Abstract
Coagulase-negative staphylococci (CNS), components of the normal flora of neonates, have emerged as important opportunistic pathogens of nosocomial infections that occur in neonatal intensive care units. Some authors have reported the ability of some CNS strains, particularly Staphylococcus epidermidis, to produce a toxin similar to S. aureus delta toxin. This toxin is an exoprotein that has a detergent action on the membranes of various cell types resulting in rapid cell lysis. The objectives of the present study were to standardize the Polymerase Chain Reaction (PCR) technique for the detection of the gene responsible for the production of delta toxin (hld gene) in staphylococcal species isolated from catheters and blood cultures obtained from neonates, and to compare the results to those obtained with the phenotypic synergistic hemolysis method. Detection of delta toxin by the phenotypic and genotypic method yielded similar results for the S. aureus isolates. However, in S. epidermidis, a higher positivity was observed for PCR (97.4%) compared to the synergistic hemolysis method (86.8%). Among CNS, S. epidermidis was the most frequent isolate and was a delta toxin producer. Staphylococcus simulans and S. warneri tested positive by the phenotypic method, but their positivity was not confirmed by PCR for the hld gene detection. These results indicate that different genes might be responsible for the production of this toxin in different CNS species, requiring highly specific primers for their detection. PCR was found to be a rapid and reliable method for the detection of the hld gene in S. aureus and S. epidermidis.
delta toxin; PCR; Staphylococcus; coagulase-negative staphylococci
ORIGINAL PAPER
Standardization of the PCR technique for the detection of delta toxin in Staphylococcus spp.
Marconi C.I; Cunha M. L. R. S.I; Araújo Jr J. P.I; Rugolo L. M. S. S.II
IDepartment of Microbiology and Immunology, Institute of Biosciences, São Paulo State University, Botucatu, São Paulo, Brazil
IIDepartment of Pediatrics, Botucatu School of Medicine, São Paulo State University, Botucatu, São Paulo, Brazil
Correspondence Correspondence to M. L. R. S. Cunha Departamento de Microbiologia e Imunologia, Instituto de Biociências, Universidade Estadual Paulista Caixa Postal 510 18618-000, Botucatu, SP, Brasil Phone: 55 14 3811 6058. Fax: 55 14 3815 3744 E-mail: cunhamlr@ibb.unesp.br
ABSTRACT
Coagulase-negative staphylococci (CNS), components of the normal flora of neonates, have emerged as important opportunistic pathogens of nosocomial infections that occur in neonatal intensive care units. Some authors have reported the ability of some CNS strains, particularly Staphylococcus epidermidis, to produce a toxin similar to S. aureus delta toxin. This toxin is an exoprotein that has a detergent action on the membranes of various cell types resulting in rapid cell lysis. The objectives of the present study were to standardize the Polymerase Chain Reaction (PCR) technique for the detection of the gene responsible for the production of delta toxin (hld gene) in staphylococcal species isolated from catheters and blood cultures obtained from neonates, and to compare the results to those obtained with the phenotypic synergistic hemolysis method. Detection of delta toxin by the phenotypic and genotypic method yielded similar results for the S. aureus isolates. However, in S. epidermidis, a higher positivity was observed for PCR (97.4%) compared to the synergistic hemolysis method (86.8%). Among CNS, S. epidermidis was the most frequent isolate and was a delta toxin producer. Staphylococcus simulans and S. warneri tested positive by the phenotypic method, but their positivity was not confirmed by PCR for the hld gene detection. These results indicate that different genes might be responsible for the production of this toxin in different CNS species, requiring highly specific primers for their detection. PCR was found to be a rapid and reliable method for the detection of the hld gene in S. aureus and S. epidermidis.
Key words: delta toxin, PCR, Staphylococcus, coagulase-negative staphylococci
INTRODUCTION
Coagulase-negative staphylococci (CNS) have emerged over the last few years as important opportunistic pathogens of nosocomial infections that occur in neonatal intensive care units (NICU) (8). The high incidence of infection by CNS in neonates can be explained by the fact that the immunological system of these children, especially premature and low birth weight infants, is not yet completely developed (5). The application of invasive procedures such as mechanical ventilation, parenteral nutrition for more than two weeks and insertion of umbilical catheters, as well as the extensive use of antibiotics and prolonged hospitalization in NICU are factors that contribute to the development of CNS-induced neonatal infections (6, 10, 18, 22).
Some authors have reported the ability of some CNS strains, particularly Staphylococcus epidermidis, to produce a toxin similar to S. aureus delta toxin. This toxin has a detergent action on cell membranes resulting in cell lysis. It acts on various cell types, including red blood cells, and has also been called delta hemolysin, but since its action is not restricted to blood cells it should better be called cytotoxin. Among the cytotoxins produced by Staphylococcus, delta toxin is characterized by its thermostability, neutralization by lectin, and synergism with beta toxin (16). Hemolytic methods, such as synergistic hemolysis of beta hemolysin, have been used for the detection of delta cytotoxin, but its production can be influenced in vitro by components present in the culture medium (9).
Based on the above considerations, the importance of developing a genotypic method for the identification of the gene responsible for producing delta toxin in Staphylococcus species becomes evident. In S. aureus, the hld gene responsible for delta toxin production is situated within the RNAIII locus of the accessory gene regulator (agr gene), which controls the expression of most exoproteins of S. aureus. The hld gene of S. aureus encodes a 44-amino acid peptide (21). The same activity is mediated in S. epidermidis by a peptide highly homologous to S. aureus delta toxin, and whose gene is also located within the RNAIII locus of the agr gene (4), but the hld gene of S. epidermidis encodes a 26-amino acid peptide that differs in only three amino acids from the hld gene of S. aureus (4, 21).
No reports using a specific primer for the detection of the hld gene in S. aureus and in CNS by Polymerase Chain Reaction (PCR) assay are available in the national and international literature, but only studies such as that of Donvito et al. (4), in which the presence of the hld gene in Staphylococcus species was determined by hybridization methods. We therefore emphasize the importance of the present study, whose objectives were to standardize the PCR technique for the hld gene detection in Staphylococcus species, and to compare the results with those obtained with the phenotypic synergistic hemolysis method described by Hébert and Hancock (9).
MATERIAL AND METHODS
Isolates
Microorganisms isolated from catheter tips and blood cultures obtained from neonates hospitalized at the Neonatal Unit of the University Hospital, Botucatu Medical School, UNESP - São Paulo State University, from 2001 to June 2003 were studied. Samples were isolated from catheter tips according to the semiquantitative method proposed by Maki et al. (15), which consists of seeding a sample by rolling the catheter tip on the surface of a blood agar plate. Microorganisms originating from blood cultures incubated using the automated BACTEC system were isolated as described by Koneman et al. (13).
Identification of Staphylococcus spp.
Microorganisms grown in culture were Gram stained to verify their purity and to determine their morphology and specific color. After confirmation of these characteristics, catalase and coagulase tests were carried out (13). The genus Staphylococcus was differentiated from Micrococcus based on the oxidation and fermentation of glucose; on the resistance to bacitracin (0.04 U), indicated by the absence or presence of an inhibition halo measuring up to 9 mm; and on the sensitivity to furazolidone (100 µg), characterized by inhibition halos measuring 15 to 35 mm in diameter (1).
Identification of coagulase-negative staphylococci
Coagulase-negative staphylococci were identified using the criteria proposed by Kloos and Schleifer (11) and Kloos and Bannerman (12) based on a simplified scheme of biochemical tests that determine the utilization of the sugars xylose, arabinose, sucrose, trehalose, mannitol, maltose, lactose, xylitol, ribose, and fructose, as well as the production of hemolysins, nitrate reduction, presence of urease and ornithine decarboxylase, and sensitivity to novobiocin.
Delta toxin production
The production of delta toxin by the isolated strains was determined by using the synergistic hemolysis method described by Hébert and Hancock (9). One beta-hemolytic S. aureus strain was seeded vertically onto a 5% sheep blood agar plate and the samples to be tested were seeded perpendicularly at a distance of approximately 1 cm from the S. aureus strain. The plates were incubated at 37ºC for 20 h under aerobic conditions and kept at room temperature for 4 to 6 h before analysis. Strains showing an increase in their hemolysis area at the extremity close to the beta-hemolytic S. aureus were considered to be toxin producers. Staphylococcus aureus (ATCC 19095), S. epidermidis (ATCC 12228), S. xylosus (ATCC 29979), and S. warneri (ATCC 10209) were used as positive controls, and S. saprophyticus (ATCC 15305) as negative control.
Detection of the delta toxin gene
DNA extraction
Total DNA was extracted from Staphylococcus strains cultured on blood agar for 24 h at 37ºC, individually inoculated into Brain Heart Infusion broth and incubated at 37ºC for 24 h. The GFX kit (Amersham Biosciences) was used for DNA extraction, which consists of initial digestion of the staphylococcal cells with lysozyme (10 mg/ml) and proteinase K (20 mg/ml). Then, 500 µl of the extraction solution was added and the mixture was centrifuged at 5,000 g for 1 min. The supernatant was then transferred to a GFX column and centrifuged at 5,000 g for 1 min. The collected eluent was discarded and 500 µl of the extraction solution was again added to the column. After centrifugation and disposal of the collected eluent, 500 µl of the wash solution was added to the column, which was centrifuged at 20,817 g for 3 min. The column was then transferred to a 1.5-ml tube and 200 µl Milli-Q water heated to 70ºC was used for elution under centrifugation at 5,000 g for 1 min.
PCR
Polymerase Chain Reaction was carried out in 0.5-ml microcentrifuge tubes in a total volume of 25 µl containing 20 pmol of each primer (Table 1), 2.5 U Taq DNA polymerase, 200 µM dNTPs, 20 mM Tris-HCl, pH 8.4, 2.0 mM MgCl2, and 3 µl of the sample. A negative control, in which DNA was replaced with water, was run in parallel with all reactions. Staphylococcus aureus (ATCC 19095) and S. epidermidis (ATCC 12228) were used as positive controls. Amplification of the hld gene of S. aureus was performed in MJ Research PTC-100 Thermocycler, and the cycle conditions used were: one cycle at 94ºC for 5 min, denaturation at 94ºC for 2 min, annealing of the primers at 50ºC, and extension at 72ºC for 1 min, followed by 4 cycles during which the annealing temperature was reduced by 1ºC per cycle. During the sixth cycle, the annealing temperature was reduced to 45ºC, followed by 25 cycles of denaturation at 94ºC for 2 min, annealing at 45ºC, and extension at 72ºC for 1 min. At the end of the 30 cycles, the tubes were incubated at 72ºC for 5 min before being stored at 4ºC. The same parameters were used for the amplification of the S. epidermidis gene, except for the annealing temperature, which, during the first 6 cycles, ranged from 45 to 40ºC, with a reduction of 1ºC per cycle, and was then maintained at 40ºC during the following 24 cycles.
The primers were designed based on the S. aureus delta toxin sequence described by Takeuchi et al. (19) and the S. epidermidis sequence published by Tegmark et al. (20) using the GeneRunner program.
Visualization of the amplified products
Amplification efficiency was determined by electrophoresis on 2% agarose gels in 1X TBE buffer stained with ethidium bromide. The size of the amplified products was compared with a 50-kb standard and the gels were photographed under UV transillumination.
RESULTS
Strains
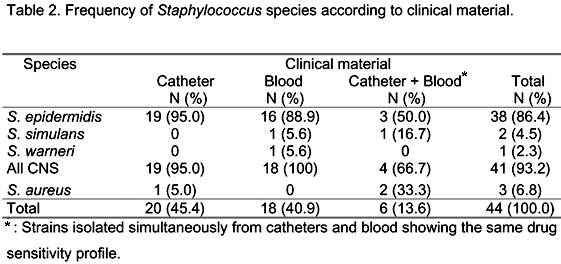
Forty-four strains belonging to the genus Staphylococcus were isolated. Twenty samples were isolated from catheter tips, 18 from blood, and 6 were simultaneously isolated from catheters and blood obtained from neonates hospitalized at the NICU of the University Hospital, Botucatu Medical School, UNESP - São Paulo State University, from September 2001 to June 2003.
Identification of Staphylococcus spp.
Coagulase-negative staphylococci were the most frequent microorganisms, accounting for 41 isolates (93.2%) compared to three (6.8%) S. aureus isolates. Among CNS, S. epidermidis was the most predominant species with 38 strains (86.4%), followed by two S. simulans (4.5%) isolates and one S. warneri (2.3%) isolate.
Table 2 shows the frequency of Staphylococcus isolates according to clinical material. Staphylococcus epidermidis was the predominant species in blood and catheters.
Delta toxin detection
Synergistic hemolysis of beta hemolysin (Figure 1) revealed production of delta toxin in 39 (88.6%) of all staphylococcal strains, including 36 (87.8%) CNS and three (100%) S. aureus isolates. Staphylococcus epidermidis was the toxin producer among 33 (86.8%) of the 38 strains studied. The two S. simulans and one S. warneri isolates were also positive for toxin production.
PCR for detection of the gene responsible for production of delta toxin demonstrated the presence of the hld gene in all three S. aureus strains studied and in 37 (97.4%) of the S. epidermidis isolates (Figure 2). On the other hand, PCR amplification did not show the presence of the hld gene in the two S. simulans samples and in the only S. warneri isolate (Table 3).
Comparison of delta toxin production determined by the synergistic hemolysis method and by PCR for the gene detection showed that, although four S. epidermidis strains had the hld gene, toxin production could not be confirmed by the phenotypic method.
DISCUSSION
The advances made in neonatology over the last few decades have led to a significant improvement in the survival of premature and low birth weight infants; however, as a consequence, a progressive increase has been observed in the diagnosis of nosocomial infections in NICU (7). This increase might be explained by the larger number of immunocompromised patients and the longer duration of hospitalization in these units, in addition to invasive procedures to which these patients are often submitted (12). Coagulase-negative staphylococci, the main components of the skin and mucosal flora, are the most frequent etiological agents involved in these infections in neonates (18).
In the present study, CNS accounted for 93.2% of all staphylococcal isolates. Staphylococcus epidermidis was the most frequent species isolated from both catheter tips (95.0%) and blood (88.9%), followed by S. aureus, which was identified in 5.0% of cultured catheters, confirming the data reported by Hudome and Fisher (10) and Li-Yin et al. (14).
Six strains were simultaneously isolated from catheter tips and blood, including three S. epidermidis isolates (50.0%), two S. aureus strains (33.3%), and one S. simulans isolate (16.7%), a finding emphasizing the importance of CNS of the newborn's normal flora in the colonization of catheters (5). According to D'Angio et al. (3), S. epidermidis becomes the predominant species in the microbiota of neonates about the fourth day of life. This predominance in the colonization of individuals and the high pathogenicity of some strains might explain the fact that S. epidermidis is the species most commonly associated with infectious processes in neonates, as reported by Cunha et al. (2).
In the present study, detection of delta toxin in S. aureus isolates by the phenotypic and genotypic method yielded similar results, while in the case of S. epidermidis a higher frequency of positive isolates was obtained by PCR (97.4%) compared to the synergistic hemolysis method (86.8%). The frequency of isolates positive for the production of delta toxin obtained in the present study was similar to those reported by others (4, 17).
Comparison of the methods revealed the presence of the hld gene in four S. epidermidis isolates, which did not show toxin production by the phenotypic method. In these cases, in vitro toxin production was either insufficient to be detected by the method used or the genes responsible for toxin production were inactive. In clinical practice, Staphylococcus isolates testing positive for the toxin gene can be considered to have the potential for producing these toxins, since toxin production in vivo cannot be excluded.
Furthermore, our results showed the production of delta toxin in two S. simulans samples and in the only S. warneri isolate included in the study, which were not confirmed to be positive when submitted to PCR for the hld gene detection. These results demonstrate differences in the gene responsible for delta toxin production in such species or the occurrence of gene insertions and deletions. Complementary studies and highly specific primers will be necessary to detect the gene responsible for delta toxin production in these species. Donvito et al. (4), identifying the hld gene by hybridization, also observed the absence of hybridization in S. simulans, which was shown to be a delta toxin producer by the phenotypic method.
Although Tegmark et al. (20) have demonstrated the existence of homology between the hld gene of S. epidermidis, S. warneri, and S. simulans, the primers designed in the present study for S. epidermidis were unable to detect the hld gene in these species.
According to Donvito et al. (4), the existence of at least two distinct molecular supports for a common synergistic hemolysis phenotype suggests that this characteristic is important in the interaction between staphylococci and their hosts. The detailed distribution and regulation of the different loci involved might clinically influence important facts such as virulence and affinity for host tissue, especially in CNS.
The results of the present study confirm that PCR is a rapid and reliable method for detecting the hld gene in S. aureus and S. epidermidis. Although S. simulans and S. warneri produced a delta toxin-like exotoxin, further studies regarding the genes responsible for its production are necessary.
ACKNOWLEDGEMENTS
We thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support.
Received: April 16, 2004
Accepted: August 11, 2004
Published online: March 3, 2005
- 1 BAKER JS. Comparison of various methods for differentiation of staphylococci and micrococci. J. Clin. Microbiol., 1984, 19, 875-9.
- 2 CUNHA MLRS., LOPES CAM., RUGOLO LMSS., CHALITA LVAS. Significância clínica de estafilococos coagulase-negativa isolados de recém-nascidos. J. Pediatr., 2002, 78, 279-88.
- 3 D'ANGIO CT., MCGOWAN KL., BAUMGART S., GEME J., HARRIS MC. Surface colonization with coagulase-negative staphylococci in premature neonates. J. Pediatr., 1989, 114, 1029-34.
- 4 DONVITO B., ETIENNE J., GREENLAND T., MOUREN C., DELORME V., VANDENESH F. Distribution of synergistic haemolysin genes hld and slush with respect to agr in human staphylococci. FEMS Microbiol. Lett, 1997, 151, 139-44.
- 5 ESHALI H., RINGERTZ S., NYSTRÖM S., FAXELIUS G. Septicaemia with coagulase-negative staphylococci in a neonatal intensive care unit. Acta Paediatr. Scan. Suppl, 1989, 360, 127-34.
- 6 FINELLI L., LIVENGOOD JR., SAIMAN L. Surveillance of pharyngeal colonization: detection and control of serious bacterial illness in low birth weight infants. Pediatr. Infect. Dis. J., 1994, 13, 854-9.
- 7 GUYER B., STROBINO DM., VENTURA SJ., SINGH GK. Annual summary of vital statistics 1994. Pediatric, 1995, 96, 1029-39.
- 8 HALL SL. Coagulase-negative staphylococcal infections in neonates. Pediatr. Infect. Dis. J., 1991, 10, 57-67.
- 9 HÉBERT GA., HANCOCK G. Synergistic hemolysis exhibited by species of Staphylococci. J. Clin. Microbiol., 1985, 22, 409-15.
- 10 HUDOME SM., FISHER MC. Nosocomial infections in the neonatal intensive care unit. Curr. Opin. Infect. Dis., 2001, 14, 303-7.
- 11 KLOOS WE., SCHLEIFER KH. Simplified scheme for routine identification of human Staphylococcus species. J. Clin. Microbiol., 1975, 1, 82-8.
- 12 KLOOS WE., BANNERMAN TL. Staphylococcus and Micrococcus In: MURRAY PR., BARON EJ., PFALLER MA., TENOVER FC., YOLKEN RH. Eds. Manual of clinical microbiology. Washington: American Society Microbiology, 1995: 282-98.
- 13 KONEMAN EW., ALLEN SD., JANDA WM., SCHRECKENBERGER PC., WINN JR WC. Color atlas and textbook of diagnostic microbiology Philadelphia: JB Lippincott, 1997.
- 14 LI-YIN C., MACNAB Y., AZIZ K., ANDREWS W., MCMILLAN DD., LEE S. Variations in central venous catheter-related infection risk among Canadian neonatal intensive care units. Pediatr. Infect. Dis. J., 2002, 21, 505-11.
- 15 MAKI DG., WEISE CE., SARAFIN HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N. Engl. J. Med., 1977, 296, 1305-9.
- 16 SCHEIFELE DW., BJORNSON GL. Delta toxin activity in coagulase-negative staphylococci from the bowels of neonates. J. Clin. Microbiol., 1988, 26, 279-82.
- 17 SCHEIFELE DW., BJORNSON GL., DYER RA., DIMMICK JE. Delta-like toxin produced by coagulase-negative staphylococci is associated with neonatal necrotizing enterocolitis. Infect. Immun, 1987, 55, 2268-73.
- 18 STOLL BJ., GORDON T., KORONES SB., SHANKARAN S., TYSON JE., BAUER CR., FANAROFF AA., LEMONS JA., DONOVAN EF., OH W., STEVENSON DK., EHRENKRANZ RA., PAPILE LA., VERTER J., WRIGHT LL. Late-onset sepsis in very low weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr., 1996, 129, 63-71.
- 19 TAKEUCHI S., MAEDA T., HASHIMOTO N., IMAIZUMI K., KAIDON T., HAYAKADA Y. Variation of the agr locus in Staphylococcus aureus isolates from cows with mastitis. Vet. Microbiol., 2001, 79, 267-74.
- 20 TEGMARK K., MORFELDT E., ARVIDSON S. Regulation of agr-dependent virulence genes in Staphylococcus aureus by RNAIII from coagulase-negative staphylococci. J. Bacteriol., 1998, 180, 3181-6.
- 21 VERMONT CL., HARTWIG NG., FLEER A., MAN P., VERBRUGH H., ANKER J., GROOT R., BELKUM A. Persistence of clones of coagulase-negative staphylococci among premature neonates in neonatal intensive care units: two center study of bacterial genotyping and patient risk factors. J. Clin. Microbiol., 1998, 36, 2485-90.
- 22 WRIGHT PF., WRIGHT PF. Infectious disease in early life in industrialized countries. Vaccine, 1998, 16, 1355-9.
Correspondence to
Publication Dates
-
Publication in this collection
03 May 2005 -
Date of issue
June 2005
History
-
Received
16 Apr 2004 -
Accepted
11 Aug 2004