Abstracts
OBJECTIVE: To study the brain areas which are activated when normal subjects make moral judgments. METHOD: Ten normal adults underwent BOLD functional magnetic resonance imaging (fMRI) during the auditory presentation of sentences that they were instructed to silently judge as either "right" or "wrong". Half of the sentences had an explicit moral content ("We break the law when necessary"), the other half comprised factual statements devoid of moral connotation ("Stones are made of water"). After scanning, each subject rated the moral content, emotional valence, and judgment difficulty of each sentence on Likert-like scales. To exclude the effect of emotion on the activation results, individual responses were hemodynamically modeled for event-related fMRI analysis. The general linear model was used to evaluate the brain areas activated by moral judgment. RESULTS: Regions activated during moral judgment included the frontopolar cortex (FPC), medial frontal gyrus, right anterior temporal cortex, lenticular nucleus, and cerebellum. Activation of FPC and medial frontal gyrus (BA 10/46 and 9) were largely independent of emotional experience and represented the largest areas of activation. CONCLUSIONS: These results concur with clinical observations assigning a critical role for the frontal poles and right anterior temporal cortex in the mediation of complex judgment processes according to moral constraints. The FPC may work in concert with the orbitofrontal and dorsolateral cortex in the regulation of human social conduct.
frontal lobes; moral judgment; acquired sociopathy; psychopathy
OBJETIVO: Estudar, com ressonância magnética funcional (RMf), as áreas cerebrais normalmente ativadas por julgamentos morais em tarefa de verificação de sentenças. MÉTODO: Dez adultos normais foram estudados com RMf-BOLD durante a apresentação auditiva de sentenças cujo conteúdo foram instruídos a julgar como "certo" ou "errado". Metade das sentenças possuía um conteúdo moral explícito ("Transgredimos a lei se necessário"), enquanto a outra metade era constituída de afirmativas factuais desprovidas de conotação moral ("Pedras são feitas de água"). Depois do estudo funcional, cada indivíduo aferiu o conteúdo moral, a valência emocional, e a dificuldade de julgamento de cada sentença em escalas de Likert. Para excluir o efeito da emoção nos resultados da ativação, as respostas individuais foram hemodinamicamente modeladas para análise de RMf relacionada a eventos. O modelo linear geral foi empregado na avaliação das áreas cerebrais ativadas pelos julgamentos morais. RESULTADOS: As regiões ativadas pelo julgamento moral compreenderam o córtex frontopolar, o giro frontal medial, o córtex temporal anterior direito, o núcleo lenticular, e o cerebelo. As ativações frontopolar e médio-frontal (áreas 10/46 e 9 de Brodmann) mostraram-se independentes da experiência emocional e representaram as maiores áreas de ativação. CONCLUSÃO: Estes resultados vão ao encontro de observações clínicas que atribuem papel crítico aos pólos frontais e ao córtex temporal anterior direito na regulação do comportamento social. O sistema frontopolar-ântero-temporal descrito no presente trabalho pode representar sistema neural relativamente independente, que opera em harmonia com os córtices orbitário e dorsolateral durante decisões baseadas em julgamentos morais.
lobos frontais; julgamento moral; sociopatia adquirida; psicopatia
FRONTOPOLAR AND ANTERIOR TEMPORAL CORTEX ACTIVATION IN A MORAL JUDGMENT TASK
Preliminary functional MRI results in normal subjects
Jorge Moll1 1 Grupo de Neuroimagem e Neurologia do Comportamento (GNNC), LABS & Rede D'Or Hospitais, Rio de Janeiro RJ, Brazil; 2 Departments of Medicine (Division of Neurology and Behavioral Science), Pensylvania State University College of Medicine, The Milton S. Hershey Medical Center, Hershey PN, USA; 3 Grupo de Neuroimagem e Neurologia do Comportamento (GNNC), LABS & Rede D'Or Hospitais, Rio de Janeiro RJ, Brazil, Assistant Professor, Hospital Universitário Gaffrée e Guinle (HUGG-UNI-Rio). , Paul J. Eslinger2 1 Grupo de Neuroimagem e Neurologia do Comportamento (GNNC), LABS & Rede D'Or Hospitais, Rio de Janeiro RJ, Brazil; 2 Departments of Medicine (Division of Neurology and Behavioral Science), Pensylvania State University College of Medicine, The Milton S. Hershey Medical Center, Hershey PN, USA; 3 Grupo de Neuroimagem e Neurologia do Comportamento (GNNC), LABS & Rede D'Or Hospitais, Rio de Janeiro RJ, Brazil, Assistant Professor, Hospital Universitário Gaffrée e Guinle (HUGG-UNI-Rio). , Ricardo de Oliveira-Souza3 1 Grupo de Neuroimagem e Neurologia do Comportamento (GNNC), LABS & Rede D'Or Hospitais, Rio de Janeiro RJ, Brazil; 2 Departments of Medicine (Division of Neurology and Behavioral Science), Pensylvania State University College of Medicine, The Milton S. Hershey Medical Center, Hershey PN, USA; 3 Grupo de Neuroimagem e Neurologia do Comportamento (GNNC), LABS & Rede D'Or Hospitais, Rio de Janeiro RJ, Brazil, Assistant Professor, Hospital Universitário Gaffrée e Guinle (HUGG-UNI-Rio).
ABSTRACT - OBJECTIVE: To study the brain areas which are activated when normal subjects make moral judgments. METHOD: Ten normal adults underwent BOLD functional magnetic resonance imaging (fMRI) during the auditory presentation of sentences that they were instructed to silently judge as either "right" or "wrong". Half of the sentences had an explicit moral content ("We break the law when necessary"), the other half comprised factual statements devoid of moral connotation ("Stones are made of water"). After scanning, each subject rated the moral content, emotional valence, and judgment difficulty of each sentence on Likert-like scales. To exclude the effect of emotion on the activation results, individual responses were hemodynamically modeled for event-related fMRI analysis. The general linear model was used to evaluate the brain areas activated by moral judgment. RESULTS: Regions activated during moral judgment included the frontopolar cortex (FPC), medial frontal gyrus, right anterior temporal cortex, lenticular nucleus, and cerebellum. Activation of FPC and medial frontal gyrus (BA 10/46 and 9) were largely independent of emotional experience and represented the largest areas of activation. CONCLUSIONS: These results concur with clinical observations assigning a critical role for the frontal poles and right anterior temporal cortex in the mediation of complex judgment processes according to moral constraints. The FPC may work in concert with the orbitofrontal and dorsolateral cortex in the regulation of human social conduct.
KEY WORDS: frontal lobes, moral judgment, acquired sociopathy, psychopathy.
Ativação do córtex frontopolar e temporal anterior em uma tarefa de julgamento moral: resultados preliminares de ressonância magnética funcional em indivíduos normais
RESUMO - OBJETIVO: Estudar, com ressonância magnética funcional (RMf), as áreas cerebrais normalmente ativadas por julgamentos morais em tarefa de verificação de sentenças. MÉTODO: Dez adultos normais foram estudados com RMf-BOLD durante a apresentação auditiva de sentenças cujo conteúdo foram instruídos a julgar como "certo" ou "errado". Metade das sentenças possuía um conteúdo moral explícito ("Transgredimos a lei se necessário"), enquanto a outra metade era constituída de afirmativas factuais desprovidas de conotação moral ("Pedras são feitas de água"). Depois do estudo funcional, cada indivíduo aferiu o conteúdo moral, a valência emocional, e a dificuldade de julgamento de cada sentença em escalas de Likert. Para excluir o efeito da emoção nos resultados da ativação, as respostas individuais foram hemodinamicamente modeladas para análise de RMf relacionada a eventos. O modelo linear geral foi empregado na avaliação das áreas cerebrais ativadas pelos julgamentos morais. RESULTADOS: As regiões ativadas pelo julgamento moral compreenderam o córtex frontopolar, o giro frontal medial, o córtex temporal anterior direito, o núcleo lenticular, e o cerebelo. As ativações frontopolar e médio-frontal (áreas 10/46 e 9 de Brodmann) mostraram-se independentes da experiência emocional e representaram as maiores áreas de ativação. CONCLUSÃO: Estes resultados vão ao encontro de observações clínicas que atribuem papel crítico aos pólos frontais e ao córtex temporal anterior direito na regulação do comportamento social. O sistema frontopolar-ântero-temporal descrito no presente trabalho pode representar sistema neural relativamente independente, que opera em harmonia com os córtices orbitário e dorsolateral durante decisões baseadas em julgamentos morais.
PALAVRAS-CHAVE: lobos frontais, julgamento moral, sociopatia adquirida, psicopatia.
The recognition that certain cerebral structures are essential for social cognition and behavior is one of the outstanding achievements of contemporary neuroscience1. As originally conceived, the "social brain" encompasses the superior temporal sulcus, amygdala, and orbitofrontal cortex (OFC), and is purported to work as a functional unit in the evaluation of socially meaningful stimuli and in the regulation of interpersonal behaviors2. Clinico-anatomic observations established that damage to neural structures included, but not limited to, the social brain often leads to changes in personality and higher order social behavior3. The severity of symptoms in individual cases depends on factors such as age of onset, extension and side of injury, and rate of progression of the underlying etiology4,5. Patients with acquired sociopathy may be clinically indistinguishable from individuals with the developmental personality disorder subsumed under the rubric of "psychopathy" in the forensic literature6.
Moral knowledge and judgment are core elements of social cognition7. One intriguing aspect of acquired sociopathy is the fact that some patients retain their knowledge of moral norms8,9. Analogous dissociations between knowing how to behave and actually behaving in socially desirable ways is found in developmental psychopaths as well10. These observations suggest that the cerebral organization of moral knowledge is relatively independent of the structures that mediate the suppression of socially untoward impulses and actions. The aforementioned case studies indicate that lesions restricted to the OFC which give rise to acquired sociopathy may preserve moral knowledge, suggesting that the ability to ponder over moral questions depends on circuits outside the OFC.
Although several aspects of social cognition have been explored by functional neuroimaging11-13, the neural correlates of moral judgment have been relatively neglected by both clinical and neuroimaging researchers. We undertook the present investigation to identify which cerebral areas are activated when normal adults reason on moral issues. Part of this work has already been presented in preliminary form14.
METHOD
Ten normal adult volunteers (six men and four women, age range = 24-43 years) participated in the study. All were right-handed (Edinburgh Inventory = 80 ± 3015) and had more than 15 years of formal education. No subject fulfilled criteria for a DSM-IV Axis I or II disorder16. Written informed consent was obtained from all subjects after a complete description of the study procedure.
Moral judgment eliciting procedures - Moral and factual sentences (Appendix 1 Appendix 1 ) were auditorily presented by way of a MR-compatible headphone system while subjects lay quietly with eyes closed in the MR scanner. Sentences were presented every 8 seconds, with an average time of presentation of 2 seconds. Thus, 6 seconds were left for the judgment process until the next sentence was delivered. Blocks of three moral or factual sentences alternated every 24 seconds. Subjects were asked to think about each statement and to judge whether they found them right or wrong. Subjects were instructed to think over the content of each sentence until the presentation of the next one. We conscientiously chose the words "certo" and "errado", the Portuguese equivalents of "right" and "wrong" because, as in English, they allow a moral connotation absent in the words "true" and "false". Moral sentences invoked judgments of value, of rights, or responsibilities, while factual sentences required judgments of facts17.
Immediately after the scanning session the whole list was presented a second time. Subjects were then instructed to recall to their impressions while in the MR scanner and to rate each sentence on 4-point Likert scales as right or wrong, as well as the degree of moral content, emotional valence (positive or negative), and judgment difficulty (Appendix 2 Appendix 2 ).
To remove the effects of emotional valence on judgments18-20, regressor wave functions were modeled on the individual ratings of emotional valence for positive or negative emotions and entered as confounding variables in a multiple regression matrix for event-related fMRI (ER-fMRI) analysis21. ER-fMRI allows the modeling of individual neural responses to isolated stimuli and has been increasingly employed in studies of emotional22 and cognitive23,24 processing.
MRI acquisition and data analysis - Functional MRI was performed with a 1.5 MRI scanner (Siemens Vision, Germany) and a quadrature head coil. Anatomic data consisted of 128 high-resolution gradient-echo T1-weighted volumetric images (slice thickness = 1.25 mm). Functional images covering the whole brain were acquired across 16 slices parallel to the plane of the anterior and posterior commissures with echoplanar BOLD technique (TR/TE = 4000/66 ms, FA = 90°, matrix size = 96 x 128, FOV = 250 mm, thickness/gap = 5/0.25 mm). Head movements were restrained by foam padding. A total of 128 functional volumes were acquired for each subject in a single run. During the presentation of each sentence, two functional volumes were obtained. Functional datasets were motion-corrected in 3D space using a rigid-body transformation algorithm. Bandpass filtering in the frequency domain (3-32 Hz) and linear trend removal were performed at the voxel level. Spatial normalization with a 9 mm FWHM Gaussian kernel was used to adjust for interindividual anatomical variability. Structural and functional volumes were transformed into standard Talairach stereotactic space.
Statistical analyses - We used unpaired t-tests to compare moral and factual sentences in terms of judgment difficulty, number of right/wrong decisions, emotional valence, and number of syllables. A 0.05 significance threshold (a), two-tailed, was set for all tests. Activations were computed according to statistical parametric methods25 using BrainVoyager v. 3.9 (Brain Innovation, Germany 1999). Univariate and multivariate correlation and regression analyses were performed independently for the time-course of each voxel. To adjust for intersubject variability, the mean level of the signal time-course at each voxel was z-transformed and included in the multiple regression model as a separate predictor. Regressors representing the main condition (moral vs. factual) and positive and negative emotions were modeled with a hemodynamic response filter (delta = 2.5 s, theta = 1.25 s) for ER-fMRI analysis21,24,25. Significance was assessed with a random-effects analysis. The threshold for displaying activations was set to an uncorrected p < 0.00001 for all contrasts, adjusted according to a 3D cluster-size extent threshold of 200 cubic millimiters26. The resulting color-coded statistical parametric maps were overlaid on averaged anatomical data from all subjects and on a reference segmented brain for visualization of sulcal and gyral anatomy.
We first investigated the effect of moral vs. factual judgments. Ten regressors representing the moral-factual condition for each subject and their corresponding means were included in the design matrix. Secondly, the relative contribution of regressors representing moral vs. factual judgments was assessed by covarying these regressors with those representing positive and negative emotional valence, condition means, and linear trends of each subject.
RESULTS
For all subjects, the degree of moral content was higher in moral than in factual sentences (68 ± 12 vs. 0; t = 17.2, df = 9, p < 0.0001), indicating that moral and factual sentences were judged as intended. Also, moral sentences were more frequently associated with emotional valence (40 ± 7.9 vs. 14.6 ± 3.5; t = 4.76, df = 9, p < 0.001). There were otherwise no significant differences in moral as compared to factual sentences in judgment difficulty (2.5 ± 1.0 vs. 3.6 ± 1.0; t = 1.38, df = 9, p = 0.20), right (12.3 ± 2.1 vs. 13.2 ± 2.2) and wrong (17.6 ± 3.2 vs. 18.0 ± 2.6; t = 0.30, df = 9, p = 0.29) ratings, and mean number of syllables (9.5 ± 3.5 vs. 8.8 ± 1.4; t = 1.64, df = 61, p = 0.11).
Brain activation in response to moral judgment
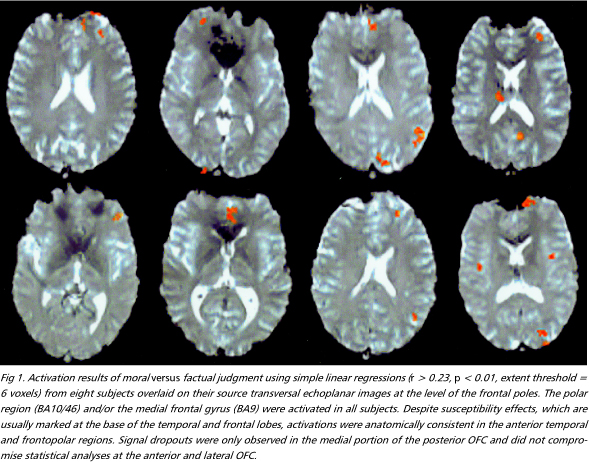
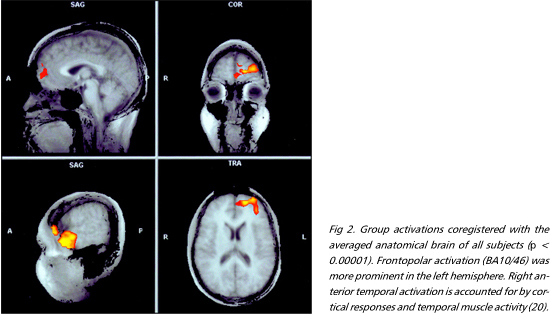
Moral versus factual judgments - The FPC and the medial frontal gyrus, corresponding to Brodmann areas (BA) 10/46 and 9, were activated bilaterally. These were the most consistently activated regions across subjects (Figs 1 e 2). Activation was more prominent in the left hemisphere in six individuals, bilateral in three, and right-sided in one. The right anterior temporal lobe was also extensively activated. A more discrete focus of activation was observed posteriorly, in the superior temporal sulcus. Activations were also observed in the external division of left globus pallidus, right cerebellar cortex and left precuneus (Table 1).
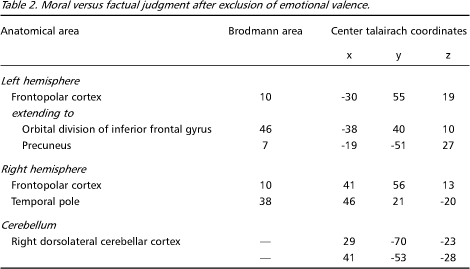
Moral versus factual judgments covarying emotional valence - Figures 3 e 4 shows activated areas related to moral judgment after adjusting for the effects of emotional valence. The frontopolar and medial frontal activations remained almost unchanged, still representing the largest areas of activity (Table 2). There was a marked reduction in the extent of the anterior temporal lobe activations and the pallidum was no longer activated, suggesting that these regions involved emotional valence.
DISCUSSION
The main result of this study was the finding that moral judgment activates the FPC and the right anterior temporal cortex. These frontal regions lay midway between OFC and dorsolateral frontal cortex, and account for a sizable portion of the human frontal lobes27. So far, the FPC had not been specifically implicated in social cognition in functional neuroimaging studies28-30. Activation of the medial frontal gyrus (BA9), on the other hand, has been observed in studies of cognitive empathy28,29 and attention to subjective emotional states30. Although our subjects were not required to infer the mental states of others or to assess their own emotions introspectively, the possibility that cognitive and affective empathy were embedded in the moral judgment process cannot be ruled out. In fact, these processes likely act in concert to promote mature moral judgments.
It is unlikely that our findings could be primarily explained by the recognition of mental state terms and abstract nouns, semantic monitoring, degree of mental effort, phonological and semantic processing, verb versus noun recognition, or helplessness induced by unsolvable anagram tasks11,31-33. Likewise, since we avoided the use of proverbs and metaphors, and since an fMRI pattern distinct from ours was reported in a study of the interpretation of the figurative aspects of language34, our results cannot be explained by these cognitive processes.
The ability to distinguish between moral and conventional transgressions probably depends on different neural substrates35. We did not ask our subjects to perform moral/conventional distinctions. Instead, the task design we employed emphasized the distinction between moral and factual judgments. Therefore, the correlates of the moral/conventional distinction were not addressed in this study.
Acquired sociopathy is classically ascribed to damage to the OFC36. This is in agreement with the implication of the OFC in social behaviors which rely on biological action-patterns, such as decision-making ruled by punishment/reward and operant conditioning in humans and other mammals37-39. However, the fact that patients with OFC damage suffer from disturbances both in instinctive and complex social domains40 indicates that the human OFC is a common channel for the expression of the function of higher-order areas. The organization of behaviors grounded on moral knowledge, on the other hand, probably depends on phylogenetically novel cortical circuits41. This hypothesis is in accord with the expansion of the FPC in humans as compared to other primates, the most consistently activated region in the present study. Further support to this notion is given by the observation that sociopathy may result from FPC injuries, extending or not to other sectors of the frontal lobe4,42,43. Another interesting issue is the appearance of sociopathy in cases of unilateral prefrontal damage4,44, as well the absence of gross conduct disorders in others45. The variable degree of hemispheric asymmetry in the prefrontal activations among the individuals in our sample may help explain this fact.
So far, complex social actions requiring moral evaluations, empathy, theory of mind, and stable social bonds were conceived to rely on two main streams of processing46: one more cognitively oriented and dependent on the dorsolateral prefrontal system and the other more emotion-oriented and mediated by anterior temporal, limbic and orbito-ventromedial frontal systems. We believe that the FPC, which has been largely overlooked in current models of the brain mechanisms of social control47, provides further regulation of social conduct. Together with the clinico-anatomic evidence, our results favor the view that the prefrontal cortex needs further functional fractionation, with polar, orbital, and dorsolateral sectors mediating distinct but complementary roles in the regulation of social cognition and behavior.
In addition, the investigation of moral judgment may be expanded to parcel out the differential activation effects of tasks involving moral judgment vs. moral knowledge vs. moral action. The present investigation is an early step towards the understanding of the cerebral organization of moral judgment and the regulation of social behavior. Its relationship to the pathophysiology of antisocial disorders should be drawn with caution. One related issue which remains open for future research concerns the study of patients with acquired or developmental sociopathy with functional imaging during moral decision making and judgment tasks.
Acknowledgments - The authors are indebted to Ivanei E. Bramati for his assistance during functional MRI image acquisition and analysis
Received 12 June 2001, received in final form 10 July 2001. Accepted 12 July 2001.
Dr. Ricardo de Oliveira-Souza - Rua Pinheiro Guimarães, 22/4° andar ¾ 22281-080 Rio de Janeiro RJ ¾ Brasil. E-mail: neuropsychiatry@hotmail.com
- 1. Eisenberg L. The social construction of the human brain. Am J Psychiatry 1995;152:1563-1575.
- 2. Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci 1991;1:27-51.
- 3. Adolphs R. Social cognition and the human brain. Trends Cogn Sci 1999;3:469-479.
- 4. Eslinger PJ, Grattan LM, Damasio H, Damasio AR. Developmental consequences of childhood frontal damage. Arch Neurol 1992;49:764-769.
- 5. Miller BL, Hou C, Goldberg M, Mena I. Anterior temporal lobes: social brain. In Miller BL, Cummings JL (eds). The human frontal lobes: functions and disorders. New York: Guilford Press, 1999.
- 6. Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiat 2000;57:119-127.
- 7. Brothers L. Friday's footprints: how society shapes the human mind. New York: Oxford Univ Press, 1997.
- 8. Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology 1985;35:1731-1741.
- 9. Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia 1991;29:1241-1249.
- 10. Hare RD. Without conscience: the disturbing world of the psychopath among us. New York: Pocket Books, 1993.
- 11. Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Br J Psychiat 1994;165:640-649.
- 12. Fletcher PC, Happé F, Frith U, et al. Other minds in the brain: a functional imaging study of "theory of mind" in story comprehension. Cognition 1995;57:109-128.
- 13. Happé F, Brownell H, Winner E. Acquired "theory of mind" impairments following stroke. Cognition 1999;70:211-240.
- 14. Oliveira-Souza R, Moll J. The moral brain: functional MRI correlates of moral judgment in normal adults. Neurology 2000;54(Suppl. 3):252.
- 15. Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971;9:97-113.
- 16. American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders. 4.Ed. Washington, DC: American Psychiatric Association, 1994.
- 17. Colby A, Kohlberg L, Speicher B, Hewer A, Candee D, Gibss J. The measurement of moral judgment, Vol 1. New York: Cambridge Univer Press, 1990.
- 18. Paradiso S, Johnson DL, Andreasen NC, et al. Cerebral blood flow changes associated with attribution of emotion valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry 1999;156:1618-1629.
- 19. Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness and disgust. Am J Psychiat 1997;154:926-933.
- 20. Reiman EM, Lane RD, Ahern GL, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 1997;154:918-925.
- 21. Josephs O, Turner R, Friston K. Event-related fMRI. Hum Brain Mapping 1997;5:243-248.
- 22. Büchel C, Morris J, Dolan RR, Friston K. Brain systems mediating aversive conditioning: an event-related study. Neuron 1998;20:947-957.
- 23. Buckner RL, Goodman J, Burock M, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron 1998;20:285-296.
- 24. Clark VP, Maisog JM, Haxby J. fMRI Study of face perception and memory using random stimulus sequences. J Neurophysiol 1998;79: 3257-3265.
- 25. Friston KJ, Holmes AP, Poline J-P, et al. Analysis of fMRI time-series revisited. Neuroimage 1995;2:45-53.
- 26. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster size threshold. Magn Reson Med 1995;33:636-647.
- 27. Reep R. Relationship between prefrontal and limbic cortex: a comparative anatomical survey. Brain Behav Evol 1984;25:5-80.
- 28. Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of "Theory of Mind" in verbal and non-verbal tasks. Neuropsychologia 2000;38:11-21.
- 29. Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. Neuro Report 1995;6:1741-1746.
- 30. Lane RD, Fink GR, Chau PML, Dolan RJ. Neural activation during selective attention to subjective emotional responses. NeuroReport 1997;8:3969-3972.
- 31. Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD. Neural pathways involved in the processing of concrete and abstract words. Hum Brain Mapping 1999;7:225-233.
- 32. Schneider F, Gur RE, Alavi A, et al. Cerebral blood flow changes in limbic regions induced by unsolvable anagram tasks. Am J Psychiatry 1996;153:206-212.
- 33. Crosson B, Rao SM, Woodley SJ, et al. Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology 1999;13:171-187.
- 34. Bottini G, Corcoran R, Sterzi R, et al. The role of the right hemisphere in the interpretation of figurative aspects of language: a positron emission tomography activation study. Brain 1994 117:1241-1253.
- 35. Blair RJR. A cognitive developmental approach to morality; investigating the psychopath. Cognition 1995;57:1-29.
- 36. Tranel D. "Acquired sociopathy": the development of sociopathic behavior following focal brain damage. Prog Exp Pers Psychopathol Res 1994;3:285-311.
- 37. Elliot R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia 1999;37:403-411.
- 38. Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci 1999;19:6610-6614.
- 39. Rogers RD, Owen AM, Middleton HC, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 1999;20:9029-9038.
- 40. Starkstein SE, Robinson RG. Mechanisms of disinhibition after brain lesions. J Nerv Ment Dis 1997;185:108-114.
- 41. Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 1999;11:1011-1036.
- 42. Marlowe WB. The impact of a right prefrontal lesion in the developing brain. Brain Cogn 1992;20:205-213.
- 43. Price BH, Daffner KR, Stowe RM, Mesulam M-M. The comportmental learning disabilities of early frontal damage. Brain 1990;113:1383-1393.
- 44. Meyers CA, Berman AS, Scheibel RS, Hayman A. Acquired antisocial personality disorder associated with unilateral left orbital frontal lobe damage. J Psychiatr Neurosci 1992;17:121-125.
- 45. Hebb DO, Penfield W. Human behavior after extensive bilateral removal from the frontal lobes. Arch Neurol Psychiat 1940;40:421-428.
- 46. Eslinger PJ. Neurological and neuropsychological bases of empathy. Eur Neurol 1998;39:193-199.
- 47. Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation: a possible prelude to violence. Science 2000;289:591-594.
Appendix 1

Appendix 2

Publication Dates
-
Publication in this collection
05 Oct 2001 -
Date of issue
Sept 2001
History
-
Accepted
12 July 2001 -
Reviewed
10 July 2001 -
Received
12 June 2001