ABSTRACT
This paper describes the preparation and characterization of alginate beads coated with gelatin and containing Lactobacillus rhamnosus. Capsules were obtained by extrusion method using CaCl2 as cross linker. An experimental design was performed using alginate and gelatin concentrations as the variables investigated, while the response variable was the concentration of viable cells. Beads were characterized in terms of size, morphology, scanning electron microscopy (SEM), moisture content, Fourier Transform Infrared Spectrometry (FTIR), thermal behavior and cell viability during storage. The results showed that the highest concentration of viable cells (4.2 x 109 CFU/g) was obtained for 1 % w/v of alginate and 0.1 % w/v of gelatin. Capsules were predominantly spherical with a rough surface, a narrow size distribution ranging from 1.53 to 1.90 mm and a moisture content of 97.70 ± 0.03 %. Furthermore, FTIR and thermogravimetric analysis indicated an interaction between alginate-gelatin. Cell concentration of alginate/gelatin microcapsules was 105 CFU/g after 4 months of storage at 8 oC.
Key words:
Probiotics; biopolymers; functional foods; microencapsulation
INTRODUCTION
In recent years, consumer demands for foods that contribute directly to people’s health have increased considerably. The current trend is that food is not only for nutritional purposes, but also to prevent nutrition-related diseases and improve the physical and mental well-being of consumers (Menrad 2003MENRAD K. 2003. Market and marketing of functional food in Europe. J Food Eng 56: 181-188.). In this regard, functional foods play a significant role.
The term ‘functional foods’ was first introduced in Japan in the mid-1980s. Over the years, the concept has been modified in Japan, Europe, United States and in national and international organizations. A standard definition for functional food was established to facilitate greater communication between food experts and non-experts, scientists, government officials and the public. According to Martirosyan and Singh (2015SINGH U. 2015. Benefits from foods beyond basic nutrition. Indian J Pharm Sci Res 5: 212-219.) The Functional Food Center (FFC) announced a proposed new definition for ‘functional food’, and examples of these foods include carotenids, dietary (functional and total) fiber, fatty acids, flavonoids, isothiocyanates, minerals, phenolic acids, plant stanols/sterols, polyols, phytoestrogens, soy protein, sulfides/thiols, vitamins, prebiotics and probiotics (Singh 2015).
According to FAO/WHO (2002), probiotics are live microorganisms (bacteria or yeasts), which when ingested or locally applied in sufficient numbers, confer one or more specified demonstrated health benefits for the host. Lactic acid bacteria (LAB) are the most studied bacteria within the probiotic field (Anal and Singh 2007ANAL AK AND SINGH H. 2007. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Tech 18: 240-251.). Among the LAB, Lactobacillus rhamnosus is typically associated with the human gastro-intestinal microbiota, and is one of the most widely studied probiotic bacteria. Lact. rhamnosus is a Gram-positive, non-motile, non-sporulating rod-shaped facultative anaerobic lactic acid bacterium. A number of health benefits have been claimed for Lact. rhamnosus, such as alleviation of inflammation or pathogen-induced barrier dysfunction (Lebeer et al. 2010LEBEER S, VANDERLEYDEN J AND DE KEERSMAECKER SC. 2010. Adaptation factors of the probiotic Lactobacillus rhamnosus GG. Benef Microbes 1: 335-342., Donato et al. 2010DONATO KA, GAREAU MG, WANG YJ AND SHERMAN PM. 2010. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and proinflammatory signalling. Microbiology 156: 3288-3297.), prevention of intestinal injuries induced by rotavirus diarrhea (Liu et al. 2013aLIU F ET AL. 2013a. Lactobacillus rhamnosus GG on rotavirus-induced injury of ileal epithelium in gnotobiotic pigs. J Pediatr Gastroenterol Nutr 57: 750-758.), a decrease in the symptoms of atopic dermatitis in children aged 4-48 months (Wu et al. 2015WU YJ, WU WF, HUNG CW, KU MS, LIAO PF, SUN HL, LU KH, SHEU JN AND LUE KH. 2015. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4-48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J Microbiol Immunol Infect 1: 1-9.) and prevention of gastrointestinal infectious (Hojsak et al. 2010HOJSAK I, SNOVAK N, ABDOVIĆ S, SZAJEWSKA H, MISAK Z AND KOLACEK S. 2010. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr 29: 312-316.).
According to the International Dairy Federation, the minimum concentration of probiotic should be around 106-107 CFU ml-1 at the end of product’s shelf life (FAO/WHO 2002). In order to exert their health benefits, the minimum recommended therapeutic dose is 108-109 viable cells per day/dose (Hou et al. 2003HOU RCW, LIN MY, WANG MMC AND TZEN JTC. 2003. Increase of viability of entrapped cells of Lactobacillus delbrueckii ssp. bulgaricus in artificial sesame oil emulsions. J Dairy Sci 86: 424-428.). However, the viability of probiotic bacteria in functional foods is dependent on their survival during manufacture and storage and this is affected by several factors. Probiotics must survive in the acidic gastric environment and be able to reach the small intestine and colonize within the host (Corcoran et al. 2005CORCORAN BM, STANTON C, FITZGERALD GF AND ROSS RP. 2005. Survival of probiotic Lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol 71: 3060-3067.), should be metabolically stable and active in the product (Gilliland 1989GILLILAND SE. 1989. Acidophilus milk-products - a review of potential benefits to consumers. J Dairy Sci 72: 2483-2495.), besides factors such as presence of hydrogen peroxide, dissolved oxygen produced by starter cultures, high oxygen content and oxygen permeability through the packaging materials (Shah and Jelen 1990SHAH NP AND JELEN P. 1990. Survival of lactic acid bacteria and their lactases under acidic conditions. J Food Sci 55: 506-509., Shah and Lankaputhra 1997). The oxygen permeability of the packaging material used currently for probiotic is not recommended due the oxygen toxicity in probiotic bacteria.
In order to improve the viability and stability of probiotics, different techniques have been proposed. The development of a microencapsulation matrix can provide a physical barrier during food processing, storage and in the conditions simulating of the gastrointestinal tract (D’Orazio et al. 2015). In this regard, microencapsulation of probiotics into hydrocolloid beads prepared by extrusion (Shaharuddin and Muhamad 2015SHAHARUDDIN S AND MUHAMAD II. 2015. Microencapsulation of alginate-immobilized bagasse with Lactobacillus rhamnosus NRRL 442: Enhancement of survivability and themotolerance. Carbohyd Polym 119: 173-181.) and emulsion (Zhao et al. 2017ZHAO M, QU F, WU Z, NISHINARI K, PHILLIPS GO AND FANG Y. 2017. Protection mechanism of alginate microcapsules with different mechanical strength for Lactobacillus plantarum ST-III, Food Hydrocolloids 66: 396-402., Zheng et al. 2017ZHENG H, GAO M, REN Y, LOU R, XIE H, YU W, LIU X AND MA X. 2017. An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohyd Polym 155: 329-335.) techniques or into spray-dried microparticles (Santos et al. 2014SANTOS RC, FINKLER L AND FINKLER CLL. 2014. Microencapsulation of Lactobacillus casei by spray drying. J Microencap 1: 1-9.), has been investigated (Doleyres and Lacroix 2005DOLEYRES Y AND LACROIX C. 2005. Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int Dairy J 15: 973-988.). Alginate is often used as an encapsulating material because it has the benefits of being non-toxic and being readily available (Ding and Shah 2008DING WK AND SHAH NP. 2008. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int Food Res J 15: 219-232. , Chávarri et al. 2010CHÁVARRI M, MARAÑÓN I, ARES R, IBÁÑEZ FC, MARZO F AND VILLARÁN MC. 2010. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int J Food Microbiol 142: 185-189., Pitigraisorn et al. 2017PITIGRAISORN P, SRICHAISUPAKIT K, WONGPADUNGKIAT N AND WONGSASULAK S. 2017. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J Food Eng 192: 11-18.), besides this, it is a naturally occurring biocompatible and biodegradable linear anionic polysaccharide (Lotfipour et al. 2012LOTFIPOUR F, MIRZAEEI S AND MAGHSOODI M. 2012. Preparation and characterization of alginate and psyllium beads containing Lactobacillus acidophilus. Sci World J, 8 p.).
Different types of Lactobacillus were encapsulated using an alginate hydrogel matrix as encapsulating agent, such as Lactobacillus bulgaricus (Meng-Yan et al. 2014), Lact. plantarum (Wang et al. 2016WANG L, YU X, XU H, AGUILAR ZP AND WEI H. 2016. Effect of skim milk coated inulin-alginate encapsulation beads on viability and gene expression of Lactobacillus plantarum during freeze-drying. LWT - Food Sci Technol 68: 8-13., Chen et al. 2012CHEN S, ZHAO Q, FERGUSON LR, SHU Q, WEIR I AND GARG S. 2012. Development of a novel probiotic delivery system based on microencapsulation with protectants. Appl Microbiol Biotechnol 93: 1447-1457.), Lact. fermentum (Martin et al. 2013MARTIN MJ, LARA-VILLOSLADA F, RUIZ MA AND MORALES ME. 2013. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT - Food Sci Technol 53: 480-486.) and Lact. acidophilus (Etchepare et al. 2016ETCHEPARE MA, RADDATZ GC, CICHOSKI AJ, FLORES EMM, BARIN JS, ZEPKA LQ, JACOB-LOPES E, GROSSO CRF AND MENEZES CR. 2016. Effect of resistant starch (Hi-maize) on the survival of Lactobacillus acidophilus microencapsulated with sodium alginate. J Funct Food 21: 321-329.). Recent works have studied encapsulation of Lact. rhamnosus using whey protein and isomaltooligosaccharide (Liu et al. 2016LIU L, LI X, ZHU Y, BORA AFM, ZHAO Y, DU L, LI D AND BI W. 2016. Effect of microencapsulation with Maillard reaction products of whey proteins and isomaltooligosaccharide on the survival of Lactobacillus rhamnosus. LWT - Food Sci Technol 73: 37-43.), silica (Zhao et al. 2016ZHAO Z, XIE X, WANG Z, TAO Y, NIU X, HUANG X, LIU L AND LI Z. 2016. Immobilization of Lactobacillus rhamnosus in mesoporous silica-based material: An efficiency continuous cell-recycle fermentation system for lactic acid production. J Biosci Bioeng 121: 645-651.), pectin (Li et al. 2016), chitosan-alginate (Gandomi et al. 2016GANDOMI H, ABBASZADEH S, MISAGHI A, BOKAIE S AND NOORI N. 2016. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT - Food Sci Technol 69: 365-371.), whey protein (Doherty et al. 2012DOHERTY SB, AUTY MA, STANTON C, ROSS RP, FITZGERALD GF AND BRODKORB A. 2012. Survival of entrapped Lactobacillus rhamnosus GG in whey protein micro-beads during simulated ex vivo gastro-intestinal. Int Dairy J 22: 31-43.) and alginate (Pirarat et al. 2015PIRARAT N, PINPIMAI K, RODKHUM C, CHANSUE N, OOI EL, KATAGIRI T AND MAITA M. 2015. Viability and morphological evaluation of alginate-encapsulated Lactobacillus rhamnosus GG under simulated tilapia gastrointestinal conditions and its effect on growth performance, intestinal morphology and protection against Streptococcus agalactiae. Anim Feed Sci Tech 207: 93-103.).
According to Mokarram et al. (2009MOKARRAM RR, MORTAZAVI SA, NAJAFI MBH AND SHAHIDI F. 2009. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res Int 42: 1040-1045.), problems related to susceptibility to disintegration in the presence of excess monovalent ions, Ca2+ chelating agents, and harsh chemical environments have been related with using alginate. Thus, coating the encapsulated beads with other polymers can improve their chemical and mechanical stability and the effectiveness of the encapsulation system (Krasaekoopt et al. 2006KRASAEKOOPT W, BHANDARI B AND DEETH HC. 2006. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT- and conventionally treated milk during storage. LWT - Food Sci Technol 39: 177-183.).
The objective of the present work was to optimize the conditions of microencapsulation of the probiotic strain Lact. rhamnosus ATCC 7469 using the extrusion method to obtain alginate/gelatin capsules and then to evaluate the survival of the encapsulated cells during storage at 8 oC over a period of 4 months.
MATERIALS AND METHODS
MATERIALS
Lact. rhamnosus ATCC 7469 was obtained from the culture collection of the Antibiotics Department of the Federal University of Pernambuco, Brazil. Sodium alginate was purchased from Danisco (Denmark), pectin type LM was purchased from CP Kelco (USA), gelatin, Na2HPO4.2H2O and KH2PO4.2H2O were obtained from Vetec (Brazil), Na3C6H5O7, CaCl2, NaCl and KCl were purchased from Quimica Moderna (Brazil), MRS broth was purchased from Merck (Germany), glucose was obtained from Nuclear (Brazil) and Agar was purchased from AgarGel (Brazil).
MICROORGANISM
Lact. rhamnosus was grown in De Man, Rogosa and Sharpe (MRS) broth at 37 oC using 250 ml Erlenmeyer flasks containing 50 ml of medium. Cultivation was conducted under stationary conditions for 24 h. Culture was harvested by centrifugation (Excelsa II Centrifuge Mod. 206 BL) at 1,000 g for 10 minutes. The pellet was re-suspended in 30 ml of lyophilization medium (sucrose 10 % w/v and gelatin 1 % w/v) and the cell suspension was divided into 3 ml portions in vials. Samples were stored at - 80 oC for 12 h and lyophilized for 24 hrs under vacuum (1,720 mT) at - 50 oC. Culture was stored at 8 oC and bacterial viability was determined using pour plate technique in MRS agar supplemented with 2 % (w/v) glucose in triplicate by cell resuspension in 3 ml saline solution (NaCl 0.85 % w/v).
CULTIVATION CONDITIONS
Lact. rhamnosus was cultured in MRS broth at 37 oC for 24 hrs using 500 ml schott-flasks containing 100 ml of medium and an inoculum concentration of 5 % (v/v). For the preparation of inoculum, the content of one vial of lyophilized bacterium (107 CFU g-1) was resuspended in 2 ml of 0.85 % w/v saline solution. Samples were withdrawn at various time intervals for analysis of concentration of viable cells with the objective of identifying the exponential phase of growth (data not shown), in order to obtain cells for encapsulation tests.
PREPARATION OF BEADS BY EXTRUSION
In order to define the conditions for Lact. rhamnosus microencapsulation and maximize the viability of the cells in the microcapsules, two independent variables (alginate concentration and gelatin concentration) were evaluated using a full 22 factorial design, with three central points (level 0), totaling seven experiments. The tests were performed randomly, and the data was analyzed using Statistica® 8.0 software (Statsoft, Tulsa, OK), with a 95 % confidence level. The experimental error was obtained from the mean and standard deviation of the central points. Table I shows the experimental conditions investigated for the experimental design:
Initially, cells were grown as described above for 14 hrs (late exponential phase). The cells were harvested by centrifugation at 3,500 rpm for 10 min, the pellets were washed once with 0.1M phosphate buffer saline (PBS) and resuspended in 5 ml of PBS so that the cell concentration in the final PBS/cell suspensions was approximately 109 CFU ml-1.
The extrusion technique for the preparation of the beads, as described by Nualkaekul et al. (2013NUALKAEKUL S, COOK MT, KHUTORYANSKIY VV AND CHARALAMPOPOULOS D. 2013. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res Int 53: 304-311.), was modified by altering the volume proportions of cell suspension and alginate. 5 ml of cell suspension was mixed with 5 ml of sodium alginate solution. The concentration of the sodium alginate solution varied according to Table I. Before use, polymer solutions were pasteurized by heating at 72 °C for 30 secs on a hot plate and then immediately put on ice to cool; it was subsequently used for coating. Pasteurisation was preferred to sterilisation at 121 °C, as it is conducted at a lower temperature and reduces the degree of polymer hydrolysis, which takes place during sterilization (Nualkaekul et al. 2013).
The cell/alginate mixture (1 ml) was extruded through a 0.8 mm diameter needle into sterile 0.15M CaCl2 (20 ml) using a peristaltic pump (Gilson) at 1.6 ml min-1. The beads were allowed to harden for 30 min and were then harvested using a sieve.
Coated beads were prepared by adding the beads produced previously into 30 ml of gelatin solution, according to experimental planning. Gelatin solutions were pasteurised as described above (72 °C, 30 s) and immediately put on ice to cool. The suspensions were mixed at 300 rpm for 10 min using an orbital shaker and the coated beads were harvested and washed with PBS. Beads were stored at 8 oC in penicillin tubes containing sterile water for further characterizations assays.
VIABLE CELL COUNTS
In order to count viable cells of Lact. rhamnosus in the beads, portions (0.2 g) of beads were solubilised in a solution of 1.5 % w/v sodium citrate and serially diluted in tubes containing sterile saline solution (NaCl, 0.85% w/v). 200 µL of the samples were plated on MRS agar supplemented with 2 % (w/v) glucose, using the pour plate technique. The plates were incubated at 37 oC for 48-72 h and the results of viable and cultivable cell counts were expressed by CFU/g.
BEADS CHARACTERIZATION
Beads obtained using the selected encapsulation conditions were characterized according to the methods described in the following sections.
Size
Direct observation of beads was carried out as follows: Twelve randomly selected beads were placed on dark paper and photographed with a digital photo camera (Canon EOS 70D) with a 100 mm macro lens. The scale bar was added from a calibrated digital image using the software ImageJ 1.47v.
Size measurements of beads were also evaluated under a microscope (Primostar, Zeiss) and photographed with a digital photo camera (Axiocam ERc5s, Zeiss). This procedure is robust since it works using the calibrated optical microscopy method for evaluation of the prepared microcapsules, as proposed by other authors (Abdelbary et al. 2012ABDELBARY A, EL-GENDY NA AND HOSNY A. 2012. Microencapsulation approach for orally extended delivery of glipizide: In vitro and in vivo evaluation. Indian J Pharm Sci 74: 319-330.).
Micromorphological analysis
For micromorphological analysis, unused microscope slides were cleaned by soaking in 70 % alcohol for 10 minutes. Next, alginate beads were stained by 1 ml of 1 % aqueous toluidine blue (Sigma-Aldrich, St. Louis, MO) and rinsed with distilled water for 30 minutes and 5 minutes, respectively. The stained capsules were placed on the glass slides, covered with a coverslip and photographed using a digital camera (Axiocam ERc5s, Zeiss) coupled to an optical microscope (Primostar, Zeiss), under oil immersion with x1000 magnification.
Scanning electron microscopy
The topographical properties of beads were investigated by scanning electron microscopy (SEM) (EVO LS15) at an accelerating voltage potential of 20 KV. Prior to examination, beads were prepared on aluminum stubs and coated with gold under an atmosphere of argon.
The moisture content
The moisture content of the beads (Xp) was determined according to the methodology described in agreement with the Association of Official Analytical Chemists (AOAC 2016). A 5 g portion of the beads was heated for 3 h at 105 oC. After cooling in a desiccator, the beads were weighed and the procedure was repeated until a constant weight (cw) was obtained. The moisture content (Xp%) was calculated using Equation 1. The tests were performed in triplicate.
FTIR and thermogravimetric analysis
Fourier Transform Infrared (FTIR) spectra were recorded with a spectrometer (Varian model 640-IR) using KBr discs and collecting data from 400-4000 cm−1.
Thermal analysis was performed by differential thermal analysis (DTA) and Thermogravimetry Analysis (TGA) using a thermoanalyzer (Shimadzu 60WS, Tokyo, Japan). All measurements employed a linear heating rate of 10 oC min-1, nitrogen as carrier gas and a platinum empty pan as reference material. Portions of sample (10 mg) were heated linearly from 30 oC to 800 oC. The analyses were carried out in the Laboratory of Physics and Chemistry of Polymeric Materials at the Federal Rural University of Pernambuco.
Viability of encapsulated Lact. rhamnosus during storage
Beads of Lact. rhamnosus were stored in penicillin tubes containing sterile distilled water that were hermetically sealed and stored at 8 oC. Probiotic survival was evaluated by performing viable cell counts immediately after the beads’ preparation and after 30, 60, 90 and 120 days of storage.
RESULTS
THE EFFECT OF DIFFERENT BEAD FORMULATIONS ON THE VIABILITY OF Lact. rhamnosus
Figure 1 (a and b) shows the response surface and the contour curve, respectively, for the concentration of viable cells for experimental design as a function of the alginate and gelatin concentrations. The highest concentration of viable cells (4.2 x 109 CFU g-1) was obtained for 1 % w/v of alginate and 0.1 % w/v of gelatin. The Pareto graph (Figure 1c) revealed that the alginate showed a statistically significant negative effect (- 4.59), indicating that higher viable cell counts were obtained at lower alginate concentrations. The variables of gelatin concentration and the factor of interaction between alginate/gelatin were not statistically significant, suggesting that the coating of the capsules with gelatin did not exert a positive effect on the cell viability of beads.
Response surface (a), contour curvature (b) and Pareto diagram (c) of viable cell concentration of Lact. rhamnosus as a function of the alginate and gelatin concentrations.
CHARACTERIZATION OF PREPARED BEADS: SIZE, MICROMORPHOLOGICAL ANALYSIS AND SCANNING ELECTRON MICROSCOPY
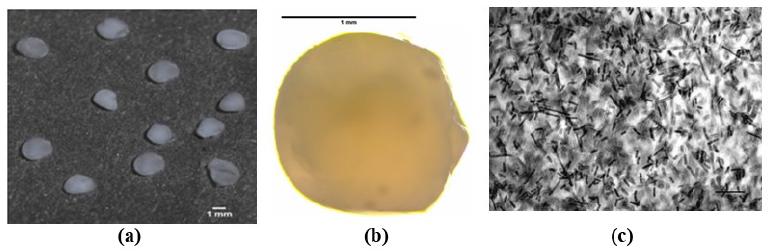
The alginate/gelatin beads for the selected formulation (1 % w/v of alginate and 0.1 % w/v of gelatin) were regular and spherical in shape and a white, opaque color (Figures 2a and 2b) and ranged in size from 1.53 to 1.90 mm. A cross section of bead (x1000 magnification) shows the integrity of Lact. rhamnosus cells entrapped within the capsule (Figure 2c). The presence of cells confirms that this technique is effective for microencapsulating this Lactobacillus.
Photographic images of alginate/gelatin beads (1 % w/v of alginate and 0.1 % w/v of gelatin). (a) Direct observation; (b) Microscopy image (x40 magnification); (c) Cross section of bead (x1000 magnification).
Scanning electron microscopy images for capsules prepared using 1 % w/v of alginate and 0.1 % w/v of gelatin show that most of the capsules were predominantly spherical, although some were found to be elongated or irregular (Figure 3a) and exhibited a rough surface, not homogeneous and were constituted of polyhedral particles (Figure 3b). These characteristics are compatible with shrinkage occurring during the drying process (Sriamornsak et al. 2007SRIAMORNSAK P, SUNGTHONGJEEN S AND PUTTIPIPATKHACHORN S. 2007. Use of pectin as a carrier for in tragastric floating drug delivery: carbonate salt contained beads. Carbohyd Polym 67: 436-445.).
SEM pictures of alginate/gelatin beads prepared with 1 % w/v of alginate and 0.1 % w/v of gelatin. (a) External aspect of the bead. Magnification of x101; (b) and (c) Internal aspect of the bead. Magnification of x10.19 K and x952, respectively.
THE MOISTURE CONTENT
The moisture content can be defined as the percentage weight of water in relation to the dry weight and is an important factor in evaluating the viability of microorganisms. The alginate/gelatin microcapsules containing Lact. rhamnosus presented a moisture content of 97.70 ± 0.03 %. Similar results were found by Belščak-Cvitanović et al. (2015), being characteristic of microparticles produced from polysaccharides that form gels and have the capacity to retain water.
FOURIER TRANSFORM INFRARED SPECTROMETRY (FTIR) AND THERMAL ANALYSIS
Figure 4 shows the IR spectra of sodium alginate (4a) and humid (4b) and dehydrated (4c) alginate/gelatin microcapsules. Thermal stability of alginate/gelatin microcapsules was determined from Thermogravimetry Analysis (TGA) and Differential Thermal Analysis (DTA), as shown in Figure 5.
FTIR spectra of sodium alginate (curve a), alginate/gelatin microcapsules humid (curve b) and dehydrated (curve c).
TGA (a) and DTA (b) curves obtained for alginate/gelatin microcapsules humid and dehydrated.
EVALUATION OF THE VIABILITY OF ENCAPSULATED BACTERIA DURING STORAGE
In this study, we tested the cell viability of alginate/gelatin microcapsules containing Lact. rhamnosus for the long-term storage stability at 8 oC for 120 days (Figure 6). We found that within four months of storage, viability was reduced from 109 to 105 CFU g-1, which indicated around 5 log reductions in the bacterial population.
Concentration of viable cells of Lact. rhamnosus in alginate capsules covered with gelatin for 120 days of storage at 8 oC.
DISCUSSION
The results indicated that the concentration of viable cells decreases with an increase in the concentrations of the polymers. According to Chandramouli et al. (2004CHANDRAMOULI V, KAILASAPATHY K, PEIRIS P AND JONES M. 2004. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J Microbiol Meth 56: 27-35. ), alginate concentrations less than 1% (w/v) are quite difficult to encapsulate because of the decreased viscosity of the alginate solution and concomitantly less ion sites for the cross-linking. Also, concentrations more than 2 % (w/v) are too viscous and cause difficulties in the process of encapsulation. Moreover, the majority of previous reports in literature suggest that sodium alginate has generally been used at a concentration varying from 0.75 % to 2 % (w/v).
The beads characteristics are similar to those observed by Lotfipour et al. (2012LOTFIPOUR F, MIRZAEEI S AND MAGHSOODI M. 2012. Preparation and characterization of alginate and psyllium beads containing Lactobacillus acidophilus. Sci World J, 8 p.), who observed results for diameters of alginate beads containing Lact. acidophilus ranging from 1.59 to 1.67 mm.
According to Totosaus et al. (2013TOTOSAUS A, ARIZA-ORTEGA TJ AND PÉREZ-CHABELA ML. 2013. Lactic acid bacteria microencapsulation in sodium alginate and other gelling hydrocolloids mixtures. J Food Nutr Res 52: 107-120.), alginate microparticles usually had a core due to the heterogeneous gelation mechanism. This effect was reported by Skjak-Brak et al. (1989), who described that during the mechanism of gelification, the polymer concentration is much higher on the surface than in the centre of the gels, which results in uneven surfaces.
Sodium alginate (curve 4a) presented a band centered at 3430 cm-1 that corresponds to the functional group O-H. The band at 2929 cm-1 is attributed to the symmetrical and asymmetrical stretching of C-H. The peak observed at 2150 cm-1 is attributed to the group CO2 group. The increase in the peak intensity at 1611 cm-1 corresponds the symmetrical stretching of COO-. Peaks observed at 1416 cm-1 and 1304 cm-1 are attributed to the asymmetrical stretching of the COO- and C-O group. Peaks at 1093 cm-1 and 1031 cm-1 are associated with the stretching with regards to the C-O group. Besides, peaks at 946 cm-1, 890 cm-1 and 818 cm-1 are attributed to the C-H vibration of the pyranose (ring of the alginate) group (Falkeborg et al. 2015FALKEBORG M, PAITAID P, SHU AN, PÉREZ B AND GUO Z. 2015. Dodecenyl succinylated alginate as a novel material for encapsulation and hyperactivation of lipases. Carbohyd Polym 133: 194-202., Bekhit et al. 2016BEKHIT M, SÁNCHEZ-GONZÁLEZ L, MESSAOUD GB AND DESOBRY S. 2016. Design of microcapsules containing Lactococcus lactis subsp. lactis in alginate shell and xanthan gum with nutrients core. LWT - Food Sci Technol 68: 446-453.).
FTIR spectrum of alginate/gelatin microcapsules humid (curve 4b) showed a peak at 3443 cm-1 (O-H vibration) due to the presence of the great amount of water. A broad band centered around 2218 cm-1 is attributed to the CO2 group. The increase in peak intensity at 1639 cm-1 is characteristic of the -CONH2 group and indicates that the negative group of the alginate could be associated with the positive load of the gelatin. The gelatin, an amphoteric polymer that presents pH below his point isoelectric, could form a complex with anionic polysaccharides, such as the alginate, through electrostatic interactions (Saravanan and Rao 2010SARAVANAN M AND RAO KP. 2010. Pectin-gelatin and alginate-gelatin complex coacervation for controlled drug delivery: Influence of anionic polysaccharides and drugs being encapsulated on physicochemical properties of microcapsules. Carbohyd Polym 80: 808-816.), leading to the formation of a polyelectrolyte complex, as described by Nualkaekul et al. (2013NUALKAEKUL S, COOK MT, KHUTORYANSKIY VV AND CHARALAMPOPOULOS D. 2013. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res Int 53: 304-311.).
The peak at 1420 cm-1 is attributed to the asymmetrical stretching of COO-. Peaks at 1090 cm-1 and 1036 cm-1 are associated to the C-H group of guluronic units, and the peak at 943 cm-1 corresponds to the C-H group of the ring pyranose (El-Ghaffara et al. 2012, Xiao et al. 2014XIAO Q, GU X AND TAN S. 2014. Drying process of sodium alginate films studied by two-dimensional correlation ATR-FTIR spectroscopy. Food Chem 164: 179-184.).
Alginate/gelatin microcapsules dehydrated (curve 4c) showed a peak at 3454 cm-1 regarding the functional group O-H linked to the hydrogen. The peak observed at 2185 cm-1 is attributed to the CO2 group. The increase in the peak intensity at 1637 cm-1 corresponding to CONH2 (C=O) indicates that the negative group of the alginate could be associated with the positive load of the gelatin. The peak at 1422 cm1 is due to the asymmetrical stretching of the COO- linked to the hydrogen of the alginate. Signals around 1310 cm-1, 1254 cm-1/1031 cm-1 and 942 cm-1 were attributed to vibrations of the C-N group of the gelatin, C-O and C-H groups, respectively. With the drying of the capsules, the molecules of water were affected and the bands decreased (Devi and Kakat 2013DEVI N AND KAKAT DK. 2013. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J Food Eng 117: 193-204. ).
Comparing the FTIR spectrum profile of pure alginate with the microcapsules spectrum it can be observed that the FTIR spectrum of pure alginate showed a peak at 2929 cm-1 characteristic of C-H stretching, while FTIR spectrum of microcapsules presented group C-H associated to peaks 1090 cm-1 and 1036 cm-1 corresponding to guluronic units for microcapsules humid and a peak at 942 cm-1 for microcapsules dehydrated. In addition, the peaks at 1639 cm-1/1637 cm-1 for microcapsules humid and dehydrated, respectively, are characteristic of the -CONH2 group and indicate the interaction between alginate and gelatin.
TGA curves (5a) are typical of weight loss, and the major mass loss refers to a thermal event characteristically endothermic (between 30 °C and 80 °C - curve 5b). As this event begins at room temperature, it is assumed that this mass loss is related to moisture. The mass loss of the dehydrated capsules is due to residual moisture, and the overlapping curves suggest that even with different initial contents of moisture this phenomenon occurs in the same way for both samples. The end of the thermal event occurs at a temperature above 100 oC, and this event may be associated with a bound water molecule. Therefore, by analyzing the results, sodium alginate has two types of water in its structure, a portion of unbound water related to moisture and another portion of water bound to the polymer.
The results indicate that up to 100 ºC the samples contained a low relative tenor of the organic matter, and starting from this temperature the samples degraded quickly during the heating up to 800 ºC.
Alginate/gelatin microcapsules showed an exothermic decomposition peak at 450 °C, a temperature higher than that observed by Sarmento et al. (2006SARMENTO B, FERREIRA D, VEIGA F AND RIBEIRO AN. 2006. Characterization of insulin loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohyd Polym 66: 1-7.) and Anbinder et al. (2011ANBINDER PS, DELADINO L, NAVARRO AS, AMALVY JI AND MARTINO MN. 2011. Yerba mate extract encapsulation with alginate and chitosan systems: Interactions between active compound encapsulation polymers. J Encap Adsorp Sci 1: 80-87.), who observed exothermic peaks for sodium alginate at 240 and 247 °C, respectively. This is probably due the presence of gelatin in the composition of microcapsules in this work, demonstrating that the presence of gelatin improve the chemical stability of the microcapsules. The oxidative degradation of the polymers can supply important information about behavior of polymeric materials under different atmospheric conditions (Liu et al. 2013bLIU X, WANG Y, YU L, TONG Z, CHEN L, LIU H AND LI X. 2013b. Thermal degradation and stability of starch under different processing conditions. Starch 65: 48-60.).
According to Stojanovic et al. (2012STOJANOVIC R, BELSCAK-CVITANOVIC A, MANOJLOVIC V, KOMES D, NEDOVIC V AND BUGARSKI B. 2012. Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads. J Sci Food Agr 92: 685-696.), the thermal decomposition of polymers includes the steps of dehydration and depolymerization accompanied by the rupture of C-O and C-C bonds and formation of CO, CO2 and H2O. Decomposition could be due to the combustion or even crystallization of the amorphous material (Gooch 2011GOOCH JW. 2011. Encyclopedic dictionary of polymers. Springer-Verlag, USA, 520 p.).
Since that during the processing and/or storage of probiotics, cellular damage and loss of cell viability could occur, an appropriate microencapsulation assures that the microorganisms survive processing and remain viable throughout storage (Thomas et al. 2014THOMAS MB, VAIDYANATHAN M, RADHAKRISHNAN K AND RAICHUR AM. 2014. Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan-dextran sulfate polyelectrolytes. J Food Eng 136: 1-8.). In this work, beads maintained the cell survival of Lact. rhamnosus, reaching 105 CFU g-1 after 4 months. On the contrary, when Trabelsi et al. (2014TRABELSI I, AYADI D, BEJAR W, BEJAR S, CHOUAYEKH H AND SALAH RB. 2014. Effects of Lactobacillus plantarum immobilization in alginate coated with chitosan and gelatin on antibacterial activity. Int J Biol Macromol 64: 84-89.) studied the efficiency of immobilizing Lactobacillus plantarum TN9 strain in alginate using gelatin as a coating material, verified that the viability of encapsulated Lact. plantarum could be preserved more than 5.8 log CFU ml-1 after 35 days of incubation at 4 °C, and no effect was observed when gelatin was used. Chávarri et al. (2010CHÁVARRI M, MARAÑÓN I, ARES R, IBÁÑEZ FC, MARZO F AND VILLARÁN MC. 2010. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int J Food Microbiol 142: 185-189.) microencapsulated Lactobacillus gasseri in alginate-chitosan capsules and they observed that after 28 days of storage, the cellular viability decreased from 109 to 107 CFU ml-1.
In conclusion, alginate/gelatin beads encapsulating probiotic Lact. rhamnosus at concentrations of 1 % w/v of alginate and 0.1 % w/v of gelatin resulted in formation of beads containing 109 CFU g-1. The microparticles were spherical with slightly roughened surfaces, and interaction between polymers was confirmed by FTIR and thermal analysis. Beads maintained the cell survival of Lact. rhamnosus, reaching 105 CFU g-1 after 4 months. Thus, this formulation could be considered promising in the production of microcapsules containing probiotics because even after 4 months of storage a mass of 10 g of microcapsules would be enough to reach the minimum quantity recommended by FAO.
ACKNOWLEDGMENTS
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for the financial support.
REFERENCES
- ABDELBARY A, EL-GENDY NA AND HOSNY A. 2012. Microencapsulation approach for orally extended delivery of glipizide: In vitro and in vivo evaluation. Indian J Pharm Sci 74: 319-330.
- ANAL AK AND SINGH H. 2007. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Tech 18: 240-251.
- ANBINDER PS, DELADINO L, NAVARRO AS, AMALVY JI AND MARTINO MN. 2011. Yerba mate extract encapsulation with alginate and chitosan systems: Interactions between active compound encapsulation polymers. J Encap Adsorp Sci 1: 80-87.
- AOAC - ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTS. 2016. Official methods of analysis. 20th edition. Arlington, USA, 2214 p.
- BEKHIT M, SÁNCHEZ-GONZÁLEZ L, MESSAOUD GB AND DESOBRY S. 2016. Design of microcapsules containing Lactococcus lactis subsp. lactis in alginate shell and xanthan gum with nutrients core. LWT - Food Sci Technol 68: 446-453.
- BELŠČAK-CVITANOVIĆ A, KOMES D, KARLOVIĆ S, DJAKOVIĆ S, ŠPOLJARIĆ I, MRŠIĆ G AND JEŽEK D. 2015. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem 167: 378-386.
- CHANDRAMOULI V, KAILASAPATHY K, PEIRIS P AND JONES M. 2004. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J Microbiol Meth 56: 27-35.
- CHÁVARRI M, MARAÑÓN I, ARES R, IBÁÑEZ FC, MARZO F AND VILLARÁN MC. 2010. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int J Food Microbiol 142: 185-189.
- CHEN S, ZHAO Q, FERGUSON LR, SHU Q, WEIR I AND GARG S. 2012. Development of a novel probiotic delivery system based on microencapsulation with protectants. Appl Microbiol Biotechnol 93: 1447-1457.
- CORCORAN BM, STANTON C, FITZGERALD GF AND ROSS RP. 2005. Survival of probiotic Lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol 71: 3060-3067.
- DEVI N AND KAKAT DK. 2013. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J Food Eng 117: 193-204.
- DING WK AND SHAH NP. 2008. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int Food Res J 15: 219-232.
- DOHERTY SB, AUTY MA, STANTON C, ROSS RP, FITZGERALD GF AND BRODKORB A. 2012. Survival of entrapped Lactobacillus rhamnosus GG in whey protein micro-beads during simulated ex vivo gastro-intestinal. Int Dairy J 22: 31-43.
- DOLEYRES Y AND LACROIX C. 2005. Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int Dairy J 15: 973-988.
- DONATO KA, GAREAU MG, WANG YJ AND SHERMAN PM. 2010. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and proinflammatory signalling. Microbiology 156: 3288-3297.
- D’ORAZIO G, GENNARO PD, BOCCARUSSO M, PRESTI I, BIZZARO G, GIARDINA S, MICHELOTTI A, LABRA M AND LA FERLA B. 2015. Microencapsulation of new probiotic formulations for gastrointestinal delivery: in vitro study to assess viability and biological properties. Appl Microbiol Biotechnol 99: 9779-9789.
- EL-GHAFFARA MAA, HASHEMA MS, EL-AWADYB MK AND RABIEC AM. 2012. pH-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohyd Polym 89: 667-675.
- ETCHEPARE MA, RADDATZ GC, CICHOSKI AJ, FLORES EMM, BARIN JS, ZEPKA LQ, JACOB-LOPES E, GROSSO CRF AND MENEZES CR. 2016. Effect of resistant starch (Hi-maize) on the survival of Lactobacillus acidophilus microencapsulated with sodium alginate. J Funct Food 21: 321-329.
- FALKEBORG M, PAITAID P, SHU AN, PÉREZ B AND GUO Z. 2015. Dodecenyl succinylated alginate as a novel material for encapsulation and hyperactivation of lipases. Carbohyd Polym 133: 194-202.
- FAO/WHO. 2002. Guidelines for the evaluation of probiotics in food. Food and Agriculture Organization of United Nations and World Health Organization. World Health Organization, London, 11 p.
- GANDOMI H, ABBASZADEH S, MISAGHI A, BOKAIE S AND NOORI N. 2016. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT - Food Sci Technol 69: 365-371.
- GILLILAND SE. 1989. Acidophilus milk-products - a review of potential benefits to consumers. J Dairy Sci 72: 2483-2495.
- GOOCH JW. 2011. Encyclopedic dictionary of polymers. Springer-Verlag, USA, 520 p.
- HOJSAK I, SNOVAK N, ABDOVIĆ S, SZAJEWSKA H, MISAK Z AND KOLACEK S. 2010. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr 29: 312-316.
- HOU RCW, LIN MY, WANG MMC AND TZEN JTC. 2003. Increase of viability of entrapped cells of Lactobacillus delbrueckii ssp. bulgaricus in artificial sesame oil emulsions. J Dairy Sci 86: 424-428.
- KRASAEKOOPT W, BHANDARI B AND DEETH HC. 2006. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT- and conventionally treated milk during storage. LWT - Food Sci Technol 39: 177-183.
- LEBEER S, VANDERLEYDEN J AND DE KEERSMAECKER SC. 2010. Adaptation factors of the probiotic Lactobacillus rhamnosus GG. Benef Microbes 1: 335-342.
- LI R, ZHANG Y, POLK DB, TOMASULA PM, YAN F AND LIU L. 2016. Preserving viability of Lactobacillus rhamnosus GG in vitro and in vivo by a new encapsulation system. J Control Release 230: 79-87.
- LIU F ET AL. 2013a. Lactobacillus rhamnosus GG on rotavirus-induced injury of ileal epithelium in gnotobiotic pigs. J Pediatr Gastroenterol Nutr 57: 750-758.
- LIU L, LI X, ZHU Y, BORA AFM, ZHAO Y, DU L, LI D AND BI W. 2016. Effect of microencapsulation with Maillard reaction products of whey proteins and isomaltooligosaccharide on the survival of Lactobacillus rhamnosus. LWT - Food Sci Technol 73: 37-43.
- LIU X, WANG Y, YU L, TONG Z, CHEN L, LIU H AND LI X. 2013b. Thermal degradation and stability of starch under different processing conditions. Starch 65: 48-60.
- LOTFIPOUR F, MIRZAEEI S AND MAGHSOODI M. 2012. Preparation and characterization of alginate and psyllium beads containing Lactobacillus acidophilus. Sci World J, 8 p.
- MARTIN MJ, LARA-VILLOSLADA F, RUIZ MA AND MORALES ME. 2013. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT - Food Sci Technol 53: 480-486.
- MARTIROSYAN DM AND SINGH J. 2015. A new definition of functional food by FFC: what makes a new definition unique? Funct Foods Health Dis 5: 209-223.
- MENG-YAN C, WEI Z, QIU-YUE D, ZHEN-HUA L, LU-E S AND ZHEN-XING T. 2014. Activity of encapsulated Lactobacillus bulgaricus in alginate-whey protein microspheres. Braz Arch Biol Technol 57: 736-741.
- MENRAD K. 2003. Market and marketing of functional food in Europe. J Food Eng 56: 181-188.
- MOKARRAM RR, MORTAZAVI SA, NAJAFI MBH AND SHAHIDI F. 2009. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res Int 42: 1040-1045.
- NUALKAEKUL S, COOK MT, KHUTORYANSKIY VV AND CHARALAMPOPOULOS D. 2013. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res Int 53: 304-311.
- PIRARAT N, PINPIMAI K, RODKHUM C, CHANSUE N, OOI EL, KATAGIRI T AND MAITA M. 2015. Viability and morphological evaluation of alginate-encapsulated Lactobacillus rhamnosus GG under simulated tilapia gastrointestinal conditions and its effect on growth performance, intestinal morphology and protection against Streptococcus agalactiae. Anim Feed Sci Tech 207: 93-103.
- PITIGRAISORN P, SRICHAISUPAKIT K, WONGPADUNGKIAT N AND WONGSASULAK S. 2017. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J Food Eng 192: 11-18.
- SANTOS RC, FINKLER L AND FINKLER CLL. 2014. Microencapsulation of Lactobacillus casei by spray drying. J Microencap 1: 1-9.
- SARAVANAN M AND RAO KP. 2010. Pectin-gelatin and alginate-gelatin complex coacervation for controlled drug delivery: Influence of anionic polysaccharides and drugs being encapsulated on physicochemical properties of microcapsules. Carbohyd Polym 80: 808-816.
- SARMENTO B, FERREIRA D, VEIGA F AND RIBEIRO AN. 2006. Characterization of insulin loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohyd Polym 66: 1-7.
- SHAH NP AND JELEN P. 1990. Survival of lactic acid bacteria and their lactases under acidic conditions. J Food Sci 55: 506-509.
- SHAH NP AND LANKAPUTHRA WEV. 1997. Improving viability of Lactobacillus acidophilus and Bifidobacterium spp. in yogurt. Int Dairy J 7: 349-356.
- SHAHARUDDIN S AND MUHAMAD II. 2015. Microencapsulation of alginate-immobilized bagasse with Lactobacillus rhamnosus NRRL 442: Enhancement of survivability and themotolerance. Carbohyd Polym 119: 173-181.
- SINGH U. 2015. Benefits from foods beyond basic nutrition. Indian J Pharm Sci Res 5: 212-219.
- SKJAK-BRAK G, GRASDALE KH, DRAGET KI AND SMIDSROD O. 1989. Inhomogeneous polysaccharide ionic gels. Carbohyd Polym 10: 31-54.
- SRIAMORNSAK P, SUNGTHONGJEEN S AND PUTTIPIPATKHACHORN S. 2007. Use of pectin as a carrier for in tragastric floating drug delivery: carbonate salt contained beads. Carbohyd Polym 67: 436-445.
- STOJANOVIC R, BELSCAK-CVITANOVIC A, MANOJLOVIC V, KOMES D, NEDOVIC V AND BUGARSKI B. 2012. Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads. J Sci Food Agr 92: 685-696.
- THOMAS MB, VAIDYANATHAN M, RADHAKRISHNAN K AND RAICHUR AM. 2014. Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan-dextran sulfate polyelectrolytes. J Food Eng 136: 1-8.
- TOTOSAUS A, ARIZA-ORTEGA TJ AND PÉREZ-CHABELA ML. 2013. Lactic acid bacteria microencapsulation in sodium alginate and other gelling hydrocolloids mixtures. J Food Nutr Res 52: 107-120.
- TRABELSI I, AYADI D, BEJAR W, BEJAR S, CHOUAYEKH H AND SALAH RB. 2014. Effects of Lactobacillus plantarum immobilization in alginate coated with chitosan and gelatin on antibacterial activity. Int J Biol Macromol 64: 84-89.
- WANG L, YU X, XU H, AGUILAR ZP AND WEI H. 2016. Effect of skim milk coated inulin-alginate encapsulation beads on viability and gene expression of Lactobacillus plantarum during freeze-drying. LWT - Food Sci Technol 68: 8-13.
- WU YJ, WU WF, HUNG CW, KU MS, LIAO PF, SUN HL, LU KH, SHEU JN AND LUE KH. 2015. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4-48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J Microbiol Immunol Infect 1: 1-9.
- XIAO Q, GU X AND TAN S. 2014. Drying process of sodium alginate films studied by two-dimensional correlation ATR-FTIR spectroscopy. Food Chem 164: 179-184.
- ZHAO M, QU F, WU Z, NISHINARI K, PHILLIPS GO AND FANG Y. 2017. Protection mechanism of alginate microcapsules with different mechanical strength for Lactobacillus plantarum ST-III, Food Hydrocolloids 66: 396-402.
- ZHAO Z, XIE X, WANG Z, TAO Y, NIU X, HUANG X, LIU L AND LI Z. 2016. Immobilization of Lactobacillus rhamnosus in mesoporous silica-based material: An efficiency continuous cell-recycle fermentation system for lactic acid production. J Biosci Bioeng 121: 645-651.
- ZHENG H, GAO M, REN Y, LOU R, XIE H, YU W, LIU X AND MA X. 2017. An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohyd Polym 155: 329-335.
Publication Dates
-
Publication in this collection
31 Aug 2017 -
Date of issue
Jul-Sep 2017
History
-
Received
31 Jan 2017 -
Accepted
03 Apr 2017