Abstract
Ferricyanide Fe(CN) 6 3- is one of the most stable cyanometallic complexes present in the gold mining effluents. This cyanocomplex is hard to degrade by natural attenuation and generates a negative impact on aquatic environments. Although free cyanide (CN-) can be obtained by acidifying the solution, the CN- is lethal for all forms of life. The oxidation of Fe(CN) 6 3- in a typical photocatalitic system was evaluated with the addition of H2O2. To verify the degradation, chemical parameters, such as free cyanide, the formation of ammonia, nitrate, and total iron were analyzed at the end of the process. Different parameters were evaluated to analyze the behavior of the degradation: 1. dark stage adsorption using the catalyst, 2. the TiO2 dosage, 3. Addition of H2O2, 4. UV radiation power (120 and 200W) and finally a test of TiO2 with solar radiation.
The photolysis effect from a ferricyanide solution at 100 mg L-1 at alkaline pH 13, showed that the complex studied is highly stable since under UV irradiation conditions (l> 300 nm), a low rate of dissociation was observed. After 24h of irradiation, the cyanocomplex was under 20%, whereas degradations up to 70% were obtained in a heterogeneous photocatalysis system with TiO2.
The best result was achieved with the H2O2 and TiO2 photocatalytic system, and the stoichiometric concentration was about 2.5 times less than the peroxide used in the gold mining industry, reaching 83% degradation. The photocatalytic process obtained less toxic byproducts than the original synthetic ferricyanide used as mining wastewater.
keywords:
cyanide; heterogeneous photocatalysis; Titanium dioxide; UV radiation; gold wastewater
1. Introduction
The mining activity generates large amounts of contaminated water in the most diverse mineral extraction and processing operations. For technical and economic reasons, cyanide is the main hydrometallurgical technique for recovering gold in the world. Cyanide is one of the few reagents that dissolves gold in an aqueous medium. Its efficiency in the process and quality of effluents depends on the mineralogical composition, and the final treatment of the effluents as well. This wastewater may contain high levels of free cyanide up to 4000 mg L-1 de CN- (Arévalo Sánchez, 2011ARÉVALO SÁNCHEZ, C. Control de efluentes cianurados mediante la oxidación con Peroxido de Hidrogeno en un laboratorio de análisis de minerales. 2011. Tesis (Profesional de Ingeniero Químico) - Facultad de Química e Ingeniería Química, Universidad Nacional Mayor de San Marcos, Lima, 2011. ) and does not always receive pretreatments before discharging into water bodies. This free cyanide concentration leads to the formation of different cyanometallic complexes (Johnson, 2014JOHNSON, C. A. The fate of cyanide in leach wastes at gold mines: An environmental perspectiveApplied Geochemistry. Applied Geochemistry journal. Denver, v. 57, p 194-205, 2014.). Disposal of effluents containing free cyanide and its complexes into the environment, affects the water quality of the receiving bodies, as well as the terrestrial and aerial ecosystems, due to the blockade of oxygen transport to the metabolism that leads to death.
Although complexed CN- can be released by altering the acidity of the solution, this represents a danger of death to all forms of life, by the release of CN(l) or HCN(g). The lethal doses for humans is 1-3 mg/Kg body weight if ingested, 100-300 ppm if inhaled and 100 mg/Kg of body weight if absorbed. If the wastewater effluent of the extraction process is alkaline, the major cyanocomplexes present in it are of iron and cobalt, which represent the most difficult cyanocomplexes to dissociate and degrade (Osathaphan et al., 2008OSATHAPHAN, K. et al. Photocatalytic oxidation of cyanide in aqueous titanium dioxide suspensions: Effect of ethylenediaminetetraacetate. Solar Energy, v. 82, n. 11, p. 1031-1036, 2008.; Arellano; Martínez, 2010ARELLANO, C. A. P.; MARTÍNEZ, S. S. Effects of pH on the degradation of aqueous ferricyanide by photolysis and photocatalysis under solar radiation. Solar Energy Materials and Solar Cells, v. 94, n. 2, p. 327-332, fev. 2010.).
Generally, these wastewaters are subjected to natural attenuation via photolysis and natural evaporation, however, there is the risk of accidental dumping or leakage into natural water bodies. Several oxidative processes have been applied to the treatment of this wastewater (Ritcey, 2005RITCEY, G. M. Tailings management in gold plants. Hydrometallurgy, v. 78, n. 1-2, p. 3-20, jul. 2005.). One of the less examined alternatives among the Advanced Oxidation Processes for gold mining wastewater treatments is the heterogeneous photocatalysis using TiO2. The photocatalytic process is based on the activation of a semiconductor using light at a more energetic wavelength than the band-gap of the conduction and valence band of the catalyst. This promotes one electron from the valence band to the conduction band, so that it creates hole-electron pairs and subsequent redox reactions on the surface of TiO2. The main radicals generated are hydroxyl radical (OH●), which are capable of degrading organic matter and electrons, and are also able to reduce other adsorbed species on the TiO2 surface (Souza et al., 2011SOUZA, J. et al. Utilização de dióxido de titânio em processos fotocatalíticos para degradação de halofenóis. Vivências. v.7, n.12, p. 91-104, 2011.; Pichat, 2013PICHAT P. (ed.) Photocatalysis and water purification. 1 ed. Weinheim, Germany:Wiley-VCH Verlag GmbH & Co, 2013. ). The application of several photocatalytic degradation processes has been widely compiled and reviewed. Suspended and silica-supported TiO2 has been studied to evaluate their effect on the degradation of free cyanide, achieving a degradation of 100% (Van Grieken et al., 2005VAN GRIEKEN, R. et al. Photocatalytic degradation of iron-cyanocomplexes by TiO2 based catalysts. Applied Catalysis B: Environmental, v. 55, n. 3, p. 201-211, fev. 2005.). TiO2 synthesized was also evaluated in the degradation of the cyanometallic complex Fe(CN) 6 3- by the sol-gel method, reaching degradations greater than 55% in 4h (Aguado et al., 2002AGUADO, J. et al. Removal of cyanides in wastewater by supported TiO2-based photocatalysts. Catalysis Today, v. 75, n. 1-4, p. 95-102, jul. 2002.). Likewise, the use of low power UV LED reactors reached 60% of the degradation of this cyanometallic complex (Devia-Orjuela et al., 2019DEVIA-ORJUELA, J. S.; BETANCOURT-BUITRAGO, L. A.; MACHUCA-MARTINEZ, F. CFD modeling of a UV-A LED baffled flat-plate photoreactor for environment applications: a mining wastewater case. Environmental Science and Pollution Research, v. 26, n. 5, p. 4510-4520, 2 fev. 2019.). The degradation of hexacyanoferrate Fe(CN) 6 3- was also evaluated using a CPC UV-LED mini-photorator in absence of oxygen, eliminating the metal by precipitation in 40%. However, the effect of the addition of H2O2 with TiO2 is not known for the treatment of this Iron complex.
This study evaluates the degradation of ferricyanide [Fe(CN) 6 3-] by the activation of TiO2 with UV radiation by means of a lamp and by solar radiation. The influence of H2O2 concentration from the minimum stoichiometric concentration required was also studied.
2. Materials and methods
2.1 Reagents
The potassium ferricyanide K3Fe(CN)6 98% pure analytical grade powdered salt provided by Neon(r) was used as the cyano-metal complex. This complex is the one that is found in greater proportion in the wastewater from gold mining. The semiconductor used was the TiO2 (P25) powder supplied by Evonik under the trade name Aeroxide(r) TiO2 P25, with a ratio of Anatase/Rutile crystalline structures: 80/20, surface area 35-65 m2 / g, with a mean particle size of 21 nm. Hydrogen peroxide, supplied by Dynamics(r), 9.47 mol L-1, was also used to assist photocatalytic degradation. In a typical experiment, a solution of 100 mg L-1 Fe(CN) 6 3- prepared from a stock solution of 1000 mg L-1 was used. The stock solution was prepared with deionized water, and used was a 10M NaOH solution in the ratio of 1 mL of NaOH per 99 mL of water to ensure a pH alkaline between 12 and 13, thereby preventing the formation of HCN (g).
2.2 Measuring techniques
The parameters for monitoring the degradation of the cyanometallic complex were performed by the following analyzes: 1) ammonia (NH3): Nesler Colorimetric Method, Standard Method 4500A NH3, 2) nitrate (NO3 -): Colorimetric Method - Standard Method 4500B NO3, 3) Total Fe: according to Standard Methods 3030B by the atomic absorption, and 4) free cyanide (CN-): by titration method, Standard Method 4500D CN-, carried out by the Institute of Biosciences at the Ecology Center of the Rio Grande do Sul Federal University. To identify and follow the degradation of the cyanometallic complexes, the UV-VIS spectrophotometry technique was used. For the hexacyanoferrate, Fe(CN)6 3-, the wavelength of maximum absorption was 302 nm.
2.3 Photocatalytic evaluation
In this study, the process was done with suspended TiO2 using a suitable batch Pyrex reactor with a quartz window, transparent for wavelength l>300nm. The lamp was of high-pressure Xe-Hg of 300W with a UVA-VIS emission spectrum. The methodology consisted of several steps) 1. Adsorption test of the TiO2 catalyst, 2) Effect of lamp power, 3) Effect of catalyst concentration, 4) Effect on the addition of H2O2 and 5) Test with solar radiation.
All solutions with TiO2 prior to the irradiation process were evaluated in the dark phase and under constant agitation for 40 min to ensure the same adsorption time on the surface of the catalyst (TiO2). Oxygen was continuously injected by an air compressor at a rate of 4 L.min-1, with the pH kept constant at 13. In the tests with solar radiation, UV-VIS spectrophotometry, temperature and photon fluxes were measured hourly by means of two ultraviolet radiometers with a photosensor for UVA- UVB. All assays were done at least in duplicate to ensure repeatability in the data. In the subsequent treatment of the samples, each aliquot of approx. 6 ml of the TiO2 suspension was withdrawn only for UV-VIS analysis, previously centrifuged for 15 min, and then 3 ml of the supernatant was withdrawn with a syringe which was filtered on a 0.22 µM PVDF membrane to remove any TiO2 particles.
2.3.1 Adsorption tests
In a beaker coated with aluminum foil to protect the solution from light, 500 ml of ferricyanide 100 mg L-1 and 0.6 g of powdered TiO2 was mixed and stirred. From the initial adsorption time at the dark phase, aliquots of 5ml were taken for UV-VIS analysis of one in one hour during the first five hours and finally in the 24 hours of contact.
2.3.2 Variation of lamp power
To determine the incidence of lamp power in the photocatalysis process, the ferricyanide solution was subjected to UV irradiation for 6 hours at two lamp powers: 110W and 200W.
2.3.3 Effect of catalyst dosing on photocatalytic tests
The catalyst doses of 0.5, 1.0, 1.2, 1.5 and 2.0 g L-1 of TiO2 were tested. For this, independent assays were performed with each catalyst dosage using 100 mg L-1 of ferricyanide for both lamp powers, 110W and 200W. The degradation efficiency was determined by Equation 1:
Where C0 is the initial concentration of the solution and C is the concentration after irradiation at time t.
2.3.4 Effect of time on photocatalysis
For the kinetic study of the dissociation of the cyanometallic complex, the power and the dosage of TiO2 were fixed at the best condition in the previous tests. The working volume was 350ml of the suspension, which was continuously irradiated for 6 hours, aliquots were withdrawn hourly to measure degradation efficiency by means of UV-VIS, and then returned to the process. To follow the chemical parameters, the total sample was collected for the NO3 -, NH3, and CN- measurement.
2.3.5 Effect of hydrogen peroxide concentration addition- H2O2
In the peroxide oxidation study, it was defined to work with the minimum concentration necessary for the degradation of free CN-. Different studies (Leiva, 2012LEIVA, P. Degradación de cianuros mediante oxidación química en efluentes industriales. 2012. 96 f. Dissertação (Máster en Química y Desarrollo Sostenible) - Universidad de Oviedo, Oviedo, 2012. ; Remu et al., 2013REMU, R. et al. Best available techniques (BAT) reference document for iron and steel production. Luxembourg: Publications Office of the European Union, 2013. 597p.) report that the following ratio of 1 mol of CN-: 1.04 mol of H2O2, is required for direct cyanide oxidation by H2O2. Thus, in a 0.3 mM solution of ferricyanide, 102 µl of H2O2 (9.4738 mol L-1) was added, which represents 50% of the excess.
2.3.6 Study with solar radiation
The effect of solar radiation in the activation of the photocatalyst to degrade the solution of ferricyanide was analyzed. In this case, it was used the best concentration obtained in the previous assay was used.
3. Results and discussion
3.1 Adsorption study of Ferricyanide on TiO2
The evaluation of the interaction between the catalyst and the solution of the cyanometallic complex was carried out in a dark phase adsorption test, at a constant pH 13 (Figure 1a). In a 24-hour period, 15% of the complex was found adsorbed by the catalyst. Thus, for the treatment of aqueous effluents contaminated with ferricyanide, TiO2 will not be a good adsorbent because its contribution will be insufficient. On the other hand, TiO2 presents pHpzc (zero charge point) between 5.6 and 6.4. This means that in alkaline pHs, the TiO2 surface has a negative surface charge that will repel the anion Fe(CN)6 3- . This could result in slow photocatalytic kinetics with a lower dissociation rate of the cyanometallic complex, and lower oxidation of free cyanide to cyanate, ammonia, nitrite and then nitrate. However, the mechanism of degradation is not always superficial but in the bulk of the fluid, so an indirect photocatalytic degradation is not ruled out. (Oliveira, 2003OLIVEIRA, E. Desinfecção de efluentes sanitários tratados através da radiação ultravioleta. 2003. 110 f. Dissertação (Mestrado em Engenharia Ambiental) - Universidade Federal de Santa Catarina, Florianópolis, 2003. ) reports that the higher efficiency of TiO2 occurs when the pH of the solution to be treated is close to the values of the pHzpc of the photocatalyst for superficial degradations.
a) photolysis, b) incidence of UV radiation on ferricyanide, c) Concentration of degradation products after 6h of UV-200W irradiation; initial sample> time zero, final sample> time 6h, pH 13.
Photolysis is the process called for the interaction of the light radiation with the molecules causing the rupture of chemical bonds. These photochemical modifications occur commonly associated with radiation having wavelengths between 200-1200nm (Oliveira, 2003OLIVEIRA, E. Desinfecção de efluentes sanitários tratados através da radiação ultravioleta. 2003. 110 f. Dissertação (Mestrado em Engenharia Ambiental) - Universidade Federal de Santa Catarina, Florianópolis, 2003. ). The power of the lamp is directly linked to the power of degradation by the number of photons that will stimulate the system and cause the rupture of the chemical bonds of the cyanide. In this case it can be observed in Figure 1b that the larger the lamp power (200W), the more the chemical bonding between CN- and Fe3+ is broken. However, there is a best steady state after 5h, but the first change of adsorption ratio occurs after 3h.
The low dissociation reached is also compatible with the chemical analyses of the products of the degradation and subsequent formation of the cyanide anion. In Figure 1c, it is possible to see that there is a dissociation of the complex followed by the increase of free cyanide CN-, but not a considerable formation of nitrate NO3 -, nor ammonia NH3. (Rader et al., 1995RADER, W. S. et al. Photocatalytic detoxification of cyanide and metal cyano-species from precious-metal mill effluents. Environmental Pollution, v. 90, n. 3, p. 331-334, 1995.) demonstrated that the cyanide ion, released by photodissociation from cyan-iron species thermodynamically stable, can be oxidized by the cyanate and nitrite route to nitrate.
3.2 Study of TiO2 catalyst concentration
Determining the optimal dosage of the catalyst is necessary both to ensure the efficient absorption of photons and to avoid the excess of the catalyst. A phenomenon known as "scattering" occurs when the concentration of TiO2 is very high and there is a decrease in the reaction rate due to the excessive opacity of the dissolution. This impacts on the optical path of the radiation and results in a reduction in the efficiency of the photocatalytic process (Pichat, 2013PICHAT P. (ed.) Photocatalysis and water purification. 1 ed. Weinheim, Germany:Wiley-VCH Verlag GmbH & Co, 2013. ).
Figure 2 shows the rate of dissociation of the pollutant as a function of the concentration change of the catalyst, using two lamp powers, 120W and 200W. It is possible to observe that the increase in catalyst concentration enhances the rate of complex dissociation when the catalyst concentrations of 1.5 g · L-1 (120 W) and 1.2 g · L-1 (200 W) were used. For the lowest power of the lamp, the dissociation rate of the complex was only 17% (1.5 g L-1 of TiO2). On the other hand, with a power of 200 W and a catalyst dosage of 1.2 g · L-1, the dissociation of the complex reached 52%. For catalyst dosages greater than these values, the dissociation decreased. Therefore, it was decided to use a dosage of the catalyst of 1.2 g·L-1. Another relevant factor in the efficiency of the photocatalytic process is the way in which the electromagnetic radiation is applied to the reactor due to energy utilization. In this study, a system with an external lamp was used. The optical distance to cross the light flux in the outer part of the reactor was of approximately 8 cm.
The incidence of radiation on ferricyanide in different concentrations of TiO2 with 120W and 200W of power. Initial concentration 100 mg/L Fe(CN)6 3-, pH 13.
(Blesa, 2001BLESA, M. (ed.) Eliminación de contaminantes por fotocatálisis heterogénea: usos de óxidos semiconductores y materiales relacionados para aplicaciones ambientales y ópticas. Buenos Aires: Comisión Nacional de Energía Atómica, 2001.) concluded that, in order to obtain a high yield in photocatalysis using TiO2, the optical path should be minimal; in this case with 2 cm distance between the light source and the reactor, whereupon, the highest efficiency was achieved using 2 g·L-1 TiO2, whereas for greater distances of the lamp, hundreds of g · L-1 of the catalyst were required. Thus, the greater the distance from the excitation source to the reactor, the higher the catalyst dosage required to obtain a similar performance.
3.3 Kinetics of Ferricyanide dissociation and formation of byproducts
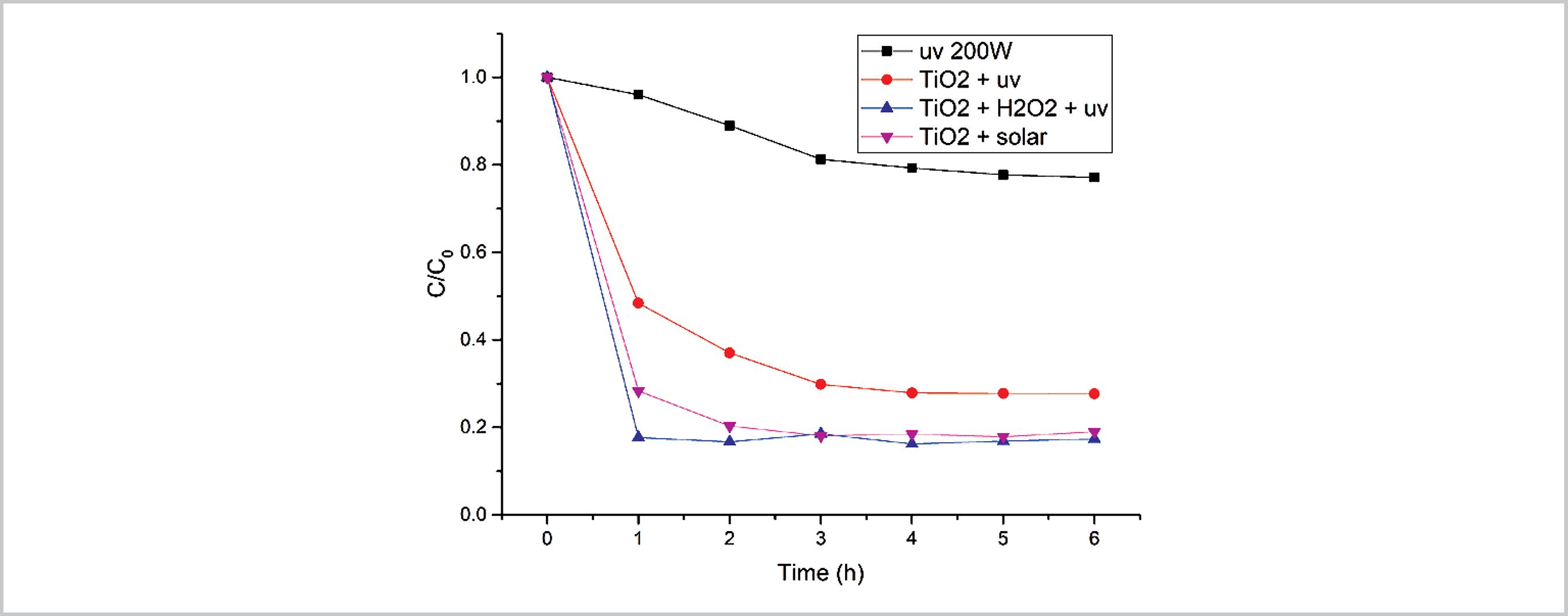
In this item, the different behavior found in the degradation of ferricyanide by photocatalysis with TiO2 will be analyzed, Figure 3. As can be seen, photolysis with a 200W UV lamp only decreases the ferricyanide 20% in 6h. In contrast with TiO2+UV, the degradation of the complex decreased the concentration in 70% in 3h. For the TiO2+solar and TiO2+H2O2+UV, the degradation followed a similar behavior, and achieved the largest value of 80% of the cyanocomplex decomposed in 1h. This could be explained by the addition of H2O2 and the presence of other wavelengths of solar light, interacting with the iron cyanocomplex. Thus, the photocatalytic degradation could be stronger in the order TiO2+UV<TiO2+solar<TiO2+H2O2+UV.
Dissociation kinetics of ferricyanide in different combinations with UV-200W, 1.2 g.L-1 TiO2, 1mM H2O2. Aparent kinetic values: k´uv 200 =0.471 h-1, k´TiO2+UV=0.3897 h-1, k´TiO2+H2O2+UV=0.546 h-1, k´TiO2+SOLAR=1.7322 h-1, pH 13.
In the first case, Figure 4a, it is observed that in TiO2 photocatalysis, the initial rate of dissociation of ferricyanide reached 50% of dissociation in the first hour, but after that time, the degradation decreases, following an exponential trend that is typical of any catalytic process, as can be seen in the kinetic apparent constants k´TiO2+SOLAR>k´TiO2+H2O2+UV>k´uv 200 >k´TiO2+UV. According to (Toor et al., 2006TOOR, A. P. et al. Photocatalytic degradation of Direct Yellow 12 dye using UV/TiO2 in a shallow pond slurry reactor. Dyes and Pigments, v. 68, n. 1, p. 53-60, jan. 2006.), there is a correlation between the amount of adsorbed substrate and the initial rate of degradation. The higher the adsorption, the higher the initial rate of degradation. Even if the adsorption of ferricyanide on the catalyst was relatively low, a high amount of pollutant adsorbed on the catalyst could also decrease the generation of the hydroxyl radical as a surface phenomenon (Tang and Huren AN, 1995TANG, W. Z.; HUREN AN. UV/TiO2 photocatalytic oxidation of commercial dyes in aqueous solutions. Chemosphere, v. 31, n. 9, p. 4157-4170, nov. 1995.). However, the degradation also can be done in the bulk of the fluid and not as a superficial phenomenon.
a) Kinetic degradation of ferricyanide with 1.2 g L-1 of TiO2; b) Concentration of degradation products after 6h of UV-200W irradiation with 1.2 g · L-1 of TiO2, pH 13.
The maximum rate of dissociation of the complex was reached in 4 hours of photocatalysis, reaching values up to 72%. After 3 hours of degradation, asymptotic behavior was observed. This behavior can be attributed to the loss of the photocatalytic power of the catalyst as a function of time. According to (Fogler, 2001), the deactivation of the catalysts can be caused by several mechanisms, such as: 1. sintering, which occurs when there is loss of surface area caused by the decrease or closure of the pores inside the catalyst particle, 2. agglomeration of crystals and 3. the growth of metal particles deposited on the surface of the catalyst. In this case, there could be traces of metallic iron or clogging caused by the deposition of impurities of the reactants on the catalyst, blocking the mass transfer between the sinus of the reaction and the active surface of TiO2.
In a visual verification, it was noticed that the initial staining of the solution from intense yellow ferricyanide underwent changes over time, migrating to much paler colors. This represents signs that the complex Fe(CN)6 3- was being degraded to a different complex (Kuhn and Young 2005KUHN, D. D.; YOUNG, T. C. Photolytic degradation of hexacyanoferrate (II) in aqueous media: the determination of the degradation kinetics. Chemosphere, v. 60, n. 9, p. 1222-1230, set. 2005.).
The degradation of the complex was verified by analyzing the products formed in the cyanide ion degradation process. In Figure 4b, the dissociation of the complex with the free cyanide parameter and byproduct formation can be seen. In all subsequent analyses, it can be observed that there is a continuous formation of free cyanide and depending on the operating conditions, different rates of byproducts are obtained. Few studies refer to the percentage of the products generated. However, (Kim et al., 2015KIM, S. H. et al. TiO 2 -catalytic UV-LED photo-oxidation of cyanide contained in Mine Wastewater. Journal of the Mineralogical Society of Korea, v. 27, n. 4, p. 223-233, 2015.) conclude that the formation of these that became cyanate OCN- ) after cyanide oxidation (CN- ), are products that are divided into two large groups of nitrogen and carbon. In the first case, with their highest proportion in nitrites, nitrates, and nitrogen (gas) and the carbon side in more inert elements, divided into 80% carbon dioxide (gas) and bicarbonate.
The behavior observed in Figure 5a shows the oxidation of the complex with UV radiation in the presence of hydrogen peroxide and in the absence of TiO2. It is known, that the cyanocomplex, decomposes to Fe(CN)x n -, which represents a mixture of cyanide complex ions with an unknown but diverse stoichiometry (Kunh, 2005). Hydrogen peroxide assists in the dissociation of cyanide ions. However, the reduction in the dissociation of the complex may be underestimated, since different products oxidized by peroxide addition are being formed and these also absorb at the ferricyanide wavelength, 302 nm. This behavior of dissociation and possible formation of different elements was verified in the analysis of products, as observed in Figure 5b. In this analysis, it is possible to verify the dissociation of the complex by the great liberation of free cyanide, but the formation of nitrate is not high. As shown by (Chen et al., 2014CHEN, F. et al. Reaction of Cu(CN)32- with H2O2 in water under alkaline conditions: cyanide oxidation, Cu+/Cu2+ catalysis and H2O2 decomposition. Applied Catalysis B: Environmental, v. 158-159, p. 85-90, 2014.), in a dissociation study of a cyanide complex with H2O2, the reactivity of H2O2 is higher with the complex than with free cyanide. This result is based on the greater capability of dissociation of the complex, than on the oxidation of cyanide to cyanate. In that study, the use of hydrogen peroxide in the treatment of iron cyanide complex did not show a complex degradation (Young et al., 1995YOUNG, C.A.; JORDAN, T.S. Cyanide remediation: current and past tecnologies. In: ANNUAL CONFERENCE ON HAZARDOUS WASTE RESEARCH, 10th, 1995, Kansas, USA. Proceedings [...]. USA: HSRC, 1995. p. 104-129.; Kitis et al., 2005KITIS, M. et al. Destruction of cyanide by hydrogen peroxide in tailings slurries from low bearing sulphidic gold ores. Minerals Engineering, v. 18, n. 3, p. 353-362, mar. 2005.).
a) Kinetics of ferricyanide degradation with 1 mM H2O2 + UV; b) Concentration of the degradation products after 6h UV-200W irradiation with 1mM H2O2, pH 13.
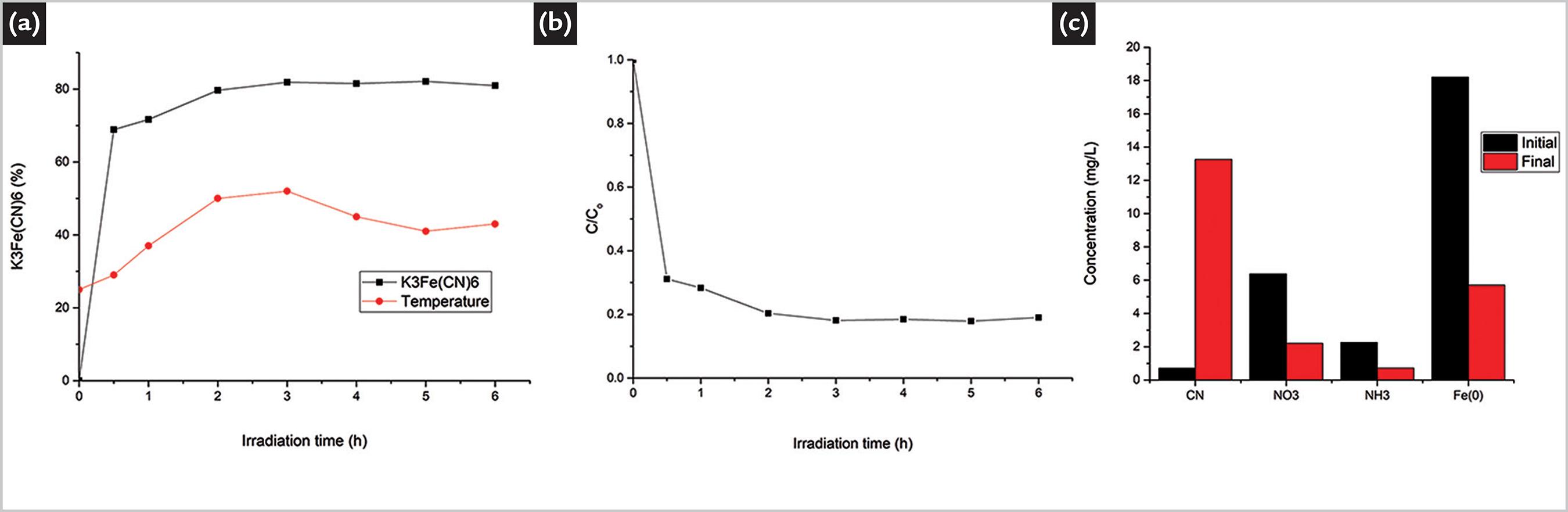
In Figure 6a, the contrast of the kinetic behavior of TiO2 with H2O2 addition can be seen. Compared to the analysis of the addition of H2O2 of Figure 5a, the highest dissociation without inverse alterations through time can be seen, which is attributed to the photocatalytic synergy of TiO2 and H2O2 by the formation of the hydroxyl radical and to the oxidative power of O2. According to (Hirakawa et al., 2009HIRAKAWA, T. et al. An approach to elucidating photocatalytic reaction mechanisms by monitoring dissolved oxygen: effect of H2O2 on photocatalysis. Applied Catalysis B: Environmental, v. 87, n. 1-2, p. 46-55, 2009.), oxygen plays two major roles in the process: it improves the photocatalytic activity by promoting the active site of the electron in the conduction band and it accepts electrons generated in TiO2 to reduce to O2 •-, causing a continuous cyanocomplex dissociation and the generation of products less toxic than free CN-.
a) Kinetic degradation of ferricyanide with 1.2 g L-1 TiO2 and 1mM H2O2 + UV. b) Concentration of degradation products after 6h of UV-200W irradiation with 1.2 g · L-1 of TiO2 and 1mM of H2O2, pH 13.
Figure 6a shows the highest dissociation content of the complex from the first hour of irradiation, and Figure 6b confirms the continuous formation of byproducts, with a higher proportion of ammonia, nitrate, precipitation of metallic iron by reducing Fe(3+) to Fe(0) and a reduction of the free cyanide concentration. This phenomenon was not observed in any of the other conditions, and the cyanide decrease confirms the degradation and possible completion of the complex dissociation process. In agreement with (Kuhn and Young 2005KUHN, D. D.; YOUNG, T. C. Photolytic degradation of hexacyanoferrate (II) in aqueous media: the determination of the degradation kinetics. Chemosphere, v. 60, n. 9, p. 1222-1230, set. 2005.), the photogenerated electrons should be sufficient to supply the energy to the cyanocomplex and thus transforming it into less stable bonds, conferred by the decrease in the release of the cyanide ion and formation of nitrate.
It is important to note that heterogeneous photocatalysis with TiO2 can act on the first objective, dissociation of the complex for the release of free cyanide (Equation 2) and proceed with the oxidation of cyanide to cyanate (OCN-), which is 1000 times less toxic than free CN- (Anderson et al. 1990ANDERSON, P. M.; SUNG, Y.; FUCHS, J. A. The cyanase operon and cyanate metabolism. FEMS Microbiology Letters, v. 87, n. 3-4, p. 247-252, dez. 1990.). After the appearance of (OCN-), the oxidation continues in less toxic species such as nitrates, ammonia, carbonates, shown in Equations 3 to 7, described below, with a schematic representation in Figure 7.
In the last test, 1.2 g·L-1 of TiO2 was activated using direct solar radiation (Figure 8). In this assay, the temperature oscillated between 25°C and 52°C and no significant changes were observed in the dissociation process. The heterogeneous photocatalysis does not require heating for photonic activation and can operate in optimal conditions at room temperature. It has been confirmed that the ideal temperature range for the photocatalytic process generally occurs between 20 and 80°C (Gogate & Pandit, 2004GOGATE, P. R.; PANDIT, A. B. A review of imperative technologies for wastewater treatment II: hybrid methods. Advances in Environmental Research, v. 8, n. 3-4, p. 553-597, mar. 2004.)(Gogate and Pandit 2004). However, this condition is not a general rule for all photolytic processes and must be checked on a case-by-case basis.
a) Temperature trendand dissociation of the complex with solar radiation; b) Kinetic degradation of ferricyanide with 1.2 g · L-1 of TiO2 + solar radiation c) Concentration of degradation products after 6 h of solar irradiation with 1.2 g·L-1 of TiO2, pH 13.
The highest dissociative activity of ferricyanide was reached with solar radiation (Figure 8b), reaching up to 82% of dissociation in the first 3 hours of irradiation. It can be seen that the high dissociation is congruent to the formation of free cyanide in Figure 8c, but the process with solar radiation did not reach the formation of nitrate or ammonia, meaning that there was only photodissociation of the complex, but no mineralization. This is an unfavorable result obtained for the UV lamp photocatalysis assays. Different pollutants are easy to eliminate by photocatalytic degradation with solar radiation, but being the ferricyanide a very stable complex, a considerable rate of degradation for less toxic byproducts was not reached. However, UV light did increase the photodegradation when the UV Lamp was applied. This implies that more energy is required to remediate iron cyanocomplexes from wastewater.
The direct UV Lamp radiation conditions showed better results than solar radiation as I) continuous incidence of energy by the constant value of the power is directly related to the number of photons emitted; II) a constant emission angle keeps at 45 °, while in the process with solar radiation, it is a variable with the highest efficiency only about 12h (45 °); in addition, the interference by the climatic conditions or clouds avoids a constant absorption of energy; III) by the amount of energy, solar light is less energetic than UV light of halogen lamps.
For this study, the energy in both UV Lamp and solar light was measured. In the first case, a constant UV radiation emission of 24. 2W/m2 was measured, and 2.5 W/m2 average UV emission for solar radiation. This shows that sunlight is only 10% of the energy supplied by the lamp, and that could explain the lack of byproducts when solar light was applied to the hexacyanoferrate. This confirms the need to activate TiO2 with UV light instead of direct solar light. Using the Electric Efficient of the oxidation indicator defined by the IUPAC (Samarghandi, Yang, Giahi, & Shirzad-Siboni, 2015SAMARGHANDI, M. R.; YANG, J. K.; GIAHI, O.; SHIRZAD-SIBONI, M. Photocatalytic reduction of hexavalent chromium with illuminated amorphous FeOOH. Environmental Technology (United Kingdom), v. 36, n.9, p.1132-1140, 2015.) and (Daneshvar, Aleboyeh, & Khataee, 2005DANESHVAR, N.; ALEBOYEH, A.; KHATAEE, A. R. The evaluation of electrical energy per order (EEo) for photooxidative decolorization of four textile dye solutions by the kinetic model. Chemosphere, v. 59, n.6, p. 761-767, 2005.), the estimated EEo for each process is TiO2+UV > UV 200 > TiO2+H2O2+UV > TiO2+SOLAR which is 56.3 > 46.6 > 40.2 > 12.7kWh/m3 order-1 respectively.
4. Conclusions
The photocatalytic activity of TiO2 was evaluated in a simple solution of potassium ferrycyanide, simulating the majority of gold mining wastewater using artificial and solar light. The cyanocomplex exhibited a sensitive behavior to photolytic effects when lamp power was about 200W, enhancing the chemical bond rupture by photolysis.
For the photolysis test, the dissociation of the complex was low, as well as the release of free cyanide, with no formation of byproducts. The heterogeneous photocatalysis obtained a high dissociation-mineralization rate, which was confirmed with the ammonia generation and afterwards, the final formation of nitrate.
The addition of H2O2 to the photocatalytic degradation resulted in better degradation than the single photocatalysis, and total peroxide used was 2.5 times lower than that used in the industry in typical direct chemical oxidation.
The best result in terms of dissociation and degradation was reached in the peroxide assisted photocatalysis system, reaching 83% degradation from the first hour, with a free cyanide decrease and nitrate and ammonia appearance at the end of the test.
Acknowledgements
To the Federal University of Rio Grande do Sul, to the Laboratory of Photochemistry and Surfaces (LAFOS); To the Laboratory of Environmental and Analytical Technology (LATAMA); To the Laboratory of Mineral Processing (LAPROM), and CAPES and the CNPQ for the financial support.
References
- AGUADO, J. et al. Removal of cyanides in wastewater by supported TiO2-based photocatalysts. Catalysis Today, v. 75, n. 1-4, p. 95-102, jul. 2002.
- ANDERSON, P. M.; SUNG, Y.; FUCHS, J. A. The cyanase operon and cyanate metabolism. FEMS Microbiology Letters, v. 87, n. 3-4, p. 247-252, dez. 1990.
- ARELLANO, C. A. P.; MARTÍNEZ, S. S. Effects of pH on the degradation of aqueous ferricyanide by photolysis and photocatalysis under solar radiation. Solar Energy Materials and Solar Cells, v. 94, n. 2, p. 327-332, fev. 2010.
- ARÉVALO SÁNCHEZ, C. Control de efluentes cianurados mediante la oxidación con Peroxido de Hidrogeno en un laboratorio de análisis de minerales 2011. Tesis (Profesional de Ingeniero Químico) - Facultad de Química e Ingeniería Química, Universidad Nacional Mayor de San Marcos, Lima, 2011.
- BETANCOURT-BUITRAGO, et al. Recent developments in the photocatalytic treatment of cyanide wastewater: an approach to remediation and recovery of metals. Processes, v. 7, v. 4, p 225, April. 2019.
- BLESA, M. (ed.) Eliminación de contaminantes por fotocatálisis heterogénea: usos de óxidos semiconductores y materiales relacionados para aplicaciones ambientales y ópticas. Buenos Aires: Comisión Nacional de Energía Atómica, 2001.
- CHEN, F. et al. Reaction of Cu(CN)32- with H2O2 in water under alkaline conditions: cyanide oxidation, Cu+/Cu2+ catalysis and H2O2 decomposition. Applied Catalysis B: Environmental, v. 158-159, p. 85-90, 2014.
- DANESHVAR, N.; ALEBOYEH, A.; KHATAEE, A. R. The evaluation of electrical energy per order (EEo) for photooxidative decolorization of four textile dye solutions by the kinetic model. Chemosphere, v. 59, n.6, p. 761-767, 2005.
- DEVIA-ORJUELA, J. S.; BETANCOURT-BUITRAGO, L. A.; MACHUCA-MARTINEZ, F. CFD modeling of a UV-A LED baffled flat-plate photoreactor for environment applications: a mining wastewater case. Environmental Science and Pollution Research, v. 26, n. 5, p. 4510-4520, 2 fev. 2019.
- FLOGUER, H. S. Elementos de engenharia das reações químicas 3. ed. Rio de Janeiro: LTC, 2002.
- GOGATE, P. R.; PANDIT, A. B. A review of imperative technologies for wastewater treatment II: hybrid methods. Advances in Environmental Research, v. 8, n. 3-4, p. 553-597, mar. 2004.
- HIRAKAWA, T. et al. An approach to elucidating photocatalytic reaction mechanisms by monitoring dissolved oxygen: effect of H2O2 on photocatalysis. Applied Catalysis B: Environmental, v. 87, n. 1-2, p. 46-55, 2009.
- JOHNSON, C. A. The fate of cyanide in leach wastes at gold mines: An environmental perspectiveApplied Geochemistry. Applied Geochemistry journal Denver, v. 57, p 194-205, 2014.
- KIM, S. H. et al. TiO 2 -catalytic UV-LED photo-oxidation of cyanide contained in Mine Wastewater. Journal of the Mineralogical Society of Korea, v. 27, n. 4, p. 223-233, 2015.
- KITIS, M. et al. Destruction of cyanide by hydrogen peroxide in tailings slurries from low bearing sulphidic gold ores. Minerals Engineering, v. 18, n. 3, p. 353-362, mar. 2005.
- KUHN, D. D.; YOUNG, T. C. Photolytic degradation of hexacyanoferrate (II) in aqueous media: the determination of the degradation kinetics. Chemosphere, v. 60, n. 9, p. 1222-1230, set. 2005.
- LEIVA, P. Degradación de cianuros mediante oxidación química en efluentes industriales 2012. 96 f. Dissertação (Máster en Química y Desarrollo Sostenible) - Universidad de Oviedo, Oviedo, 2012.
- OLIVEIRA, E. Desinfecção de efluentes sanitários tratados através da radiação ultravioleta 2003. 110 f. Dissertação (Mestrado em Engenharia Ambiental) - Universidade Federal de Santa Catarina, Florianópolis, 2003.
- OSATHAPHAN, K. et al. Photocatalytic oxidation of cyanide in aqueous titanium dioxide suspensions: Effect of ethylenediaminetetraacetate. Solar Energy, v. 82, n. 11, p. 1031-1036, 2008.
- PICHAT P. (ed.) Photocatalysis and water purification. 1 ed. Weinheim, Germany:Wiley-VCH Verlag GmbH & Co, 2013.
- RADER, W. S. et al. Photocatalytic detoxification of cyanide and metal cyano-species from precious-metal mill effluents. Environmental Pollution, v. 90, n. 3, p. 331-334, 1995.
- REMU, R. et al. Best available techniques (BAT) reference document for iron and steel production Luxembourg: Publications Office of the European Union, 2013. 597p.
- RITCEY, G. M. Tailings management in gold plants. Hydrometallurgy, v. 78, n. 1-2, p. 3-20, jul. 2005.
- SAMARGHANDI, M. R.; YANG, J. K.; GIAHI, O.; SHIRZAD-SIBONI, M. Photocatalytic reduction of hexavalent chromium with illuminated amorphous FeOOH. Environmental Technology (United Kingdom), v. 36, n.9, p.1132-1140, 2015.
- SOUZA, J. et al. Utilização de dióxido de titânio em processos fotocatalíticos para degradação de halofenóis. Vivências v.7, n.12, p. 91-104, 2011.
- TANG, W. Z.; HUREN AN. UV/TiO2 photocatalytic oxidation of commercial dyes in aqueous solutions. Chemosphere, v. 31, n. 9, p. 4157-4170, nov. 1995.
- TOOR, A. P. et al. Photocatalytic degradation of Direct Yellow 12 dye using UV/TiO2 in a shallow pond slurry reactor. Dyes and Pigments, v. 68, n. 1, p. 53-60, jan. 2006.
- VAN GRIEKEN, R. et al. Photocatalytic degradation of iron-cyanocomplexes by TiO2 based catalysts. Applied Catalysis B: Environmental, v. 55, n. 3, p. 201-211, fev. 2005.
- YOUNG, C.A.; JORDAN, T.S. Cyanide remediation: current and past tecnologies. In: ANNUAL CONFERENCE ON HAZARDOUS WASTE RESEARCH, 10th, 1995, Kansas, USA. Proceedings [...] USA: HSRC, 1995. p. 104-129.
Publication Dates
-
Publication in this collection
20 Dec 2019 -
Date of issue
Jan-Mar 2020
History
-
Received
22 Mar 2019 -
Accepted
23 Aug 2019