Abstracts
Considerable success has been obtained in the use of classical breeding to control economically important plant diseases, such as the coffee leaf rust and the coffee berry disease (CBD). There is a strong consensus that growing genetically resistant varieties is the most appropriate cost effective means of managing plant diseases and is one of the key components of crop improvement. It has also been recognized that a better knowledge of both, the pathogens and the plant defence mechanisms will allow the development of novel approaches to enhance the durability of resistance. After a brief description of concepts in the field of plant disease resistance, we attempt to give a view of the research progress on coffee leaf rust and CBD concerned with the pathogens infection and variability, coffee breeding for resistance and coffee resistance mechanisms.
Coffea; Colletotrichum kahawae; Hemileia vastatrix; coffee breeding; resistance
Sucesso considerável tem sido obtido no uso do melhoramento clássico para o controle de doenças de plantas economicamente importantes, tais como a ferrugem alaranjada das folhas e a antracnose dos frutos do cafeeiro (CBD). Há um grande consenso de que o uso de plantas geneticamente resistentes é o meio mais apropriado e eficaz em termos de custos do controle das doenças das plantas, sendo também um dos elementos chave do melhoramento da produção agrícola. Tem sido também reconhecido que um melhor conhecimento do agente patogênico e dos mecanismos de defesa das plantas permitirá o desenvolvimento de novas abordagens no sentido de aumentar a durabilidade da resistência. Após uma breve descrição de conceitos na área da resistência das plantas às doenças, nesta revisão tentou-se dar uma idéia do progresso na investigação da ferrugem alaranjada do cafeeiro e do CBD relativamente ao processo de infecção e variabilidade dos agentes patogênicos, melhoramento do cafeeiro para a resistência e mecanismos de resistência do cafeeiro.
Coffea; Colletotrichum kahawae; Hemileia vastatrix; melhoramento do cafeeiro; resistência
MINIREVIEW
Coffee resistance to the main diseases: leaf rust and coffee berry disease
Resistência do cafeeiro para suas principais doenças: ferrugem alaranjada das folhas e antracnose dos frutos
Maria do Céu SilvaI,* * Corresponding author: mceusilva@hotmail.com ; Victor VárzeaI; Leonor Guerra-GuimarãesI; Helena Gil AzinheiraI; Diana FernandezII; Anne-Sophie PetitotII; Benoit BertrandIII; Philippe LashermesII; Michel NicoleII
IInstituto de Investigação Científica Tropical (IICT) - Centro de Investigação das Ferrugens do Cafeeiro (CIFC), Quinta do Marquês, 2784-505 Oeiras, Portugal
IIInstitut de Recherche pour le Développement (IRD), UMR DGPC, Résistance des Plantes, BP 64501 - 34394 Montpellier Cedex 5, France
IIICentre de Coopération Internationale en Recherche pour le Développement (CIRAD), Département des Cultures Pérennes, UMR DGPC, Résistance des Plantes, BP 64501 - 34394 Montpellier Cedex 5, France
ABSTRACT
Considerable success has been obtained in the use of classical breeding to control economically important plant diseases, such as the coffee leaf rust and the coffee berry disease (CBD). There is a strong consensus that growing genetically resistant varieties is the most appropriate cost effective means of managing plant diseases and is one of the key components of crop improvement. It has also been recognized that a better knowledge of both, the pathogens and the plant defence mechanisms will allow the development of novel approaches to enhance the durability of resistance. After a brief description of concepts in the field of plant disease resistance, we attempt to give a view of the research progress on coffee leaf rust and CBD concerned with the pathogens infection and variability, coffee breeding for resistance and coffee resistance mechanisms.
Key words:Coffea, Colletotrichum kahawae, Hemileia vastatrix, coffee breeding, resistance.
RESUMO
Sucesso considerável tem sido obtido no uso do melhoramento clássico para o controle de doenças de plantas economicamente importantes, tais como a ferrugem alaranjada das folhas e a antracnose dos frutos do cafeeiro (CBD). Há um grande consenso de que o uso de plantas geneticamente resistentes é o meio mais apropriado e eficaz em termos de custos do controle das doenças das plantas, sendo também um dos elementos chave do melhoramento da produção agrícola. Tem sido também reconhecido que um melhor conhecimento do agente patogênico e dos mecanismos de defesa das plantas permitirá o desenvolvimento de novas abordagens no sentido de aumentar a durabilidade da resistência. Após uma breve descrição de conceitos na área da resistência das plantas às doenças, nesta revisão tentou-se dar uma idéia do progresso na investigação da ferrugem alaranjada do cafeeiro e do CBD relativamente ao processo de infecção e variabilidade dos agentes patogênicos, melhoramento do cafeeiro para a resistência e mecanismos de resistência do cafeeiro.
Palavras-chave:Coffea, Colletotrichum kahawae, Hemileia vastatrix, melhoramento do cafeeiro, resistência.
1. INTRODUCTION
Although coffee is not a food crop, it represents for most coffee-growing countries the major source of revenue for foreign exchange. Limiting factors of coffee production include major diseases, such as the coffee leaf rust (or orange rust) and the coffee berry disease (CBD) caused by the fungi Hemileia vastatrix Berkeley and Broome and Colletotrichum kahawae Bridge and Waller, respectively.
Other coffee rust diseases (powdery, yellow rust or grey rust), caused by the fungus Hemileia coffeicola Maubl and Rog., have not been considered so important economically as leaf rust. The symptoms of the disease are characterised by a dusty or powdery coating of yellow uredosori covering the underside of the coffee leaves, in contrast to H. vastatrix that forms distinct blotches or pustules (Rodrigues Jr. 1990; Adejumo, 2005). According to Waller (1985), H. coffeicola occurs only in West Africa where it can be serious for some cultivars of C. canephora grown in very wet areas and it is favoured by hot humid conditions which are unsuitable for Arabica coffees.
Other fungal diseases like coffee wilt disease or tracheomycosis caused by Fusarium xylarioides Steyaert (teleomorph: Gibberella xylarioides Heim and Saccas) is becoming important in some regions of Central and West Africa, not only in Robusta but also for Arabica. It is a vascular disease causing yellowing and wilting of the trees. Fusarium stilboides Wollenw (telemorph: Gibberella stilboides), the causal agent of the coffee bark disease, is also present in some African countries, particularly in Ethiopia, Kenya, Malawi and Tanzania. Its characteristic symptom is a scaling of the bark leading to stem cankers and a progressive dying back of the whole tree. The brown eye spot or berry blotch (Cercospora coffeícola Berk and Cooke) is a disease of widespread occurrence in nurseries and plantations, infecting the coffee leaves and the fruits as well. The American leaf spot of coffee caused by Mycena citricolor (Berk and Curt.) Sacc. has been reported exclusively in the American continent in cool, moist areas at higher altitudes (Waller 1985; Wrigley 1988; Chen, 2002; Geiser et al., 2005).
The two known bacterial coffee diseases are the halo blight of coffee incited by Pseudomonas syringae pv. garcae Amaral et al. (Amaral et al., 1956) and the coffee leaf scorch caused by the polyphagous bacteria Xilella fastidiosa Wells et al. (Wells et al., 1987). The former disease has been described in Brazil, Kenya, Uganda and China where it is becoming of some concern due to its higher incidence and severity (Wen and Chen 1995; Chen, 2002). The bacterium infects the coffee leaves, branch-tips and young fruits, in some instances with symptoms (irregular dark necrotic lesions) identical to Phoma sp. However, it can be distinguished from the fungus by the presence of bacterial exudates in the young lesions. The bacteria persist, in a large number, on all healthy surfaces of the tree, and during cool and wet weather they multiply and initiate the epidemics. Coffee leaf scorch has been recorded so far only in Brazil and Costa Rica where the bacterium X. fastidiosa attacks several crops with high incidence, particularly in citrus and prunes. A strain of the bacterium, able to infect coffee, is transmitted by the sharpshooter leafhopper Dilobopterus cortalimai (Cicadellidae: Cicadellinae) and was recorded for the first time in 1995 in the State of S. Paulo (Leite Jr. et al., 1999; Li et al., 2001). Symptoms in affected coffee plants include shortened internodes, premature loss of older leaves, terminal clusters of small pale green to yellow deformed leaves, lateral shoot dieback, and overall plant stunting (De Lima et al., 1998; Li et al., 2001).
Some records of virus diseases in coffee have sporadically been made (leaf rugosity, leaf curl) in Brazil and Colombia but with no economic significance and of eventual misinterpreted diagnostic (Chen, 2002). However, the coffee ringspot virus CoRSV associated with the presence of the mite Brevipalpus phoenicis Geijskes has been reported in several Brazilian states (São Paulo, Paraná, Minas Gerais, and Distrito Federal) and recently found in Costa Rica. It causes conspicuous ringspot symptoms on leaves, berries, and less frequently on twigs. A similar disease is known in the Philippines, but no information exists about its relationship to CoRSV. Coffee ringspot had no economical significance until recently when a large-scale infection was reported in Minas Gerais that resulted in yield loss (Chagas et al., 2003).
The extremely high costs of fungicides to control diseases in coffee plantations, particularly leaf rust and CBD, as well as the difficulties in its application, have led the coffee experimental centres to give priority in their coffee improvement programmes to select for resistance to these diseases, as the main part of an integrated global protection approach (Bettencourt and Rodrigues Jr., 1988). Improvement of coffee resistance to leaf rust and CBD has been mainly focused on finding new sources of resistance and a better understanding of both the pathogens diversity and the host defence mechanisms. In view of the complexity of plant disease resistance to pathogens, which is becoming increasingly apparent as new tools in genetics, cell biology, biochemistry and molecular biology are being used, this review will focus on pathogen recognition, signal transduction pathways and subsequent defence responses, as well as on the specificity of host resistance. Particular attention will be given to developments that have been made in coffee leaf rust and CBD, including aspects related to pathogen infection and variability, coffee breeding for resistance and host resistance mechanisms.
2. CONCEPTS OF DISEASE RESISTANCE
Plants have the ability to recognize potential invading pathogens and have developed various elaborate defence mechanisms to ward off pathogen attack (Heath 1997a, Mansfield et al., 1997; Staskawicz, 2001). At the same time, pathogens have developed strategies to compromise plant resistance mechanisms in what must have been an evolutionary game of "ping-pong" (Keen 1999). Some of these mechanisms are constitutively expressed and provide physical and chemical barriers while others are induced only after pathogen attack which involves a network of signal transduction and the rapid activation of gene expression (Dixon and Lamb 1990; Bradley et al., 1992; Heath 1997a).
2.1. Pathogen recognition and signal transduction
The perception and rapid response to the presence of most pathogens by plants determine whether or not it will survive. Perception involves the detection of molecules from the pathogen or from the wounded plant itself (Keen 1999). These molecules have come to be called elicitors (non-race-specific or race-specific) and may be peptides or proteins, fatty acid derivatives, sterols or other low molecular weight chemicals, with diverse structures and present in very low concentrations (Ebel and Scheel 1997; Somssich and Hahlbrock 1998; Yamaguchi et al., 2000; Peck, 2003). Whatever the source of the elicitor, the mechanism of signal perception appears to rely on the presence of specific receptors on the plant cell surface (Ebel and Scheel 1997; Pontier et al., 2002). Several of the receptor-like protein kinase (RLK) family are transmembrane proteins with cytoplasmic serine/threonine kinase domains and divergent extracellular domains (Morris and Walker, 2003). Some of the rapidly activated kinases have been identified as mitogen-activated protein kinases (MAPKs) (Jonak et al., 2002), calcium-dependent protein kinases (CDPK) (Romeis et al., 2001) and wall-associated kinase (WAK) (He et al., 1998). As yet, however, little is known about how these kinases transmit information to the cell (Peck, 2003).
Upon recognition, signalling events are initiated resulting in numerous cellular changes, such as an inward flux of Ca2+, the production of reactive oxygen species (ROS), actin cytoskeleton involvement, changes in the levels of a subset of transcripts (Grant and Mansfield 1999; Guest et al., 1999; Heath, 2000a, Dangl and Jones, 2001; Peck, 2003), involvement of plasma-membrane-bound NADPH oxidases, cell-wall-bound peroxidases, amino oxidases in the apoplast and phosphoproteins (Bestwick et al., 1997; Heath, 2000a, Laloi et al., 2004). The activation of signalling cascades at the plasma membrane leads to the induction of a complex network of other signal molecules, such as salicylic acid, jasmonic acid, ethylene, nitric oxide, that will induce, within minutes, a general defence response that will kill/limit the spread of the pathogen through the plant (Alvarez, 2000; Dixon et al., 2002; Turner et al., 2002; Harrison and Baldwin, 2004; Delledonne, 2005).
2.2. Defence responses
2.2.1. Preformed defences
Protection from the initial invasion of the pathogen is achieved via passive defences, such as physical and/or chemical barriers. Physical barriers largely involve properties of the plant surface, that is, the amount and quality of wax and cuticle that cover the epidermal cells, the structure of the epidermal cell walls and the size, location and shape of stomata (Agrios 1997). Some plants invest in very thick walls and/or cuticles, and bark (where present) can also provide a physical impediment to infection. Chemical barriers include compounds that have antimicrobial activity and compounds that affect the vectors of plant viruses, such as, phenols, quinines, lactones, cyanogenic glucosides, saponins, terpenoids, stilbenes and tannins (Keen 1999). Evidence is accumulating to show that plant cell wall, apart from being a structural barrier against pathogen attack, is capable of triggering multiple signalling pathways. These signal properties are essential not only for the role of the cell wall in defence but also for the co-ordination of wall synthesis and expansion between adjacent cells (Pilling and Hofte, 2003).
2.2.2. Induced defences
The hypersensitive response (HR), a rapid localised death of one or a few host plant cells in response to invasion by an avirulent isolate of a pathogen, is the most common response of gene-for-gene interactions. The HR is characterized by a rapid loss of membrane integrity in the infected host cells and the accumulation of brown phenolic oxidation products (Goodman and Novacky 1994; Hammerschmidt and Nicholson 1999; Heath, 2000a). Although the HR may be an effective defence against biotrophic pathogens, which depend on the living host cells for their survival (Heath 1997b, Mansfield et al., 1997; Richael and Gilchrist 1999), it is likely that this host response is only a single part of the overall defensive strategy of the plant (Hammerschmidt and Nicholson 1999). HR is now almost universally accepted as a form of programmed cell death (PCD) (Ryerson and Heath 1996; De Wit 1997; Heath 1999, 2000a) and differs from developmental PCD in its consistent association with induction of local and systemic resistance (Costet et al., 1999; Alvarez, 2000; Heath, 2000a). How the HR directly contributes to host resistance and how the onset of the HR is related to the expression of the other putative defences needs further clarification. The oxidation of phenolic compounds as cells undergo the HR suggests that there is an increase in phenol oxidizing enzymes as well as the production of reactive oxygen species (ROS) (Goodman and Novacky, 1994; Bolwell 1999; Hammerschmidt and Nicholson 1999). However, it is not at all clear that the production of ROS (oxidative burst) is required for the development of the HR for all plant-pathogen interactions (Heath 1998, 2000b). It has been suggested that ROS produced during the oxidative burst may function in the defence-signalling pathways leading to different defence responses against pathogens (Neill et al., 2002; Laloi et al., 2004). These molecules also have the potential to act directly as antimicrobial agents (Baker and Orlandi 1995).
Another early event in the interaction between a pathogen and a host plant cell is the expression of genes encoding enzymes of the phenylpropanoid pathway, such as phenylalanine ammonia-lyase - PAL (Dorey et al., 1997). PAL has been shown to play an important role in plant resistance, namely by its involvement in the biosynthetic pathway providing isoflavonoids and phenylpropanoids with phytoalexin activity and as a precursor of lignin-like compounds, suberin or other types of phenolic materials that are often deposited in the host cell walls at the point of fungal invasion (e.g. papilla formation), and appear to block the progression of the hyphae (Hammerschmidt 1999; Dixon et al., 2002; Caño-Delgado et al., 2003). PAL has also been implicated in the biosynthetic pathway for salicylic acid (SA), another defence-related compound and a key signalling component required for the activation of PR-genes, catalases, receptor-like protein kinases and transcription factors (MauchMani and Slusarenko 1996; Klessig et al., 2000; Dixon et al., 2002; Way et al., 2002; Mould et al., 2003).
Resistance expression is often accompanied by the activation of the phenol-oxidising enzymes peroxidase and phenoloxidase and the lipid peroxidizing enzyme lipoxygenase (Goodman and Novacky, 1994). Peroxidase activity often increases in response to infection, and may function in defence through production of antimicrobial quantities of hydrogen peroxide as well as in cell wall lignification and crosslinking (Peng and Kúc, 1992; Rasmussen et al., 1995; Chittor et al., 1999; Do et al., 2003). Increase in activity of phenoloxidases and its interaction with endogenous phenols have been correlated with the onset of HR (Hammerschmidt and Nicholson 1999). Lipoxygenase contributes to the HR via disruption of cell membrane lipids, to defence through the formation of toxic lipid oxidation products, as well as in the formation of lipid-derived signals (Croft et al., 1993; Jalloul et al., 2002; Montillet et al., 2005).
Another induced response is the synthesis of pathogenesis-related proteins (PR proteins), which do not only accumulate locally in the infected tissues, but are also induced systemically (Van Loon et al., 1994). Most of the existing 17 families include components that are exported to the extracellular space, where they are believed to have a role in resistance (Fritig et al., 1998; Christensen et al., 2002). A collective set of PR proteins (e.g. chitinases and b-1,3-glucanases) may be effective in inhibiting pathogen growth, multiplication and/or spread (Van Loon and Van Strien, 1999). Others like PR-9 proteins (with peroxidase activity) are enzymes with possible implications in the oxidative cross-linking of plant cell wall components to prevent the pathogen from penetrating (Thordal-Christensen et al., 1992). Furthermore, oxalate oxidase (Zhou et al., 2000) and some chitinases are PR proteins that have been suggested to be involved in the release of chemical signals, from fungal cell walls that will act as elicitors of other plant-defence responses (Schlumbaum et al., 1986; Ryan, 1987; Lawrence et al., 2000). It is very likely that novel extracellular PR proteins play a role within one of these three classes of phenomena, namely antimicrobial activity, cell wall biology and signal transduction (Van Loon 1999; Tuzun, 2001; Christensen et al., 2002).
2.3. Specificity of host resistance
Host resistance is usually parasite-specific in that it is restricted to a particular pathogen species, and commonly is expressed against specific pathogen genotypes (i.e., race- or pathotype-specific resistance) (Heath 1997a, 2000b). This is the case of several plant-pathogen interactions, particularly those involving biotrophic parasites such as rusts, which are governed by specific interactions between pathogen avirulence (avr) loci and alleles of the corresponding plant disease resistance (R) locus (Flor, 1942). This gene-for gene resistance has been validated in the past decade with the cloning of up to a dozen of R and avr genes, and with in-depth analyses of several incompatible (resistance-eliciting) plant-pathogen interactions (Bogdanove, 2002). Race-specific monogenic (or oligogenic) resistance, also described as "vertical resistance (Van der Plank 1963) usually gives clear phenotypes with a qualitative effect like no sporulation, necrotic spots or complete lack of symptoms and, is generally associated with the rapid host cell death (Hypersensitive Response HR) whereby a number of biochemical reactions occur, such as the oxidative burst, PR protein accumulation, production of phytoalexins, etc (Lindhout, 2002). Only in few cases, durable resistance, i.e. resistance that remains effective for a long period, where application is made on a large scale in an environment conducive to the pathogen (Johnson, 1981) has been achieved using single R genes. In general, the rapid evolution of new virulent pathotypes, on previously resistant cultivars, forced breeders into producing replacement cultivars with new R genes (Pink, 2002). Thus, although this type of resistance is the most frequently deployed in plant breeding, the great disadvantage is that it is often non-durable (Vale et al., 2001; Lindhout, 2002; Niks and Rubiales, 2002; Parlevliet, 2002).
Another type of host resistance is the race non-specific resistance, which appears to be specific for one pathogen species only, although more or less equally effective against all genotypes of the pathogen (Parlevliet, 1983). This type of resistance refers to plant disease resistance generated via interactions between the products of multiple plant genes, not a single R gene (Simmonds, 1991). This multigenic (or polygenic) resistance is generally quantitative. With the onset of molecular technologies, the identification of chromosome regions that are involved in quantitative resistance has become feasible. These regions are designated "quantitative trait loci" (QTLs) and, as they are defined by the position on the genome and the quantitative effect on resistance, they are not informative of the function. Hence QTLs may be involved in any resistance mechanism and hence also in HR (Lindhout, 2002). The genetics of this type of resistance, also described as "field-resistance, horizontal resistance, partial resistance, etc. (Van der Plank, 1963) is hard to study, as the effect of each gene is small and often influenced by the environment or by the interaction with other genes (epistasis). As QTLs can confer a high level of resistance by their combined forces, the adaptation of a pathogen to render each QTL involved in the polygenic resistance ineffective is theoretically more complex than to render just one gene ineffective in the case of monogenic resistance (Lindhout, 2002). Thus, in some pathosystems with long experience of non-durable race-specific monogenic resistance associated with HR, the polygenic quantitative resistance offers a good alternative, and marker assisted breeding will facilitate the exploitation of this resistance for commercial purposes (Lindhout, 2002)
3. COFFEE RESISTANCE TO THE MAIN DISEASES
3. 1. Coffee leaf rust
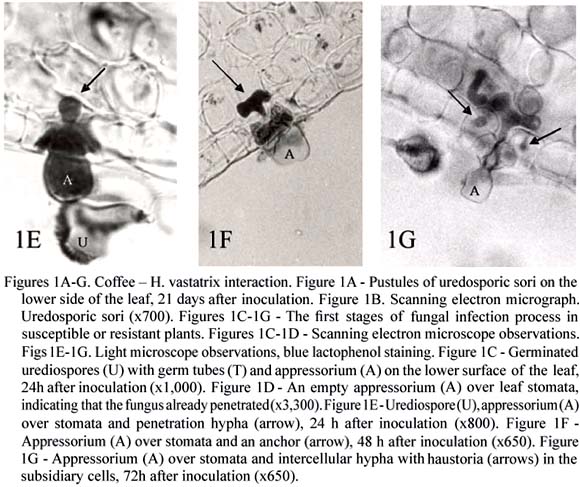
H. vastatrix, the causal agent of coffee leaf rust, produces the uredinal, telial, and basidial stages, but only the dycariotic urediospores are responsible for the disease. H. vastatrix infects the lower surface of the leaves where it produces large, orange colonies of uredosori (figures 1A-B), leading to premature leaf fall and yield losses. The leaf rust was apparently recorded for the first time in 1861; near Lake Victoria, but it was in Sri-Lanka that, in 1868, it caused the first great economic impact (Wellman, 1957). Leaf rust is spread throughout coffee-growing countries and may cause up to 10-40 % of losses.
3.1.1. Fungal infection process
Biotrophic fungi, such as rusts are entirely dependent on plant living cells for their growth and reproduction (Heath 1997b, Shulze-Lefert and Panstruga, 2003). The following properties appear to be hallmarks of these fungi: (i) highly developed infection structures; (ii) limited secretory activity, especially of lytic enzymes; (iii) carbohydrate-rich and protein-containing interfacial layers that separate fungal and plant plasma membranes; (iv) haustoria, which are specialized hyphae for nutrient absorption and metabolism; (v) both long-term suppression of host defences and induction of specific host genes for the establishment of biotrophy (Mendgen and Hahn, 2002; Shulze-Lefert and Panstruga, 2003; Voegele and Mendgen, 2003).
The initiation of the dycariotic phase of H. vastatrix on coffee leaves, as with other rust fungi (Heath 1995, 1997a, Mendgen and Hahn, 2002; Mendgen and Voegele, 2005) involves specific events including appressorium formation over stomata and penetration by inter- and intracellular colonization (Rodrigues Jr. et al., 1975; Rijo and Rodrigues Jr., 1977; Tiburzy et al., 1983; Coutinho et al., 1993; Martins and Moraes, 1996; Silva et al., 1999a, 2002). Thus, in susceptible coffee leaves, after urediospore germination and appressorium differentiation over stomata (figure 1C), the fungus penetrates (from 12h after inoculation) (figure 1D) forming a penetration hypha (figures 1E) that grows into the substomatal chamber. This hypha produces at the advancing tip two thick lateral branches; each hypha and its branches resemble an anchor (figure 1F). Each lateral branch of the anchor bears a hypha (haustorial mother cell HMC), the subsidiary cells being the first invaded by haustoria (figure 1G), whose formation starts around 36h after inoculation. The fungus pursues its growth with formation of more intercellular hyphae, including HMCs, and a large number of haustoria in the cells of the spongy and the palisade parenchyma and even of the upper epidermis. A dense mycelium is observed below the penetration area and a uredosporic sorus protrudes like a "bouquet" through the stomata about 20 days after inoculation.
After adhesion of rust urediospores to the plant surface, the development of infection structures results from a sophisticated host-surface recognition system. The tip of the dicaryotic germ tube is able to follow topographical features of the plant cuticle and thus increase the probability of encountering a stomatal opening (Mendgen and Voegele, 2005). Host specific features, like the dimension of the outer lip of stomatal guard cells serve as inductive signals, perhaps through synergistic interaction with chemicals such as leaf alcohols (Collins et al., 2001; Wiethölter et al., 2003). To control further fungal development within the plant a successive sequence of signals is also required (Heath, 1997b, Mendgen and Voegele, 2005). In order to understand which signals and signalling pathways are involved in the differentiation of H. vastatrix infection structures, different artificial membranes were tested. With oil collodion membranes the percentages of urediospore germination and appressoria formation did not differ significantly from the values obtained on coffee leaves, indicating the role of topographic signals in the induction of pre-penetration structures. However, subsequent infection structures only developed at a very low rate (1-2 %) and in the majority of cases with no septum to delimitate the penetration hypha from the apressorium (Azinheira et al., 2001; Azinheira, 2005). Thus, it seems that for H. vastatrix a second stimulus is required to continue the fungal differentiation, although the nature of the signal is still unknown (Azinheira, 2005). Molecular studies, using RT-PCR strategy showed the presence of a gene homologue of pmk1 from Magnaporthe grisea which belongs to the MAPK family. Despite its expression in urediospores and germ tubes a slight increase observed in the expression of the MAPK during appressorium formation may suggest that this protein is important in signalling mechanisms leading to the formation of that structure (Azinheira, 2005).
H. vastatrix contains b-1,3-glucans and chitin as major components of the cell walls of urediospores, and pre- and post-penetration structures (Maxemiuc-Naccache and Dietrich, 1981; Silva et al., 1999a). Both polymers are regularly distributed over the walls of pre-penetration fungal structures (Silva et al., unpublished). Over the cell walls of intercellular hyphae a regular labelling for b-1,3-glucans was observed, while chitin accumulated preferentially over the internal part, likely to be less exposed to the eventual action of host chitinases (Silva et al., 1999a). For different rusts, Deising et al. (1996) infer that on the infection structures formed in the intercellular space of the leaf, chitin is difficult to detect by FITC-labelled WGA. They suggested that restriction of chitin exposition to fungal structures which are not in direct contact with inter- and intracellular host defence enzymes may be a general rule in rust fungi.
The ultrastructure of intercellular hyphae and haustoria of H. vastatrix in susceptible coffee leaves, described by Rijkenberg and Truter (1973), Rijo and Sargent (1974), Silva and Nogueira (1977), Benac (1981) and Silva et al. (1999a) is similar to that observed in other compatible host-rust interactions (review of Littlefield and Heath, 1979). Cytochemical studies made by Silva et al. (1999a) showed that during haustorium formation, plant cell wall degradation was restricted to the site of penetration with minimal damage to host cells, a common goal of biotrophic fungi (Mendgen and Hahn, 2002; Schulze-Lefert and Panstruga, 2003).
3.1.2. Fungal variability
The first race differentiation of H. vastatrix was carried out by Mayne (1932) in India, who differentiated the local rust samples into four physiologic races. No other studies were made on the physiological specialization of H. vastatrix until D'Oliveira initiated a world survey of coffee rust races in 1952 in Portugal (D'Oliveira 1954-1957, 1965). The work carried out at the Coffee Rusts Research Center (CIFC) enabled the characterization of about 45 rust races (D'Oliveira, 1965; D'Oliveira and Rodrigues, 1961; Rodrigues et al., 1965; Bettencourt et al., 1965; Rodrigues et al., 1975; Rodrigues et al., 1993; Várzea et al., 2002a).
Molecular studies to detect genetic diversity in H. vastatrix were carried out by Nandris et al. (1998). The RAPD (Random Amplified Polymorphic DNA) method revealed polymorphism between individuals. However, a linkage between the molecular markers obtained and the pathotypes used was not established. In recent studies at CIFC, using RAPD and MSP-PCR (Microsatellite-Primed Polymerase Chain Reaction), a considerable degree of variability among the populations studied was observed, although no clear relationship was obtained between host, geographical origin and physiologic race (Gouveia et al., 2005).
3.1.3. Coffee resistance to leaf rust
3.1.3.1. Inheritance of resistance
The inheritance studies of rust resistance carried out at CIFC have demonstrated that the gene-for-gene theory is applicable to coffee-rust interactions (Noronha-Wagner and Bettencourt 1967) where the resistance of coffee plants is conditioned by at least nine major dominant genes (SH1-SH9), singly or associated. By the same theory, it was possible to infer 9 genes of virulence (v1-v9) in H. vastatrix. (Rodrigues Jr. et al., 1975; Bettencourt and Rodrigues Jr. 1988). Major genes SH1, SH2, SH4 and SH5 were found in pure Arabicas from Ethiopia origin, the gene SH3 is considered to derive from C. liberica, and SH6, SH7, SH8 and SH9 were only found in "Hibrido de Timor" - HDT (C. arabica x C. canephora) derivatives, therefore supposedly coming from the Robusta side of the hybrid (Rodrigues Jr. et al., 1975; Bettencourt and Rodrigues Jr., 1988). Besides these SH genes it is likely that other major and minor genes might condition the coffee-rust interactions (Bettencourt and Rodrigues Jr., 1988).
The coffee genotypes are classified in physiological groups which are distinguished from each other essentially by responses involving either complete resistance or susceptibility (low and high infection type) to several rust races. Group A, characterized by resistance to all the known rust races, has been found in hybrids between C. arabica x C. canephora, either spontaneously as in the HDT or man-made as in Icatú (D'Oliveira and Rodrigues Jr. 1961; Marques and Bettencourt 1979). Plants of group A have also been found in C. liberica, C. dewevrei, C. eugenioides, C. congensis, etc. (D'Oliveira and Rodrigues Jr. 1961) while the E-group, characterized by susceptibility to almost all known races, includes the traditional Typica and Bourbon cultivars (Bettencourt and Rodrigues Jr., 1988).
Non-specific polygenic resistance has been assessed at CIFC and more extensively in other countries (Kushalappa and Eskes 1989), mainly under laboratory conditions using different parameters, such as latency period, percentage of sporulating lesions, and spore production per lesion. Eskes (1983), Leguizamon (1983), Várzea et al. (1985), Fagioli et al. (1990), and Holguin (1993), among others, indicated the existence of sources of this type of resistance in C. canephora and interspecific hybrids and also in some C. arabica genotypes, but the inheritance of this type of resistance remains unknown.
3.1.3.2. Coffee breeding for resistance
Since 1927 the Central Coffee Research Institute (CCRI) in Balehonnur, in India, has carried out an important national breeding programme. However, it was after the creation of CIFC that coffee breeding for rust resistance received a decisive impulse.
A major breakthrough in the CIFC's plant breeding programme for obtaining resistant varieties was the discovery in the late 1950s of the Hibrído de Timor (HDT), a single plant found in the ex-Portuguese colony of Timor (now Timor Lorosae). Remarkably, most of the HDT offspring offered resistance to all or some of the known rust races. It has been demonstrated that HDT is supposed to be a natural hybrid between C. arabica and C. canephora and to have received from the latter the genes responsible for rust resistance. This interspecific hybrid has the same number of chromosomes as C. arabica and the crosses are fertile. The breeding programme of CIFC was essentially based on the utilization of HDT as a resistant parent. The main hybrids produced at CIFC with HDT were: HW26 = Caturra Vermelho x HDT 832/1; H 46 = Caturra Vermelho x HDT 832/2; H361 = Villa Sarchi x HDT 832/2; H528 = Catuaí Amarelo x HW26/13; H529 = Caturra Amarelo x H361/3.
The fundamental and applied research developed at CIFC on leaf rust, as well as the research it originated in several coffee-growing countries, led to substantial advances towards obtaining durable resistance to this disease in Arabica. This progress has been possible as a result of a joint co-operation with several coffee Experimental Centers in different countries, namely Kenya (Coffee Research Station), Brazil (Instituto Agronômico de Campinas, Instituto Brasileiro do Café, Sistema Estadual de Pesquisa Agropecuária de Minas Gerais, Instituto Agronômico do Paraná, Universidade Federal de Viçosa and Universidade Federal de Lavras); Colombia (Centro Nacional de Investigaciones de Café); Central America (Mexico, Panama and the Dominican Republic) under the project Promecafe (PROMECAFE: Programa Cooperativo para la Protección, Modernización de la Caficultura en Centroamerica, México, Panama Y República Dominicana) (Bettencourt, 1981; Bettencourt, 1983; Rodrigues Jr. et al., 2000)
In the pedigree selections, the F3 and F4 generations of HW26 and H46 received the designation of Catimor by the Universidade Federal de Viçosa. The hybrids H361, H528 and H529 were introduced in the American Continent in 1970, and F3 and further generations received the designations of Sarchimor, Cavimor and Cachimor (Bettencourt, 1983). Catimor and Sarchimor, the most advanced selections, have been widely distributed in the coffee-growing countries, not only in Latin America but also in Africa (Malawi), Asia (India) and Oceania (Papua New Guinea). After local selection for several years, Catimor received regional designations such as Oeiras, Tupi, Obata, Iapar59 (Brazil), Catrenic (Nicaragua), Costa Rica 95 (Costa Rica), Ihcafe-90 and Lempira (Honduras), Catisic (el Salvador) and Mida 96 (Panama). In Colombia, HDT 1343 was crossed with Caturra to produce the original hybrid from which variety "Colombia" has derived (Rodrigues Jr. et al., 2000)
3.1.3.3. Durability of resistance
In the last few years, some improved commercial varieties from HDT and other interspecific tetraploid hybrids, like Icatú are gradually loosing their resistance to leaf rust in some countries, due to the appearance of new virulent races (Rodrigues et al., 2000; Várzea et al., 2004; Várzea and Marques, 2005). Some genotypes of the referred coffee varieties, however, maintain their resistance and others, although infected in the field, present an incomplete type of resistance with others heavily infected, suggesting that they probably possess a polygenic type of resistance, like the variety Colombia (Alvarado, 2005). On the other hand, some Arabica varieties like Rume Sudan and Tafarikella with low yields and classified at CIFC as belonging to the susceptible group E, showed a very high partial resistance in the field for many years (Várzea et al., 2000; Várzea et al., 2002a). At CIFC the level of resistance of HDT derivatives and some lines of Rume Sudan are now being re-evaluated. However, it is interesting to note that when the yield is totally suppressed, the susceptible cultivar Caturra appears partially resistant under conditions of strong infection (Bertrand et al., unpublished data). Moreover in a F2 population resulting from a cross between 'Resistant x Susceptible', the same authors observed that plants with low productivity appear with very high frequency, partially or totally resistant and plants with high productivity appeared resistant or susceptible and rarely partially resistant. Consequently, some partial resistance might be explained by the physiological status of the plant.
3.1.3.4. Cytological and biochemical resistance mechanisms
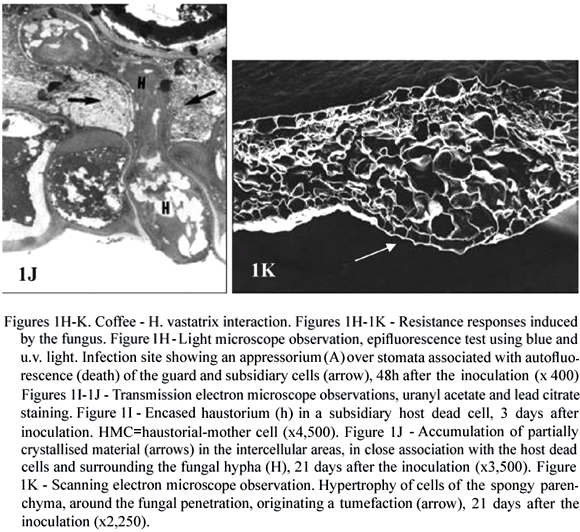
There is no evidence for the existence of preformed defences in coffee, which could limit the growth of H. vastatrix although several resistance mechanisms are induced after infection (Rodrigues et al., 1975; Kushalappa and Eskes, 1989; Rodrigues et al., 2000; Várzea et al., 2002a, Várzea et al., 2004). H. vastatrix urediospores usually germinate and differentiate the appressoria over stomata equally well on susceptible and resistant coffee plants (Silva 1996; Silva, et al., 2002). For a number of coffee (Coffea spp.) genotypes, the resistance is post-haustorial (in that the fungus ceases its growth at different stages of the infection, but more frequently after the formation of the first haustorium) and is expressed by the rapid hypersensitive cell death (HR) (figure 1H) recognized by the presence of autofluorescent and/or browning cells or by deep blue staining with Evans blue. Cell death began to be observed around 2 days post-inoculation, in the guard cells only, or in both the guard and subsidiary cells at the infection sites in which the fungus reached the stage of appressorium or penetration hypha (Silva 1996; Silva et al., 2000, 2002). Death of subsidiary and mesophyll cells invaded by a haustorium was observed from the 3rd day after inoculation. During the time course of infection, cell death spread to adjacent epidermal and mesophyll non-invaded cells, as has been generally described for other coffee resistant genotypes (Martins et al., 1985; Rijo et al., 1991) and in plants resistant to other rust fungi and to other obligate biotrophs (Heath, 2000a, Koga et al., 1990; Huang et al., 1998). In susceptible coffee plants, death of guard and subsidiary cells was observed from the 3rd day after inoculation, but only in a small percentage of infection sites (generally less than 20 %), in which the fungus had stopped its growth at early stages (Silva, 1996; Silva et al., 2002). Transmission electron microscope observation of host cells undergoing HR revealed membrane breakdown at the level of the plasma membrane and in different organelles, namely chloroplasts, nucleus and mitochondria, with a change in the chloroplast and nucleus appearance and coagulation of cytoplasm (Silva et al., unpublished data).
Another signal of incompatibility detected early at the cytological level was the haustoria encasement (figure 1I). This host response was also observed in compatible interactions, but latter in the infection process (from the 7th day post-inoculation) and only in a small number of haustoria (Rijo and Vasconcelos, 1984; Silva, 1996; Silva et al., 1999a, 2002). The haustoria encased material in resistant or susceptible leaves reacted positively for callose and b-1,4-glucans, as indicated by the use of polyclonal antibodies raised against b-1,3-glucans and an exoglucanase-gold complex, respectively. The use of anti-galacturonic acid monoclonal antibodies (JIM7) allowed the localization of pectins in the encasing material around the penetration pegs, but not around haustorial bodies (Silva et al., 1999a, 2002). In several plants resistant to rust and other obligate biotrophs, haustorium encasement has been regarded as one expression of incompatibility (Littlefield and Heath 1979; Cohen et al., 1990; Skalamera et al., 1997). Callose, the major compound of haustorial encasements, has been reported to be less permeable to small molecules than other cell wall components (Heslop-Harrison, 1966) and may therefore restrict the passage of nutrients to the fungus and consequently to slow the fungal growth (Rijo and Vasconcelos, 1984).
In resistant coffee leaves, the early detected epifluorescence and/or browning of cells was followed by the lignification of their walls, which occurred from the 7th day post-inoculation, as indicated by the phloroglucinol-HCl test (Tiburzy et al., 1983; Rijo and Vasconcelos, 1984; Martins et al., 1985; Silva et al., 2002). Biochemical studies, with coffee resistant genotypes, revealed an early increase of phenylalanine ammonia-lyase (PAL) and peroxidase activity just before or at the same time as the beginning of the observation of cell death, which may indicate the involvement of these enzymes in HR. By 4-5 days post-inoculation a second increase of PAL and peroxidase activity was observed which can be related with the later accumulation of phenolic compounds and lignification of the host cell walls detected cytologically (Silva et al., 2002, 2003a,b). The isoenzyme pattern for peroxidases obtained by IEF gels showed an increase in activity of anionic isoenzymes and a new cationic isoform at the same time as the first peak of peroxidase activity detected in the incompatible interactions (Silva et al., 2003a,b). At that time, peroxidase activity, cytochemically localized using DAB (Diaminobenzidine), was detected at the interface host cuticle-fungal pre-penetration structures, as well as in the walls, middle lamella, cytoplasmic contents, chloroplasts and endoplasmic reticulum of stomatal and spongy cells, at infection and penetration sites. The treatment of resistant coffee leaves with 2,4-dichlorophenol, an activator of peroxidases and other oxidases, significantly increased cell death. On the contrary, salicyl hydroxamic acid, an inhibitor of the same enzymes and diphenyleneiodonium chloride, an inhibitor of NADPH oxidases decreased cell death. These results suggested that the peroxidases, NADPH oxidases and eventually other oxidases are involved in the HR of the coffee-rust interaction. The same kind of treatments using scavengers of active oxygen species (catalase, superoxide dismutase and manitol) showed that only the superoxide dismutase significantly inhibited the cell death, also suggesting the involvement of the superoxide anion radical O2- in the HR (Silva et al., 2001, 2003b). On the other hand, studies made by Rojas et al. (1993) revealed a rise in lipoxygenase activity during an incompatible coffee-rust interaction, whereas the activity of the enzyme remained fairly constant in the compatible interaction.
An early increase of chitinase and glucanase activity in coffee-leaf rust incompatible interactions, but not in the compatible ones was observed by Maxemiuc-Naccache et al. (1992) using crude extracts of coffee leaves. Similar results were obtained when studying chitinase activity in intercellular fluids (IF) of incompatible coffee-rust interactions. Although basic isoforms of chitinases, from IF of coffee leaves, were present in both compatible and incompatible interactions, they were detected earlier in the incompatible ones. Immunodetection analyses performed with antibodies specific to class I chitinases revealed the importance of these isoforms in the incompatible interactions (Guerra-Guimarães et al., 2001, 2003).
Ultrastructural observations of different coffee resistant genotypes revealed the accumulation of a material partially crystallised in the intercellular spaces around the senescent hyphae, next to dead host cells and in close association with the middle lamella (figure 1J), around 5-7 days post-inoculation. However, such material was never detected in healthy or susceptible tissue. Cyto- and immunocytochemical tests showed that at the beginning of accumulation the material contained weakly esterified pectins. It also contained polysaccharides and phenolic-like compounds. Cellulose, hemicellulose, extensins, hydroxyproline-rich glycoproteins and proteins were not detected. Although the role of this material is unknown it might be the result of plant cell death associated with the slowdown of tissue invasion by the pathogen (Silva et al., 2002, 2005). Another response observed, around 12 days post-inoculation in different coffee resistant genotypes was the hypertrophy of the host mesophyll cells in the infection area (Rijo et al., 1990; Silva et al., 2002) suggesting the possible involvement of growth regulators (figure 1K). These larger cells surrounding the intercellular hyphae gave rise to a localized tumefaction and corresponded macroscopically to the reaction type flt, the most common reaction type of incompatible coffee-rust interactions.
3.1.3.5. Molecular disease resistance responses of C. arabica
3.1.3.5.1. Identification of coffee genes expressed during the HR to H. vastatrix
To gain insights into defence and resistance gene activation in coffee, a catalogue of genes expressed early in coffee leaves when challenged by the rust pathogen was established (Fernandez et al., 2004; Santos et al., 2004). Two subtractive cDNA libraries were constructed and Expressed Sequence Tags (ESTs) were generated by random sequencing of cDNA clones. Library 1 contained subtracted cDNAs obtained from coffee leaves inoculated with the rust fungus for 12 h. Library 2 contained subtracted cDNAs derived from a pool of mRNAs obtained from coffee leaves collected 24 and 48 h post-inoculation (Fernandez et al., 2004). Genes associated with expression of early resistance mechanisms of coffee plants to parasites were isolated from the two cDNA libraries (Fernandez et al., 2004) (figure 1L). At least 13 % of the ESTs may represent genes involved in plant defence reactions (disease resistance proteins, stress- and defence-proteins, components of resistance signal pathways), 13 % in cell signalling processes (ionic channels, MAP kinases, receptor kinases), and 13 % in gene regulation (transcription factors, proteasome machinery). The highest proportion of cDNAs (34 %) were homologous to plant genes of unknown function and might be an additional source of genes participating in the expression of coffee defence responses to parasites. Although far from being exhaustive, the ESTs isolated may provide a significant set of data for improving our knowledge of coffee resistance to pathogens. A lot of the genes isolated showed homology to known plant genes suggesting conservation of signalling pathways and resistance mechanisms against pathogens in C. arabica and other plants.
When comparing libraries, quantitatively more ESTs were assigned to the cell/organism defence category in library 2 (15 %) than in library 1 (10 %). On the other hand, more ESTs were related to the general metabolism category in library 1 (18 %) than in library 2 (12 %). Evolution of the percentage of ESTs in these categories during infection may reflect reorganization of the plant host metabolism in order to fight off pathogen attack. It has been recently shown that a shift from housekeeping to pathogen defence metabolism in Arabidopsis thaliana correlated with induction of HR to Pseudomonas syringae (Scheideler et al., 2002). In addition to the quantitative difference, we also observed qualitative differences in the cell/organism defence category between the two libraries. As an example, ESTs with similarity to known R proteins (Hammond-Kosack and Jones, 1997) were only found in library 2.
3.1.3.5.2. Coffee gene expression in biotic and abiotic stresses
Coffee molecular responses to rust infection and to abiotic treatments were examined by semi-quantitative RT-PCR (table 1). A set of candidate genes were chosen on the basis of their homology to known plant genes involved in resistance to parasites, and on the basis of a differential screening of the cDNA clones of the two libraries (Fernandez et al., 2004). Among them were genes putatively encoding disease resistance signalling proteins, stress response proteins, disease resistance and defence proteins, transcription factors, as well as gene coding proteins of unknown function (table 1). Transcript accumulation was examined during both compatible and incompatible rust interactions, and upon salicylic acid (SA) treatment (500 µM) or wounding (leaf cuttings). A lot of genes clearly showed enhanced transcript accumulation in rust inoculated plants (compatible or incompatible interaction), over the time-course experiment as compared with control plants (mockinoculated). In addition, 9 genes also showed transient enhanced transcript accumulation during the incompatible interaction (HR) when comparing with the compatible interaction (Fernandez et al., 2004). Gene expression patterns upon abiotic treatments showed that about half of the genes examined also responded to SA or to wounding.
Based on sequence similarity to known genes, 4 out of the 9 HR-upregulated sequences putatively encoded stress-related proteins or components of disease resistance signalling pathways (table 1). Two HR-upregulated sequences matched cytochromes P450 and HSP70. Cytochrome P450 genes may be involved in the biosynthesis of defence-related compounds, such as the Arabidopsis PAD3 gene required for camalexin synthesis in the resistance response to Alternaria brassicicola (Zhou et al., 1999). Recently, Kanzaki et al. (2003) showed that members of the HSP70 and 90 families were essential components of the plant defence signal transduction pathway. With regard to HR-upregulated sequences encoding resistance-signalling components, two coffee sequences best matched the A. thaliana DND1 and NDR1 proteins. The DND1 (defence, no death) protein is a cyclic nucleotide-gated ion channel (AtCNGC2) involved in the HR signalling pathway to P. syringae (Clough et al., 2000). The NDR1 (non race-specific disease resistance) protein is a key component of the signalling pathway of many CC-NBS-LRR resistance proteins (Century et al., 1997). Isolation of ESTs displaying similar expression patterns and sequence homology to DND1 and NDR1 suggest they might have a potential role in the resistance of C. arabica to H. vastatrix and indicate the conservation of some components of R-gene-mediated resistance signalling pathways in coffee plants.
Finally, 4 other HR-upregulated cDNA clones were of potential interest regarding defence mechanisms (table 1). One EST putatively encoded a receptor-like kinase. This class of signal proteins is involved in a diverse array of developmental and defence functions (Du and Chen, 2000; Morris and Walker, 2003). Another gene best matched an UDP-glucose: salicylic acid glucosyltransferase. Glucosyltransferases catalyze the transfer of glucose residues to numerous substrates and regulate the activity of compounds that play important roles in plant defence against pathogens, such as salicylic acid (Chong et al., 2002). Two ESTs putatively encoded an AP2-type transcription factor and a WRKY transcription factor. A number of gain-of-function studies have shown the direct implication of several transcription factors in potentiating the plant responses to pathogen infection. Particularly involved are several WRKY proteins which are implicated in the regulation of several biological processes, including pathogen defence (Dong et al., 2003; Ülker and Somssich, 2004).
3.1.3.5.3. Time-course of gene induction during coffee rust infection
Real-time quantitative RT-PCR experiments were conducted to quantify gene expression levels during coffee rust infection. Three candidate genes were selected: CaNDR1 (EST CO773976), CaWRKY1 (CO773974) and EST CaR111 had homology to a protein of unknown function, and may be a novel and interesting component of plant disease resistance. The Ubiquitin gene chosen as internal reference of gene expression was assayed in parallel with the candidate genes. The relative changes in gene expression estimated using the 2 -DDCt method (Livak and Schmittgen, 2001) are presented in Figure 1M.
For the 3 candidate genes tested, enhancement of the mRNA levels was observed at early times of pathogen infection (12-20 hpi). Analysis of time-course experiments showed that the 3 genes were transiently induced during the plant/fungus interaction. Statistically significant differences (P<0.05) in the relative expression were found between the compatible and incompatible interactions for CaR111 and CaWRKY1. CaR111 was induced by the avirulent H. vastatrix strain around 12-16 hpi but weakly activated by the virulent strain. Activation of the CaWRKY1 gene occurred during both interactions, but was higher in the incompatible samples around 16 hpi and higher in the compatible samples at 24 hpi. With regard to the CaNDR1 gene, only a poor induction (< 3-fold) was observed at 12-16 hpi and occurred in response to both virulent and avirulent pathogen (no statistically significant difference). The 3 analyzed genes returned to their basal expression level at 24 hpi for CaR111 and CaNDR1 (figure 1M), and 48 hpi for CaWRKY1 (data not shown).
Coffee gene induction that we observed around 12-18 hpi shows that recognition of the pathogen may occur soon after penetration of the fungus into the substomatal chamber. In several plant-rust interactions, host specific resistance responses are typically expressed concurrently with the formation of the first haustorium (Heath, 1997b; Mould et al., 2003). In the cowpea (Vigna unguiculata) / Uromyces vignae pathosystem, specific upregulation of cowpea genes in the resistant cultivar occurred soon after the penetration of the epidermal cell wall by the fungus (Mould et al., 2003). In the cereal rusts Puccinia graminis tritici and P. graminis avenae, activation of defencerelated genes was observed in barley (Rostoks et al., 2004) and in oat when haustorial mother cells (HMCs) were formed in almost all infection sites (Lin et al., 1998). In the coffeeH. vastatrix interaction, the haustorium stage may be reached by H. vastatrix between 24-48 hpi (Martins and Moraes 1996; Silva et al., 1999a, 2002). Cytological observations of resistant coffee leaves revealed that in many infection sites (stomata) the fungus had stopped its growth at a pre-haustorium stage (HMC) (Silva et al., 2002), suggesting that early host resistance responses may be expressed. The gene activation observed around 12-18 hpi may be part of the coffee resistance responses and may determine the outcome of the coffee-rust interaction.
The genes we isolated might therefore participate in the variety of expression of the plant defence responses to H. vastatrix. With the availability of high-density cDNA filters technology, the expression profiles of hundreds ESTs will be monitored simultaneously in several coffee-rust interactions to help determine the mechanisms of these biological processes. In addition, identification of candidate genes for disease resistance would allow allelicmining for exploiting coffee genetic resources and the development of molecular markers to assist coffee genetics programmes.
3.2. COFFEE BERRY DISEASE (CBD)
Three species of Colletotrichum have been isolated from coffee berries, leaves and branches: C. kahawae (the only parasitic species, originally designated as C. coffeanum), C. gloeosporioiedes Penz and C. acutatum Simmond (Hindorf, 1970; Masaba and Waller, 1992; Waller et al., 1993). Colletotrichum kahawae infect all stages of the crop from flowers to ripe fruits and occasionally leaves, but maximum crop losses occur following infection of green berries with the formation of dark sunken lesions with sporulation (acervuli) (figures 2A-B), causing their premature dropping and mummification. This disease, first reported in Kenya in1922 (McDonald, 1926) may cause up to 70-80 % of losses if no control measures are adopted (McDonald, 1926; Saccas and Charpentier, 1969; Kibani, 1982; Waller, 1985). Although CBD is so far restricted to Africa, in other coffee-growing areas precautions should be taken to prevent the introduction of this disease.
3.2.1. Fungal infection process
The pathogenesis of Colletotrichum diseases is varied, arising from the fundamental nutritional and ecological diversities within the genus (Latunde-Dada, 2001). The early stages of fungal development on the plant surface are essentially the same for all Colletotrichum species. Conidia adhere to the cuticle and germinate to produce germ tubes, forming thereafter appressoria which penetrate the cuticle directly (Perfect et al., 1999; O'Connell et al., 1996, 2000). Maturation of the appressorium involves formation of a penetration pore in the base of the cell, deposition of new wall layers, and secretion of extracellular matrix materials. Subsequently, melanin is deposited in a layer of the cell wall close to the plasma membrane (Bailey et al., 1992; O'Connell et al., 2000). Fungal penetration into the hosts may be based on mechanical pressure exerted by melanized appressoria, the secretion of cutin degrading enzymes, or by a combination of both processes (Bailey et al., 1992). The conidia of C. kahawae germinate and differentiate melanised appressoria both "in vitro" and "in vivo" (figure 2C) and the penetration of different coffee organs (hypocotyls, leaves and green berries) also occurs directly through the epidermal cell walls (Garcia et al., 1997; Garcia, 1999; Várzea et al., 2002b, Chen, 2002; Chen et al., 2003, 2004b). Chen (2002) and Chen et al. (2004b) concluded that the C. kahawae appressorium turgor pressure of about 44 bars was 4 times higher that the osmotic pressure of the coffee green berries. On the other hand, unmelanized appressoria induced by the use of the fungicide tricyclazole showed turgor pressures as low as one quarter as melanized ones and, as a consequence, the percentage of infection on coffee leaves and green berries was much lower. They also showed that cutinase was present in C. kahawae conidial mucilage and in extracellular fluids of germinated conidia in vitro and in vivo. The use of diisopropyl fluorophosphates, a cutinase inibitor, totally inhibited cutinase activity of culture filtrates and extracellular fluids but did not prevent infection. Based on these results they concluded that turgor pressure of C. kahawae appressoria might play a major role in coffee cuticle penetration.
Following penetration, Colletotrichum species use two main strategies to successfully colonise host tissues and avoid host defence responses: subcuticular intramural colonization and intracellular colonization (Benhamou et al., 1991; O'Connell and Bailey, 1991; Bailey et al., 1992; Pring et al., 1995; Latunde-Dada et al., 1996; O'Connell et al., 1996; Latunde-Dada et al., 1997; O'Connell et al., 2000; Latunde-Dada, 2001; Mendgen and Hahn, 2002; Diéguez-Uribeondo et al., 2005). For Colletotrichum species exhibiting a subcuticular intramural mode of infection, penetration is followed by growth of hyphae beneath the cuticle and within the periclinal and anticlinal walls of epidermal cells in a necrotropic manner, which involve the massive secretion of cell wall-degrading enzymes. Thus, these pathogens obtain the nutrients from the host cells that they have killed, being classified as necrotrophs (e.g. C. capsici). By contrast, most Colletotrichum species, including C. kahawae (Garcia et al., 1997; Garcia, 1999; Silva et al., 1999b, Várzea et al., 2002b) are hemibiotrophs, as they exhibit a transient post-penetrative asymptomatic biotrophy that is rapidly succeeded by a phase of destructive necrotrophy culminating in the appearance of symptoms of disease and pathogen reproduction. During the symptomless biotrophic phase, the pathogen invades host cells without killing them and feeds on living cells. Subsequently, the pathogen switches to a necrotrophic mode of nutrition, feeding on dead host tissues. The intracellular hemibiotrophic Colletotrichum species can be further subdivided in two groups: i) those in which the biotrophic phase extends to many host cells, as for example C. lindemuthianum on Phaseolus vulgaris and C kahawae on Coffee spp., and those in which the biotrophic phase occurs only inside the initially infected epidermal cell, for example C. destructivum on Medicago sativa. Few Colletotrichum species (mostly C. gloeosporioides and C. acutatum) are either mutualistic endophytes or quiescent endophytes which resort to necrotrophy after a spell of latency (Prusky and Plumbley, 1992; Latunde-Dada et al., 1999). Endophytic species often penetrate host leaves directly or through stomatal openings and then ramify the mesophyll intercellularly without development of symptoms. Quiescent species have a prolonged phase of penetrative growth arrested in synchrony with the physiological state of the infected organ.
According to Garcia et al. (1997), Garcia (1999), Silva et al. (1999b) and Várzea et al. (2002b), in green berries, hypocotyls and leaves of coffee infected by C. kahawae, after penetration of epidermal cell wall the infection peg swells to form a globose infection vesicle inside the cell lumen (figure 2D). The susceptibility involves the intra- and intercelullar ramification of the infection vesicle in the living host cells (figure 2D). This period where the fungus feeds on living cells (biotrophy) (Fig 2D) may last 48h or 72h after the inoculation, depending, respectively, on the greater or lesser degree of aggressiveness of the fungal isolates (Varzea et al., 1999). At this initial stage of the infection process no macroscopic symptoms are visible. Transmission electron microscope observations showed that the intracellular infection vesicles and hyphae remained external to the plant plasma membrane, which became invaginated around the fungus. The biotrophy was followed by necrotrophic growth. In the latter, the fungus colonization was associated with severe cell wall alterations and death of the host protoplast (Figs 2E-F). In some host cells the cytoplasm was markedly reduced, and appeared often as fine strands in which membranous debris were the only recognizable features of pre-existing organelles. The biotrophic phase was repeated as the fungus started the colonisation of new host cells. Consequently, it was possible to observe hyphal growth simultaneously in dead and living host cells. As shown by light and electron microscopic observations (Garcia et al., 1997; Garcia, 1999 and Várzea et al., 2002b), during the necrotrophic phase of susceptible hypocotyls, green fruits and leaves, callose was present, but only around some intracellular hyphae (figures 2G-H) of epidermal and cortex cells. This host response in the susceptible tissues seemed to occur too late to prevent fungal growth and sporulation (Garcia et al., 1997; Garcia, 1999 and Várzea et al., 2002b).
An evaluation of the activity of several cell wall-degrading enzymes (polygalacturonases, polymetylgalacturonase, pectate lyase, pectin lyase, carboxymethylcellulase) was carried out in extracts of susceptible green berries inoculated with C. kahawae (Chen, 2002; Chen et al., 2004a). The activity of pectate lyase, measurable on the 3rd day after inoculation (with the fungus in the biotrophic phase) almost doubled by the 5th day (when necrotic lesions were already observed) and reached the maximum at the 9th day (the last day of the experiment). These results indicate that this enzyme may have a role in the pathogenicity of C. kahawae.
3.2.2. Fungal variability
Research carried out on CBD in Kenya has provided much valuable information. Many different coffee genotypes were tested with local C. kahawae isolates and differential pathogenicity was never observed in this country (van der Vossen et al., 1976). Moreover, differential interactions between host and pathogen population were seldom found in Ethiopia (van der Graaff, 1981). The latter author mentions that the positive effects were, however, small and it is improbable that they were caused by gene-for-gene specificity.
Many authors agree that the aggressiveness of the pathogen population shows variability between isolates from the same or different geographic origins (Firman and Waller, 1977; Omondi et al., 1997; Masaba and van der Vossen, 1978; van der Vossen, 1985; Manga et al., 1998; Manga, 1999; Varzea et al., 2002b, Varzea et al., 2001).
For the first time, Rodrigues et al. (1992) and Várzea et al. (1993) mention evidence for the existence of physiologic races of C. kahawae. Differential reactions on selections of Sarchimor and Catimor from CATIE were also found by Bieysse et al. (1995). In more exhaustive studies differences were only found in the aggressiveness in C. kahawae isolates (Manga et al., 1998). Omondi et al. (1997, 2000) working with local Kenyan isolates concluded that variation in pathogenicity among isolates of C. kahawae was predominantly due to aggressiveness, and differential pathogenicity, although small, was highly significant and could not be ignored. However they did not find positive evidence of physiologic races for C. kahawae. Bieysse et al. (unpublished data, INCO-project ICA4-CT-2001-10008), in a study with strains collected in East Africa and Central Africa, concluded that differences between strains were predominantly due to aggressiveness.
Molecular studies carried out in the population of C. kahawae from different geographic origins did not show the existence of polymorphism within this species (Beynon et al., 1995; Sreenivasaprasad and Mills, 1993; Manga et al., 1998; Loureiro et al., 2004, 2005). However, isoenzymatic characterisation using PAGE (non-denaturating electrophoresis in polyacrylamide gel) and IEF (isoelectric focusing) techniques showed polymorphism among the isolates studied (Loureiro et al., 2004; Loureiro et al., 2005). Recently, Bieysse et al. (unpublished data - INCO-project ICA4-CT-2001-10008) using microsatellite primers have analysed 140 strains collected from all the geographical zones (Cameroon, Kenya, Burundi, Tanzania, Rwanda, Zimbabwe, Malawi, Angola, Ethiopia). They concluded that the strains studied might be classified into two groups - East African and Cameroon. Each geographical population showed a strong homogeneity between the isolates suggesting a clonal multiplication of the pathogen.
3.2.3. Coffee resistance to CBD
3.2.3.1. Inheritance of resistance
The genetic resistance in coffee appeared complete in C. canephora and partial in C. Arabica. For Arabica, studies carried out in Kenya by van der Vossen & Walyaro (1980) concluded that coffee resistance to CBD appears to be controlled by major genes on three different loci. The highly resistant variety Rume Sudan carries the dominant R- and the recessive K-genes. The variety Pretoria also has the K-gene. The moderately resistant variety K7 carries only the recessive K-gene. Hibrido de Timor carries one gene for CBD resistance on the T-locus with intermediate gene action. Robinson (1974) and van der Graaff (1981, 1985) suggested that CBD resistance is horizontal/quantitative. Ameha and Belachew (1982) mentioned that 3-5 recessive genes control resistance to CBD in non-introgressed Arabicas.
3.2.3.2. Coffee breeding for resistance
Differences in resistance of coffee trees to CBD are frequently observed under field and laboratory conditions. In Kenya, "Geisha 10" and "Blue Mountain" K7, Rume Sudan, and also some progenies of "Hibrido de Timor" (HDT) have more resistance than "Harar" and "Bourbon". The high levels of resistance were found in Rume Sudan (Rayner, 1952; Nutman and Roberts, 1960; Firman, 1964; Gibs, 1968; Firman and Waller, 1977; van der Vossen, 1985; van der Vossen et al., 1976; van der Vossen and Walyaro, 1980). The rapid outbreaks of CBD in Eastern Africa prompted some countries to carry out hybridisation programmes with the objective of combining yield with resistance to CBD and coffee leaf rust (CLR) as in Kenya and Tanzania. In 1989, a new research line aimed at supporting breeding programmes on CBD resistance in different coffee- growing countries was initiated at CIFC. Thousand of progenies of different coffee genotypes were tested against CBD isolates from different geographic origins. No coffee genotypes showed 100 % resistance to all the isolates used in the hypocotyl pre-screening tests. However some lines of Rume Sudan showed high levels of resistance to the majority of the studied isolates as well as some derivatives of HDT CIFC 1343. Intermediate levels of resistance can also be found in some derivatives of interspecific tetraploid hybrids from different origins.
In the coffee-growing countries, among progenitors for disease resistance were Hibrido de Timor, Rume Sudan, Kaffa and Geisha which were crossed to varieties SL 28, SL 34, N 39, KP 423 and H 66 (Walyaro, 1983; van der Vossen and Walyaro, 1980, 1981). In Zambia, a pure line, likely derived from the Colombia variety, exhibited a high level of resistance compared with Caturra or Catuai. With these latter varieties, 80 % of crop losses were observed when chemical control was not applied, while losses corresponded only to 15-20 % for the pure line derived from the Colombia variety (C. Hemmings, personal communication). In Tanzania, 8 new clones with high levels of resistance have been selected and multiplied vegetatively by somatic embryogenesis (Teri et al., 2004). In Kenya, the complex hybrid Ruiru 11 revealed a good level of resistance. In Ethiopia (based on 'the coffee land races programme' developed by the EARO Institute) results were obtained very quickly and around twenty pure coffee lines displaying good tolerance to CBD, under conditions of strong parasite pressure, were selected and distributed to farmers from 1978 onwards throughout the coffee-growing areas in the country.
3.2.3.3. Cytological and biochemical resistance mechanisms
In Arabica coffees resistance mechanisms to C. kahawae are both preformed and induced, and operate at different stages of pathogenesis (Gichuru, 1997). The coffee berry cuticle could act as a physical barrier to the penetrating pathogen. Nutman and Roberts (1960) among others observed that removal of the epidermis of green coffee berries, in comparison with unwounded ones, transformed resistant varieties, such as Blue Mountain and Kent, as susceptible as the var. Harar. Wounding allows direct leaching of nutrients to the infection peg and also the direct access of the fungus to the internal tissues (Nutman ad Roberts, 1960; Gichuru, 1997).
Several investigations on the occurrence and possible role in CBD resistance of preformed antifungal compounds in the cuticle have been carried out, although the chemical nature of these compounds was not identified. Nutman and Roberts (1960) found that extracts from the resistant variety Blue Mountain had a stimulatory effect on the infection process in contrast to what happened with the susceptible variety Harar. However, Steiner (1972) concluded that surface wax extracted with chloroform from green berries of Rume Sudan and Blue Mountain contain substances that significantly decreased conidial germination and may contribute towards the high levels of field resistance to C. kahawae shown by these varieties. Lampard and Carter (1973) also reported the presence of antifungal compounds in the cuticular wax layers of green berries of coffee and they found a correlation between the degree of activity of cuticular wax extracts from many cultivars of arabica coffee and their field resistance to C. kahawae. However, the activities obtained in cuticle extracts from other cultivars did not correlate well with their reported susceptibility. Masaba and Helderman (1985) also found that hypocotyls of Rume Sudan and SL 28 formed compounds that inhibited the conidia germination and the mycelial growth of C. kahawae, 2 days after the inoculation.
According to Masaba and van der Vossen (1982), the resistance to CBD in Arabica coffee may, to a certain extent, be based on the formation of cork barriers. Phellogen was rapidly formed in some cells below the infection site and the progress of the fungal invasion was blocked by a complete barrier of suberised cells. These cork barriers corresponded macroscopically to the scab lesion, the common macroscopic expression of resistance to CBD (Gichuru, 1997). Such a resistance mechanism is likely to be stable (race-nonspecific) and depends apparently on actively metabolizing plant tissues (Masaba and van der Vossen, 1982; Masaba and Waller, 1992). In fact, cork barrier formation was observed in both attached berries and hypocotyls but not in detached berries, which quickly lost their ability to respond to C. kahawae infection. The formation of a cork barrier is temperature dependent (Masaba, 1982). Differences in cork barrier formation in resistant and susceptible varieties were greatest between 19ºC and 22ºC. On the other hand, temperatures below 16ºC and over 28ºC depressed barrier formation.
Biochemical studies showed a significant increase in proteolytic enzymes and in peroxidase activity in coffee varieties after inoculation with C. kahawae (Gichuru, 1993; Gichuru et al., 1997). Peroxidase activity of extracts obtained from non-inoculated hypocotyls and detached and attached berries did not reveal differences between the resistant and susceptible varieties, but the highest increases in enzyme activity were associated with susceptibility rather than with resistance (Gichuru et al., 1997).
Histological studies made with resistant hypocotyls of Hibrido de Timor (HDT) derivatives, in comparison with those of the susceptible cultivar Caturra revealed no differences in conidia germination and in differentiation of melanised appressoria, but the hyphal length inside the host tissues was significantly lower in the resistant genotypes, from 41h after inoculation. (Silva et al., 1999b; Várzea et al., 2002b). In the resistant coffee genotypes the fungal hyphae were confined to epidermal cells or to the first layers of the cortex cells. At these sites, signs of incompatibility were observed: (i) hypersensitive host cell death (monitored by cell autofluorescence and/or browning); (ii) modifications in the cell walls (thickness and autofluorescence) and (iii) early accumulation of phenolic compounds (indicated by the epifluorescence test) (figures 2I-K). These host responses corresponded macroscopically to the scab lesion. The same pattern of responses was observed in resistant hypocotyls of C. racemosa (Silva et al., unpublished data), as well as in C. canephora, in which no symptoms were detected in the majority (>90 %) of the hypocotyls tested (Loureiro et al., unpublished data).
The rapid hypersensitive death of host infected cells is typical of incompatible interactions of C. lindemuthianum with P.vulgaris and Vigna unguiculata, C. trifolii with Medicago sativa and Medicago truncatula and, some interactions involving C. gloeosporioides and Stylosanthes spp. (O'Connell and Bailey, 1986; Trevorrow et al., 1988; Bailey et al., 1990; Mould et al., 1991; Torregrosa et al., 2004). In the bean anthracnose pathosystem, this early HR does not occur in the compatible interactions, thereby indicating a high correlation between the HR and the race-cultivar specificity (Esquerré-Tugayé et al., 1992). The host cell wall modifications, namely the increase in the levels of hydroxyproline-rich glycoproteins (HRGPs), as well as the early accumulation of phenolic compounds in the infected host cells have also been associated with host resistance to Colletotrichum spp. (Esquerré-Tugayé et al., 1992; Skipp et al., 1995; Torregrosa et al., 2004). In the M. truncatula - C. trifolli incompatible interaction, one of the earlier phenolic compounds accumulated is the phytoalexin medicarpin, which is synthesized through the mobilization of several enzymes of the phenylpropanoid pathway. Several genes of this pathway were induced in the resistant varieties before the maximum level of autoflourescence (indicative of the accumulation of phenolic compounds) (Torregrosa et al., 2004). The identification of the phenolic compounds involved in the coffee - C. kahawae interaction is currently under study and, preliminary results revealed an accumulation of flavonoides and hydroxycinnamic acid derivatives at infection sites. These compounds were detected earlier in resistant and partially resistant genotypes than in susceptible ones.
In resistant hypocotyls of certain HDT derivatives, another early host response detected was the deposition of callose around intracellular hyphae of epidermal cells (figure 2L) and sometimes also of cortex cells, as indicated by the aniline blue test. What is frequently reported is the formation of wall appositions (papillae), deposited between the plasma membrane and outer wall of epidermal cells, at sites of attempted penetration by Colletotrichum spp.. Papillae produced in response to Colletotrichum spp. are described in living cells of both resistant and susceptible hosts as being largely composed by callose, HRGPs and phenolics (O'Connell and Bailey, 1986; Skipp et al., 1995). In the coffee - C. kahawae compatible and incompatible interactions cytochemical tests will be performed to identify the nature of the compounds involved in the interface between the host and the pathogen.
3.3. CONCLUSIONS
In the last decades, considerable success has been attained in the use of classical breeding for resistance to control economically important plant diseases, such as coffee leaf rust and CBD. The discovery of "Hibrido de Timor" (HDT) with a good genetic background for rust and CBD resistance was a breakthrough in coffee research. However, in the last few years, some improved commercial varieties from HDT, and other interspecific tetraploid hybrids like Icatú, are gradually loosing their resistance to rust in some countries, due to the increase of H. vastatrix virulence. This shows the importance of re-evaluating existing varieties with the new rust races and the care needed before the release of new ones. Priority must also be given to the identification of sources of more durable resistance.
It has long been recognized that more effective durable forms of disease control might be devised if we had a better knowledge of both the dynamics of the pathogen populations and the factors that determine host resistance or susceptibility. In bringing different research approaches together (genetics, molecular biology, cytology and biochemistry), important advances have been made recently in coffee resistance, particularly against leaf rust. In the future, significant contributions can be expected to improve the durability of coffee resistance to both diseases, that take into account the environmental needs and the development of a sustainable coffee economy.
Várzea VMP, Rodrigues Jr. CJ, Marques DV, Silva MC (2004) International survey of coffee leaf pathotypes. Evolution of virulence in H. vastatrix detected in resistant coffee varieties. In: Proceedings of the 20th International Conference on Coffee Science (ASIC), Bangalore, India. (in press).
- Adejumo TO (2005) Crop protection strategies for major diseases of cocoa, coffee and cashew in Nigeria. Afric. J. Biotechnol. 4:143-150.
- Agrios GN (1997) Plant Pathology. 4th Ed, Academic Press, San Diego, London, Boston, New York, Sydney, Tokyo, Toronto.
- Alvarado GA (2005) Evolution of Hemileia vastatrix virulence in Colombia. In: Zambolim L, Zambolim EM, Várzea VMP (eds), Durable Resistance to Coffee Leaf Rust, pp.99-115. Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brasil.
- Alvarez ME (2000) Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant. Mol. Biol. 44:429-442.
- Amaral JF, Teixeira C, Pinheiro ED (1956) A bactéria causadora da mancha aureolada do cafeeiro. Arq. Inst. Biol. 23:151.
- Ameha A, Belachew B (1982) Resistance of the F1 to CBD in six-parent diallel crosses in coffee. In: First regional workshop on coffee berry disease. Addis Ababa, Ethiopia, pp.167-177.
- Azinheira HG (2005) Estudos celulares bioquímicos e moleculares da diferenciação de Hemileia vastatrix Universidade Técnica de Lisboa (Instituto Superior de Agronomia). PhD Thesis.
- Azinheira HG, Silva MC, Guerra-Guimarães L, Mendgen K, Rodrigues Jr. CJ, Ricardo CP (2001) Development of infection structures of Hemileia vastatrix on artificial membranes. In: Proceedings of the 11th Congress of the Mediterranean Phytopathological Union and 3rd Congress of the Sociedade Portuguesa de Fitopatologia, Évora, Portugal, pp.353-355.
- Bailey JA, Nash C, O'Connell RJ, Skipp RA (1990) Infection process and host specificity of a Colletotrichum species causing anthracnose disease of cowpea Vigna unguiculata Mycol. Res. 94:810 -814.
- Bailey JA, O'Connell RJ, Pring RJ, Nash C (1992) Infection strategies of Colletotrichum species. In: Bailey JA, Jeger MJ (eds), Colletotrichum: Biology, Pathology and Control, pp.88-120. Wallingford UK: CAB International.
- Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33:299-321.
- Benac R (1981) La rouille commune du caféier (Coffea sp.). Étude du champignon Hemileia vastatrix Berk & Br. dans les tissues foliaires. Caryol. 34:141-177.
- Benhamou N, Lafitte C, Barthe J-P, Esquerré-Tugayé M-T (1991) Cell surface interactions between bean leaf cells and Colletotrichum lindemuthianum Plant Physiol. 97:234-244.
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv. phaseolicola. Plant Cell 9:209-221.
- Bettencourt AJ (1981) Melhoramento genético do cafeeiro. Transferência de factores de resistência à Hemileia vastatrix Berk & Br. para as principais cultivares de Coffea arabica L. Centro de Investigação das Ferrugens do Cafeeiro (CIFC/IICT), Lisboa.
- Bettencourt AJ (1983) Características agronómicas de selecções derivadas de cruzamentos entre Híbrido de Timor e as variedades Caturra, Villa Sarchi e Catuaí. In: Simpósio sobre Ferrugens do Cafeeiro, Oeiras, Portugal, pp.353-373.
- Bettencourt AJ, Rodrigues Jr. CJ (1988) Principles and practice of coffee breeding for resistance to rust and other diseases. In: Clarke RJ, Macrae R (eds), Coffee Agronomy Vol. 4, pp.199-234. Elsevier Applied Science Publishers LTD, London and New York.
- Bettencourt AJ, Rodrigues Jr. CJ, Lopes J (1965) Study of the physiologic specialization of the coffee rust H. vastatrix and selection of coffee clones for the establishment of a standard range of differential hosts for this rust. In: Progress report 1960-65, pp.28-46, Coffee Rusts Research Center, Oeiras, Portugal.
- Beynon SM, Coddington A, Lewis BG, Várzea VMP (1995) Genetic variation in the coffee berry disease pathogen, Colletotrichum kahawae Physiol. Mol. Plant Pathol. 46:457-470.
- Bieysse D, Bompard E, Manga B, Roussel V, Vergnes C (1995) Diversité génétique et variabilité du pouvoir pathogène chez Colletotrichum kahawae, agent de lanthracnose des baies de Coffea arabica In: Proceedings of the 16 th International Conference on Coffee Science (ASIC), Kyoto, Japan, pp.745-749.
- Bogdanove AJ (2002) Protein-protein interactions in pathogen recognition by plants. Plant. Mol. Biol. 50:981-989.
- Bowell GP (1999) Role of active oxygen species an NO in plant defense responses. Curr. Opin. Plant Biol. 2:287-294.
- Bradley DJ, Kjellbom P, Lamb CJ (1992) Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70:21-30.
- Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana Plant J. 34:351-362.
- Century KS, Shapiro AD, Repetti PP, Dahlbeck D, Holub E, Staskawicz BJ (1997) NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278:1963-1965.
- Chagas CM, Kitajima EW, Rodrigues JC (2003) Coffee ringspot virus vectored by Brevipalpus phoenicis (Acari: Tenuipalpidae) in coffee. Exp. Appl. Acarol. 30:203-213.
- Chen Z (2002) Morphocultural and Pathogenic Comparisons between Colletotrichum kahawae and Colletotrichum gloeosporioides isolated from coffee berries. Universidade Técnica de Lisboa, Instituto Superior de Agronomia, PhD thesis.
- Chen Z, Guerra-Guimarães L, Rodrigues Jr. CJ (2004a) Pectate lyase inhibitors may condition non-pathogenicity of Colletotrichum gloeosporioides to green coffee berries. In: Actas do 4ş Congresso da Sociedade Portuguesa de Fitopatologia. Universidade do Algarve, Faro, Portugal, pp.111-115.
- Chen Z, Nunes MA, Silva MC, Rodrigues Jr. CJ (2004b) Apressorium turgor pressure of Colletotrichum kahawae may have a role in coffee cuticle penetration. Mycologia 96:11991208.
- Chen ZJ, Ribeiro A, Silva MC, Santos P, Guerra-Guimarães L, Gouveia M, Fernandez D, Rodrigues Jr. CJ (2003) Heat shock-induced susceptibility of green coffee leaves and berries to Colletotrichum gloeosporioides and its association to PR and hsp70 gene expression. Physiol. Mol. Plant Pathol. 63:181-190.
- Chittoor JM, Leach JE, White FF (1999) Induction of peroxidase during defense against pathogens. In: Datta SK, Muthukrishnan S (eds), Pathogenesis-Related Proteins in Plants, pp.171-193. CRC Press, Boca Raton, London, New York, Washington DC.
- Chong J, Baltz R, Schmitt C, Beffa R, Fritig B, Saindrenan P (2002) Down regulation of a pathogen-responsive tobacco UDP-Glc: phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 14:1093-107.
- Christensen AB, Cho BH, Naesby M, Gregersen PL, Brandt J, Madriz-Ordeñana K, Collinge DB, Thordal-Christensen H (2002) The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 3:135-144.
- Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Bent AF (2000) The Arabidopsis dnd1 "defense, no death" gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Nat. Acad. Sci. USA 97:9323-8-9323-8.
- Cohen Y, Eyal H, Hanania J (1990) Ultrastructure, autofluorescence, callose deposition and lignification in susceptible and resistant muskmelon leaves infected with the powdery mildew fungus Sphaerotheca fuliginea Physiol. Mol. Plant Pathol. 36:191-204.
- Collins TJ, Moerschbacher BM, Read ND (2001) Synergistic induction of wheat stem rust appressoria by chemical and topographical signals. Physiol. Mol. Plant Pathol. 58:259-266.
- Costet L, Cordelier S, Dorey S, Baillieul F, Fritig B, Kauffmann S (1999) Relationship between localized acquired resistance (LAR) and the hypersensitive response (HR): HR is necessary for LAR to occur and salicylic acid is not sufficient to trigger LAR. Mol. Plant-Microbe Interact. 12:655-662.
- Coutinho TA, Rijkenberg FHJ, Van Asch MAJ (1993) Development of infection structures by Hemileia vastatrix in resistant and susceptible selections of Coffea and in Phaseolus vulgaris Can. J. Bot. 71:1001-1008.
- Croft KPC, Juttner F, Slusarenko AJ (1993) Volatile products of the lipoxygenase pathway evolved from Phaseolus vulgaris L. leaves inoculated with Pseudomonas syringae pv. phaseolicola Plant Physiol. 101:13-24.
- D'Oliveira B (1954-1957) As ferrugens do cafeeiro. Ver. Café Português 1(4):5-13; 2(5):5-12; 2(6):5-15; 2(7):9-18; 2(8):5-22; 4(16):5-15.
- D'Oliveira B (1965) Introduction. Progress Report 1960-65, pp.1-20. Coffee Rusts Research Center, Oeiras, Portugal
- D'Oliveira B, Rodrigues Jr. CJ (1961) O problema das ferrugens do cafeeiro. Rev. Café Português 8:5-50.
- Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826-833.
- De Lima JEO, Miranda VS, Hartung JS, Brlansky RH, Coutinho A, Roberto SR, Carlos EF (1998) Coffee leaf scorch bacterium: Axenic culture, pathogenicity, and comparison with Xylella fastisdiosa of citrus. Plant Dis. 82:94-97.
- De Wit PJGM (1997) Pathogen avirulence and plant resistance: a key role for recognition. Trends Plant Sci. 2:452-458.
- Delledonne M (2005) NO news is good news for plants. Curr. Opin. Plant Biol. 8:390-396.
- Diéguez-Uribeondo J, Forster H, Soto-Estrada A, Adaskaveg JE (2005) Subcuticular-intracellular hemibiotrophic and intercellular necrotrophic development of Colletotrichum acutatum on almond. Phytopathology 95:751-758.
- Deising H, Heiller S, Rauscher M, Xu H, Mendgen K (1996) Cellular aspects of rust infection structure differentiation. In: Nicole M, Gianinazzi-Pearson V (eds), Histology, Ultrastructure and Molecular Cytology of Plant-Microorganism Interaction, pp.135-156. Kluwer Academic Publishers, The Netherlands.
- Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MSS, Wang L (2002) The phenylpropanoid pathway and plant defence a genomics perspective. Mol. Plant Pathol. 3:371-390.
- Dixon RA, Lamb CJ (1990) Molecular communication in interactions between plant and microbial pathogens. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41:339-367.
- Do HM, Hong JK, Jung HW, Kim SH, Ham JH, Hwang BK (2003) Expression of peroxidase-like genes, H2O2 production, and peroxidase activity during the hypersensitive response to Xanthomonas campestris pv. vesicatoria and Capsicum annuum Mol. Plant-Microbe Interact. 16:196-205.
- Dong J, Chen C, Chen Z (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 51:21-37.
- Dorey S, Baillieul F, Pierrel MA, Saindrenan P, Fritig B, Kauffmann S (1997) Spatial and temporal induction of cell death, defense genes, and accumulation of salicylic acid in tobacco leaves reacting hypersensitively to a fungal glycoprotein elicitor. Mol. Plant-Microbe Interact. 10:646-655.
- Du L, Chen Z (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis Plant J. 24:837-847.
- Ebel J, Scheel D (1997) Signals in host-parasite interactions. In: Carrol GC, Tudzynski P (eds), The Mycota, Vol. V Part A, pp. 85-105. Springer-Verlag, Berlin, Heidelberg, New York.
- Eskes AB (1983) Incomplete resistance to coffee leaf rust Hemileia vastatrix University of Wageningen. PhD thesis.
- Esquerré-Tugayé M-T, Mazau D, Barthe J-P, Lafitte, Touzé A (1992) Mechanisms of resistance to Colletotrichum species. In: Bailey JA, Jeger MJ (eds), Colletotrichum: Biology, Pathology and Control, pp.121-133. Wallingford UK: CAB International.
- Fagioli SLG, Berry D, Bieysse D (1990) Recherche sur la résistance incomplète à Hemileia vastatrix Berk. et Br. dans un groupe de génotypes de Coffea arabica L. d'origine Éthiopienne. Café Cacao Thé XXXIV:105-133.
- Fernandez D, Santos P, Agostini C, Bon M-C, Petitot A-S, Silva MC, Guerra-Guimaraes L, Ribeiro A, Argout X, Nicole M (2004) Coffee (Coffea arabica L.) genes early expressed during infection by the rust fungus (Hemileia vastatrix). Mol. Plant Pathol. 5:527-536.
- Firman ID (1964) Screening of coffee for resistance to coffee berry disease. East Afr. Agric. For. J. 29:192-194.
- Firman ID, Waller JM (1977) Coffee Berry Disease and other Colletotrichum diseases of coffee. Phytopathological papers nş20. Commonwealth Mycological Institute, Kew.
- Flor HH (1942) Inheritance of pathogenicity in Melampsora lini Phytopathology 32:653-669.
- Fritig B, Heitz T, Legrand M (1998) Antimicrobial proteins in induced plant defense. Curr. Opin. Immunol. 10:16-22.
- Garcia I (1999) Histologia e ultrastrutura do processo de infecção de Colletotrichum kahawae e Colletotrichum gloeosporioides em Coffea arabica Universidade Técnica de Lisboa, Instituto Superior de Agronomia. MSc Thesis.
- Garcia I, Silva MC, Rijo L, Rodrigues Jr. CJ (1997) Histology and Fine Structure of the infection process of Colletotrichum kahawae and Colletotrichum gloeosporioides in Coffea arabica Resumo da XXXII Reunião Anual da Sociedade Portuguesa de Microscopia Electrónica e Biologia Celular. Coimbra, Portugal, pp.45.
- Geiser DM, Ivey MLL, Hakiza G, Juba JH, Miller SA (2005) Gibberella xylarioides (anamorph: Fusarium xylarioides), a causative agent of coffee wilt disease in Africa, is a previously unrecognized member of the G. fujikuroi species complex. Mycologia 97:191-201.
- Gibs JN (1968) Annual report 1967-68, pp 44-46. Coffee Research Foundation. Kenya
- Gichuru EK (1993) Isozyme patterns and enzyme activities of Coffea arabica L. cultivars susceptible and resistant to coffee berry disease (Colletotrichum kahawae Waller and Bridge), University of Nairobi, Kenya. M.Sc. thesis.
- Gichuru EK (1997) Resistance mechanisms in Arabica coffee to coffee berry disease (Colletotrichum kahawae Sp. Nov.) A Review. Kenya Coffee 727:2441-2444.
- Gichuru EK, Mwang'ombe AW, Gupta VK (1997) Peroxidase activity in Coffea arabica cultivars resistant and susceptible to coffee berry disease. Afr. Crop. Sci. J. 2:223-232.
- Goodman RN, Novacky AJ (1994) The Hypersensitive Reaction in Plant to Pathogen: a resistance phenomenon. APS Press, St. Paul, Minnesota.
- Gouveia MMC, Ribeiro A, Várzea VMP, Rodrigues Jr. CJ (2005) Genetic diversity in Hemileia vastatrix based on RAPD markers. Mycologia 97:396-404.
- Grant M, Mansfield J (1999) Early events in host-pathogen interactions. Curr. Opin. Plant Biol. 2:312-319.
- Guerra-Guimarães L, Silva MC, Nicole M, Azinheira HG, Rodrigues Jr. CJ, Ricardo CP (2001) Chitinase activity associated with coffee-rust interaction. In: Proceedings of the 11th Congress of the Mediterranean Phytopathological Union and 3rd Congress of the Sociedade Portuguesa de Fitopatologia, Évora, Portugal, pp.356-358.
- Guerra-Guimarães L, Silva MC, Struck C, Loureiro A, Nicole M, Rodrigues Jr. CJ, Ricardo CP (2003) Chitinase activity in the intercellular fluid of coffee-rust interactions. In: 8th International Congress of Plant Pathology, Christchurch, New Zealand. Abstract, pp.199.
- Guest DI, Sutherland M, McDonald KL (1999) Sacrificial cell death in plants. Today's Life Sci. 22:50-53.
- Hammerschmidt R (1999) Induced disease resistance: how do induced plants stop pathogens? Physiol. Mol. Plant Pathol. 55:77-84.
- Hammerschmidt R, Nicholson RL (1999) A survey of plant defense responses to pathogens. In: Agrawal AA, Tuzun S, Bent E (eds) Induced Plant Defenses Against Pathogens and Herbivores, pp. 5571. APS Press, St. Paul, Minnesota.
- Hammond-Kosack KE, Jones JDG (1997) Plant disease resistance genes. Annu Rev Plant Physiol. Mol Biol 48:575-607.
- Harrison MJ, Baldwin IT (2004) Biotic interactions: Ploy and counter-ploy in the biotic interactions of plants. Curr. Opin. Plant Biol. 7:353-355.
- He ZH, He D, Kohorn B (1998) Requirement for the induced expression of a cell wall associated receptor kinase for survival during the pathogen response. Plant J. 14:55-63.
- Heath MC (1995) Signal exchange between higher plants and rust fungi. Can. J. Bot. 73 (Suppl. 1):S616-S623.
- Heath MC (1997a) Evolution of plant resistance and susceptibility to fungal parasites. In: Carrol GC, Tudzynski P (eds), The Mycota, Vol. V Part B, pp.257-276. Springer-Verlag, Berlin, Heidelberg, New York.
- Heath MC (1997b) Signalling between pathogenic rust fungi and resistant or susceptible host plants. Ann. Bot. 80:713-720.
- Heath MC (1998) Apoptosis, programmed cell death and the hypersensitive response. Eur. J. Plant Pathol. 104:117-124.
- Heath MC (1999) The enigmatic hypersensitive response: induction, execution, and role. Physiol. Mol. Plant Pathol. 55:1-3.
- Heath MC (2000a) Hypersensitive response-related death. Plant Mol. Biol. 44:321-334.
- Heath MC (2000b) Nonhost resistance and non-specific plant defenses. Curr. Opin. Plant Biol. 3:315-319.
- Heslop-Harrison J (1966) Cytoplasmic continuities during spore formation in flowering plants. Endeavour 25:65-72.
- Hindorf H (1970) Colletotrichum spp. isolated from Coffea arabica L. in Kenya. Z Planzenkr Pflanzenschutz 77:328-331.
- Holguin MF (1993) Contribution à la recherche d'une résistance durable du caféier (Coffea spp.) à la rouille orangée (Hemileia vastatrix Berk and Br.). Étude de la variabilité genétique du pathogène. Université Montpellier. PhD Thesis.
- Huang C, Groot T, Meijer-Dekens F, Niks RE, Lindhout P (1998) The resistance of powdery mildew (Oidium lycopersicum) in Lycopersicon species is mainly associated with hypersensitive response. Eur. J. Plant Pathol. 104:399-407.
- Jalloul A, Montillet JL, Assigbetsé K, Agnel JP, Delannoy E, Daniel JF, Marmey P, Geiger JP, Nicole M (2002) Lipid peroxidation in cotton Xanthomonas interactions. Role of lipoxygenases during the hypersensitive reaction and leaf blight. Plant J. 32:1-12.
- Johnson R (1981) Durable resistance: Definition of, genetic control, and attainment in plant breeding. Phytopathology 71:567-568.
- Jonak C, Okresz L, Bogre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 5:415-424.
- Kanzaki H, Saitoh H, Ito A, Fujisawa S, Kamoun S, Katou S, Yoshioka H, Terauchi R (2003) Cytosolic HSP90 and HSP70 are essential components of INF1-mediated hypersensitive response and non-host resistance to Pseudomonas cichorii in Nicotiana benthamiana Mol. Plant Pathol. 4:383-391.
- Keen NT (1999) Plant Disease Resistance: Progress in basic understanding and practical application. In: Callow JA (eds), Advances in Botanical Research, pp.291-328. Academic Press, San Diego, London, Boston, New York, Sydney, Tokyo and Toronto.
- Kibani THM (1982) The nature of the coffee berry disease in Tanzania. In: First Regional Workshop on Coffee Berry Disease, Addis Ababa, Ethiopia, pp.131-136.
- Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang SQ, Kachroo P, Trifa Y, Pontier D, Lam E, Silva H (2000) Nitric oxide and salicylic acid signalling in plant defence. Proc. Natl. Acad. Sci. USA 97:8849-8855.
- Koga H, Bushnell WR, Zeyen RJ (1990) Specificity of cell type and timing of events associated with papilla formation and the hypersensitive reaction in leaves of Hordeum vulgare attacked by Erysiphe graminis f.sp. hordei Can. J. Bot. 68:2344-2352.
- Kushalappa AC, Eskes AB (1989) Coffee Rust: Epidemiology, Resistance, and Management. CRC Press, Inc. Boca Raton, Florida.
- Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr. Opin. Plant Biol. 7:323-328.
- Lampard JF, Carter GA (1973) Chemical investigations on resistance to coffee berry disease in Coffea arabica An antifungal compound in coffee cuticular wax. Ann. Appl. Biol. 73:31-37.
- Latunde-Dada AO (2001) Colletotrichum: tales of forcible entry, stealth, transient confinement and breakout. Mol. Plant Pathol. 2:187-198.
- Latunde-Dada AO, Bailey JA, Lucas JA (1997) Infection process of Colletotrichum destructivum O'Gara from Lucerne (Medicago sativa L.). Eur. J. Plant Pathol. 103:35-41.
- Latunde-Dada AO, O'Connell RJ, Nash C, Lucas JA (1999) Stomatal penetration of cowpea (Vigna unguiculata) leaves by a Colletotrichum species causing latent anthracnose. Plant Pathol. 48:777-785.
- Latunde-Dada AO, O'Connell RJ, Nash C, Pring RJ, Lucas JA, Bailey JA (1996) Infection process and identity of the hemibiophic anthracnose fungus (Colletotrichum destructivum) from cowpea (Vigna unguiculata). Mycol. Res 100:1133-1141.
- Lawrence CB, Singh NP, Qiu J, Gardner RG, Tuzun S (2000) Constitutive hydrolytic enzymes are associated with polygenic resistance of tomato to Alternaria solani and may function as an elicitor release mechanism. Physiol. Mol. Plant Pathol. 57:211-220.
- Leguizamon JC (1983) Contribution à la connaissance de la resistance incompléte du cáfeier à Hemileia vastatrix Berk and Br. Université Montpellier. PhD Thesis.
- Leite Jr. RP, Mehta A, Carvalho FMS, Ueno B (1999) Genetic analysis of Brazilian strains of Xylella fastidiosa associated with citrus and coffee. In. Proceedings of III International Seminar on Biotechnology in the Coffee Agrindustry. Londrina, Brasil, pp.151-154.
- Li W-B, Pria WD, Teixeira DC, Miranda VS, Ayres AJ, Franco CF, Costa MG, He C-X, Hartung JS (2001) Coffee leaf scorch caused by a strain of Xylella fastidiosa from citrus. Plant Dis. 85:501-505.
- Lin KC, Bushnell WR, Smith AG, Szabo LJ (1998) Temporal accumulation patterns of defense response gene transcripts in relation to resistant reactions in oat inoculated with Puccinia graminis Physiol. Mol. Plant Pathol. 52:95-114.
- Lindhout P (2002) The perspectives of polygenic resistance in breeding for durable resistance. Euphytica 124:217-226.
- Littlefield LJ, Heath MC (1979) Ultrastructure of Rust Fungi. Academic Press, New York, San Francisco, London.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-DDC(T)) method. Methods 25:402-408.
- Loureiro A, Várzea VMP, Ribeiro A, Rodrigues Jr. CJ (2004) Caracterização morfocultural, patogénica, bioquímica e molecular da variabilidade de Colletotrichum kahawae. In: Actas do 4ş Congresso da Sociedade Portuguesa de Fitopatologia. Universidade do Algarve, Faro, Portugal, Abstract P2, pp.29.
- Loureiro A, Várzea VMP, Silva MC, Guerra-Guimarães L, Ribeiro A, Nicole M, Bertrand B (2005) Colletotrichum kahawae on coffee pathogen diversity and host resistance. In: Workshop Cryo Methods in Analytical and Immuno-Electron Microscopy, Lisboa, Portugal, Abstract.
- Manga B (1999) Etude de la diversité de Colletotrichum kahawae responsable de lanthracnose des baies et characterisation de la résistance du caféier arabica à cet agent pathogène. Université Montpellier. PhD Thesis.
- Manga B, Bieysse D, Mouen Bedimo JA, Akalay I, Bompard E, Berry D (1998) Observations sur la diversité de la population de Colletotrichum kahawae agent de lanthracnose des baies du caféier Arabica. Implications pour laméloration génétique. In: Proceedings of the 17th International Conference on Coffee Science (ASIC), Nairobi, Kenya, pp.604-612.
- Mansfield J, Bennett M, Bestwick C, Woods-Toer A (1997) Phenotypic expression of gene-for-gene interaction involving fungal and bacterial pathogens: variation from recognition to response. In: Crute IR, Holub EB, Burdon JJ (eds), The Gene-For-Gene Relationship in Plant-Parasite Interactions, pp.265-291. CAB International.
- Marques DV, Bettencourt AJ (1979) Resistência à H. vastatrix numa população de Icatú. Garcia de Orta (Série de Estudos Agronómicos) 6:19-24.
- Martins EMF, Moraes WBC (1996) Development of Hemileia vastatrix in coffee plants with genetic and induced resistance. J. Phytopathol. 144:519-526.
- Martins EMF, Tiburzy R, Moraes WBC (1985) Histological studies of compatible and incompatible interactions of coffee leaves and Hemileia vastatrix Fitopatol. Bras. 10:627-636.
- Masaba DM (1982) Mechanisms of resistance. Annual Report 1977/78, pp72. Coffee Research Foundation, Kenya.
- Masaba DM, Helderman K (1985) Mechanisms of resistance to CBD: Annual Report 1984/85, pp. 101, Coffee Research Foundation, Kenya.
- Masaba DM, Van der Vossen HAM (1978) Differential pathogenicity of isolates of the CBD pathogen. In: Annual Report 1977/78, pp.72. Coffee Research Foundation, Kenya.
- Masaba DM, Van der Vossen HAM. (1982) Evidence of cork barrier formation as a resistance mechanism to berry disease (Colletotrichum coffeanum) in arabica coffee. Neth. J. Plant Pathol. 88:19-32.
- Masaba DM, Waller JM (1992) Coffee berry disease: the current status. In: Bailey JA, Jeger MJ (eds), Colletotrichum: Biology, Pathology and Control, pp.237-249. Wallingford UK: CAB International.
- Mauch-Mani B, Slusarenko AJ (1996) Production of salicylic acid precursors is a major function of phenylalanine amonia lyase in the resistance of Arabidopsis to Peronospora parasitica Plant Cell 8:203-212.
- Maxemiuc-Naccache V, Braga MR, Dietrich SMC (1992) Chitinase and b-1,3-glucanase changes in compatible and incompatible combinations between coffee leaf disks and coffee rust (Hemileia vastatrix). Rev. Bras. Bot. 15:145-150.
- Maxemiuc-Naccache V, Dietrich SMC (1981) Cell wall composition of spores of Hemileia vastatrix (coffee rust). Rev. Microbiol. 12:61-64.
- Mayne WW (1932) Physiologic specialization of Hemileia vastatrix B. et Br. Nature 129:510.
- McDonald J (1926) A preliminary account of a disease of green coffee berries in Kenya Colony. Trans. Br. Mycol. Soc. 11:145-154.
- Mendgen K, Voegele RT (2005) Biology of rusts and mechanisms of infection. In: Zambolim L, Zambolim E, Várzea VMP (eds), Durable Resistance to Coffee Leave Rust, pp.233-248. Universidade Federal de Viçosa, Viçosa, Brasil.
- Mendgen K, Hahn M (2002) Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 7:352-356.
- Montillet JL, Chamnongpol S, Rustérucci C, Dat J, de Cotte B, Agnel JP, Battesti C, Inzé D, van Breusegem F, Triantaphylidès C (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant J. 138:1516-1526.
- Morris E, Walker J (2003) Receptor-like protein kinases, the keys to response. Curr. Opin. Plant Biol. 4:339-342.
- Mould MJ, Xu T, Barbara M, Iscove NN, Heath MC (2003) cDNAs generated from individual epidermal cells reveal that differential gene expression predicting subsequent resistance or susceptibility to rust fungal infection occurs prior to the fungus entering the cell lumen. Mol. Plant-Microbe Interact. 16:835-45.
- Mould MJR, Boland GJ, Robb J (1991) Ultrastructure of the Colletotrichum trifolii-Medicago sativa pathosystem. II. Post-penetration events. Physiol. Mol. Plant Pathol. 38:195 - 210.
- Nandris D, Kohler F, Fernandez D, Lashermes P, Rodrigues Jr. CJ, Pellegrini PF (1998) Coffee pathosystems modelling: 2. Assessment pathogen biodiversities. In: Proceedings of the 7th International Congress of Plant Pathology, Edinburgh, Scotland. Abstract 2.2.119.
- Neill S, Dasikan R, Hancock J (2002). Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 5:388-395.
- Niks RE, Rubiales D (2002) Potentially durable resistance mechanisms in plants specialised fungal pathogens. Euphytica 124:201-216.
- Noronha-Wagner H, Bettencourt AJ (1967) Genetic study of resistance of Coffea sp. to leaf rust. I. Identification and behaviour of four factors conditioning disease reaction in Coffea arabica to twelve physiologic races of Hemileia vastatrix Can. J. Bot. 45:2021-2031.
- Nutman JF, Roberts FM (1960) Investigations on a disease of Coffea arabica caused by a form of Colletotrichum coffeanum Noack. I. Some factors affecting infection by the pathogen. Trans. Br. Mycol. Soc. 43:489-505.
- O'Connell RJ, Bailey JA (1986) Cellular interactions between Phaseolus vulgaris and the hemibiotroph Colletotrichum lindemuthianum In: Bailey JSA (ed), Biology and Molecular Biology of Plant-Pathogen Interactions, pp.39-48. SpringerVerlag, Berlin.
- O'Connell RJ, Bailey JA (1991) Hemibiotrophy in Colletotrichum lindemuthianum In: Mendgen K, Lesemann D-E (eds), Electron Microscopy of Plant Pathogens, pp.211-222. Springer-Verlag, Berlin, Heidelberg.
- O'Connell RJ, Perfect S, Hughes B, Carzaniga R, Bailey J, Green J (2000) Dissecting the cell Biology of Colletotrichum Infection Processes. In: Prusky D, Freeman S, Dickman MB (eds), Colletotrichum Host specificity, Pathology, and Host-Pathogen Interaction, pp.57-77. The American Phytopathological Society, St. Paul, MN, USA.
- O'Connell RJ,Pain NA, Bailey JA, Mendgen K, Green JR (1996) Use of monoclonal antibodies to study differentiation of Colletotrichum infection structures. In: Nicole M, Gianinazzi-Pearson V (eds), Histology, Ultrastructure and Molecular Cytology of PlantMicroorganisms Interactions, pp.79-97. Kluwer Academic Publishers, Dordrecht, Boston, London.
- Omondi CO, Ayiecho PO, Myangombe AW, Hindorf H (2000) Reaction of some Coffee arabica genotypes to strains of Colletotrichum kahawae, the causal of Coffee Berry Disease. J. Phytopathol. 148:61-63.
- Omondi CO, Hindorf H, Welz HG, Saucke D, Ayiecho PO, Miwang'Ombe AW (1997) Genetic diversity among isolates of Colletotrichum kahawae causing coffee berry disease. In: Proceedings of 17th International Conference on Coffee Science (ASIC), Nairobi, Kenya, pp.800-803.
- Parlevliet JE (1983) Models explaining the specificity and durability of host resistance derived from the observations on the barley Puccinia hordei system. In: Lamberti F, Wallen JM, Van Graaff NA (eds), Durable Resistance in Crops, pp.57-80. Plenum Press, USA, NY.
- Parlevliet JE (2002) Durability of resistance against fungal, bacterial and viral pathogens; present situation. Euphytica 124:147-156.
- Peck SC (2003) Early phosphorylation events in biotic stress. Curr. Opin. Plant Biol. 6:334-338.
- Peng M, Kúc J (1992) Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 82:696-699.
- Perfect SE, Hughes HB, O'Connell RJ (1999) Colletotrichum: a model genus for studies on pathology and fungal-plant interactions. Fungal Genet. Biol. 27:186-198.
- Pilling E, Hofte H (2003) Feedback from the wall. Curr. Opin. Plant Biol. 6:611-616.
- Pink DAC (2002) Strategies using genes for non-durable disease resistance. Euphytica 124:227-236.
- Pontier D, Mittler R, Lam E (2002) Mechanism of cell death and disease resistance induction by transgenic expression of bacterio-opsin. Plant J. 30:499-509.
- Pring RJ, Nash C, Zakaria C, Bailey JA (1995) Infection process and host range of Colletotrichum capsici Physiol. Mol. Plant Pathol. 46:137-152.
- Prusky D, Plumbley RA (1992) Quiescent infections of Colletotrichum in Tropical and subtropical fruits. In: Bailey JA, Jeger MJ (eds), Colletotrichum: Biology, Pathology and Control, pp.289-307. Wallingford UK: CAB International.
- Rasmussen JB, Smith JA, Williams S, Burkhart W, Ward E, Sumerville SC, Ryals J, Hammerschmidt R (1995) cDNA cloning and systemic expression of acidic peroxidases associated with systemic acquired resistance to disease in cucumber. Physiol. Mol. Plant Pathol. 46:389-400.
- Rayner RW (1952) Coffee Berry Disease. A survey of investigations carried out up to 1950. East Afr. Agric. For. J. 17:130-158
- Richael C, Gilchrist D (1999) The hypersensitive response: a case of hold or fold. Physiol. Mol. Plant Pathol. 55:5-12.
- Rijkenberg FHJ, Truter SJ (1973) Haustoria and intracellular hyphae in the rusts. Phytopathology 63:281-286.
- Rijo L, Rodrigues Jr. CJ (1977) The infection process of Hemileia vastatrix in susceptible and resistant cultivars of Coffea arabica. In: Proceedings of the 8th International Conference on Coffee Science (ASIC), Abidjan, Ethiopia, pp.509-510.
- Rijo L, Rodrigues Jr. CJ, Silva MC, Vasconcelos MI (1991) Does gene SH5 confer to certain coffee-rust associations a reaction near immunity? A histopatological study. Café Cacao Thé XXXV:167-176.
- Rijo L, Sargent JA (1974) The fine structure of the coffee leaf rust, Hemileia vastatrix Can. J. Bot. 52:1363-1367.
- Rijo L, Silva MC, Vasconcelos MI (1990) Alguns aspectos morfológicos e citológicos do uredósporo e tubo germinativo de Hemileia vastatrix e da reacção de incompatibilidade da associação Coffea spp. - H. vastatrix Rev Ciências Agrárias XIII:169-178.
- Rijo L, Vasconcelos MI (1984) Formação de calose e lenhina em combinações incompatíveis Coffea spp. - H. vastatrix In: Simpósio sobre Ferrugens do Cafeeiro, Oeiras, Portugal, pp.269-279.
- Robinson RA (1974) Terminal report of the FAO coffee pathologist to the government of Ethiopia, FAO Rome, AGO/74/443
- Rodrigues Jr. CJ (1990) Coffee Rusts: history, taxonomy, morphology, distribution and host resistance. Fitopatol. Bras. 15:5-9.
- Rodrigues Jr. CJ, Várzea V, Silva MC, Guerra-Guimarães L, Rocheta M, Marques DV (2000) Recent advances on coffee leaf rust. In: Proceedings of the International Scientific Symposium on Coffee, Bangalore, India, pp.179-193.
- Rodrigues Jr CJ, Várzea VMP, Medeiros E (1992) Evidence for the resistance of physiological races of Colletotrichum coffeanum Noack sensu Hindorf. Kenya Coffee 57:1417-1420.
- Rodrigues Jr. CJ, Bettencourt AJ, Lopes J (1965) Study of the physiologic specialization of the coffee rust H. vastatrix and selection of coffee clones for the establishment of a standard range of differential hosts for this rust. In: Progress report 1960-65, pp.121-27. Coffee Rusts Research Center, Oeiras, Portugal.
- Rodrigues Jr. CJ, Bettencourt AJ, Rijo L (1975) Races of the pathogen and resistance to coffee rust. Annu. Rev. Phytopathol. 13:49-70.
- Rodrigues Jr. CJ, Várzea VMP, Godinho IL, Palma S, Rato RC (1993) New physiologic races of Hemileia vastatrix In: Proceedings of the 15th International Conference on Coffee Science (ASIC), Montpellier, France, pp 318-321.
- Rojas ML, Montes de Gómez V, Ocampo CA (1993) Stimulation of lipoxygenase activity in cotyledonary leaves of coffee reacting hypersensitively to the coffee leaf rust. Physiol. Mol. Plant Pathol. 43:209-219.
- Romeis T, Ludwig AA, Martin R, Jones JDG (2001) Calcium-dependent protein kinase plays an essencial role in the plant defense response. EMBO J 20:5556-5567.
- Rostoks N, Steffenson BJ, Kleinhofs A (2004) Structure and expression of the barley stem rust resistance gene Rpg1 messenger RNA. Physiol. Mol. Plant Pathol. 64:91-101.
- Ryan CA (1987) Oligosaccharide signalling in plants. Annu Rev Cellular Biol 3:295-317.
- Ryerson DE, Heath MC (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8:393-402.
- Saccas AM. Charpentier J (1969) L'anthracnose des caféiers robusta et excelsa due à Colletotrichum coffeanum Noack en République Centrafricaine. Café Cacao Thé 13:221-230.
- Santos P, Silva MC, Guerra-Guimarães L, Ribeiro A, Fernandez D (2004) Identificação de genes envolvidos na resistência do cafeeiro (Coffea arabica) à ferrugem alaranjada (Hemileia vastatrix). In: Actas do 4ş Congresso da Sociedade Portuguesa de Fitopatologia, Universidade do Algarve, Faro, Portugal, pp.20-23.
- Scheideler M, Schlaich NL, Fellenberg K, Beissbarth T, Hauser NC, Vingron M, Slusarenko AJ, Hoheisel JD (2002) Monitoring the switch from housekeeping to pathogen defense metabolism in Arabidopsis thaliana using cDNA arrays. J. Biol. Chem. 277:10555-61.
- Schlumbaum A, Mauch F, Vögeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324:365-367.
- Schulze-Lefert P, Panstruga R (2003) Establisment of biotrophy by parasitic fungi and reprogramming of host cells for disease resistance. Annu. Rev. Phytopathol. 41:641-667.
- Silva DM, Nogueira NL (1977) Observações ao microscópio electrónico do processo de infecção do cafeeiro pela Hemileia vastatrix Berk & Br. Summa Phytopatol 3:89-92.
- Silva MC (1996) Estudos Histológicos e de Ultrastrutura em Interacções de Coffea spp. e Espécies não Hospedeiras com Hemileia vastatrix, e de Coffea arabica com Ferrugens não Patogénicas. Universidade Técnica de Lisboa (Instituto Superior de Agronomia). PhD thesis.
- Silva MC, Guerra-Guimarães L, Nicole M (2005) Cytological and biochemical mechanisms involved in coffee leaf rust resistance. In: Zambolim L, Zambolim E, Várzea VMP (eds), Durable Resistance to Coffee Leave Rust, pp.249-283. Universidade Federal de Viçosa, Viçosa, Brasil.
- Silva MC, Guerra-Guimarães L, Nicole M, Loureiro A, Fernandez D, Ribeiro A, Santos P, Rodrigues Jr. CJ (2003a) Involvement of peroxidases in the hypersensitive reaction of coffee (Coffea arabica) plants to orange rust (Hemileia vastatrix). In: 8th International Congress of Plant Pathology, Christchurch, New Zealand. Abstract, pp.199.
- Silva MC, Guerra-Guimarães L, Nicole M, Loureiro A, Fernandez D, Santos P, Ribeiro A Rodrigues Jr. CJ (2003b) Early defense responses in coffee-rust interactions. In: Workshop on Plant Stress Biology, University of Minho, Braga. Abstract. PL6 (Plenary Lecture).
- Silva MC, Loureiro A, Guerra-Guimarães L, Nicole M, Valente P, Rodrigues Jr. CJ (2001) Active oxygen metabolism in the hypersensitive response of coffee-rust interaction. In: Proceedings of the 11th Congress of the Mediterranean Phytopathological Union and 3rd Congress of the Sociedade Portuguesa de Fitopatologia, Évora, Portugal, pp.346-348.
- Silva MC, Nicole M, Guerra-Guimarães L, Rodrigues Jr. CJ (2002). Hypersensitive cell death and post-haustorial defense responses arrest the orange rust (Hemileia vastatrix) growth in resistant coffee leaves. Physiol. Mol. Plant Pathol. 60:169-183.
- Silva MC, Nicole, M, Rijo L, Geiger JP, Rodrigues Jr. CJ (1999a) Cytochemistry of plant-rust fungus interface during the compatible interaction Coffea arabica (cv. Caturra)-Hemileia vastatrix (race III). Int. J. Plant Sci. 160:79-91.
- Silva MC, Várzea V, Marques DV, Guerra-Guimarães L, Nicole M, Coelho D, Geiger JP, Rodrigues Jr. CJ (2000). The role of peroxidase activity in the hypersensitive reaction of Coffea arabica plants with complete resistance to orange rust (Hemileia vastatrix). In: International Symposium on Durable resistance: Key to sustainable agriculture, Ede-Wageningen, Holanda. Abstract, pp.59
- Silva MC, Várzea VMP, Rijo L, Rodrigues Jr. CJ, Moreno G (1999b) Cytological studies in Hibrido de Timor derivatives with resistance to Colletotrichum kahawae In: Proceedings of the 18th International Conference on Coffee Science (ASIC), Hensinki, Finland, Abstract, A130.
- Simmonds NW (1991) Genetics of horizontal resistance to disease of crops. Biol. Rev. 66:189-241.
- Skalamera D, Jibodh S, Heath MC (1997) Callose deposition during the interaction between cowpea (Vigna unguiculata) and the monokaryotic stage of the cowpea rust fungus (Uromyces vignae). New Phytol. 136:511-524.
- Skipp RA, Beever RE, Sharrock KR, Rikkerink EHA, Templeton MD (1995) Colletotrichum. In: Kohmoto K, Singh US, Singh RP (eds), Pathogenesis and Host Specificity in Plant Diseases. Histological, Biochemical and Molecular Bases. Volume II. Eukaryotes, pp.119-143. Pergamon, Elsevier Science Ltd.
- Somssich IE, Hahlbrock K (1998). Pathogen defence in plants a paradigm of biological complexity. Trends Plant Sci. 3:86-90.
- Sreenivasaprasad S, Brown AE, Mills PR (1993) Coffee berry disease pathogen in Africa: genetic structure and relationship to the group species Colletotrichum gloeosporioides. Mycol. Res. 97:995-1000.
- Staskawicz B (2001) Genetics of plant-pathogen interactions specifying plant disease resistance. Plant Physiol. 125:73-76.
- Steiner KG (1972) The influence of surface wax obtained from green berries of six selections of Coffea arabica on conidia of Colletotrichum coffeanum. Kenya Coffee 37:107-108.
- Teri JM, Kilambo DL, Mtenga DJ, Nyange NE, Nzallawahe TS, Chipungahelo GS, Kipokola TP, Kullaya IK (2004) Improved Arabica varieties for the benefit of Tanzania coffee growers. In: Proceedings of the 20th International Conference on Coffee Science (ASIC), Bangalore, India, pp. 1187-1191.
- Thordal-Christensen H, Brandt J, Cho BH, Rasmussen SK, Gregersen PL, Smedegaard-Peterson V, Collinge DB (1992) cDNA cloning and characterization of two barley peroxidase transcripts induced differentially by the powdery mildew fungus Erysiphe graminis Physiol. Mol. Plant Pathol. 30:395-409.
- Tiburzy R, Martins EMF, Moraes WBC (1983) Visualization of Hemileia vastatrix structures in coffee leaves by fluorescence microscopy. Fitopatol. Bras. 8:461-466.
- Torregrosa C, Cluzet S, Fournier J, Thierry H, Gamas P, Prospéri J-M, Ésquerré-Tugayé M-T, Dumas B, Jacquet C (2004) Cytological, genetic and molecular analysis to characterize compatible and incompatible interactions between Medicago truncatula and Colletotrichum trifolii Mol. Plant-Microbe Interact. 8:909-920.
- Trevorrow PR, Irwin JAG, Cameron DF (1988) Histopathology of compatible and incompatible interactions between Colletotrichum gloeosporioides and Stylosanthes scabra Trans. Br. Mycol. Soc. 90:421-429.
- Turner JG, Ellis C, Devoto A (2002). The jasmonate signal pathway. Plant Cell: S153-S164.
- Tuzun S (2001) The relationship between pathogen-induced systemic resistance (ISR) and multigenic (horizontal) resistance in plants. Eur. J. Plant Pathol. 107:85-93.
- Ülker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological functions. Curr. Opin. Plant Biol. 7:491498.
- Vale FXR, Parlevliet JE, Zambolim L (2001) Concepts in Plant Disease Resistance. Fitopatol. Bras. 26:577-589.
- Van der Graaff NA (1981) Selection for Arabica coffee types resistant to CBD in Ethiopia. Wageningen, The Netherlands. PhD Thesis.
- Van der Graaff NA (1985) A decade of resistance breeding FAO's international programme on horizontal resistance. Plant Prot. Bull. 33:139-145.
- Van der Plank JE (1963) Plant Diseases: Epidemics and Control. Academic Press, New York and London.
- Van der Vossen HAM (1985) Coffee selection and breeding. In: Clifford MN, Wilson KC (eds), Coffee: Botany, biochemistry and production of beans and beverage. Croom Helm, London.
- Van der Vossen HAM, Cook RT, Murakaru GNW (1976) Breeding for resistance to coffee berry disease caused by Colletotrichum coffeanum Noack (sensu Hindorf) in Coffea arabica L. I. Methods of preselection for resistance. Euphytica 25:733-745
- Van der Vossen HAM, Walyaro DJ (1980) Breeding for resistance to coffee berry disease in Coffee arabica II. Inheritance of resistance. Euphytica 29:777-791.
- Van der Vossen, HAM, Walyaro DJ (1981) The coffee breeding programme in Kenya. A review of progress made and plan of action for the coming years. Kenya Coffee 46:113-130.
- Van Loon LC (1999) Occurrence and properties of plant pathogenesis-related proteins. In: Datta SK, Muthukrishnan S (eds), Pathogenesis-Related Proteins in Plants, pp.1-19. CRC Press, Boca Raton, London, New York, Washington DC.
- Van Loon LC, Pierpoint WS, Boller T, Conejero V (1994) Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 12:245-264.
- Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 55:85-97.
- Várzea VMP, Marques DV (2005) Population variability of Hemileia vastatrix vs coffee durable resistance. In: Zambolim L, Zambolim E, Várzea VMP (eds), Durable resistance to coffee leaf rust. pp.53-74. Universidade Federal de Viçosa, Viçosa, Brasil.
- Várzea VMP, Pedro JNM, Rodrigues Jr. CJ, Marques DV (2001) Cultural variants in Colletotrichum kahawae In: Proceedings of the 11th Congress of the Mediterranean Phytopathological Union and 3rd Congress of the Sociedade Portuguesa de Fitopatologia, Évora, Portugal, pp.269-271.
- Várzea VMP, Rodrigues Jr. CJ, Mexia JT (1985) Evaluation of the level of horizontal resistance to Hemileia vastatrix of some arabica plants of different physiologic groups when confronted with virulent races. In: Proceedings of the 11th International Conference on Coffee Science (ASIC), Lomé, Togo, pp.625-633.
- Várzea VMP, Rodrigues Jr. CJ, Marques D, Silva MC. (2000) Loss of resistance in interspecific tetraploid coffee varieties to some pathotypes of Hemileia vastatrix In: International Symposium on Durable resistance: Key to sustainable agriculture, Ede-Wageningen, Holanda. Abstract, pp.34.
- Várzea VMP, Rodrigues Jr. CJ, Medeiros E (1993) Different pathogenicity of CBD isolates on coffee genotypes. In: Procedings of the 15th International Conference on Coffee Science (ASIC), Montpellier, France, pp.303-308.
- Várzea VMP, Rodrigues Jr. CJ, Silva MC, Gouveia M, Marques DV, Guerra-Guimarães L, Ribeiro A (2002a) Resistência do cafeeiro à Hemileia vastatrix. In: Zambolim L (ed.), O Estado da Arte de Tecnologias na Produção de Café, pp.297-320. Universidade Federal Viçosa, Departamento de Fitopatologia, Brasil.
- Várzea VMP, Rodrigues Jr. CJ, Silva MC, Pedro JP, Marques DM. (1999) High virulence of a Colletotrichum kahawae isolate from Cameroon as compared with other isolates from other regions. In: Proceedings of the 18th International Conference on Coffee Science. Helsinki, Finland. Abstract, A 131.
- Várzea VMP, Silva MC, Rodrigues Jr. CJ (2002b) Resistência à antracnose os frutos verdes do cafeeiro. In: Zambolim L (ed.), O Estado da Arte de Tecnologias na Produção de Café, pp.321-366. Universidade Federal Viçosa, Viçosa, Brasil.
- Voegele RT, Mendgen K (2003) Rust haustoria: nutrient uptake and beyond. New Phytol. 159:93-100.
- Waller JM (1985) Control of coffee diseases. In: Coffee: Clifford MN, Willson RC (eds), Botany, Biochemistry and Production of Beans and Beverage, pp.219-229. Croom Helm Ltd.
- Waller JM, Bridge PD, Black R, Hakiza G (1993) Characterization of the coffee berry pathogen, Colletotrichum kahawae sp. Nov. Mycol. Res 97:989-994.
- Walyaro DJ (1983) Considerations in breeding for improved yield and quality in arabica coffee (Coffee arabica L.), Wageningen, The Netherlands. PhD Thesis.
- Way HM, Kazan K, Mitter N, Goulter KC, Birch RG, Manners JM (2002) Constitutive expression of a phenylalanine ammonia-lyase gene from Stylosanthes humilis in transgenic tobacco leads to enhanced disease resistance but impaired plant growth. Physiol. Mol. Plant Pathol. 60:275-282.
- Wellman FL (1957) Hemileia vastatrix Federation Cafetalera de America, San Salvador.
- Wells JM, Raju BC, Hung H, Weisburg WG, Mandelco-Paul, Brenner DJ (1987) Xylella fastidiosa gen. Nov., sp. Nov: Gram-negative, xylem-limited fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Bacteriol. 37:136-143.
- Wen YT, Chen ZJ (1995) Identification of the causal organism of three bacterial diseases on tropical crops. Chin. J. Trop. Crops 16 (2):93-97.
- White O, Kerlavage AR (1996) TDB: New databases for biological discovery. Methods Enzymol. 266:27-40.
- Wiethölter N, Horn S, Reisige K, Beike U, Moerschbacher B (2003) In vitro differentiation of haustorial mother cells of the wheat stem rust fungus, Puccinia graminis f. sp. tritici, triggered by the synergistic action of chemical and physical signals. Fungal Genet. Biol. 38:320-326.
- Wrigley G (1988) Coffee. Tropical Agricultural Series. Longman Scientific & Technical. Copublished in the United States with John Wiley & Sons, Inc., New York.
- Yamaguchi T, Ito Y, Shibuya N (2000) Oligosaccharide elicitors and their receptors for plant defense responses. Trends Glycosci. Glycotechnol. 12:113-120.
- Zhou FS, Andersen CH, Burhenne K, Fischer PH, Collinge DB, Thordal-Christensen H (2000) Proton extrusion is an essencial signalling component in the HR of epidermal single cells in the barley-powdery mildew interaction. Plant J. 23:245-254.
- Zhou N, Tootle TL, Glazebrook J (1999) Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11:2419-28.
Publication Dates
-
Publication in this collection
14 June 2006 -
Date of issue
Mar 2006