Abstract
Dental caries and periodontal disease are associated with oral pathogens. Several plant derivatives have been evaluated with respect to their antimicrobial effects against such pathogenic microorganisms. Lippia sidoides Cham (Verbenaceae), popularly known as "Alecrim-pimenta" is a typical shrub commonly found in the Northeast of Brazil. Many plant species belonging to the genus Lippia yield very fragrant essential oils of potential economic value which are used by the industry for the commercial production of perfumes, creams, lotions, and deodorants. Since the leaves of L. sidoides are also extensively used in popular medicine for the treatment of skin wounds and cuts, the objective of the present study was to evaluate the composition and antimicrobial activity of L. sidoides essential oil. The essential oil was obtained by hydro-distillation and analyzed by GC-MS. Twelve compounds were characterized, having as major constituents thymol (56.7%) and carvacrol (16.7%). The antimicrobial activity of the oil and the major components was tested against cariogenic bacterial species of the genus Streptococcus as well as Candida albicans using the broth dilution and disk diffusion assays. The essential oil and its major components thymol and carvacrol exhibited potent antimicrobial activity against the organisms tested with minimum inhibitory concentrations ranging from 0.625 to 10.0 mg/mL. The most sensitive microorganisms were C. albicans and Streptococcus mutans. The essential oil of L. sidoides and its major components exert promising antimicrobial effects against oral pathogens and suggest its likely usefulness to combat oral microbial growth.
Lippia sidoides; Essential oil; Antimicrobial activity; Oral pathogens
Braz J Med Biol Res, March 2007, Volume 40(3) 349-356
Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens
 Correspondence and Footnotes
Correspondence and Footnotes
 M.A. Botelho1, N.A.P. Nogueira2, G.M. Bastos2, S.G.C. Fonseca3, T.L.G. Lemos4, F.J.A. Matos4, D. Montenegro5, J. Heukelbach5, V.S. Rao6 and G.A.C. Brito7
M.A. Botelho1, N.A.P. Nogueira2, G.M. Bastos2, S.G.C. Fonseca3, T.L.G. Lemos4, F.J.A. Matos4, D. Montenegro5, J. Heukelbach5, V.S. Rao6 and G.A.C. Brito7
1Programa de Pós-Graduação em Ciências Médicas, 2Departamento de Análises Clínicas e Toxicológicas, 3Departamento de Farmácia, 4Departamento de Química Orgânica e Inorgânica, 5Departamento de Saúde Comunitária, 6Departamento de Fisiologia e Farmacologia, 7Departamento de Morfologia, Universidade Federal do Ceará, Fortaleza, CE, Brasil
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Dental caries and periodontal disease are associated with oral pathogens. Several plant derivatives have been evaluated with respect to their antimicrobial effects against such pathogenic microorganisms. Lippia sidoides Cham (Verbenaceae), popularly known as "Alecrim-pimenta" is a typical shrub commonly found in the Northeast of Brazil. Many plant species belonging to the genus Lippia yield very fragrant essential oils of potential economic value which are used by the industry for the commercial production of perfumes, creams, lotions, and deodorants. Since the leaves of L. sidoides are also extensively used in popular medicine for the treatment of skin wounds and cuts, the objective of the present study was to evaluate the composition and antimicrobial activity of L. sidoides essential oil. The essential oil was obtained by hydro-distillation and analyzed by GC-MS. Twelve compounds were characterized, having as major constituents thymol (56.7%) and carvacrol (16.7%). The antimicrobial activity of the oil and the major components was tested against cariogenic bacterial species of the genus Streptococcus as well as Candida albicans using the broth dilution and disk diffusion assays. The essential oil and its major components thymol and carvacrol exhibited potent antimicrobial activity against the organisms tested with minimum inhibitory concentrations ranging from 0.625 to 10.0 mg/mL. The most sensitive microorganisms were C. albicans and Streptococcus mutans. The essential oil of L. sidoides and its major components exert promising antimicrobial effects against oral pathogens and suggest its likely usefulness to combat oral microbial growth.
Key words: Lippia sidoides, Essential oil, Antimicrobial activity, Oral pathogens
Introduction
The early stage of dental caries is characterized by a destruction of superficial dental structures caused by acids which are by-products of carbohydrate metabolism by cariogenic bacteria (1). Candida albicans is found in infections of the mouth, vagina, lungs, and in skin lesions (2). It is by far the fungal species most commonly isolated from infected root canals, showing resistance to intracanal medication (3). The ability to form a biofilm and to invade dentinal tubules may help to explain the association with cases of persistent root canal infections (3-5).
Bacterial plaque accumulated on dental surfaces and composed of native oral flora is the primary etiologic agent of periodontal disease and dental caries. Colonization of teeth by cariogenic bacteria is one of the most important risk factors in the development of dental diseases with Streptococcus mutans being the primary species associated with the early dental caries process (1).
The prevalence and incidence of oral diseases, coupled with the resultant social and economic implications, has led to a constant striving to produce safer substances for the development of new natural antimicrobial agents (6,7). In fact there is an overwhelming number of studies on the biological activities of plants and their natural product derivatives (8-10). Essential oils and their derivatives are one such example (11-13).
In Brazil tooth decay and periodontal disease are a major public health issue (14) which, and according to latest WHO report on dental disorders, affects about 60% of the world adult population (15).
Natural products have been recently investigated more thoroughly as promising agents for the prevention of oral diseases, especially plaque-related diseases such as dental caries (10,16). The increasing resistance to available antimicrobials has attracted the attention of the scientific community regarding a search for new cost-effective drugs of natural or synthetic origin (10,11). Essential oils in general demonstrate antimicrobial activity against cariogenic microbes (12) and fungal filaments as well (17). Some studies have pointed out that plant-derived essential oils may be an effective alternative to overcome microbial resistance (12,13).
Lippia sidoides Cham (Verbenaceae), popularly known as "Alecrim-pimenta", is a typical shrub commonly growing in the Northeast of Brazil. This species produces an essential oil rich in thymol and carvacrol, which has a potent antimicrobial activity against fungi and bacteria (6,18). This oil is one of the substances widely used in Brazilian traditional medicine, especially by poor local communities in the Northeast of Brazil for skin cuts, insect bites and sore throat (6).
Previous studies have described the larvicidal property of L. sidoides essential oil (LSEO) (19). Recently, quinones from LSEO have been described to possess cytotoxic activity (20). Popular and scientific evidence also suggests that the essential oil may be useful for oral hygiene and in the prevention of dental disorders such as caries and gingivitis (21). However, the in vitro activity of the oil against pathogens related to caries and aphthae has not been reported.
Any treatment that would eliminate or substantially reduce colonization by cariogenic bacteria would be likely to have a strong impact on caries development (22,23).
The aim of the present study was to investigate the antibacterial and antifungal activity of LSEO and its components thymol and carvacrol against oral pathogens related to caries (24) and to oral fungal infection (2,5), since these organisms have now gained more importance due to the increased incidence of AIDS/HIV (25). In addition, we studied the phytochemical composition of the essential oil by gas chromatography-mass spectrometry (GC-MS) analysis.
Material and Methods
Plant material
L. sidoides leaves were collected in August 2004 in the Medicinal Plants Garden Prof. Francisco José de Abreu Matos, Fortaleza, CE, Brazil, with the authorization of the Federal University of Ceará Ethics Committee (#551/04). Taxonomic identification of the plants was performed by botanists of the Prisco Bezerra Herbarium, Department of Biology, Federal University of Ceará, where a voucher specimen has been deposited (#25149).
Essential oil extraction and compounds tested
Leaf essential oil was extracted by the hydro-distillation technique using a modified Clevenger apparatus (26). After extraction, the volume of essential oil obtained in both extractions was measured and the essential oil stored in hermetically sealed glass containers with rubber lids, covered with aluminum foil to protect the contents from light and kept under refrigeration at 8ºC until used. Carvacrol (W224502) and thymol (T0501) were purchased from Sigma-Aldrich Chemical Co., St. Louis, MO, USA.
Gas chromatography-mass spectrometry analysis
The chemical composition of the essential oil was determined at the Technological Development Center (PADETEC) of the Federal University of Ceará by GC-MS using a Hewlett-Packard 5971 GC/MS apparatus (Avondale, PA, USA) under the following conditions: a 0.25 mm x 30 m polydimethylsiloxane DB-1 fused silica capillary column, with a film thickness of 0.10 µm; helium as the carrier gas helium (1 mL/min), injector temperature of 250ºC, and detector temperature of 200ºC. The column temperature ranged from 35 to 180oC/min, at 4oC V/min, and then from 180 to 280ºC, at 20oC V/min; mass spectra were obtained by electronic impact 70 eV. The constituents were identified by a computer-based library search, with retention indices and visual interpretation of the mass spectra (27).
Antimicrobial assay
Microbial strains. S. mutansss-980 was kindly donated by the Brazilian Microbiology Institute (Federal University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil). C. albicans ATCC 10239, donated by FIOCRUZ (Reference Material Laboratory, São Paulo, SP, Brazil), was also included in the study. The other three cariogenic strains (Streptococcus mitis, S. sanguis, and S. salivarius) were clinically isolated by rubbing a sterile cotton-tipped swab over the teeth, gums and tongue of a group of patients at the Dental Clinics of the Federal University of Ceará. The swabs were then placed in a glass tube containing 1 mL phosphate-buffered saline solution. The tube was mixed thoroughly using a vortex mixer and the bacterial suspension was inoculated onto a range of selective and non-selective culture media, and then incubated microaerobically at 37oC for 2 days, with pure cultures being obtained. Isolates were identified using biochemical profiles and other standard microbiological methods (28).
Antimicrobial screening by the disk diffusion method. The antimicrobial activity of LSEO,thymol and carvacrol against oral pathogens was determined by standard disk susceptibility tests (29) in the Department of Clinical Analysis, Pharmacy College, Federal University of Ceará.
The clinically isolated bacterial and fungal inoculum of each strain was obtained from fresh colonies grown on Muller-Hinton agar plates. Each strain was inoculated into 5 mL of Muller-Hinton broth in order to obtain a concentration of 1.5 x 108 CFU/mL (0.5 Macfarland). The inoculum was then inoculated into Muller-Hinton agar (Merck, Darmstadt, Germany) and Sabouraud agar (Merck), with a sterile swab.
Sterile filter paper disks (Schleicher, Schüll, Dassel, Germany, 6 mm in diameter) were impregnated with 10 µL LSEO, thymol and carvacrol diluted in DMSO at 2% working concentrations (LSEO 435-6.79, thymol 400-6.25, and carvacrol 400-6.25 mg/mL). The plates were incubated at 35ºC for 18 h after standing at ambient temperature (28ºC) for 30 min. After incubation, all plates were examined for any growth inhibition zones. The diameters of the inhibition zones were measured in millimeters using a Fisher-Lilly Antibiotic Zone Reader (Fisher Scientific Co., Union, NJ, USA). Clear inhibition zones around the discs indicate the presence of antimicrobial activity. All the tests were performed in triplicate and the mean values were calculated. DMSO at 2% showed no inhibitions in preliminary studies and therefore was used as negative control. Vancomycin (3 mg/mL) and ketoconazole (5 mg/mL) were used as reference antimicrobial compounds in order to control the sensitivity of the bacteria and yeast strains, respectively. The discs were obtained from Oxoid (Hampshire, UK).
Determination of minimum inhibitory concentration, minimum bactericidal concentration, and minimum fungicidal concentration
A broth dilution method was used to determine the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) (30). A serial doubling dilution of LSEO (80-0.625 mg/mL), thymol (10-0.078 mg/mL) and carvacrol (10-0.078 mg/mL) was prepared. The BHI broth (Merck)
was supplemented with DMSO (Merck) at a 2% concentration in order to enhance sample solubility. Antimicrobial assays were performed with different concentrations of LSEO, thymol and carvacrol. The cultures with visible turbidity adjusted to approximately 106 CFU/mL in BHI broth were supplemented with different concentrations of the compounds tested and incubated at 35ºC/24 h. To determine MBC/MFC, 100 µL aliquots of inoculum were removed aseptically from tubes that had not presented visible turbidity used to determine MIC values, and then plated onto agar by the pour-plate method using Agar Plate Count (Merck) and incubated for 24 h at 37ºC. MBC/MFC is defined as the lowest concentration of the essential oil at which 99.99% or more of the initial inoculum was killed. The number of surviving organisms was determined by viability counts. All tests were performed in triplicate.
Results
Chemical constituents
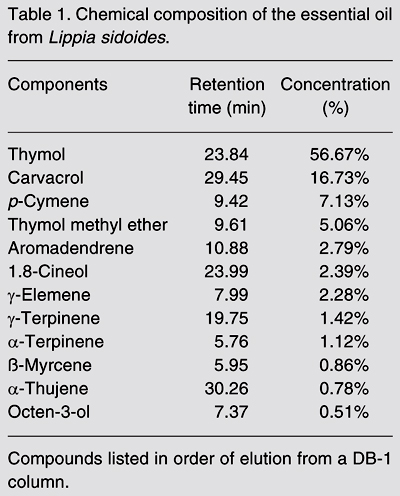
Analysis of the chemical composition of the essential oil by GC-MS facilitated the identification of oil components (Table 1). The major compounds identified were thymol (56.7%) and carvacrol (16.7%) (Figure 1). The other compounds were p-cymene (7.1%), ß-myrcene (0.86%), a-terpinene (1.1%), and the oxygen-containing compounds thymol methyl ether (5.06%) and 1,8-cineol (2.39%).
Antimicrobial activity
The antibacterial activity of the LSEO and the phenolic compounds (thymol and carvacrol) was initially evaluated by the disk diffusion method using four strains of cariogenic bacteria (S. mutans, S. sanguis, S. salivarius, and S. mitis) and one yeast strain (C. albicans). The three tested compounds exhibited relatively strong antibacterial and antifungal activity. The results obtained in the disk diffusion assay regarding the growth inhibition zones of the tested microbes are shown in Table 2. The inhibition zones determined by LSEO, thymol and carvacrol were concentration dependent for all strains.
The inhibition zones for cariogenic bacteria ranged from 8.5 to 18.7 mm for LSEO, 7.7 to 16 mm for thymol, and 7.5 to 15 mm for carvacrol. The inhibition zones for C. albicans were 10.6 and 9 mm for thymol and carvacrol, respectively. The LSEO was not effective in inhibiting bacterial or fungal growth at the same concentration of the phenolic monoterpenes (which were four times diluted in comparison to LSEO; Table 2).
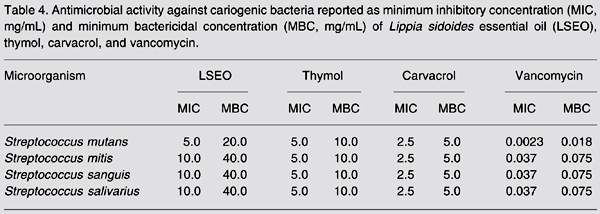
The pure major components, thymol and carvacrol, were more active, with MIC values ranging from 2.5 to 5.0 mg/mL, compared to LSEO (MIC = 5-10 mg/mL), thus showing a significant effect against cariogenic bacteria.
Although the MICs and MBCs varied between test organisms, in most cases the MIC was close to the MBC, indicating a good antibacterial action of the phenolic monoterpene compounds.
Concerning the antifungal property (against C. albicans), while the MFC value for LSEO was 5 mg/mL, carvacrol showed a value two times lower (2.5 mg/mL). The lowest MBC value obtained with LSEO represents a significant antimicrobial activity, similar to those of standard compounds such as thymol and carvacrol. These results are presented in Table 3.
The LSEO presented higher MBC values against cariogenic bacteria (ranging from 20 to 40 mg/mL) when compared to thymol (MBC = 10.0 mg/mL) and carvacrol (MBC = 5.0 mg/mL; Table 4).
Chemical structures of the major compounds of Lippia sidoides essential oil tested. A) thymol; B) carvacrol.
Antimicrobial activity of Lippia sidoides essential oil (LSEO), thymol and carvacrol determined by the agar dish diffusion method (Ref. 29).
Antimicrobial activity against Candida albicans reported as minimum inhibitory concentration (MIC, mg/mL) and minimum bactericidal concentration (MBC, mg/mL) of Lippia sidoides essential oil (LSEO), thymol, carvacrol, and ketoconazole.
Antimicrobial activity against cariogenic bacteria reported as minimum inhibitory concentration (MIC, mg/mL) and minimum bactericidal concentration (MBC, mg/mL) of Lippia sidoides essential oil (LSEO), thymol, carvacrol, and vancomycin.
Discussion
Our data show that the essential oil tested and its major phenolic compounds (thymol and carvacrol) showed good antimicrobial activity against the main group of cariogenic bacteria and C. albicans as well. In our study, among the microorganisms related to dental caries, S. mutans presented the highest sensitivity to the compounds tested. The yeast C. albicans, frequently associated with infections in HIV(+) patients, was the most sensitive among all tested microorganisms. It is interesting to report that the oil extracted was collected at noon and therefore its chemical composition showed an appreciable amount of monoterpenes, a therapeutical potential that should not be ignored.
Oral pathogens have been recently tested for antimicrobial susceptibility to a number of plant extracts and natural substances (7,8). To the best of our knowledge, this is the first report on antimicrobial activity of L. sidoides essential oil against oral pathogens.
Several natural and synthetic agents including chlorhexidine, cetyl pyridinium chloride and phenol derivatives have been used in dentistry to inhibit bacterial growth. Nevertheless, the dental community is still searching for new therapeutic drugs to prevent or treat dental plaque-related diseases (9,10).
Individuals heavily colonized by cariogenic bacteria are considered to be at high risk for dental caries. Hence, eradication of these microorganisms is important for dental treatment (31).
L. sidoides (Verbenaceae), popularly known as "alecrim-pimenta", grows virtually anywhere in Northeastern Brazil. This plant has been historically used by indigenous people in the treatment of a variety of human diseases of bacterial and fungal origin (6). However, few studies have been conducted on its potential bioactive compounds (18,21). Natural products, such as LSEO, are receiving increased attention due to their diverse range of biological properties, representing sources for the discovery of novel bioactive compounds (19,20).
Due to its low cost, the use of LSEO in the prevention and treatment of oral conditions could be of benefit to low-socioeconomic level urban and rural communities. A mouth rinse formulation may also be advantageous in regions where L. sidoides is a culturally accepted plant (16).
In previous studies, a mouth rinse prepared with LSEO was able to reduce gingivitis and plaque indexes in humans and dogs with mild gingivitis (16,21). Within the limitation of the present study, it was demonstrated that LSEO, thymol and carvacrol are effective in inhibiting the growth of oral pathogens, supporting the traditional use of this plant in the treatment of oral infections (18).
The present results demonstrate that not only the essential oil, but also its isolated phenolic compounds (thymol and carvacrol) inhibited all the oral pathogens tested with different levels of activity. A recent study has suggested an antagonism of the essential oils with high percentages of p-cymene and phenolic monoterpenes (thymol and carvacrol), reducing the antimicrobial activity of the essential oil (32). This may explain in part the higher activity of thymol and carvacrol when evaluated separately. Our data show that LSEO has a lower antibacterial potential when compared to thymol and carvacrol, suggesting that the minor components may contribute to an antagonistic effect on the activity of the essential oil.
There are two important differences between our study and those in which LSEO was reported to be devoid of antimicrobial activity (6,18). First, our study focused on a comparative evaluation of the activity of separate and combined compounds against microorganisms related to caries and oral infection, whereas the previous studies focused on essential oil activity alone or against other microorganisms. Second, our study provides a comparative evaluation of the antibacterial and antifungal activity of the tested compounds.
The preventive strategy against dental disorders is based on an appropriate control of the pathogens and LSEO seems to be important for this preventive approach as a natural compound for a novel mouth rinse (21,16).
Our results suggest that LSEO, thymol and carvacrol have antibacterial activity against S. mutans, one of the most important cariogenic bacteria which leads to demineralization of dental enamel (1,11), and may be useful for maintaining oral hygiene by reducing bacterial growth. Nevertheless, further studies are needed to identify and purify other active LSEO ingredients for future use in trials using toothpaste and mouth rinse formulations. In addition, longer-term studies will be required to evaluate the usefulness of these substances.
Acknowledgments
We wish to thank Dinalva de Brito Queiroz (Farmácia Evidence, Fortaleza, CE, Brazil) for technical support. Thanks are also due to Prof. Juarez Braga Soares (Departamento de Biologia, Universidade Federal do Ceará) and Ary Marques da Silva (PADETEC).
 Address for correspondence: M.A. Botelho, Programa de Pós-Graduação em Ciências Médicas, Faculdade de Medicina, UFC, R. Prof. Costa Mendes, 1608, 4º andar, 60430-170 Fortaleza, CE, Brasil. Fax: +55-85-3366-8056. E-mail: marcobotelho1@gmail.com
Address for correspondence: M.A. Botelho, Programa de Pós-Graduação em Ciências Médicas, Faculdade de Medicina, UFC, R. Prof. Costa Mendes, 1608, 4º andar, 60430-170 Fortaleza, CE, Brasil. Fax: +55-85-3366-8056. E-mail: marcobotelho1@gmail.com
Research supported by CNPq (No. 820370/92-4) and by a grant to M.A. Botelho (No. 08246) from Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP). Received March 3, 2006. Accepted January 12, 2007.
- 1. Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev 1986; 50: 353-380.
- 2. Odds FC. Candida and candidosis: A review and bibliography 2nd edn. London: Bailiere Tindall; 1988.
- 3. Oztan MD, Kiyan M, Gerceker D. Antimicrobial effect, in vitro, of gutta-percha points containing root canal medications against yeasts and Enterococcus faecalis Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 102: 410-416.
- 4. Sena NT, Gomes BP, Vianna ME, Berber VB, Zaia AA, Ferraz CC, et al. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int Endod J 2006; 39: 878-885.
- 5. Siqueira JF Jr, Rocas IN, Lopes HP, Magalhaes FA, de Uzeda M. Elimination of Candida albicans infection of the radicular dentin by intracanal medications. J Endod 2003; 29: 501-504.
- 6. Lemos TL, Craveiro AA, Alencar JW, Matos FJ, Clarck AM, MacChesney JD. Antimicrobial activity of essential oil of Brazilian plants. Phytother Res 1990; 4: 82-84.
- 7. Iauk L, Lo Bue AM, Milazzo I, Rapisarda A, Blandino G. Antibacterial activity of medicinal plant extracts against periodontopathic bacteria. Phytother Res 2003; 17: 599-604.
- 8. Hebbar SS, Harsha VH, Shripathi V, Hegde GR. Ethnomedicine of Dharwad district in Karnataka, India - plants used in oral health care. J Ethnopharmacol 2004; 94: 261-266.
- 9. Bakri IM, Douglas CW. Inhibitory effect of garlic extract on oral bacteria. Arch Oral Biol 2005; 50: 645-651.
- 10. Pai MR, Acharya LD, Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel - a 6-week clinical study. J Ethnopharmacol 2004; 90: 99-103.
- 11. Fine DH, Furgang D, Barnett ML, Drew C, Steinberg L, Charles CH, et al. Effect of an essential oil-containing antiseptic mouthrinse on plaque and salivary Streptococcus mutans levels. J Clin Periodontol 2000; 27: 157-161.
- 12. Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol 2004; 19: 61-64.
- 13. Didry N, Dubreuil L, Pinkas M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm Acta Helv 1994; 69: 25-28.
- 14. Ministério da Saúde. Programa Brasil Sorridente - Levantamento das condições de saúde bucal da população brasileira - SB Brasil. http://dtr2004.saude.gov.br/dab/saudebucal/brasil_sorridente.php. Accessed May 23, 2006.
- 15. Petersen PE. Challenges to improvement of oral health in the 21st century - the approach of the WHO Global Oral Health Programme. Int Dent J 2004; 54: 329-343.
- 16. Fernandes-Filho ES, Morais SM, Fonseca SG, Mota OM. Preparação e avaliação clínica do óleo essencial da planta medicinal Lippia sidoides Cham (Alecrim-pimenta). Rev Ass Bras Odonto 1998; 6: 323-325.
- 17. Prashar A, Hili P, Veness RG, Evans CS. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae Phytochemistry 2003; 63: 569-575.
- 18. Lacoste E, Chaumont JP, Mandin D, Plumel MM, Matos FJ. Antiseptic properties of essential oil of Lippia sidoides Cham. Application to the cutaneous microflora. Ann Pharm Fr 1996; 54: 228-230.
- 19. Carvalho AF, Melo VM, Craveiro AA, Machado MI, Bantim MB, Rabelo EF. Larvicidal activity of the essential oil from Lippia sidoides Cham. against Aedes aegypti Linn. Mem Inst Oswaldo Cruz 2003; 98: 569-571.
- 20. Costa SM, Lemos TL, Pessoa OD, Pessoa C, Montenegro RC, Braz-Filho R. Chemical constituents from Lippia sidoides and cytotoxic activity. J Nat Prod 2001; 64: 792-795.
- 21. Girao VC, Nunes-Pinheiro DC, Morais SM, Sequeira JL, Gioso MA. A clinical trial of the effect of a mouth-rinse prepared with Lippia sidoides Cham essential oil in dogs with mild gingival disease. Prev Vet Med 2003; 59: 95-102.
- 22. Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc 2000; 131: 887-899.
- 23. Svensater G, Borgstrom M, Bowden GH, Edwardsson S. The acid-tolerant microbiota associated with plaque from initial caries and healthy tooth surfaces. Caries Res 2003; 37: 395-403.
- 24. Lindquist B, Emilson CG. Colonization of Streptococcus mutans and Streptococcus sobrinus genotypes and caries development in children to mothers harboring both species. Caries Res 2004; 38: 95-103.
- 25. Samaranayake LP. Introduction and historical aspects. In: Samaranayake LP, MacFarlane TW (Editors), Oral candidosis London: Butterworth & Co., Ltd.; 1990. p 1-9.
- 26. Craveiro AA, Matos FJ, Alencar JW. A simple and inexpensive steam generator for essential oils extraction. J Chem Ed 1976; 53: 652.
- 27. Craveiro AA, Matos FJ, Alencar JW. Kovats indices as pre-selection routine in mass spectra library search of volatiles. J Nat Prod 1984; 47: 890-892.
- 28. Tappuni AR, Challacombe SJ. Distribution and isolation frequency of eight streptococcal species in saliva from predentate and dentate children and adults. J Dent Res 1993; 72: 31-36.
- 29. NCCLS (National Committee for Clinical Laboratory Standards). Performance Standards for Antimicrobial Disk Susceptibility Tests 6th edn. Wayne: Approved Standards; 1998.
- 30. NCCLS (National Committee for Clinical Laboratory Standards). Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically 5th edn. Wayne: Approved Standard; 2000.
- 31. Rodis OM, Shimono T, Matsumura S, Hatomoto K, Matsuo K, Kariya N, et al. Cariogenic bacteria and caries risk in elderly Japanese aged 80 and older with at least 20 teeth. J Am Geriatr Soc 2006; 54: 1573-1577.
- 32. Bassole IH, Ouattara AS, Nebie R, Ouattara CA, Kabore ZI, Traore SA. Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry 2003; 62: 209-212.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
12 Feb 2008 -
Date of issue
Mar 2007
History
-
Accepted
12 Jan 2007 -
Received
03 Mar 2006