Abstracts
INTRODUCTION: Obsessive-compulsive disorder (OCD) is a heterogeneous condition, in which subtypes have been proposed. Previous studies suggested that gender plays a relevant role in OCD phenotypic expression. This study aimed to review the literature on gender differences in clinical, genetic or familial aspects of OCD. METHOD: A conventional review was conducted, including all papers that investigated demographic, clinical, and genetic aspects of OCD according to gender. The search was based on data available in Medline and PsycINFO databases in the last 20 years, using as keywords: obsessive-compulsive disorder; and: gender, sex, male, female, demographic characteristics, clinical features, clinical characteristics, genetic, genes, genetics gender OCD, genes OCD, genes OCD males, genes OCD females. RESULTS: Sixty three of 487 phenotypical and genetics studies were selected. Most studies indicate that male patients are more likely than females to be single, present early onset of symptoms and chronic course of the disorder, greater social impairment, more sexual-religious and aggressive symptoms, and greater comorbidity with tic and substance use disorders. Female patients present more contamination/cleaning symptoms and greater comorbidity with eating and impulse-control disorders. Genetic and family studies are inconclusive, but suggest that gender may play a role in the disease expression. CONCLUSIONS: Gender is a relevant factor that should be taken into account when evaluating OCD patients. More studies are necessary to determine whether in fact it defines a homogeneous and particular group in OCD.
Obsessive-compulsive disorder; Gender identity; Sex; Phenotype; Genetics
INTRODUÇÃO: O transtorno obsessivo-compulsivo (TOC) é um quadro heterogêneo, no qual subtipos têm sido propostos. Estudos anteriores sugerem que gênero desempenha papel relevante na expressão fenotípica. O objetivo foi realizar uma revisão convencional da literatura sobre diferenças de gênero em relação a aspectos clínicos e genéticos ou familiares do TOC. MÉTODO: Realizou-se uma revisão convencional da literatura incluindo todos os artigos que investigaram aspectos sociodemográficos, clínicos e genéticos do TOC, de acordo com o gênero. A pesquisa foi baseada em publicações disponíveis nas bases de dados Medline e PsycInfo nos últimos 20 anos, usando como palavras-chave: obsessive-compulsive disorder (OCD), e: gender, sex, male, female, demographic characteristics, clinical features, clinical characteristics, genetic, genes, genetics gender OCD, genes OCD, genes OCD males, genes OCD females. RESULTADO: Sessenta e três artigos de fenótipo e genética foram selecionados. Na maioria dos estudos, o sexo masculino associou-se mais que o feminino com: ser solteiro, apresentar início mais precoce dos sintomas, maior prejuízo social, mais sintomas sexuais, religiosos e de agressão, e mais comorbidade com transtorno de tiques e abuso de substâncias. Pacientes do sexo feminino apresentam mais sintomas de contaminação/limpeza e mais comorbidade com transtornos alimentares e do controle de impulsos. Estudos genéticos e familiares são controversos, mas indicam que o gênero pode desempenhar um papel na expressão da doença. CONCLUSÃO: Gênero é um fator relevante a ser considerado na avaliação de pacientes com TOC. São necessários mais estudos para determinar se este fator define de fato um grupo homogêneo e particular de TOC.
Transtorno obsessivo-compulsivo; Identidade de gênero; Sexo; Fenótipo; Genética
REVIEW ARTICLE
Gender differences in obsessive-compulsive disorder: a literature review
Diferenças de gênero no transtorno obsessivo-compulsivo: uma revisão da literatura
Maria Alice de MathisI; Pedro de AlvarengaI; Guilherme FunaroI ; Ricardo Cezar TorresanII; Ivanil MoraesI; Albina Rodrigues TorresII; Monica L. ZilbermanI; Ana Gabriela HounieI
IDepartment of Psychiatry, Universidade de São Paulo (USP), São Paulo, Brazil
IIDepartment of Neurology, Psychology and Psychiatry, Botucatu Medical School, Universidade Estadual Paulista (Unesp), Brazil
Corresponding author Corresponding author: Maria Alice de Mathis Departamento de Psiquiatria, Faculdade de Medicina da USP Rua Dr. Ovídio Pires de Campos, 785, CEAPESQ, sala 7 05403-010 São Paulo, SP, Brazil Phone: (+55 11) 30697896; Fax: (+55 11) 30697895 E-mail: alicedemathis@gmail.com
ABSTRACT
INTRODUCTION: Obsessive-compulsive disorder (OCD) is a heterogeneous condition, in which subtypes have been proposed. Previous studies suggested that gender plays a relevant role in OCD phenotypic expression. This study aimed to review the literature on gender differences in clinical, genetic or familial aspects of OCD.
METHOD: A conventional review was conducted, including all papers that investigated demographic, clinical, and genetic aspects of OCD according to gender. The search was based on data available in Medline and PsycINFO databases in the last 20 years, using as keywords: obsessive-compulsive disorder; and: gender, sex, male, female, demographic characteristics, clinical features, clinical characteristics, genetic, genes, genetics gender OCD, genes OCD, genes OCD males, genes OCD females.
RESULTS: Sixty three of 487 phenotypical and genetics studies were selected. Most studies indicate that male patients are more likely than females to be single, present early onset of symptoms and chronic course of the disorder, greater social impairment, more sexual-religious and aggressive symptoms, and greater comorbidity with tic and substance use disorders. Female patients present more contamination/cleaning symptoms and greater comorbidity with eating and impulse-control disorders. Genetic and family studies are inconclusive, but suggest that gender may play a role in the disease expression.
CONCLUSIONS: Gender is a relevant factor that should be taken into account when evaluating OCD patients. More studies are necessary to determine whether in fact it defines a homogeneous and particular group in OCD.
Descriptors: Obsessive-compulsive disorder; Gender identity; Sex; Phenotype; Genetics.
RESUMO
INTRODUÇÃO: O transtorno obsessivo-compulsivo (TOC) é um quadro heterogêneo, no qual subtipos têm sido propostos. Estudos anteriores sugerem que gênero desempenha papel relevante na expressão fenotípica. O objetivo foi realizar uma revisão convencional da literatura sobre diferenças de gênero em relação a aspectos clínicos e genéticos ou familiares do TOC.
MÉTODO: Realizou-se uma revisão convencional da literatura incluindo todos os artigos que investigaram aspectos sociodemográficos, clínicos e genéticos do TOC, de acordo com o gênero. A pesquisa foi baseada em publicações disponíveis nas bases de dados Medline e PsycInfo nos últimos 20 anos, usando como palavras-chave: obsessive-compulsive disorder (OCD), e: gender, sex, male, female, demographic characteristics, clinical features, clinical characteristics, genetic, genes, genetics gender OCD, genes OCD, genes OCD males, genes OCD females.
RESULTADO: Sessenta e três artigos de fenótipo e genética foram selecionados. Na maioria dos estudos, o sexo masculino associou-se mais que o feminino com: ser solteiro, apresentar início mais precoce dos sintomas, maior prejuízo social, mais sintomas sexuais, religiosos e de agressão, e mais comorbidade com transtorno de tiques e abuso de substâncias. Pacientes do sexo feminino apresentam mais sintomas de contaminação/limpeza e mais comorbidade com transtornos alimentares e do controle de impulsos. Estudos genéticos e familiares são controversos, mas indicam que o gênero pode desempenhar um papel na expressão da doença.
CONCLUSÃO: Gênero é um fator relevante a ser considerado na avaliação de pacientes com TOC. São necessários mais estudos para determinar se este fator define de fato um grupo homogêneo e particular de TOC.
Descritores: Transtorno obsessivo-compulsivo; Identidade de gênero; Sexo; Fenótipo; Genética.
Introduction
Obsessive-Compulsive Disorder (OCD) is the fourth most prevalent psychiatric disorder in the United States.1 Epidemiological studies conducted in several countries reported current prevalence around 1% and lifetime prevalence ranging from 2% to 3%.2 Regarding gender, a bimodal distribution of age of onset has been described. Studies assessing pediatric samples report male preponderance (70%), whereas adult studies report equal gender distribution or a slight female preponderance.3 Males usually report early-onset of obsessive-compulsive symptoms (OCS) and association with tic disorders, attention deficit, and hyperactivity disorder (ADHD).4,5
OCD is a very heterogeneous disorder, and gender differences may help identify more homogeneous subgroups. This strategy may enhance our understanding of the pathophysiology of OCD, including gene-environment interactions.6 Also, as a considerable proportion of OCD patients is not responsive to first line treatments,7 the identification of more homogeneous subgroups may lead to more tailored and effective treatments.8 Until now, some OCD subgroups have been proposed based on age of OCS onset, presence of tics, and history of streptococcal infection.8,9
Gender has been shown to play a vital role in neurobiological aspects, psychosocial factors, and behavioral patterns in several psychiatric disorders. Whereas sex is a concept focused on biological aspects, gender is a broader concept, including a range of psychosocial aspects (e.g. attitudes, feelings, values, behaviours, and activities) that, through a process of social construction, differentiate men and women.10 Recent studies of OCD clinical samples suggest that gender moderates the genetic expression of this complex disorder, resulting in specific phenotypes.11-14
The aim of this study was to conduct a two-decade conventional literature review of gender differences in demographic, clinical, and genetic aspects of OCD.
Method
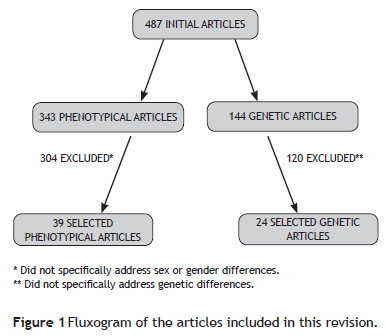
A conventional review of gender (or sex) differences in OCD patients was conducted, including all epidemiological, clinical, neuropsychological, and genetic or familial studies available in Pubmed and PsycINFO databases between January 1990 and August 2010. Only articles published in English and Portuguese were included. The phenotypical studies search strategy included the following keywords: obsessivecompulsive disorder (OCD) and: gender, sex, male, female, demographic characteristics, clinical features, clinical characteristics. Initially, 343 studies were found, but only 39 of them were included in the present review, as most articles (N = 304) did not specifically address gender differences and were therefore excluded. A few references cited in the selected articles that had not been previously found and that were considered pertinent were also included.
To investigate genetic or familial aspects, the following key words were used: obsessive-compulsive disorder (OCD) and genetic(s), genes, gender OCD, genes OCD, genes OCD males, genes OCD female, gene OCD male. Twenty-four genetic studies from a pull of 144 articles were finally included in this study (Figure 1).
Results
Phenotypical Studies
Several studies describe an earlier OCD onset among male patients, compared to femaless.14,15 Approximately one third of adult patients refer symptom onset in childhood26 and, among children, two thirds of cases are boys.3,13,27-28 Fontenelle et al. evaluated possible differences in Brazilian patients with early (up to 17 years of age) and late onset, confirming the predominance of men in the first group, which presented higher clinical severity when compared to the latter.20 In another study conducted in Brazil of 330 patients, men also prevailed in the early onset group (up to 11 years of age).29 An Italian study found a higher frequency of insidious onset and chronic course among male sufferers and more episodic course among females.19
According to Noshirvani et al.,17 similarly to schizophrenia, OCD cases with an early onset are usually more severe, reflecting the influence of cerebral lesions or constitutional aspects to which men are more vulnerable than women. Likewise, Bogetto et al.19 consider that early OCD onset is not strongly related to the impact of stressful life events but, conversely, could indicate a higher influence of biological lesions to which men are more predisposed. Biological theories propose that damage occurring during pregnancy is more global than that occurring after birth, and that the rapid growing organs are more vulnerable. Boys would be more susceptible than girls to developmental damage because among humans and other animals females are biologically stronger compared to males.30 A more accelerated growth of the prefrontal cortex (PFC) was observed between 8 and 14 years of age,31 a period in which most cases of OCD begin in childhood. This finding indicates that abnormal development of these structures could be related to early onset of symptoms.32 As the PFC continues to mature throughout adult life, abnormalities in later maturation processes could be related to late-onset OCD. Because the development of the caudate nucleus and the orbitofrontal cortex (OFC) - two central components of the OFC/ACC (anterior cingulate cortex) loop - is different for boys and girls, this could explain, at least in part, the higher prevalence of early-onset OCD among boys.32
Sobin et al.33 found some indirect indicators of a higher negative impact of OCD among male sufferers. For example, two thirds remain single versus only one third of women. Moreover, almost half of male patients continued to live with their original families or in assisted homes, compared with only 20% of women. Torresan et al.25 described earlier age of symptom interference among Brazilian men, who were also more likely than women to be single, which is consistent with findings from other countries.18,23,34 Several other authors have also described that women with OCD are more frequently married than men.14,22-24 Matsunaga et al.,34 evaluating 94 Japanese patients, found higher social interference of OCS among men, whereas women more frequently involved family members in their rituals. Therefore, an early onset among males can lead to a higher impact on several areas of daily life, including social adjustment and interpersonal relationships, which may be indirectly indicated by the lower rates of marriage in this population.
Differences in the content of OCS between genders have also been described in studies conducted in Spain, Italy, France, India, and Turkey. Women are more prone to contamination obsessions,22 usually associated with cleaning rituals;6,19,23,24 whereas sexual obsessions are more common among men.19,22,23,35 Several studies combine rates of sexual and religious symptoms, which are more frequent in males.6,24,36 Results are inconclusive regarding aggressive obsessions, with studies showing varying predominance between genders.22,36,37 In male, symmetry obsessions would be more common,23,24,38 as well as ordering,36,38 repeating,19 checking,23 and hoarding compulsions.39,40,41
Torresan et al.25 found among Brazilian men more sexual, religious, and symmetry obsessions, as well as more mental rituals. Chacon et al.42 evaluated OCD symptom dimensions in 40 pairs of Brazilian siblings with OCD. When both siblings were male, there was a significant correlation with contamination obsessions and cleaning rituals; and when both siblings were female, a correlation was found with hoarding symptoms.
The higher frequency of aggressive obsessions among women,37 which are usually characterized by fear of losing control, could be related to motherhood and fear of causing some harm to the newborn.37 Different ways of clustering symptoms hamper the comparisons between OCD studies to some extent. Some of them, for example, analyze aggressive obsession along with sexual and religious obsessions,36,38-42 which are more common among males. Such symptoms are called by some authors "forbidden" or "taboo" thoughts,43 usually manifested as ego-dystonic impulses. Aspects related to sexuality are particularly complex, involving a constant interaction between biological, psychosocial, and cultural factors, which are remarkably divergent between genders.
Likewise, the predominance of contamination-cleaning symptoms among females, a finding almost universal in OCD phenomenological studies, probably involve biological, psychological, and sociocultural aspects related to the female role in different societies. Mataix-Cols et al.,44 in a functional neuroimaging study during a provocation task of cleaning symptoms, described a greater activation of areas such as ventromedial prefrontal regions, medium temporal gyrus, right caudate nucleus, medium frontal gyrus, and left cingulus antero-dorsal gyrus in OCD patients, when compared to normal controls. A study of individuals without any psychiatric disorder suggested that the same cerebral areas contain a large number of sex hormone receptors, with relevant sexual dimorphism, with women presenting larger volumes of these structures.45 According to Labad et al.,6 this finding could at least partially explain the greater proportion of women with these symptoms.
Regarding psychiatric comorbidities in OCD, some of them seem to be more associated with one gender than the other.46 According to some authors, more women present co-occurring major depressive disorder.6,23,33 On the other hand, several studies found no gender differences regarding major depression comorbidity, which is the most common Axis I disorder in both genders.21,22,24,25,37,47 In an Italian study,19 men presented more social phobia, tic disorders, and hypomania episodes. In Brazil, Torresan et al.25 found more posttraumatic stress disorder and tic disorders among men, whereas women presented more simple phobias, eating disorders (anorexia nervosa), as well as more impulse control disorders in general, particularly skin picking and "compulsive" shopping.25 Another Brazilian study47 compared OCD patients with and without alcohol use disorders and found more men in the first group, in accordance with other studies.19,33 Men also presented more chronic tics and Tourette syndrome (TS), a clinical aspect that overlaps with early onset of OCS.9 Besides substance use disorders, men were more likely to present attention deficit, hyperactivity, social phobia, affective, and anxiety disorders in general.24,25,48
Some gender-related comorbidity findings in OCD, such as substance use and social phobia among men, and eating and impulse control disorders among women, seem to reflect a general trend of clinical samples with other mental disorders, as well as community samples. Therefore, it is noteworthy that major depression usually does not predominate among women with OCD, possibly indicating that depressive symptoms are intrinsically linked to OCS regardless of gender.
The results of the main clinical studies assessing OCD clinical features and gender are summarized in Table 1.
Genetics studies
Several genes of serotoninergic, dopaminergic, and other systems have been implicated in OCD pathogenesis, based on theories derived from treatment response to medications that exert effects on those pathways.
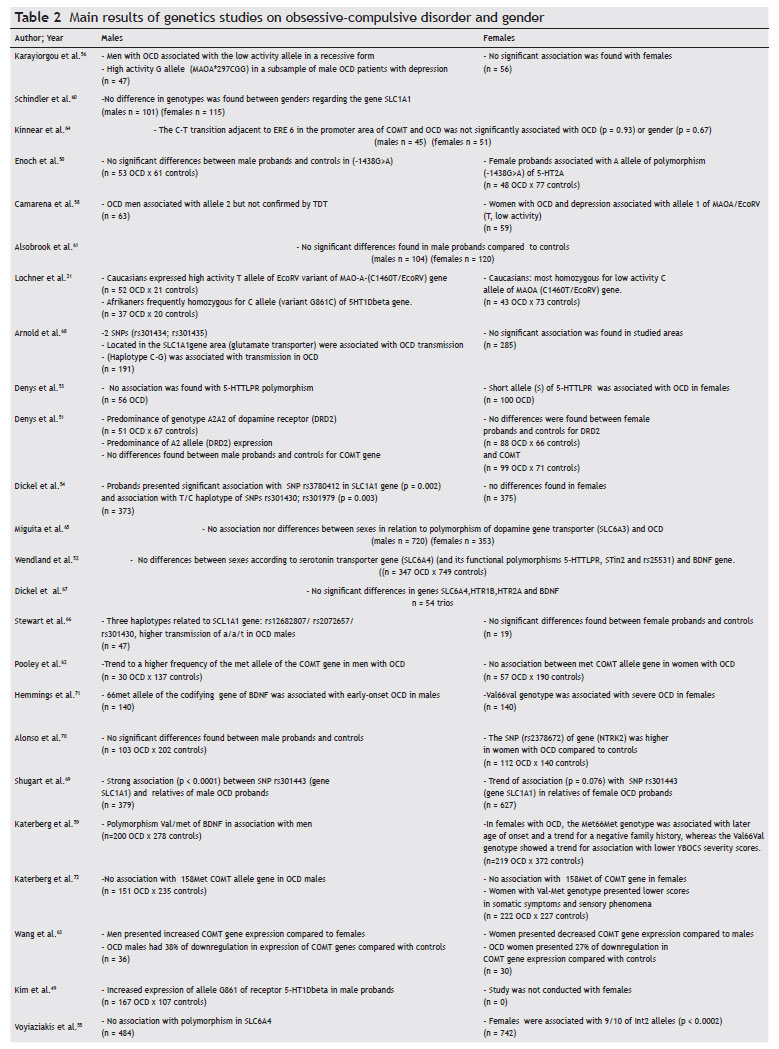
Table 2 presents the main findings for genetic differences between genders in OCD. The discussion will not focus on each individual study, as the main findings for each individual are summarized in the above-mentioned table. As for methodological details, they can be found in the original articles. For some studies, when genotypic data was available, we reanalyzed the allelic frequencies. Thus, some findings published as "trends" were not included by us as positive results (e.g., study 50) if after our reanalysis they turned to be negative (Izbicki et al., submitted).
The results of association or family-based studies are inconclusive, with some rendering positive associations with one gender, others negative and even some with positive findings for the opposite sex regarding several polymorphisms of candidate genes. Candidate genes in the serotonergic pathway were more frequently studied due to the consistent response of OCD to selective serotonin reuptake inhibitors (SSRI). However, findings have not been conclusive. For example, the same polymorphism of the gene 5HT1Dbeta [Single Nucleotide Polymorphism (SNP) G861C] was associated with OCD in males in two studies, one found association with the C allele,21 and the other with the G allele.49 However, the second study49 evaluated only males, not allowing an unequivocal association. On the other hand, OCD in women, but not in men, was associated with the A allele of the -1438G>A polymorphism of the promoter region of 5-HT2A50 and the short allele of 5-HTTLPR.51 However Wendland et al.52 found no association between OCD, gender and three other functional polymorphisms of the serotonin transporter gene (5-HTTLPR, STin2, and rs25531),52 despite using a larger sample than the one used by Denys et al.,53 which also found an association between the short allele of 5-HTTLPR and women with OCD.53
Dickel et al.54 also found an association between woman with OCD and the 5-HTTLPR long allele polymorphism, although, after correction for multiple testing, the finding lost significance.54 Although no individual study has been replicated, a pooled analysis of five TDT replication studies of the SLC6A4 5-HTTLPR polymorphism supports association (p = 0.02) between OCD and this polymorphism.
In the most recent study of SLC6A4 in OCD, Voyiaziakis et al.55 genotyped 1241 individuals from 278 families for 13 SNPs in the polymorphic region (LPR) indel (insertion/deletion) including haplotypes of the rs25531, VNTR polymorphisms in introns 2 and 7, and for a 381 bp deletion in the 3' portion of LPR. Data were analyzed with the Family Based Association Test (FBAT) and after sex stratification, women were associated with alleles 9/10 of the Int2 (p < 0.0002).55 This was the most significant finding in terms of statistical outcomes, which remained significant even after correction for multiple markers. The authors mentioned that many alleles related to the Int2 marker are associated with neuropsychiatric disorders, and as the majority of the affected individuals were women (66%) and there were differences due to gender in the prevalence of several Axis I disorders (women more affected with major depressive and panic disorders), it is plausible that the association observed with 9/10 alleles of Int2 VNTR in the sample may be explained by other Axis I disorder rather than by OCD. The most plausible explanation so far for the discrepancy in the findings lies in the small size of the sample.
An interesting finding is the association between MAOA gene variants and OCD. The monoamine oxidase is responsible for the oxidation of biogenic amines. Additionally, the gene encoding MAO-A is located on the X chromosome. There are few functional MAOA polymorphisms that have been studied in OCD: one is a 30 bp VNTR in the encoding region (intron 1); another is a T to G substitution in the exon 8 (T941G) that, despite not leading to aminoacid change, results in high enzymatic activity; and a third is a T to C silent substitution in exon 14, in which the T allele is related to lower enzymatic activity (C1460T/EcoRV). Karayiorgou et al.56 and Lochner et al.21 have found an association, in independent studies and with independent polymorphisms, between men with OCD and high activity alleles of the MAO-A gene. Camarena et al.58 and Lochner et al.,21 pointed towards an association between low activity MAO-A alleles and woman with OCD. These findings depict a probable example of sexual dimorphism, although its significance is currently not clear, as IMAOs are not as effective as SSRIs in OCD treatment and the outcomes found were based on small samples and different populations. Another explanation for the disparity of the findings is that the studied polymorphisms were close to the "true" polymorphism possibly associated with OCD, although they are not, per se, responsible for OCD.
The COMT gene (catecol-O-metil transferase) encodes a homonymous enzyme, which also metabolizes monoamines, with particular importance for dopamine in the prefrontal cortex.59 The COMT gene has several allelic variations, including the most studied polymorphism Val158Met, which is a functional SNP(G158A) of this gene, resulting in a substitution of valine (high activity allele) to methionine (low activity allele). In a study of 110 OCD families, Karayiorgou et al.56 found a sexually dimorphic association between a low enzymatic activity variant of the COMT gene and OCD males. This finding is consistent with a previous study11 in which the same association between OCD and Met allele was mentioned, although in a smaller sample. However Schindler et al.,60 Alsobrook et al.,61 Katerberg et al.,59 and Denys et al.51 have not found differences due to gender in the allelic or genotypic frequencies, although Katerberg et al.,59 found that women with the val-met genotype had lower scores in somatic- sensorial phenomena. Pooley et al.62 found a trend towards a higher frequency of the Met allele in OCD males, although genotypic data were not published, which did not allow us to confirm the finding.62 But this meta-analyses, based on data from all case-control studies published (n = 1,908 patients) showed association between the 158met allele and OCD in males (OR = 1.88, 95% CI 1.45-2.44, p < 0.001). Wang et al. (2009) in a COMT gene expression study found that not only did OCD patients present a lower expression of the COMT gene, in relation to controls, but also women had lower expression than men.63 The main controversial point of this study is that plasma levels of COMT do not necessarily reflect what occurs in the CNS, leaving the interpretation of the present results for future studies. Kinnear et al.64 failed to detect statistical significance in their sample stratified by gender when studying the association between a new polymorphism (Cà T transition) in the COMT gene, its levels of enzymatic activity, and the regulation by estrogen through the estrogen responsive elements in the promoter region of this gene in OCD patients.64 The positive findings are biologically plausible: compared to val-COMT, met-COMT individuals performed less well on tests of task shifting, had an electrophysiological profile indicative of decreased flexibility of prefrontal neural processing, and showed greater amygdala-orbitofrontal connectivity in response to negative emotional stimuli suggestive of inflexible emotional processing. It may be speculated that the stability and relative inflexibility of neural processing associated with met-COMT may become excessive, and thereby render the individual vulnerable to obsessions and compulsions. Anatomically, although COMT functionality is thought to be predominantly cortical, the val158met polymorphism also influences striatal dopamine activity, and may thus influence both cortical and subcortical levels, signaling within the corticostriatal loops that underlie the pathophysiology of OCD.
Denys et al.,53 studying dopamine gene receptors in a sample of 300 patients, half of them with OCD and half controls, concluded that male OCD patients expressed a predominance of A2A2 genotypes (p = 0.049), as well as the A2 DRD2 allele (p = 0.020).53 This genetic link, supporting the association of the dopaminergic system with the pathophysiology of OCD and a dimorphic pattern due to gender, was not found while investigating the association between the polymorphism located in the intron 8 of the dopamine transporter gene (SLC6A3 or DAT1) and OCD in a Brazilian sample of 208 patients and 865 healthy controls.65 The positive finding of Denys et al. (2006) needs replication as it is difficult to interpret: the association of the A2 allele with the OCD sample was unexpected and the A1 allele of the DRD2 TaqI A system has been found to be associated with a variety of addictive, impulsive, and compulsive disorders.
The studies of the genes in the glutamatergic system found a positive association between OCD, several SNPs, and haplotypes in the gene encoding the glutamate transporter, more associated with men with OCD, according to family-based studies. Although the associated SNPs have not been the same, all of them point towards the need for biological validation of the findings, as these have been the most consistent findings in the genetic research of OCD.66-69 Moreover, although the stratified analysis by gender status in Shugart et al.69 indicated that the male-only analysis showed more significant results than the female-only analysis in our samples, the pooled data set showed the strongest results. Therefore, their finding may not strongly support the results reported by the other three independent groups in which only the families with male probands contributed to the significant association of signs, which generally suggests an association with OCDl. Increasing evidence from several lines of study implicate a role for glutamatergic neurotransmission in OCD. Cerebrospinal fluid levels of glutamate have been shown to be increased in a sample of adult OCD patients compared to controls, and decreased glutamate levels have been reported following a successful paroxetine trial. Furthermore, successful preliminary open-label trials of two glutamatergic modulating agents (riluzole and n-acetylcysteine) have been reported.66
Finally, a few studies that searched for association between BDNF gene polymorphism and OCD also reported controversial findings.
Wendland et al.52 and Dickel et al.54 found no association between BDNF gene polymorphism and OCD, even after gender stratification.52,54 However, Alonso et al.70 in a sample of 215 OCD patients and 342 controls, found a significant association between the SNP (rs2378672) in the BDNF receptor gene (neurotrophic tyrosine kinase receptor type 2- NTRK2) and OCD in women (p < 0.0001).70 Katerberg et al.59 found that the BDNF's genotype Met66Met might be associated with a mild phenotype in Dutch women (but not in South Africans), and that the BDNF Val66Val genotype and the Val66 allele may have a role in sexual and religious obsessions.59
Hemmings et al.,71 who investigated the variant val66met in the BDNF gene, showed an association between the met66 allele and the male gender, as well as an association with early onset OCD. However, the genotype val66val was associated with more severe OCD in females. BDNF has been implicated in neuronal survival and in activity-dependent neuroplasticity. Studies in knockout mice as well as those using B cell lines suggest that BDNF modulates the serotonin transporter function. Although the BDNF gene is an attractive OCD candidate gene, from both brain development and neurotransmitter perspectives, the findings are still controversial.
Despite the fact that almost ten years have passed since the beginning of the Genome Project and the hope that genes involved in several diseases would be easily found, the results of genetic psychiatric studies remain inconclusive. These dissonant findings are typical of association studies of complex diseases carried out in different populations. The low rate of replication of these studies could be explained by methodological problems, such as differences in phenotypical definition and small statistical power of the studies. It has become clear over time that without establishing more homogeneous and valid subgroups and without the correct identification of the inherited phenotypes, molecular genetics by itself will not be able to identify the susceptible genes for OCD. Moreover, not only larger samples are required for association studies of the entire genome, but also a search for psychopathological and clinical inherited features and the characterization of homogeneous subgroups with consistent genetic validation. Finally, it should be borne in mind that genetic studies are the key to understanding the interaction between genes and environment, and that all efforts towards molecular studies are in vain if the phenotype and environmental factors are not carefully examined in advance.
Conclusions
This literature review points to some consistent differences between genders regarding OCD phenomenology, as well as to possible genetic differences regarding susceptible polymorphisms. The study of these differences may lead to the definition of more homogeneous phenotypes and contribute to the elucidation of etiological factors and development of new treatments. The investigation of OCS using the recently developed dimensional approach,73 which combines obsessions and compulsions that more frequently co-occur, may bring relevant contributions to the understanding of this heterogeneous disorder.6 The importance of such investigation is reinforced by studies that described considerable differences in neuroimaging results for different OCD symptom dimensions.74,75
In summary, gender may be a relevant factor to determine OCD clinical presentation and course, and may be considered to define more homogeneous subgroups. Although the reasons why OCD presentation differs between genders are not clear, one may speculate that they result from both biological and psychosocial influences. Among the biological factors, sexlinked genetic features and hormonal differences may be cited. Considering psychosocial aspects, gender-related social roles may affect the content of obsessions and compulsions. While age of onset seems to be a more genetic characteristic influenced by family history of OCD, the occurrence of sexual and aggression symptoms may be influenced by hormonal characteristics, as well as by gender role. For instance, it is widely accepted that testosterone levels influence sexual drive and preoccupations, impulsivity and aggressive behavior. Thus, regardless of the causes of gender differences found in patients, it is important to address this issue when it comes to the evaluation and treatment of OCD. Further studies are necessary to determine whether in fact gender defines a particular OCD subgroup. Phenotypical, epidemiological, genetic, family, neuroimaging, neuropsychological, and intervention studies taking into account aspects of gender differences may add to the understanding of OCD etiological mechanisms and possibly also to the development of more specific and effective therapeutic approaches.
References
1. Karno M, Golding J. Obsessive-Compulsive Disorder. In: Psychiatric Disorders in America. The epidemiological catchment area study. New York, NY: The Free Press; 1991. p. 204-19.
2. Torres AR, Lima MCP. Epidemiology of obsessive-compulsive disorder: a review. Rev Bras Psiquiatr. 2005;27(3):237-42.
3. Swedo SE, Rapoport JL, Leonard H, Lenane M, Cheslow D. Obsessive-compulsive disorder in children and adolescents. Clinical phenomenology of 70 consecutive cases. Arch Gen Psychiatry. 1989;46(4):335-41.
4. Riddle MA, Scahill L, King R, Hardin MT, Towbin KE, Ort SI, Leckman JF, Cohen DJ. Obsessive compulsive disorder in children and adolescents: phenomenology and family history. J Am Acad Child Adolesc Psychiatry. 1990;29(5):766-72.
5. Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, Alsobrook J, Peterson BS, Cohen DJ, Rasmussen SA, Goodman WK, McDougle CJ, Pauls DL. Symptoms of obsessive-compulsive disorder. Am J Psychiatry. 1997;154(7):911-7.
6. Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Jaurrieta N, Leckman JF, Vallejo J. Gender differences in obsessive-compulsive symptom dimensions. Depress Anxiety. 2008;25(10):832-8.
7. Miguel EC, Rauch SL, Jenike MA. Obsessive-compulsive disorder. Psychiatr Clin North Am. 1997;20(4):863-83.
8. Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, Chacon P, Pauls DL. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. 2005;10(3):258-75.
9. de Mathis MA, do Rosario MC, Diniz JB, Torres AR, Shavitt RG, Ferrão YA, Fossaluza V, de Bragança Pereira CA, Miguel EC. Obsessive-compulsive disorder: influence of age at onset on comorbidity patterns. Eur Psychiatry. 2008;23(3):187-94.
10. Olinto M. Reflexões sobre o uso do conceito de gênero e/ou sexo na epidemiologia: um exemplo nos modelos hierarquizados de análise. Rev Bras Epidemiol. 1998;1(2):162-9.
11. Karayiorgou M, Altemus M, Galke BL, Goldman D, Murphy DL, Ott J, Gogos JA.Genotype determining low catecholO-methyltransferase activity as a risk factor for obsessivecompulsive disorder. Proc Natl Acad Sci USA. 1997;94(9):4572-5.
12. Rasmussen SA, Eisen JL. Epidemiology of obsessive compulsive disorder. J Clin Psychiatry. 1990;51 Suppl:10-4.
13. Bellodi L, Sciuto G, Diaferia G, Ronchi P, Smeraldi E. Psychiatric disorders in the families of patients with obsessive-compulsive disorder. Psychiatry Res. 1992;42(2):111-20.
14. Castle DJ, Deale A, Marks IM. Gender differences in obsessive compulsive disorder. Aust N Z J Psychiatry. 1995;29(1):114-7.
15. Rasmussen SA, Tsuang MT. Clinical characteristics and family history in DSM-III obsessive-compulsive disorder. Am J Psychiatry. 1986;143(3):317-22.
16. Minichiello W, Baer L, Jenike MA, Holland A. Age of onset of major subtypes of obsessive-compulsive disorder. Anxiety Dis. 1990;4:147-50.
17. Noshirvani HF, Kasvikis Y, Marks IM, Tsakiris F, Monteiro WO. Gender-divergent aetiological factors in obsessive-compulsive disorder. Br J Psychiatry. 1991;158:260-3.
18. Neziroglu FA, Yaryura Tobías JA, Lemli JM, Yaryura RA. Demographic study of obsessive compulsive disorder. Acta Psiquiatr Psicol Am Lat. 1994;40(3):217-23.
19. Bogetto F, Venturello S, Albert U, Maina G, Ravizza L. Genderrelated clinical differences in obsessive-compulsive disorder. Eur Psychiatry. 1999;14(8):434-41.
20. Fontenelle LF, Mendlowicz MV, Marques C, Versiani M. Early- and late-onset obsessive-compulsive disorder in adult patients: an exploratory clinical and therapeutic study. J Psychiatr Res. 2003;37(2):127-33.
21. Lochner C, Hemmings SMJ, Kinnear CJ, Moolman-Smook JC, Corfield VA, Knowles JA, Niehaus DJ, Stein DJ. Gender in obsessive-compulsive disorder: clinical and genetic findings. Eur Neuropsychopharmacol. 2004;14(2):105-13.
22. Tükel R, Polat A, Genç A, Bozkurt O, Atli H. Gender-related differences among Turkish patients with obsessive-compulsive disorder. Compr Psychiatry. 2004;45(5):362-66.
23. Karadaĝ F, Oguzhanoglu NK, Ozdel O, Ateşci FC, Amuk T. OCD symptoms in a sample of Turkish patients: a phenomenological picture. Depress Anxiety. 2006;23(3):145-52.
24. Jaissorya T, Janardhan R, Srinath S, Thennarasu K. Sex differences in Indian patients with obsessive-compulsive disorder [Internet]. Compr Psychiatry. 2009 [citado 2010 Dez 18] .
25. Torresan RC, Ramos-Cerqueira ATDA, de Mathis MA, Diniz JB, Ferrão YA, Miguel EC, Torres AR. Sex differences in the phenotypic expression of obsessive-compulsive disorder: an exploratory study from Brazil. Compr Psychiatry. 2009;50(1):63-9.
26. Rosario-Campos MC, Leckman JF, Mercadante MT, Shavitt RG, Prado HS, Sada P, Zamignani D, Miguel EC. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry. 2001;158(11):1899-903.
27. Rapoport J, Elkins R, Langer DH, Sceery W, Buchsbaum MS, Gillin JC, Murphy DL, Zahn TP, Lake R, Ludlow C, Mendelson W. Childhood obsessive-compulsive disorder. Am J Psychiatry. 1981;138(12):1545-54.
28. Flament MF, Koby E, Rapoport JL, Berg CJ, Zahn T, Cox C, Denckla M, Lenane M. Childhood obsessive-compulsive disorder: a prospective follow-up study. J Child Psychol Psychiatry. 1990;31(3):363-80.
29. de Mathis MA, Diniz JB, Shavitt RG, Torres AR, Ferrão YA, Fossaluza V, Pereira C, Miguel E, do Rosario MC. Early onset obsessive-compulsive disorder with and without tics. CNS Spectr. 2009;14(7):362-70.
30. Lochner C, Stein D. Gender in obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Arch Women Ment Health. 2001;4:19-26.
31. Kanemura H, Aihara M, Aoki S, Araki T, Nakazawa S. Development of the prefrontal lobe in infants and children: a threedimensional magnetic resonance volumetric study. Brain Dev. 2003;25(3):195-9.
32. Maia TV, Cooney RE, Peterson BS. The neural bases of obsessivecompulsive disorder in children and adults. Dev Psychopathol. 2008;20(4):1251-83.
33. Sobin C, Blundell M, Weiller F, Gavigan C, Haiman C, Karayiorgou M. Phenotypic characteristics of Obsessive-Compulsive Disorder ascertained in adulthood. J Psychiatr Res. 1999;33(3):265-73.
34. Matsunaga H, Kiriike N, Matsui T, Miyata A, Iwasaki Y, Fujimoto K, Kasai S, Kojima M. Gender differences in social and interpersonal features and personality disorders among Japanese patients with obsessive-compulsive disorder. Compr Psychiatry. 2000;41(4):266-72.
35. Ratnasuriya RH, Marks IM, Forshaw DM, Hymas NF. Obsessive slowness revisited. Br J Psychiatry. 1991;159:273-4.
36. Hantouche EG, Lancrenon S. Modern typology of symptoms and obsessive-compulsive syndromes: results of a large French study of 615 patients. Encephale. 1 996;22 Spec No 1:9-21.
37. Lensi P, Cassano GB, Correddu G, Ravagli S, Kunovac JL, Akiskal HS. Obsessive-compulsive disorder. Familial-developmental history, symptomatology, comorbidity and course with special reference to gender-related differences. Br J Psychiatry. 1996;169(1):101-7.
38. Li Y, Marques L, Hinton DE, Wang Y, Xiao Z. Symptom dimensions in Chinese patients with obsessive-compulsive disorder. CNS Neurosci Ther. 2009;15(3):276-82.
39. Samuels J, Bienvenu OJ, Riddle MA, Cullen BAM, Grados MA, Liang KY, Hoehn-Saric R, Nestadt G. Hoarding in obsessive compulsive disorder: results from a case-control study. Behav Res Ther. 2002;40(5):517-28.
40. Fischer DJ, Himle JA, Hanna GL. Age and gender effects on obsessive-compulsive symptoms in children and adults. Depress Anxiety. 1996;4(5):237-9.
41. Stein DJ, Andersen EW, Overo KF. Response of symptom dimensions in obsessive-compulsive disorder to treatment with citalopram or placebo. Rev Bras Psiquiatr. 2007;29(4):303-7.
42. Chacon P, Rosario-Campos MC, Pauls DL, Hounie AG, Curi M, Akkerman F, Shimabokuro FH, de Mathis MA, Lopes AC, Hasler G, Miguel EC. Obsessive-compulsive symptoms in sibling pairs concordant for obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):551-5.
43. Matsunaga H, Hayashida K, Kiriike N, Maebayashi K, Stein DJ. The clinical utility of symptom dimensions in obsessive-compulsive disorder. Psychiatry Res. 2010;180(1):25-9.
44. Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61(6):564-76.
45. Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11(6):490-7.
46. Wheaton M, Timpano KR, Lasalle-Ricci VH, Murphy D. Characterizing the hoarding phenotype in individuals with OCD: associations with comorbidity, severity and gender. J Anxiety Disord. 2008;22(2):243-52.
47. Gentil AF, de Mathis MA, Torresan RC, Diniz JB, Alvarenga P, do Rosário MC, Cordioli AV, Torres AR, Miguel EC. Alcohol use disorders in patients with obsessive-compulsive disorder: the importance of appropriate dual-diagnosis. Drug Alcohol Depend. 2009;100(1-2):173-7.
48. Hounie, AG, Brotto S, Diniz J, Chacon P, Miguel, EC. Transtorno obsessivo-compulsivo: possíveis subtipos. Rev Bras Psiquiatr. 2001;23(Supl 2):13-6.
49. Kim SJ, Namkoong K, Kang JI, Kim C. Association of a 5-HT1Dbeta receptor gene polymorphism with obsessive-compulsive disorder in Korean male subjects. Neuropsychobiology. 2009;59(2):96-99.
50. Enoch MA, Greenberg BD, Murphy DL, Goldman D. Sexually dimorphic relationship of a 5-HT2A promoter polymorphism with obsessive-compulsive disorder. Biol Psychiatry. 2001;49(4):385-8.
51. Denys D, Van Nieuwerburgh F, Deforce D, Westenberg H. Association between the dopamine D2 receptor TaqI A2 allele and low activity COMT allele with obsessive-compulsive disorder in males. Eur Neuropsychopharmacol. 2006;16(6):446-50.
52. Wendland JR, Kruse MR, Cromer KR, Cromer KC, Murphy DL. A large case-control study of common functional SLC6A4 and BDNF variants in obsessive-compulsive disorder. Neuropsychopharmacology. 2007;32(12):2543-51.
53. Denys D, Van Nieuwerburgh F, Deforce D, Westenberg HGM. Association between serotonergic candidate genes and specific phenotypes of obsessive compulsive disorder. J Affect Disord. 2006;91(1):39-44.
54. Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH Jr, Hanna GL. Association studies of serotonin system candidate genes in early-onset obsessive-compulsive disorder. Biol Psychiatry. 2007;61(3):322-9.
55. Voyiaziakis E, Evgrafov O, Li D, Yoon H, Tabares P, Samuels J, Wang Y, Riddle MA, Grados MA, Bienvenu OJ, Shugart YY, Liang KY, Greenberg BD, Rasmussen SA, Murphy DL, Wendland JR, McCracken JT, Piacentini J, Rauch SL, Pauls DL, Nestadt G, Fyer AJ, Knowles JA. Mol Psychiatry. 2011;16(1):108-20.
56. Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, Ott J, Gogos JA. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol. Psychiatry. 1999;45(9):1178-89.
57. Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Jaurrieta N, Leckman JF, Vallejo J. Gender differences in obsessive-compulsive symptom dimensions. Depress Anxiety. 2008;25(10):832-8.
58. Camarena B, Rinetti G, Cruz C, Gómez A, de La Fuente JR, Nicolini H. Additional evidence that genetic variation of MAO-A gene supports a gender subtype in obsessive-compulsive disorder. Am J Med Genet. 2001;105(3):279-82.
59. Katerberg H, Lochner C, Cath DC, de Jonge P, Bochdanovits Z, Moolman-Smook JC, Hemmings SM, Carey PD, Stein DJ, Sondervan D, Boer JA, van Balkom AJ, Polman A, Heutink P. The role of the brain-derived neurotrophic factor (BDNF) val66met variant in the phenotypic expression of obsessive-compulsive disorder (OCD). Am J Med Genet B Neuropsychiatr Genet. 2009;150B(8):1050-62.
60. SchindlerKM, RichterMA, Kennedy JL, PatoMT, PatoCN. Association between homozygosity at the COMT gene locus and obsessive compulsive disorder. Am J Med Genet. 2000;96(6):721-4.
61. Alsobrook JP, Zohar AH, Leboyer M, Chabane N, Ebstein RP, Pauls DL. Association between the COMT locus and obsessivecompulsive disorder in females but not males. Am J Med Genet. 2002;114(1):116-20.
62. Pooley EC, Fineberg N, Harrison PJ. The met(158) allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case-control study and meta-analysis. Mol Psychiatry. 2007;12(6):556-61.
63. Wang Z, Xiao Z, Inslicht SS, Tong H, Jiang W, Wang X, Metzler T, Marmar CR, Jiang S. Low expression of catecholamine-Omethyl-transferase gene in obsessive-compulsive disorder. J Anxiety Disord. 2009;23(5):660-4.
64. Kinnear C, Niehaus DJ, Seedat S, Moolman-Smook JC, Corfield VA, Malherbe G, Potgieter A, Lombard C, Stein DJ. Obsessivecompulsive disorder and a novel polymorphism adjacent to the oestrogen response element (ERE 6) upstream from the COMT gene. Psychiatr Genet. 2001;11(2):85-7.
65. Miguita K, Cordeiro Q, Siqueira-Roberto J, Shavitt RG, Castillo JCR, Castillo AR, Miguel EC, Vallada H. Association analysis between a VNTR intron 8 polymorphism of the dopamine transporter gene (SLC6A3) and obsessive- compulsive disorder in a Brazilian sample. Arq Neuropsiquiatr. 2007;65(4A):936-41.
66. Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, Jenike MA, Pauls DL. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1027-33.
67. Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):778-85.
68. Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessivecompulsive disorder. Arch Gen Psychiatry. 2006;63(7):769-76.
69. Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Rasmussen SA, Cullen B, Hoehn-Saric R, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Bienvenu OJ, Riddle MA, Liang KY, Nestadt G. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(6):886-92.
70. Alonso P, Gratacòs M, Menchón JM, Saiz-Ruiz J, Segalàs C, Baca-García E, Labad J, Fernández-Piqueras J, Real E, Vaquero C, Pérez M, Dolengevich H, González JR, Bayés M, de Cid R, Vallejo J, Estivill X. Extensive genotyping of the BDNF and NTRK2 genes define protective haplotypes against obsessive-compulsive disorder. Biol Psychiatry. 2008;63(6):619-28.
71. Hemmings SMJ, Kinnear CJ, Van der Merwe L, Lochner C, Corfield VA, Moolman-Smook JC, Stein DJ. Investigating the role of the brain-derived neurotrophic factor (BDNF) val66met variant in obsessive-compulsive disorder (OCD). World J. Biol. Psychiatry. 2008;9(2):126-34.
72. Katerberg H, Cath DC, Denys DA, Heutink P, Polman A, van Nieuwerburgh FC, Deforce DL, Bochdanovits Z, van Balkom AJ, den Boer JA. The role of the COMT Val(158)Met polymorphism in the phenotypic expression of obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):167-76.
73. Rosario-Campos MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, Katsovich L, Scahill L, King RA, Woody SR, Tolin D, Hollander E, Kano Y, Leckman JF. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. 2006;11(5):495-504.
74. Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, Brammer MJ, Williams SC, Speckens A, Phillips ML. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry. 2003;53(6):482-93.
75. Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessivecompulsive disorder. Arch Gen Psychiatry. 2004;61(7):720-30.
Received on July 13, 2011; accepted on July 31, 2011
- 1. Karno M, Golding J. Obsessive-Compulsive Disorder. In: Psychiatric Disorders in America. The epidemiological catchment area study. New York, NY: The Free Press; 1991. p. 204-19.
- 2. Torres AR, Lima MCP. Epidemiology of obsessive-compulsive disorder: a review. Rev Bras Psiquiatr. 2005;27(3):237-42.
- 3. Swedo SE, Rapoport JL, Leonard H, Lenane M, Cheslow D. Obsessive-compulsive disorder in children and adolescents. Clinical phenomenology of 70 consecutive cases. Arch Gen Psychiatry. 1989;46(4):335-41.
- 4. Riddle MA, Scahill L, King R, Hardin MT, Towbin KE, Ort SI, Leckman JF, Cohen DJ. Obsessive compulsive disorder in children and adolescents: phenomenology and family history. J Am Acad Child Adolesc Psychiatry. 1990;29(5):766-72.
- 5. Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, Alsobrook J, Peterson BS, Cohen DJ, Rasmussen SA, Goodman WK, McDougle CJ, Pauls DL. Symptoms of obsessive-compulsive disorder. Am J Psychiatry. 1997;154(7):911-7.
- 6. Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Jaurrieta N, Leckman JF, Vallejo J. Gender differences in obsessive-compulsive symptom dimensions. Depress Anxiety. 2008;25(10):832-8.
- 7. Miguel EC, Rauch SL, Jenike MA. Obsessive-compulsive disorder. Psychiatr Clin North Am. 1997;20(4):863-83.
- 8. Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, Chacon P, Pauls DL. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. 2005;10(3):258-75.
- 9. de Mathis MA, do Rosario MC, Diniz JB, Torres AR, Shavitt RG, Ferrão YA, Fossaluza V, de Bragança Pereira CA, Miguel EC. Obsessive-compulsive disorder: influence of age at onset on comorbidity patterns. Eur Psychiatry. 2008;23(3):187-94.
- 10. Olinto M. Reflexões sobre o uso do conceito de gênero e/ou sexo na epidemiologia: um exemplo nos modelos hierarquizados de análise. Rev Bras Epidemiol. 1998;1(2):162-9.
- 11. Karayiorgou M, Altemus M, Galke BL, Goldman D, Murphy DL, Ott J, Gogos JA.Genotype determining low catecholO-methyltransferase activity as a risk factor for obsessivecompulsive disorder. Proc Natl Acad Sci USA. 1997;94(9):4572-5.
- 12. Rasmussen SA, Eisen JL. Epidemiology of obsessive compulsive disorder. J Clin Psychiatry. 1990;51 Suppl:10-4.
- 13. Bellodi L, Sciuto G, Diaferia G, Ronchi P, Smeraldi E. Psychiatric disorders in the families of patients with obsessive-compulsive disorder. Psychiatry Res. 1992;42(2):111-20.
- 14. Castle DJ, Deale A, Marks IM. Gender differences in obsessive compulsive disorder. Aust N Z J Psychiatry. 1995;29(1):114-7.
- 15. Rasmussen SA, Tsuang MT. Clinical characteristics and family history in DSM-III obsessive-compulsive disorder. Am J Psychiatry. 1986;143(3):317-22.
- 16. Minichiello W, Baer L, Jenike MA, Holland A. Age of onset of major subtypes of obsessive-compulsive disorder. Anxiety Dis. 1990;4:147-50.
- 17. Noshirvani HF, Kasvikis Y, Marks IM, Tsakiris F, Monteiro WO. Gender-divergent aetiological factors in obsessive-compulsive disorder. Br J Psychiatry. 1991;158:260-3.
- 18. Neziroglu FA, Yaryura Tobías JA, Lemli JM, Yaryura RA. Demographic study of obsessive compulsive disorder. Acta Psiquiatr Psicol Am Lat. 1994;40(3):217-23.
- 19. Bogetto F, Venturello S, Albert U, Maina G, Ravizza L. Genderrelated clinical differences in obsessive-compulsive disorder. Eur Psychiatry. 1999;14(8):434-41.
- 20. Fontenelle LF, Mendlowicz MV, Marques C, Versiani M. Early- and late-onset obsessive-compulsive disorder in adult patients: an exploratory clinical and therapeutic study. J Psychiatr Res. 2003;37(2):127-33.
- 21. Lochner C, Hemmings SMJ, Kinnear CJ, Moolman-Smook JC, Corfield VA, Knowles JA, Niehaus DJ, Stein DJ. Gender in obsessive-compulsive disorder: clinical and genetic findings. Eur Neuropsychopharmacol. 2004;14(2):105-13.
- 22. Tükel R, Polat A, Genç A, Bozkurt O, Atli H. Gender-related differences among Turkish patients with obsessive-compulsive disorder. Compr Psychiatry. 2004;45(5):362-66.
- 24. Jaissorya T, Janardhan R, Srinath S, Thennarasu K. Sex differences in Indian patients with obsessive-compulsive disorder [Internet]. Compr Psychiatry. 2009 [citado 2010 Dez 18]
- 25. Torresan RC, Ramos-Cerqueira ATDA, de Mathis MA, Diniz JB, Ferrão YA, Miguel EC, Torres AR. Sex differences in the phenotypic expression of obsessive-compulsive disorder: an exploratory study from Brazil. Compr Psychiatry. 2009;50(1):63-9.
- 26. Rosario-Campos MC, Leckman JF, Mercadante MT, Shavitt RG, Prado HS, Sada P, Zamignani D, Miguel EC. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry. 2001;158(11):1899-903.
- 27. Rapoport J, Elkins R, Langer DH, Sceery W, Buchsbaum MS, Gillin JC, Murphy DL, Zahn TP, Lake R, Ludlow C, Mendelson W. Childhood obsessive-compulsive disorder. Am J Psychiatry. 1981;138(12):1545-54.
- 28. Flament MF, Koby E, Rapoport JL, Berg CJ, Zahn T, Cox C, Denckla M, Lenane M. Childhood obsessive-compulsive disorder: a prospective follow-up study. J Child Psychol Psychiatry. 1990;31(3):363-80.
- 29. de Mathis MA, Diniz JB, Shavitt RG, Torres AR, Ferrão YA, Fossaluza V, Pereira C, Miguel E, do Rosario MC. Early onset obsessive-compulsive disorder with and without tics. CNS Spectr. 2009;14(7):362-70.
- 30. Lochner C, Stein D. Gender in obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Arch Women Ment Health. 2001;4:19-26.
- 31. Kanemura H, Aihara M, Aoki S, Araki T, Nakazawa S. Development of the prefrontal lobe in infants and children: a threedimensional magnetic resonance volumetric study. Brain Dev. 2003;25(3):195-9.
- 32. Maia TV, Cooney RE, Peterson BS. The neural bases of obsessivecompulsive disorder in children and adults. Dev Psychopathol. 2008;20(4):1251-83.
- 33. Sobin C, Blundell M, Weiller F, Gavigan C, Haiman C, Karayiorgou M. Phenotypic characteristics of Obsessive-Compulsive Disorder ascertained in adulthood. J Psychiatr Res. 1999;33(3):265-73.
- 34. Matsunaga H, Kiriike N, Matsui T, Miyata A, Iwasaki Y, Fujimoto K, Kasai S, Kojima M. Gender differences in social and interpersonal features and personality disorders among Japanese patients with obsessive-compulsive disorder. Compr Psychiatry. 2000;41(4):266-72.
- 35. Ratnasuriya RH, Marks IM, Forshaw DM, Hymas NF. Obsessive slowness revisited. Br J Psychiatry. 1991;159:273-4.
- 36. Hantouche EG, Lancrenon S. Modern typology of symptoms and obsessive-compulsive syndromes: results of a large French study of 615 patients. Encephale. 1 996;22 Spec No 1:9-21.
- 37. Lensi P, Cassano GB, Correddu G, Ravagli S, Kunovac JL, Akiskal HS. Obsessive-compulsive disorder. Familial-developmental history, symptomatology, comorbidity and course with special reference to gender-related differences. Br J Psychiatry. 1996;169(1):101-7.
- 38. Li Y, Marques L, Hinton DE, Wang Y, Xiao Z. Symptom dimensions in Chinese patients with obsessive-compulsive disorder. CNS Neurosci Ther. 2009;15(3):276-82.
- 39. Samuels J, Bienvenu OJ, Riddle MA, Cullen BAM, Grados MA, Liang KY, Hoehn-Saric R, Nestadt G. Hoarding in obsessive compulsive disorder: results from a case-control study. Behav Res Ther. 2002;40(5):517-28.
- 40. Fischer DJ, Himle JA, Hanna GL. Age and gender effects on obsessive-compulsive symptoms in children and adults. Depress Anxiety. 1996;4(5):237-9.
- 41. Stein DJ, Andersen EW, Overo KF. Response of symptom dimensions in obsessive-compulsive disorder to treatment with citalopram or placebo. Rev Bras Psiquiatr. 2007;29(4):303-7.
- 42. Chacon P, Rosario-Campos MC, Pauls DL, Hounie AG, Curi M, Akkerman F, Shimabokuro FH, de Mathis MA, Lopes AC, Hasler G, Miguel EC. Obsessive-compulsive symptoms in sibling pairs concordant for obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):551-5.
- 43. Matsunaga H, Hayashida K, Kiriike N, Maebayashi K, Stein DJ. The clinical utility of symptom dimensions in obsessive-compulsive disorder. Psychiatry Res. 2010;180(1):25-9.
- 44. Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61(6):564-76.
- 45. Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11(6):490-7.
- 46. Wheaton M, Timpano KR, Lasalle-Ricci VH, Murphy D. Characterizing the hoarding phenotype in individuals with OCD: associations with comorbidity, severity and gender. J Anxiety Disord. 2008;22(2):243-52.
- 47. Gentil AF, de Mathis MA, Torresan RC, Diniz JB, Alvarenga P, do Rosário MC, Cordioli AV, Torres AR, Miguel EC. Alcohol use disorders in patients with obsessive-compulsive disorder: the importance of appropriate dual-diagnosis. Drug Alcohol Depend. 2009;100(1-2):173-7.
- 48. Hounie, AG, Brotto S, Diniz J, Chacon P, Miguel, EC. Transtorno obsessivo-compulsivo: possíveis subtipos. Rev Bras Psiquiatr. 2001;23(Supl 2):13-6.
- 49. Kim SJ, Namkoong K, Kang JI, Kim C. Association of a 5-HT1Dbeta receptor gene polymorphism with obsessive-compulsive disorder in Korean male subjects. Neuropsychobiology. 2009;59(2):96-99.
- 50. Enoch MA, Greenberg BD, Murphy DL, Goldman D. Sexually dimorphic relationship of a 5-HT2A promoter polymorphism with obsessive-compulsive disorder. Biol Psychiatry. 2001;49(4):385-8.
- 51. Denys D, Van Nieuwerburgh F, Deforce D, Westenberg H. Association between the dopamine D2 receptor TaqI A2 allele and low activity COMT allele with obsessive-compulsive disorder in males. Eur Neuropsychopharmacol. 2006;16(6):446-50.
- 52. Wendland JR, Kruse MR, Cromer KR, Cromer KC, Murphy DL. A large case-control study of common functional SLC6A4 and BDNF variants in obsessive-compulsive disorder. Neuropsychopharmacology. 2007;32(12):2543-51.
- 53. Denys D, Van Nieuwerburgh F, Deforce D, Westenberg HGM. Association between serotonergic candidate genes and specific phenotypes of obsessive compulsive disorder. J Affect Disord. 2006;91(1):39-44.
- 54. Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH Jr, Hanna GL. Association studies of serotonin system candidate genes in early-onset obsessive-compulsive disorder. Biol Psychiatry. 2007;61(3):322-9.
- 55. Voyiaziakis E, Evgrafov O, Li D, Yoon H, Tabares P, Samuels J, Wang Y, Riddle MA, Grados MA, Bienvenu OJ, Shugart YY, Liang KY, Greenberg BD, Rasmussen SA, Murphy DL, Wendland JR, McCracken JT, Piacentini J, Rauch SL, Pauls DL, Nestadt G, Fyer AJ, Knowles JA. Mol Psychiatry. 2011;16(1):108-20.
- 56. Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, Ott J, Gogos JA. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol. Psychiatry. 1999;45(9):1178-89.
- 57. Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Jaurrieta N, Leckman JF, Vallejo J. Gender differences in obsessive-compulsive symptom dimensions. Depress Anxiety. 2008;25(10):832-8.
- 58. Camarena B, Rinetti G, Cruz C, Gómez A, de La Fuente JR, Nicolini H. Additional evidence that genetic variation of MAO-A gene supports a gender subtype in obsessive-compulsive disorder. Am J Med Genet. 2001;105(3):279-82.
- 59. Katerberg H, Lochner C, Cath DC, de Jonge P, Bochdanovits Z, Moolman-Smook JC, Hemmings SM, Carey PD, Stein DJ, Sondervan D, Boer JA, van Balkom AJ, Polman A, Heutink P. The role of the brain-derived neurotrophic factor (BDNF) val66met variant in the phenotypic expression of obsessive-compulsive disorder (OCD). Am J Med Genet B Neuropsychiatr Genet. 2009;150B(8):1050-62.
- 60. SchindlerKM, RichterMA, Kennedy JL, PatoMT, PatoCN. Association between homozygosity at the COMT gene locus and obsessive compulsive disorder. Am J Med Genet. 2000;96(6):721-4.
- 61. Alsobrook JP, Zohar AH, Leboyer M, Chabane N, Ebstein RP, Pauls DL. Association between the COMT locus and obsessivecompulsive disorder in females but not males. Am J Med Genet. 2002;114(1):116-20.
- 62. Pooley EC, Fineberg N, Harrison PJ. The met(158) allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case-control study and meta-analysis. Mol Psychiatry. 2007;12(6):556-61.
- 63. Wang Z, Xiao Z, Inslicht SS, Tong H, Jiang W, Wang X, Metzler T, Marmar CR, Jiang S. Low expression of catecholamine-Omethyl-transferase gene in obsessive-compulsive disorder. J Anxiety Disord. 2009;23(5):660-4.
- 64. Kinnear C, Niehaus DJ, Seedat S, Moolman-Smook JC, Corfield VA, Malherbe G, Potgieter A, Lombard C, Stein DJ. Obsessivecompulsive disorder and a novel polymorphism adjacent to the oestrogen response element (ERE 6) upstream from the COMT gene. Psychiatr Genet. 2001;11(2):85-7.
- 65. Miguita K, Cordeiro Q, Siqueira-Roberto J, Shavitt RG, Castillo JCR, Castillo AR, Miguel EC, Vallada H. Association analysis between a VNTR intron 8 polymorphism of the dopamine transporter gene (SLC6A3) and obsessive- compulsive disorder in a Brazilian sample. Arq Neuropsiquiatr. 2007;65(4A):936-41.
- 66. Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, Jenike MA, Pauls DL. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1027-33.
- 67. Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):778-85.
- 68. Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessivecompulsive disorder. Arch Gen Psychiatry. 2006;63(7):769-76.
- 69. Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Rasmussen SA, Cullen B, Hoehn-Saric R, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Bienvenu OJ, Riddle MA, Liang KY, Nestadt G. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(6):886-92.
- 70. Alonso P, Gratacòs M, Menchón JM, Saiz-Ruiz J, Segalàs C, Baca-García E, Labad J, Fernández-Piqueras J, Real E, Vaquero C, Pérez M, Dolengevich H, González JR, Bayés M, de Cid R, Vallejo J, Estivill X. Extensive genotyping of the BDNF and NTRK2 genes define protective haplotypes against obsessive-compulsive disorder. Biol Psychiatry. 2008;63(6):619-28.
- 71. Hemmings SMJ, Kinnear CJ, Van der Merwe L, Lochner C, Corfield VA, Moolman-Smook JC, Stein DJ. Investigating the role of the brain-derived neurotrophic factor (BDNF) val66met variant in obsessive-compulsive disorder (OCD). World J. Biol. Psychiatry. 2008;9(2):126-34.
- 72. Katerberg H, Cath DC, Denys DA, Heutink P, Polman A, van Nieuwerburgh FC, Deforce DL, Bochdanovits Z, van Balkom AJ, den Boer JA. The role of the COMT Val(158)Met polymorphism in the phenotypic expression of obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):167-76.
- 73. Rosario-Campos MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, Katsovich L, Scahill L, King RA, Woody SR, Tolin D, Hollander E, Kano Y, Leckman JF. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. 2006;11(5):495-504.
- 74. Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, Brammer MJ, Williams SC, Speckens A, Phillips ML. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry. 2003;53(6):482-93.
- 75. Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessivecompulsive disorder. Arch Gen Psychiatry. 2004;61(7):720-30.
Corresponding author:
Publication Dates
-
Publication in this collection
20 Dec 2011 -
Date of issue
Dec 2011
History
-
Received
13 July 2011 -
Accepted
31 July 2011