Abstract
In this work the process of desorption of hexavalent chromium, a toxic metal ion, from the marine algae Sargassum sp, following biosorption experiments 2³ factorial design was studied. A technique was applied to three eluents: HCl, H2SO4 and EDTA. Three factors of importance were evaluated: concentration of eluent, the ratio between mass of biosorbent and volume of eluent (S/L) and process time. A statistical analysis of the experimental results showed that the three variables evaluated are significant for all three eluents. The models for chromium desorption were validated, as the results agreed well with the observed values. Through use of the response surface methodology, a factorial design based optimization technique; it was possible to identify the most suitable eluent and the interval of values for the process variables that resulted in the most significant desorption of chromium, which is relevant information for work aiming at process optimization.
desorption; marine algae; chromium
A study of the process of desorption of hexavalent chromium
W.B.AmorimI; A.M.HayashiI; P.F.PimentelII; M.G.C.da SilvaI
ILEA/DTF/FEQ/UNICAMP, Laboratory of Environmental Engineering, Phone (19) 3788-3928, Fax (19) 3788-3922, Cx. P. 6066, CEP 13080-970, Campinas - SP, Brazil, E-mail:wbamorim@feq.unicamp.br
IICETEC/MG, Biotechnology and Chemical Technology Department, Av. José Cândido da Silveira 2.000 Horto, CEP 31170-000, BH - MG, Brazil, E-mail: patricia@cetec.br
ABSTRACT
In this work the process of desorption of hexavalent chromium, a toxic metal ion, from the marine algae Sargassum sp, following biosorption experiments 23 factorial design was studied. A technique was applied to three eluents: HCl, H2SO4 and EDTA. Three factors of importance were evaluated: concentration of eluent, the ratio between mass of biosorbent and volume of eluent (S/L) and process time. A statistical analysis of the experimental results showed that the three variables evaluated are significant for all three eluents. The models for chromium desorption were validated, as the results agreed well with the observed values. Through use of the response surface methodology, a factorial design based optimization technique; it was possible to identify the most suitable eluent and the interval of values for the process variables that resulted in the most significant desorption of chromium, which is relevant information for work aiming at process optimization.
Keywords: desorption, marine algae, chromium.
INTRODUCTION
The advantages of industrial development, such as improvement in the standard of living are numerous. However, this advancement does not come without harmful consequences. A side effect of this progress is the pollution caused by the presence of heavy metals in industrial effluents, which has become a serious ecological problem with both economic and social repercussions. Conventional treatments for effluents containing heavy metals involve physical and chemical processes of flocculation, precipitation, electrolysis, crystallization and adsorption, which either are expensive or produce new contaminants.
Chromium (Cr) is found in aqueous solutions in two oxidation states, trivalent chromium, Cr(III), and hexavalent chromium, Cr(VI). Neither elemental chromium nor Cr(III) is toxic, and the latter is known to be essential to mammals. On the other hand, when dissolved in water, Cr (VI) is carcinogenic, extremely irritating and toxic.
The conventional treatments for chromium-rich effluents are very expensive and consist of four stages: reduction of Cr(VI) to Cr(III), precipitation of a Cr(III) salt, sedimentation of the insoluble metal and adequate disposal of the sediment. For these reasons, new methods, including the process of biosorption based on the principle of the capacity of biological material (biomass) to retain metals from solutions through metabolism-independent processes, are being studied.
Of the algae biomasses available, marine algae are considered to be the most useful as biosorbents due to their abundance in the seas. Furthermore, dead biomass may be used in the process, thereby eliminating possible expenses in maintaining the culture, as in the case of fungi and bacteria. In addition, algae can be reused in some cycles without an appreciable reduction in their adsorptive capacity.
Seaweed cells have a large superficial area with sites which are able to provide fast and reversible bonding with cations. According to Sigg (1987), this cell surface consists of a mosaic of cation and anion exchange sites on the cell walls. The external surface of the seaweed has a fibrous composition of proteins and carbohydrates (Siegel and Siegel, 1973) with which metallic species can react.
Of the algae tested Sargassum sp, showed the best results as biosorbent. Kratochvil et al. (1998) have shown that the Sargassum biomass is a good biosorbent for Cr(VI) at pH=2 and removes Cr(III) at pH>3. The biosorbent was regenerated with acid and the Cr(III) recovered in concentrated solution. The removal of Cr(III) through biosorption suggests the possibility of reducing the cost of effluent treatment due to the lower volume of toxic sludge generated. Moreover, seaweed biomass shows potential as a reducing agent, totally or partially substituting conventionally used substances such as SO2, Na2S2O5 and FeSO4, since it can be obtained at a lower cost and is readily available.
One of the basic requirements for studies aiming at using biomass as an adsorbent is to evaluate its regenerative capability in successive sorption/desorption cycles (Volesky, 1988). The metals deposited on the biomass are washed away and the biosorbent is regenerated for application in a new cycle. A basic condition of this process must be the maintenance of the biosorption capacity as well as the physical and chemical integrity of the biosorbent.
Yang and Volesky (1999) studied the desorption of Uranium captured by Sargassum fluitans using 0.1 HCl M. Their adsorption and desorption experiments were carried out in seaweed-packed columns (diameter/length ratio of 1/15).
Volesky and Kuyucak (1988) studied the process of desorption of cobalt ions using various eluents: H2SO4, HCl, NH4OH, KHCO3, EDTA, KSCN, KCl and CaCl2 solutions. The solution of CaCl2 (0.05M) in HCl showed the best results for the desorption of the trapped metal, removing more than 96% of the bonded cobalt at pH 2-3. S/L ratios over 10 showed the best results, without reducting the capacity of the biossorvent.
Aldor et al. (1995) achieved the desorption of cadmium from algae using HCl solution at pH 1. They verified that S/L ratio is a key parameter for determination of elution efficiency, simultaneously affecting the equilibrium desorption pH, the concentration of eluted cadmium and the ratio of metals recovered.
The objective of this work was to evaluate the process of desorption of hexavalent chromium from Sargassum sp, employing a 23 factorial design technique to analyze three factors of great importance in the process: concentration of eluent, S/L ratio and process time.
METHODOLOGY
23 Factorial Design
The first step in the development of the experimental planning technique was to select the parameters that influence the process and the desired reponse. Previous studies indicate that the amount of the elutioned metal is influenced by the S/L ratio (mg of alga/ml of eluent), the concentration of eluent and the process time. Using this information, a 23 factorial design was developed. The upper and lower levels employed in this work are shown in Table 1, for the three eluents tested: HCl, H2SO4 and EDTA.
The response surface methodology, an optmization technique based on factorial design, was adopted in order to analyze the influence of the variables selected. These surfaces were plotted keeping one variable constant while allowing the others to vary. Level curves were drawn to assist in visualization of these surfaces, providing a model representation with lines where the response is constant.
Biosorption
Sargassum sp biomass collected off the coast of the state of São Paulo was used. The samples were washed, dried at 60 oC overnight and then protonated with 0.6 HCl M, following procedure described by Kratochvil et al. (1998).
Chromium Biosorption Experiments
In order to apply the 23 experimental planning technique to the process of desorption of hexavalent chromium, biosorption of the metal on seaweed was carried out through finite bath experiments. These experiments consisted in adding 0.15 g of the protonated biomass to 50 ml of 500 ppm solution of K2Cr2O7 in 125 ml Erlenmeyer flasks. Initial pH of the solution was adjusted to 2 and kept constant with the addition of 0.1N to solutions of H2SO4 or NaOH until equilibrium was observed. Duplicate experiments were conducted. The biomass containing chromium was vacuum filtered and washed with water to remove the Cr which had not been adsorbed, before being used in the desorption experiments. The concentration of the metal in the remaining solution was analyzed.
Desorption
The biomass containing the adsorbed metal was introduced into 125ml Erlenmeyer flasks containing the appropriate volume of eluent based on the factorial design. The Erlenmeyer flasks were shaken at 200 rpm for a predetermined time, again based on factoral design. The samples were then centrifuged and the metal concentration in the final solution was analyzed.
Determination of Chromium Concentration
The initial and final solutions from the adsorption and desorption processes were analyzed for total concentration of Cr by atomic absorption spectroscopy. The experimental amount of biosorption (Qads) and desorption (Qdes) of chromium was determined using equations 1 and 2, respectively:
RESULTS AND DISCUSSION
The results of the process of adsorption and desorption of Cr, used in the factorial design for HCl, H2SO4 and EDTA eluent, are shown in Table 2. Experiments 9 through 16 are duplicates of experiments 1 through 8.
Table 3 shows the duplicate experimental results for chromium desorption with HCl, H2SO4 and EDTA eluents, according to the factorial design (FD).
The data acquired from the factorial designs were used to generate response surfaces and level curves that allow for closer evaluation of the influence of each factor. The response surfaces are constructed by keeping one parameter constant while allowing the other two to vary. To aid in visualization of the surfaces, level curves were plotted to provide a bidimensional representation of the surface where constant response lines are shown.
Figure 1 shows the results of hexavalent chromium desorption for varying levels of S/L ratio and time, keeping the concentration of HCl eluent constant at 0.6 M. The best results for chromium desorption are achieved at the lower levels of S/L ratio and time.
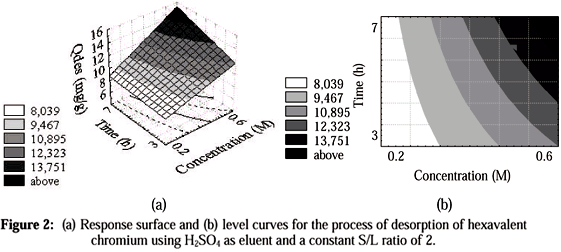
Figure 2 shows that when the S/L ratio is kept constant at its lower level, 2, the best results for desorption occur at the upper levels of process time and concentration of H2SO4 eluent.
Figure 3 shows that, for a constant S/L ratio of 2, the most significant results were obtained for the upper levels of time and concentration of EDTA eluent.
Models that relate the amount of chromium eluted with the factors studied were proposed based on the factorial design and are shown in equations 3, 4 and 5 for HCl, H2SO4 and EDTA, respectively:
It is important to note that this model uses coded variable values, i. e., values of -1 and +1. The relevance of the model can only be assured through analysis of variance of the results, which is shown in Table 4 for HCl, H2SO4 and EDTA eluent.
In the analysis of variance, the statistical significance of the regression is an important parameter. If the ratio between the quadratic average due to regression (MQR) and the quadratic average due to the residue (MQr) is greater than the statistical F distribution, in the desired confidence interval, the possibility that the factor under study is null must be discarded. According to Table 4 (ANOVA), for HCl, MQR/MQr = 8.00; for H2SO4, MQR/MQr = 15.71 and for EDTA MQR/MQr = 52.20.
For the statistical F distribution, assuming six degrees of freedom for MQR and nine for MQr, with a confidence level of 95%, it can be observed that: F6,9 = 3.37 (F distribution table, 95%, n1 = 6 and n2 = 9). For MQR/MQr > F, the regression model for each of the studied eluents under study is significant.
Another parameter observed through ANOVA is the extent of lack of fit between predicted and observed values. This is accomplished by verifying the ratio between the quadratic average due to lack of fit and the quadratic average due to pure error (MQLOF/MQPE). The bigger this ratio is in respect to the F distribution with a specified confidence level, the less likely this model is to adjust the experimental values suitably. Thus, for HCl, H2SO4 and EDTA eluents, MQLOF/MQPE = 0.8, MQLOF/MQPE = 0.30 and MQLOF/MQPE = 0.48, respectively. For the statistical F distribution, assuming one degree of freedom for MQLOF and eigth for MQPE, with a confidence level of 95%, it can be observed that F1,8 = 5.32 (F distribution table, 95%, n1 = 1 and n2 = 8). As MQLOF/MQPE < F for all the models, there is no evidence of lack of fit. Therefore, the models for desorption of chromium for the three eluents are significant and properly adjust the values observed in the experiments.
CONCLUSION
The process of desorption of hexavalent chromium was studied using the experimental planning technique to evaluate three important factors: the concentration of eluent, the S/L ratio the process time. According to the experimental results obtained, the three factors evaluated are significant for the three eluents studied, as is possible to observe through the models obtained for each eluent (HCl, H2SO4 and EDTA).
It was also observed that for each of the eluents tested, MQR/MQr > F, indicating that the regression used for the models is valid. In addition, the models efficiently predict the experimental values.
Analysis of the factors under study revealed that H2SO4 is the most effective eluent in desorbing chromium from biomass.
NOMENCLATURE
ACKNOWLEDGEMENTS
The authors would like to thank FAPESP for providing the financial aid necessary to carry out this project and CEBIMar for furnishing the algae.
Received: February 20, 2002
Accepted: February 20, 2003
- Aldor, Ilana, Fourest, Eric and Volesky, B. Desorption of Cadmium from Algal Biosorbent. The Canadian Journal of Chemical Engineering, 73: p. 516-522 (1995).
- Kratochvil, D., Pimentel P. and Volesky, B. Removal of Trivalent and Hexavalent Chromium by Seaweed Biosorbent, Environ. Sci. Technol., 32, p. 2693-2698 (1998).
- Sigg, L., In Aquatic Surface Chemistry, Chemical Processes at the Particle. Water Interface, Stumm W., Ed; Wiley-Interscience: New York, p. 319-349 (1987).
- Siegel, B. Z. and Siegel, S. M., CRC Crit. Rev. Microbiol., vol. 10 (1973).
- Volesky, B, Biotechnology, 2., p. 135-149 (1988).
- Volesky, B., and Kuyucak, N. Desorption of Cobalt-laden Algal Biosorvent, Biotechnology and Bioengineering, 33, p. 815-822 (1988).
- Yang, J. and Volesky, B. Biosorption of Elution of Uranium with Seaweed Biomass. Presented at the IBS 99 conference - Spain (1999).
Publication Dates
-
Publication in this collection
01 Sept 2003 -
Date of issue
Sept 2003
History
-
Accepted
20 Feb 2003 -
Received
20 Feb 2002