 696 mm y-1 on average in the southern part of its range (Santibáñez et al. 1993).
Microclimate and topography determine the density of the Chilean palm population (Rubinstein 1969), while the density of understory associated with palm trees is determined by the density of the palm tree population, environmental conditions, grazing intensity and the use of accompanying vegetation for fuel (Donoso 1982). The differences in height and age are mainly defined by earlier exploitation (Rubinstein 1969) and regeneration conditions. The natural structure of a properly regenerated stand is open (Vita 1989), with a high canopy of adult palms, followed by different strata of regenerating palms of different ages and associated bushy Mediterranean species.
In 1550 the Chilean palm population was estimated at 5 million trees (Hechenleitner et al. 2005). In The History of the Kingdom of Chile, the Jesuit priest Alonso De Ovalle stated that these palm trees grow so densely in mountains and ravines that from afar they appear placed there by magic (De Ovalle 1646). This enabled the species to have a continuous population throughout its natural distribution. Nowadays, the Chilean palm population is drastically reduced to approximately 123000 trees (Alvarado 2009, González et al. 2009).
The Chilean palm is a representative species of the open forests of the Chilean Mediterranean zone (Vita 1989). Its importance is reflected in the fact that vegetational formations that include it are classified as Chilean palm-type forests, according to the classification of Donoso (1981). In these formations, J. chilensis is associated, for example, with Lithrea caustica (Mol.) Hook & Am, Cryptocarya alba (Mol.) Looser, Quillaja saponaria Mol., Acacia caven Mol., Peumus boldus Mol. and Maytenus boaria Mol. (Donoso 1981). All of these species are also typical of the Mediterranean zone of Chile.
Propagation and management of the Chilean palm
In its natural environment, J. chilensis seeds take between six months to four years to germinate. However, germination often occurs within 18 months (Angulo 1985), since Chilean palm seed suffers physiologic latency that is manifested by the immaturity of the embryo (Forcelledo 2006), corresponding to morphologic latency according to the latest dormancy classification. Under nursery conditions, there is great variability in germination capacity, varying from four months at 12 °C, with 90% of seeds sprouting (Fernández, unpublished) to 21 months at 30 °C, with 68.7% of seeds sprouting (Cabello 1990). Under in vitro culture, embryos take 15 days to germinate, reaching 75% of germination capacity in 45 days at 30 °C (Cabello 1990). The woody endocarp delays water accessing the seed interior and therefore does not impede germination (Cabello and Infante 1994).
By soaking seeds in Ethrel it is possible to accelerate the germination process (Arrué 2000), obtaining sprouted seeds in 20 days (Vega 2001). This product accelerates the ripening process of the seed, and it may be beneficial to break the dormancy of the species (Forcelledo 2006). Solari (2002) proposed that the best nursery practice is to use a substrate of 70% leaf mold and 30% weathered granite, without additional fertilization, and 0% of shade. Under these conditions, 93.8% plant survival is reached. Solari (2002) proposed that the best nursery practice is to use a substrate of 70% leaf mold and 30% weathered granite, without additional fertilization, and 0% of shade.
A test establishing plants with and without irrigation in a Mediterranean environment showed no response to watering at Lolol in the Region of O'Higgins, 34° 43' 43" S, 71° 38' 41" W (Castillo 2000). However, we disagree with these results. The trial was measured during only one growing season. Later visits to the trial, by personal observation of one author, over the following 10 years have shown that plants irrigated during the summer as a local people initiative in some sections of the trial are remarkably different in size from non-irrigated plants. The irrigated plants also make an earlier transition from simple to pinnate leaves.
In other experiences, although there is no evidence about the effect of fertilization on palm survival at planting (Solari 2002 Lewin 2003), there was an increase in root biomass, which benefits plant growth and improves survival rates (Lewin 2003).
Ornamental uses of Chilean palm and palm honey harvest.
Multiple uses
Historically, each part of the Chilean palm tree has had a domestic use (Gay 1853) the extracted sap being the most important (Rodríguez et al. 1983). Other examples are the use of palm leaves in basketry and handcrafts and trunk fibers in making paper (Urban 1934, Rodríguez et al. 1983) Haynes and McLaughlin (2000) noted that the Jubaea and Parajubaea genera are par of a small group of palm species whose seeds are used as food. In contrast, the fresh fruit of the Butia species is used as food, but not its seeds. Chilean palms are currently no being harvested for honey, but the fruit i becoming an important source for profitable businesses. In relation to medicinal uses Chilean palm fruit has laxative and digestive properties, and high levels of terpene content have been found (Montenegro et al. 1994).
Salvatierra (2011) explored the production of monofloral bee (Apis mellifera L.' honey from the Chilean palm, evaluating the possibility to enhance the commercial value of the species by the production o certified monofloral honey. Unfortunately its participation in the local honey was no significant.
Palm honey harvest
One of the most well known uses of the Chilean palm is honey production, which is made from the sap and extracted by turning the palm, which ultimately kills the plant. Vicuña-Mackenna (1877) stated that it would be more profitable to extract the palm fruit than the honey.
The plants are selected for extracting sap based on accessibility, the amount of associated vegetation and the experience of the collector (Rubinstein 1969). The sap is extracted between October and April by raising the tree out of the ground and the apex is defoliated and a container is attached to collect the sap. The collected sap is concentrated at 66.6 °Brix, at which point it reaches a stable density (Rubinstein 1969). Sap yields per tree vary between 80 to 600 L and the conversion factor from sap to basic concentrated honey is 0.256 (Rubinstein 1969).
The relationship between traditional forest variables like tree height, diameter and volume, and the amount of sap produced is not clear (Rubinstein 1969). Curiously, having an experienced labor force can be a major factor in determining the yield of extracted sap (Velásquez 1995). Only 16.7% of palm species (30 species) are used for sap extraction and with five of these, including J. chilensis, it is necessary to fell the tree, while a non-destructive method is used with Parajubaea cocoides and P. torallyi (Haynes and McLaughlin 2000).
Ornamental use
Historical situation in Chile
Since colonial times, around the XVIII century, the Chilean palm has been a valuable ornamental species. At that time, its slow growth was not considered an impediment and therefore it was commonly used for decorating avenues, the entrances of important buildings, churches and mansions, among others. There are still vestiges of these uses throughout Chile as the entrance of the monastery of San Pedro de Alcántara at the Province of Cardenal Caro, Region of O'Higgins, which was built in 1725, and in a manor house at Cunaco, Province of Colchagua, Region of O'Higgins.
Current revitalization in the use of the Chilean palm
In recent decades there has been increasing use of the Chilean palm as an ornamental plant in squares, parks, and gardens (Campos 1998). Because of its size and great ornamental value, the Chilean palm is currently raised in nurseries, including for exportation, and even adult palms are transplanted for landscaping. As well, J. chilensis and Butia odorata have been used as parent plants to obtain the interspecific hybrid Jubautia and the species Jubautia splendens Hodel (Hodel 2011).
Uses around the world
Although some naturalists have reported the species in Europe, one of the most famous descriptions was that of Charles Darwin, who observed the species in Chile during his voyage on the Beagle. He wrote: "In a few places there were palms, and I was surprised to see one at an elevation of at least 4500 feet. These palms are, for their family, ugly trees. Their trunk is very large, and of a curious form, being thicker in the middle than at the base or top. They are excessively numerous in some parts of Chile, and valuable on account of a sort of treacle made from the sap... they tried to count them, but failed, after having numbered several hundred thousand. A good tree will give ninety gallons, and all this must have been contained in the vessels of the apparently dry trunk" (Fitzroy et al. 1839).
Since then, and particularly during the 19th century, J. chilensis was exported and used as an ornamental plant. In an exhaustive review of original documents, Zona (c2011) describes the journeys of J. chilensis to and within Europe, from the 19th century onward, arriving first to the Kew Gardens in London between 1843 and 1846. Unfortunately, this first specimen was cut down in 2014, despite its 164 years, due to the remodeling of the Temperate House (Kennedy 2013). Later the species was reported in Portugal, France, Italy, Germany and other countries. The first records are from botanic gardens, arboretums, greenhouses, collectors or people that could afford a specimen for their personal gardens. Because of its longevity, living examples of the original Chilean palms can still be found in different parts of Europe (Zona c2011). Nowadays, Chilean palms are widely used as ornamental plants in warm, temperate, arid, and even cold regions (Dransfield et al. 2008).
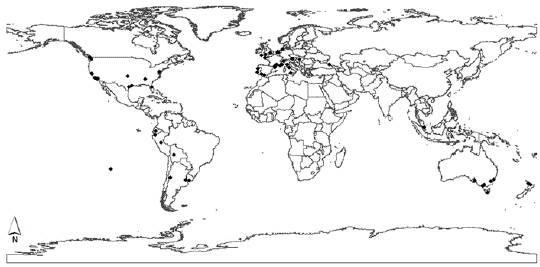
Generally, Chilean palms are used ornamentally in squares and around important buildings, but above all as exhibit pieces in botanical gardens and arboretums (Fig. 7). In Ambato (Ecuador), in addition to its ornamental use, the Chilean palm is used for coconut production (Pintaud and Ludeña 2008).
Figure 7: Map of Jubaea chilensis distribution around the world. Black dots indicate places where there are specimens of the species. As example, there are Chilean palms in cities in Australia (Intown 2014, Parsons c2013, Zona c2011), the USA (Fullerton Arboretum 2014, Goleta Valley Historical Society 2014, The Huntington c2014, Zona c2011), Spain (del Cañizo 2011, Plumed 2013, Sánchez 2011), France (Del Cañizo 2011, Dugand 1965, INRA 1998), Ireland (Kells Bay House and Gardens 2014), Italy (Plaumann 2001, Zona c2011), the Netherlands and Portugal (Zona c2011), the UK (Kew Royal Botanic Gardens 2014) and Russia (Nichols 2014).
The feasibility of using the Chilean palm has been considered and studied in other countries. For example, the Chilean palm can be used ornamentally in Turkey given its resistance to cold (Coskuner 2006 cited by Hazir and Buyukozturk 2013). Another example is a master plan of Santa Monica, California for an urban forest (USA), which involves replacing Phoenix canariensis hort. ex Chabaud with J. chilensis in streets and avenues to ensure the benefits of an urban forest for residents and tourists (Santa Monica Public Landscape Division c2011).
There are several individuals that serve an ornamental function on Helgoland Island in the German North Sea, where temperatures can fall to -2 °C. These palms are highly resistant to winter cold, with no need for protection from the cold, although they do need protection from salty sea winds. In the continent, the palm can resist temperatures as low as -15°C but does not tolerate permafrost (Jäck 2011).
Threats and conservation
Threats
From early days, Chilean palms were felled to extract sap for palm honey production (Vicuña-Mackenna 1877, Urban 1934). Vicuña-Mackenna (1877) indicated that several thousand trees were felled every year for honey and to produce liquor. In fact, in the past this was considered the prime threat for the species (González 1992). Land use change from Mediterranean forests to agricultural use has been the other main threat to the species. As the species is now legally protected, the main four threats to natural populations today are indiscriminate fruit harvests, herbivorous feeding, decreased vegetal cover and fires.
The first threat, indiscriminate fruit harvesting is the most serious and is responsible for the ageing of palm tree populations (González 1992). Indiscriminate fruit harvesting is an issue for the regeneration of species related to the Chilean palm, in particular, B. capitata (Mart.) Becc. from Uruguay and Brazil (Rivas 2005), Parajubaea sunkha Moraes from Bolivia (Enssle et al. 2006) and P. cocoides Burret from Peru (Roca 2010).
The second threat is herbivorous feeding by native and other species. The degú (Octodon degus Molina, 1782) is a native rodent that feeds on fallen palm fruit. The life cycle of this rodent fits with the phenological cycle of the plant species in the same habitat (Zunino et al. 1992). The European rabbit (Oryctolagus cuniculus Linnaeus, 1785) is a non-native species that feeds on Chilean palm seedlings (Rubinstein 1969, Marcelo et al. 2006). There is also the presence of grazing cattle (González 1992).
The third threat is decreased vegetal cover. The vegetal cover provides the necessary microhabitat for seedling establishment. This feature is of the utmost importance for the survival and growth of individuals (Vita 1989). In the palm tree population located in Tapihue, Chile, seedlings were only found under cover of a boldo tree (Peumus boldus Mol.) (Flores 2012). Marcelo et al. (2006) also determined the crucial importance of vegetal cover in the recruitment and survival of Chilean palm seedlings. Vegetative cover is lost mainly with the conversion of forests to agricultural and grazing lands (González 1992) or the use of associated vegetation for fuel.
The fourth threat is fires. Regeneration is also affected by continuous fires in the habitats of the Chilean palm due to human negligence. An example of this is the analysis of the age structure based on morphologic attributes of two palm tree populations, at the locations of Ocoa and Las Siete Hermanas, Region of Valparaíso in Chile. One of the conclusions of this analysis is that the latter should be a high priority for conservation because the population is not regenerating naturally, with only 10% of individuals as seedlings and juveniles, resulting in the aging of the population in the context of continuous fires in the area (Díaz 2009). Fires affect both associated vegetation and palm seedlings (Rubinstein 1969). Fires do not greatly affect adult palm trees because their trunks are resistant to fire. Nevertheless, Chilean palm trees regularly regenerate themselves after fires provided they are not located on bare ground (Quintanilla and Reyes 1999), and there are enough seeds available and low levels of herbivorous feeding.
Another threat are diseases, but with a minor impact. At the national level, some fungi attacks have been described in the leaves of J. chilensis (Baldini and Pancel 2002). At the international level, several pests have been described as affecting the Chilean palm. In France, J. chilensis can be affected by the caterpillars of the moth Paysandisia archon Burmeister, 1880 (Peltier 2007) and in Italy, the palm is sensitive to coleopteran Rhynchophorus ferrugineus Olivier, 1790 (Raciti et al. 2013).
Protection and conservation
Legal efforts to preserve J. chilensis began with the Supreme Decree No. 908 in 1941, which regulated the export of the plants and its products. In the 1970s a historic trend towards the complete elimination of the Chilean palm was identified, with indications of the cutting rates and age range of the remaining individuals (Rubinstein 1969). The Chilean palm is currently classified as a vulnerable species (González 1998). Experts suggest that the best way to preserve the Chilean palm over time is through its protection and sustainable use (Bañados 1991).
Among the plant species of the Chilean Mediterranean forest ecosystem, the Chilean palm is considered to have the greatest economic potential, more for its fruit than its honey (Chile Forestal 1998). Its use can be highly profitable under appropriate management, taking into account the products that can be obtained, the social benefits of preserving the species, the recreational uses, as well as direct products (Rubinstein 1969). In addition, Chilean palm crops could be profitable in 49 districts of 29 towns in Chile, comprising a total of 86,615 hectares. After evaluating the related socioeconomic and physical-ecological factors, González et al. (2001) considered Chilean palm cultivation a feasible source of sustenance for low-income families.
Final Comments
Jubaea chilensis is particularly interesting to study because of its unique features. The species is one of the largest in its family, with a limited but climatically varied natural distribution. The Chilean palm is highly resistant to extreme conditions like low temperatures. Proof of this is that the Chilean palm is the southernmost palm species in the continent, but not in the planet. Considering its beauty, longevity, feather-grass shape and color, and the products made from it, the Chilean palm is known nationally and internationally. This is also the basis for further genetic work with this species, including the hybrids obtained with Butia with features of J. chilensis of ornamental interest.
Despite all the characteristics that differentiate it from other palm species, such as size, geographic distribution, productive potential, presence around the world in places where other palm species do not survive, J. chilensis has had a low level of regeneration in its natural environment in recent years as a result of uncontrolled harvesting of its fruit. There is also greater knowledge and public recognition about the main threats to the species. However, this knowledge has not been applied in the design of new management plans. Therefore, J. chilensis remains in the category of vulnerable species.
In order to contribute to this issue, more research at the species level is highly recommended. Three broad thematic areas should be the focus for deepening our knowledge about the Chilean palm. The first theme is the biology of the species, specifically in terms of ecophysiology and reproduction. In the ecophysiology area, deeper knowledge of metabolic and internal processes is necessary to better understand species dynamics and interactions with the environment. The second theme is to determine the environmental requirements of the species. Finally, and resulting from the application of the knowledge of the first two themes, is reconstructing natural populations. To do this, it is necessary to define protocols for the reproduction and reforestation of J. chilensis, establishing the best strategies for its reintroduction.
Once advances have been made in the aforementioned themes, public management and conservation policies and plans can be defined to improve the conservation of the species. It is also fundamental to involve communities close to populations of this species in their protection, because of which information and education should always be considered fundamental elements.
AUTHORS PARTICIPATION
EG conception, design, collection of information, analysis and writing of the document. JAC and SC analysis and writing of the document. MPF conception, design, collection of information, analysis and writing of the document.
ACKNOWLEDGMENT
We are grateful to Mauricio Moreno ("Oasis de la Campana" Nursery) for allowing us to learn firsthand about the management of the species. We also thank Mauro Torres Sebastian for his support during the writing process of this research.
LITERATURA CITADA
Referencias
Alvarado C. 2009. Guía de Flora Nativa de El Salto y Forestal. Proyecto gestión urbana y territorial participativa: una llave para la cohesión social y territorial. Ilustre Municipalidad de Viña del Mar, Viña del Mar. 19 pp.
Álvarez de Araya G, Matte V. 1964. Contribución al estudio de la palma chilena. Boletín de la Universidad de Chile 053/054:40–43.
Angulo J. 1985. La palma chilena: interesante recurso natural renovable. Santiago de Chile: Sociedad Agrícola y Forestal Hacienda Las Palmas de Cocalán Ltda.
Arrué K. 2000. Ensayo de germinación de palma chilena (Jubaea chilensis (Mol.) Baillon) con fines de propagación masiva. [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias
Forestales, Universidad de Chile.
Baker WJ, Dransfield J. 2016. Beyond Genera Palmarum: progress and prospects in palm systematics. Bot. J. Linn. Soc. 182(2):207–233.
Baldini A, Pancel L. 2002. Agentes de daño en el bosque nativo. Santiago de Chile: Editorial Universitaria.
Balslev H. 2011. Palm harvest impacts in northwestern South America. Bot. Rev. 77:370–380.
Balslev H, Kahn F, Millan B, Svenning JC, Kristiansen T, Borchsenius F, Pedersen D, Eiserhardt WL. 2011. Species diversity and growth forms in tropical American palm
communities. Bot. Rev. 77:381–425.
Bañados A. 1991. Palma chilena: una especie que requiere explotación. Chile Forestal 189:12–14.
Bernal R, Henderson R. 1986. A new species of Socratea from Colombia with note on the Genus. Brittonia 38:55–59.
Borschsenius F, Borgotofot-Pedersen H, Balslev H. 1998. Manual to the Palms of Ecuador. Aarhus: AAU Reports 37.
Bruniard E. 1982. La diagonal árida argentina: un límite climático real. Revista Geográfica 95: 5–20.
Cabello A. 1990. Antecedentes sobre la germinación y el cultivo in vitro de la palma chilena (Jubaea chilensis (Mol.) Baillon). Ciencias Forestales 6:3–21.
Cabello A, Infante L. 1994. Efecto del endocarpio, de la temperatura y del periodo de remojo, sobre el contenido de humedad de semillas de palma chilena (Jubaea chilensis (Mol.) Baillon). Ciencias Forestales 9:3–10.
Cabello A. 2006. Jubaea chilensis (Mol.) Baillon. In: Donoso C, editor. Las especies arbóreas de los bosques templados de Chile y Argentina, Autoecología. Valdivia: Marisa Cuneo Ediciones. p. 285-297.
Camilo C. 2008. Análisis proximal de semillas no comunes: palma chilena (Jubaea chilensis), cilantro (Coriandrum sativum), mora (Rubus glaucus), rosa mosqueta (Rosa aff. rubiginosa) y caracterización de su aceite. [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile.
Campos J. 1998. Productos forestales no madereros en Chile. Santiago de Chile: Oficina Regional de la FAO para América Latina y el Caribe.
Castillo J. 2000. Efecto del riego sobre la sobrevivencia y biomasa inicial de plantaciones de palma chilena (Jubaea chilensis (Mol.) Baillon) en la comuna de Lolol. [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Forestales, Universidad de Chile.
Chile Forestal. 1998. Ficha forestal: palma chilena (Jubaea chilensis). Chile Forestal 258:49–50.
Climate-Data.Org. 2015. Clima: Frutillar. [last accessed: 15 Nov 2015]. http://es.climatedata.org/location/55866/
De Ovalle A. 1646. Histórica Relación Del Reyno de Chile. Roma: Francisco Cavallo.
Del Cañizo JA. 2011. Palmeras. 3º edición. Madrid: Ediciones Mundi-Prensa.
Díaz E. 2009. Estructura de poblaciones naturales de palma chilena, Jubaea chilensis (Mol.) Baillon. [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Forestales, Universidad de Chile.
Donoso C. 1981. Tipos forestales de los bosques nativos de Chile. Documento de trabajo Nº 38. Investigación y desarrollo forestal (CONAF, PNUD-FAO). Santiago de Chile: FAO.
Donoso C. 1982. Reseña ecológica de los bosques mediterráneos de Chile. Bosque 4:117–146.
Dransfield J, Flenley JR, King SM, Harkness DD, Rapu S. 1984. A recently extinct palm from Easter Island. Nature 312:750–752.
Dransfield J, Uhl NW, Asmussen CB, Baker WJ, Harley MM, Lewis CE. 2008. Genera palmarum: the evolution and classification of palms. Second edition. London: Kew Publishing.
Dugand A. 1965. Las palmeras y la tierra. Caldasia 9:187–217.
Edwards A. 1903. Ejemplares gigantescos de la palma chilena Jubaea chilensis (Mol.). Rev. Chil. Hist. Nat. 5: 254.
Enssle J, Ferrufino H, Ibisch PL. 2006. Conservation status and economic potential of Parajubaea sunkha, an endemic palm of Bolivia. Palms 50:143–151.
Evans R. 2001. Monograph of Colpothrinax. Palms 45(4): 177–195.
Fitzroy R, Darwin C, King PP. 1839. Narrative of the surveying voyages of his majesty’s ships Adventure and Beagle (vol.3): between the years 1826 and 1836: describing their examination of the southern shores of South America, and the Beagles’s circumnavigation of the globe. United Kingdom. [last accessed: 15 Nov 2015]. http://libros.uchile.cl/25
Flores M. 2012. Propuesta de lineamientos estratégicos de conservación de palma chilena en la localidad de palmas de Tapihue, comuna de Pencahue, región del Maule. [Degree
thesis]. [Santiago de Chile]: Facultad de Ciencias Agronómicas, Universidad de Chile.
Forcelledo A. 2006. Germinación y calidad de planta de palma chilena (Jubaea chilensis (Mol.) Baillon) según sustrato, periodo de siembra y procedencia de semilla. [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Forestales, Universidad de Chile.
Fullerton Arboretum. 2014. Plant database. Fullerton Arboretum, California. [last accessed: 11 Agu 2014]. http://www.fullertonarboretum.org/results.php?mode=search&bedID=176
Gay C. 1853. Historia física y política de Chile botánica. Tomo Sexto. Paris: E. Thunoy y Compañía Print.
Gentil DFO, Ferreira SAN. 2005. Morfologia da plântula em desenvolvimento de Astrocaryum aculeatum Meyer (Arecaceae). Acta Amazónica 35(3): 337–342.
Goleta Valley Historical Society. 2014. Rancho La Patera y Stow House. [last accessed: 11 Agu 2014]. http://stowhouse.com/gardens/
González L. 1992. La palma chilena: Perspectivas futuras para su uso sustentable. Revista Ambiente y Desarrollo 8:73–76.
González M. 1998. Jubaea chilensis. The IUCN Red List of Threatened Species 1998: e.T38586A10128158. [last accessed: 28 Apr 2014]. http://dx.doi.org/10.2305/IUCN.
UK.1998.RLTS.T38586A10128158.en
González L, Garfias R, Pedernera A, Toral M. 2001. Fomento del cultivo de palma chilena. Chile Forestal 283:11–16.
González L, Bustamante R, Navarro R, Herrera M, Toral M. 2009. Ecology and management of the Chilean Palm (Jubaea chilensis): history, current situation and perspectives. Palms 52(2):68–74.
Gu J-Q, Eppler M, Montenegro G, Timmins S, Timmermann B. 2005. Identification of Nematicidal Fatty Acids and Triglycerides from Seeds of Jubaea chilensis by GC-EI-MS and Chemical Transformation Methods. Z. Naturforsch. Sect. C J. Biosci. 60(7-8):527–533.
Gunn B. 2004. The Phylogeny of the Cocoeae (Arecaceae) with Emphasis on Cocos nucifera. Annals of the Missouri Botanical Garden 91:505–522.
Haynes J, McLaughlin J. 2000. Edible palms and their uses. Fact Sheet MDCE-00-50-1. University of Florida Institute of Food and Agricultural Sciences. http://www.quisqualis.com/tv01ediblepalms.html
Hazir A, Buyukozturk H. 2013. Phoenix spp. and other ornamental palms in Turkey: The threat from red palm weevil and red palm scale insects. Emir. J. Food Agric. 25(11):843–853. doi: 10.9755/ejfa.v25i11.16500.
Hechenleitner P, Gardner MF, Thomas PI, Echeverría C, Escobar B, Brownless P, Martínez C. 2005. Plantas Amenazadas del Centro-Sur de Chile. Distribución, Conservación y Propagación. Primera Edición. Chile: Universidad Austral de Chile y Real Jardín Botánico de Edimburgo.
Henderson A. 1990. Introduction and the Iriarteinae. Flora Neotropica Monograph 53.
New York: The New York Botanical Garden.
Henderson A, Galeano G, Bernal R. 1995. Field Guide to the Palms of the Americas. New Jersey: Princeton University Press.
Hodel D. 2011. A new nothospecies and two cultivars for the hybrids in cultivation between Butia odorata and Jubaea chilensis. Palms 55(2):62–71.
Hurtado F. 2013. Funciones locales de volumen de especies nativas de bosques naturales y plantaciones. Región de Valparaíso. Documento técnico 212. CONAF, Ministerio
de Agricultura. Valparaíso: CONAF, Ministerio de Agricultura.
[INRA] L’Institut national de la recherche agronomique. 1998. Jardin Botanique Villa Thuret. [last accessed: 11 Agu 2014]. http://www.biologie.uni-ulm.de/systax/infgard/indsem/antibes.html
INTOWN. 2014 The Geelong Botanical Gardens. Intown Geelong’s online entertainment, leisure, tourist & city guide. [last accessed: 11 Agu 2014]. http://www.intown.com.au/locals/geelong/attractions/geelong_botanical_gardens1.htm
Jäck J. 2011. “Exoten” und Palmen auf Helgoland: Ein Pflanzenführer der besonderen Art. First Edition, Norderstedt, Germany: Books on Demand.
Joyal E. 1996. The Use of Sabal uresana (Arecaceae) and other Palms in Sonora, Mexico. Econ. Bot. 50:429–445.
Kells Bay House and Gardens. 2014. Jubaea chilensis. [last accessed: 11 Agu 2014]. http://www.kellsgardens.ie/nursery/jubaea-chilensis
Kennedy M. 2013. Kew Gardens’s Temperate House closes for five-year restoration project. [last accessed: 15 Nov 2015]. http://www.theguardian.com/lifeandstyle/2013/aug/04/kew-gardens-temperate-house-closesrestoration
Kew Royal Botanic Gardens. 2014. Jubaea chilensis (Chilean wine palm). [last accessed: 11 Agu 2014]. http://www.kew.org/scienceconservation/plants-fungi/jubaea-chilensischilean-wine-palm
La Torre-Cuadros MA, Islebe GA. 2003. Traditional ecological knowledge and use of vegetation in southeastern Mexico: a case study from Solferino, Quintana Roo. Biodivers. Conserv. 12:2455–2476.
Lewin P. 2003. Ensayos de fertilización para el establecimiento de palma chilena (Jubaea chilensis (Mol.) Baillon). [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Forestales, Universidad de Chile.
Marcelo W, Bustamante RO, Vásquez RA. 2006. Efectos de la herbivoría, el microhábitat y el tamaño de las semillas en la sobrevivencia y crecimiento de plántulas de la palma chilena. Revista Ambiente y Desarrollo 22(2):55–62.
Martel C, Noblick L, Stauffer F. 2013. An anatomical character to support the cohesive unit of Butia species. Palms 57(1):30–35.
Masson L, Camilo C, Torija ME. 2008. Caracterización del aceite de coquito de palma (Jubaea chilensis). Grasas Aceites 59(1):33–38.
Meerow A, Noblick L, Borrone J, Couvreur T, Mauro-Herrera M, Hahn W, Kuhn D, Nakamura K, Oleas N, Schnell E. 2009. Phylogenetic Analysis of Seven WRKY Genes across the Palm Subtribe Attaleinae (Arecaceae) Identifies Syagrus as Sister Group of the Coconut. PloS ONE 4:1–10.
Mieth A, Bork HR. 2010. Humans, climate or introduced rats – which is to blame for the Woodland destruction on prehistoric Rapa Nui (Easter Island)? J. Archaeol. Sci. 37:417–426.
Montenegro G, Gómez M, Iturriaga L, Timmermann B. 1994. Potencialidad de la flora nativa chilena como fuente de productos naturales de uso medicinal. VI Congreso
Latinoamericano de Botánica, 2 al 8 de Octubre de 1994, Mar del Plata, Argentina. p. 49-66.
Moraes M, Henderson A. 1990. The genus Parajubaea (Palmae). Brittonia 42:92–99.
Moraes M. 1996. Novelties of the Genera Parajubaea and Syagrus (Palmae) from Interandean Valleys of Bolivia. Novon 6:85–92.
Muñoz C. 1971. Chile: Plantas en extinción. Santiago de Chile: Editorial Universitaria.
Muñoz-Schick M, Moreira-Muñoz A, Moreira-Espinoza S. 2012. Origen del nombre de los géneros de plantas vasculares nativas de Chile y su representatividad en Chile y el mundo.
Gayana Bot. 69:309–359.
Nichols C. 2014. Bark of Jubaea chilensis. Sochi Arboretum, Russia. [last accessed: 11 Agu 2014]. http://www.clivenichols.com/cgi-bin/stephen_johnson/database/imageFolio.cgi?act
ion=view&link=25k&image=025677.jpg&img=0&search=RUSSIA&cat=all&tt=&bool=phrase
Parish S. 1907. A contribution toward a knowledge of genus Washingtonia. Bot. Gaz. 44:408–434.
Parsons RF. 2007. The southernmost limits for palms. N.Z. J. Bot. 45:477–478.
Parsons S. c2013. Chilean Soap Bark and Chilean Wine Palm. National Arboretum Canberra. [last accessed: 11 Ago 2014]. https://www.nationalarboretum.act.gov.au/livingcollection/trees/tree_stories/chilean_soap_bark_and_chilean_wine_palm
Peltier JB. 2007. Une glu salvatrice contre le ravageur de palmiers, Paysandisia archon. INRA, France. 9 pp.
Pintaud JC, Ludeña B. 2008. Andean palms in Ecuadorean cities. Palms 52(4): 165–173.
Plaumann J. 2001. Surprises in Palermo. Chamaerops 41.
Plumed J. 2013. El Palmetum del Jardín Botánico de la Universidad de Valencia. El Botánico Revista de la AIMJB 7:11–14.
Quintanilla V, Reyes C. 1999. Modificaciones por efecto del fuego en el bosque esclerófilo de quebradas húmedas de Chile Central y su incidencia en la palma chilena. Rev. Geogr. Chile Terra Australis 44:7–18.
Raciti E, Conti F, Carta-Cerrella D, Morabito M, Li-Destri A, Malfitana S, Romano D. 2013. Palm species potentially resistant to red palm weevil attacks in sites of Eastern Sicily heavily infested. Palm Pest Mediterranean Conference, Nice, 16 - 18 January 2013.
Rivas M. 2005. Desafíos y alternativas para la conservación in situ de los palmares de Butia capitata (Mart.) Becc. Agrociencia 9(1-2):161–168.
Rioseco R, Tesser C. c2014. Cartografía interactiva de los climas de Chile. Instituto de Geografía, Pontificia Universidad Católica de Chile. [last accessed: 8 Sep 2014]. http://www7.uc.cl/sw_educ/geografia/cartografiainteractiva/
Roca F. 2010. Parajubabea cocoides, a new record for Peru. Palms 54:133–136.
Rodríguez R, Matthei O, Quezada M. 1983. Flora arbórea de Chile. Santiago de Chile: Editorial de la Universidad de Concepción.
Rubinstein A. 1969. Inventario y estudio de producción de un rodal de palma chilena, Jubaea chilensis (Mol.) Baillon (Hacienda Ocoa, Provincia de Valparaíso). [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Agronómicas, Universidad de Chile.
Rundel P, Weisser P. 1975. La Campana, a new national park in central Chile. Biol. Conserv. 8:35–46.
Salvatierra J. 2011. Manejo Sustentable de los Recursos Naturales en la Reserva Mundial de la Biosfera, Oasis de la Campana: Potencial de Jubaea chilensis para la producción de miel monofloral. [Master thesis]. [Santiago de Chile]: Facultad de Agronomía e Ingeniería Forestal, Pontificia Universidad Católica de Chile.
Sánchez M. 2011. Nuevos príncipes en el Real Jardín Botánico – CSIC: La familia Palmae. Foresta 52:238–240.
Sanin MJ, Galeano G. 2011. A revision of the Andean wax palms, Ceroxylon (Arecaceae). Phytotaxa 34:1–64.
Santa Monica Public Landscape Division.c2011. Santa Monica Urban Forest Master Plan. [last accessed: 1 Jul 2014] http://www.smgov.net/uploadedFiles/Portals/UrbanForest/
Handout%206%20-%20Urban%20Forest%20Master%20Plan.pdf
Santibáñez F, Uribe JM, Vicencio M. 1990. Atlas Agroclimático de Chile Regiones V y Metropolitana. Santiago de Chile: Ministerio de Agricultura, Fondo de Investigación Agropecuaria, Corporación de Fomento de la Producción, Universidad de Chile.
Santibáñez F, Uribe JM, Vicencio M. 1993. Atlas Agroclimático de Chile Regiones Sexta, Séptima, Octava y Novena. Santiago de Chile: Ministerio de Agricultura, Fondo de Investigación Agropecuaria, Corporación de Fomento de la Producción, Universidad de Chile.
Senerman J. 1970. Algunas consideraciones sobre la anatomía del estípite de palma chilena (Jubaea chilensis (Mol.) Baillon). [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Forestales, Universidad de Chile.
Seubert E. 1998a. Root anatomy of palms IV. Arecoideae, part 2 systematic implications. Feddes Repert. 109:231–247.
Seubert E. 1998b. Root anatomy of palms IV. Arecoideae, part 1, general remarks and descriptions on the roots. Feddes Repert.109:89–127.
Solari R. 2002. Viverización de palma chilena (Jubaea chilensis (Mol.) Baillon) bajo diferentes tratamientos de intensidad de luz, sustratos y fertilización. [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Forestales, Universidad de Chile.
The Huntington. c2014. Palm Garden. [last accessed: 11 Ago 2014]. http://www.huntington.org/WebAssets/Templates/content.aspx?id=524
Tomlinson PB. 2006. The uniqueness of palms. Bot. J. Linn. Soc. 151:5–14.
Tomlinson PB, Horn JW, Fisher JB. 2011 The anatomy of palms. Oxford: Oxford University Press.
Urban O. 1934. Botánica de las plantas endémicas de Chile. Concepción: Soc. Impr. y Litografía Concepción.
Vega M. 2001. Influencia del tiempo, tipo de almacenaje y del sustrato en la germinación de la palma chilena (Jubaea chilensis (Mol.) Baillon). [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Forestales, Universidad de Chile.
Velásquez R. 1995. Evaluación de factores que influyen en la producción de savia en palma chilena (Jubaea chilensis (Mol.) Baillon) en el sector de Cocalán, VI Región. [Degree thesis]. [Santiago de Chile]: Facultad de Ciencias Agrarias y Forestales, Universidad de Chile.
Vicuña-Mackenna B. 1877. De Valparaíso a Santiago. Santiago de Chile: Imprenta de la Librería del Mercurio.
Villagrán C, Armesto J. 2005. Fitogeografía histórica de la Cordillera de la Costa de Chile. In: Smith-Ramírez C, Armesto J, Valdovinos C, editors. Historia, biodiversidad y ecología de los bosques costeros de Chile. Santiago de Chile: Editorial Universitaria. p. 99–119.
Vita A. 1989. Ecosistemas de bosques y matorrales mediterráneos y sus tratamientos silviculturales en Chile. Santiago de Chile: Corporación Nacional Forestal.
Zona S. 1996. Roystonea (Arecaceae: Arecoideae). Flora Neotropica Monograph 71. New York: The New York Botanical Garden.
Zona S. 2000. Arecaceae. In: Flora of North America Editorial Committee, editors. Flora of North America north of Mexico. Volumen 22. New York: Oxford University Press. p. 95–123.
Zona S. 2002. A Revision of Pseudophoenix. Palms 46(1):19–38.
Zona S. c2011. The Travels of Jubaea. Pacific Horticulture. [last accessed: 5 Jul 2014]. http://www.pacifichorticulture.org/articles/thetravels-of-jubaea/
Zunino S, Saiz F, Yates L. 1992. Uso del espacio de Octodon degus y oferta de recursos en Ocoa, Parque Nacional La Campana. Rev. Chil. Hist. Nat. 65: 343–355.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Jorge J. Araujo, José L. Rojas, Héctor A. Keller, Norma Hilgert. (2021). Landscape management among the Guarani of the Atlantic Forest of Misiones, Argentina: the case of the Syagrus romanzoffiana (Cham.) Glassman (Arecaceae) palm tree. Ethnobiology and Conservation, 10 https://doi.org/10.15451/ec2021-04-10.22-1-19.
2. Sebastián Cordero, Francisca Gálvez, Francisco E. Fontúrbel. (2021). Multiple Anthropogenic Pressures Lead to Seed Dispersal Collapse of the Southernmost Palm Jubaea chilensis. Frontiers in Ecology and Evolution, 9 https://doi.org/10.3389/fevo.2021.719566.
3. Eduardo Guzmán, M. Paulina Fernández, José-Antonio Alcalde, Samuel Contreras, Pasi Raumonen, Lorenzo Picco, Cristián Montalba, Cristián Tejos. (2022). Phyllotaxis transition over the lifespan of a palm tree using Magnetic Resonance Imaging (MRI) and Terrestrial Laser Scanning (TLS): the case of Jubaea chilensis. Plant Methods, 18(1) https://doi.org/10.1186/s13007-022-00920-z.
4. Andreas Mieth, Annette Kühlem, Burkhard Vogt, Hans-Rudolf Bork. (2022). The Prehistory of Rapa Nui (Easter Island). Developments in Paleoenvironmental Research. 22, p.483. https://doi.org/10.1007/978-3-030-91127-0_19.
5. Daniel W. Ingersoll, Kathleen B. Ingersoll, Fred W. Stauffer. (2022). The Prehistory of Rapa Nui (Easter Island). Developments in Paleoenvironmental Research. 22, p.377. https://doi.org/10.1007/978-3-030-91127-0_15.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Caldasia

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).