ABSTRACT
Electron microscopy is routinely used to identify viral infections in protozoan parasites. These viruses have been described as non-enveloped and icosahedral structures with a diameter of 30-60 nm. Most of them are classified within the non-segmented dsRNA Totiviridae family. We observed virus-like particles (VLPs) through transmission electron microscopy in the cytoplasm of Trypanosoma cruzi epimastigotes grown in cultures. Clusters of electrodense enveloped VLPs having a diameter of 48 nm were also observed. These clusters appear to have been released from distended Golgi cisternae. Furthermore, a paracrystalline array of electrodense, non-enveloped VLPs (with a diameter of 32 nm) were found in distended Golgi cisternae or as smaller clusters at a distance from the RE or Golgi. We cannot rule out that the 48 nm enveloped VLPs belong to the ssRNA Flaviviridae family because they are within its size range. The localization of enveloped VLPs is consistent with the replication strategy of these viruses that transit through the Golgi to be released at the cell surface. Due to the size and shape of the 32 nm non-enveloped VLPs, we propose that they belong to the dsRNA Totiviridae family. This is the first description of cytoplasmic enveloped and non-enveloped VLPs in T. cruzi epimastigotes.
Trypanosoma cruzi; Arthropod-borne viruses; Protozoan infections; Flaviviridae; Totiviridae
INTRODUCTION
The parasite Trypanosoma cruzi is the etiological agent of Chagas disease, which is endemic to Latin America and affects approximately 6 to 7 million people11. World Health Organization. La enfermedad de Chagas (tripanosomiasis americana). Nota descriptiva N°340. [cited 2016 Dec 15]. Available from: http://www.who.int/mediacentre/factsheets/fs340/es/index.html
http://www.who.int/mediacentre/factsheet...
. This protozoan has a round nucleus, with one nucleolus and heterochromatin patches close to the nuclear envelope and a single mitochondrion with an enlarged portion (termed kinetoplast) that harbors the mitochondrial DNA (kDNA)22. Rassi A Jr, Rassi A, Marcondes de Rezende J. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am. 2012;26:275-91.
3. Motta MC, de Souza W, Thiry M. Immunocytochemical detection of DNA and RNA in endosymbiont-bearing trypanosomatids. FEMS Microbiol Lett. 2003;221:17-23.-44. Elias MC, da Cunha JP, de Faria FP, Mortara RA, Freymuller E, Schenkman S. Morphological events during the Trypanosoma cruzi cell cycle. Protist. 2007;158:147-57.. Virus-like particles (VLPs) have been described in protozoa such as Naegleria55. Schuster FL. Intranuclear virus-like bodies in the amoeboflagellate Naegleria gruberi. J Protozool. 1969;16:724-7., Entamoeba66. Diamond LS, Mattern CF, Bartgis IL. Viruses of Entamoeba histolytica. I. Identification of transmissible virus-like agents. J Virol. 1972;9:326-41. and Plasmodium77. Samso A, Lenstra R, Le Bras J, Galibert F. Isolation of virus-like particles of in vitro cultures of Plasmodium falciparum. C R Acad Sci III. 1988;307:317-22.. VLPs have also been described in the cytoplasm of Leishmania hertigi and in Endotrypanum promastigotes88. Molyneux DH. Virus-like particles in Leishmania parasites. Nature. 1974;249:588-9.
9. Croft SL, Molyneux DH. Studies on the ultrastructure, virus-like particles and infectivity of Leishmania hertigi. Ann Trop Med Parasitol. 1979;73:213-26.-1010. Croft SL, Chance ML, Gardener PJ. Ultrastructural and biochemical characterization of stocks of Endotrypanum. Ann Trop Med Parasitol. 1980;74:585-9.. Bacterial symbionts have furthermore been reported in Crithidia, in Blastochritidia1111. Chang KP. Ultrastructure of symbiotic bacteria in normal and antibiotic-treated Blastocrithidia culicis and Crithidia oncopelti. J Protozool. 1974;21:699-707., and in Trypanosoma cobitis, a fish trypanosome1212. Lewis JW, Ball SJ. Electron microscope study of the epimastigotes of a fish trypanosome, Trypanosoma cobitis, in culture. Ann Trop Med Parasitol. 1981;75:533-8.. In studies of the host-parasite relationship of T. melophagium in the hindgut, epimastigotes were found to be attached to the cuticular intima by hemidesmosomes1313. Molyneux DH, Heywood P. Evidence for the incorporation of virus-like particles into Trypanosoma. Z Parasitenkd. 1984;70:553-6.. VLPs were observed within expanded attached flagella of epimastigotes whose appearance was the same as those found in the midgut cells and lumen of Melophagus ovinus. These observations suggest that VLPs or viruses of a vector can be incorporated into parasites, probably through vesicles that can be observed when attached flagella are in contact with the insect cuticle1414. Brun R. Ultrastructure and life cycle of Herpetomonas muscarum, “Herpetomonas mirabilis” and Crithidia luciliae in Chrysomyia chloropyga. Acta Trop. 1974;32:219-90.,1515. Molyneux DH, Jenni L. Mechanoreceptors, feeding behaviour and trypanosome transmission in Glossina. Trans R Soc Trop Med Hyg. 1981;75:160-3.. There have been several reports of VLPs in vectors of trypanosomes, notably Glossina spp.1616. Jenni L. Virus-like particles in a strain of G. morsitans centralis, Machado 1970. Trans R Soc Trop Med Hyg. 1973;67:295.
17. Jenni L, Steiger R. Viruslike particles of Glossina fuscipes fuscipes Newst. 1910. Acta Trop. 1974;31:177-80.
18. Jaenson TG. Virus-like rods associated with salivary gland hyperplasia in tsetse, Glossina pallidipes. Trans R Soc Trop Med Hyg. 1978;72:234-8.-1919. Otieno LH, Kokwaro ED, Chimtawi M, Onyango P. Prevalence of enlarged salivary glands in wild populations of Glossina pallidipes in Kenya, with a note on the ultrastructure of the affected organ. J Invertebr Pathol 1980;36:113-8. and in Melophagus ovinus2020. Molyneux DH. Trypanosma (Megatrypanum) melophagium: modes of attachment of parasites to mid-gut, hindgut and rectum of the sheep ked, Melophagus ovinus. Acta Trop. 1975;32:65-74.. Other vectors of trypanosomes and Leishmania are known to be vectors of “arboviruses” (sandflies)2121. Tesh RB, Modi GB. Growth and transovarial transmission of Chandipura virus (Rhabdoviridae: Vesiculovirus) in Phlebotomus papatasi. Am J Trop Med Hyg. 1983;32:621-3.,2222. Endris RG, Tesh RB, Young DG. Transovarial transmission of Rio Grande virus (Bunyaviridae: Phlebovirus) by the sand fly, Lutzomyia anthophora. Am J Trop Med Hyg. 1983;32:862-4..
Most of the viruses currently known to infect protozoa belong to the Totiviridae, a family that has linear dsRNA genome and also infects fungi. The genome size of members of this family that infect protozoa is between 4.6 to 6.27 kb. The capsid of these viruses is icosahedral and has T=1 symmetry with 120 coat protein molecules per particle. Viral replication occurs in the cytoplasm via a headful mechanism, whereby empty particles areinitially assembled and subsequently the genome is packaged into these particles. The final size of the virions is 33-40 nm2323. Mahy BW, Van Regenmortel MH, editors. Encyclopedia of virology. 3rd ed. Amsterdam: Academic; 2008..
Single-stranded RNA viruses may also infect protozoa. A candidate virus named LR-1 was identified in L. braziliensis guyanensis, associated with 32 nm spherical particles and a genome size of approximately 6 kb. The authors conclude that LR-1 is probably a single-stranded RNA virus2424. Tarr PI, Aline RF Jr, Smiley BL, Scholler J, Keithly J, Stuart K. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci U S A. 1988;85:9572-5.. Furthermore, Leishmania guyanensis has been shown to contain 32 nm cytoplasmic LRV-1 viral particles that have both single-stranded and double-stranded RNA2525. Weeks R, Aline RF Jr, Myler PJ, Stuart K. LRV1 viral particles in Leishmania guyanensis contain double-stranded or single-stranded RNA. J Virol. 1992;66:1389-93.. Leishmania infected by the RNA virus 1 (LRV1), a double-stranded RNA virus of the Totiviridae family, have been shown to enhance the production of cytokines and chemokines (TNF-“a”, CXCL10, CCL5, IL-6) in macrophages2626. Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Schutz F, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331:775-8.. The discovery that LRV1 can change the course of Leishmania infections has heightened the search for viral hyperpathogens in other infections2727. Hartley MA, Ronet C, Zangger H, Beverley SM, Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol. 2012;2:99..
To our knowledge, no enveloped viruses that infect protozoa have been identified. The present study describes the presence of both enveloped and non-enveloped VLPs in Trypanosoma cruzi epimastigotes and the analysis of their ultrastructure.
MATERIALS AND METHODS
Parasites
Trypanosoma cruzi was isolated from feces of an infected specimen of Triatoma barberi that was obtained from the State of Queretaro, Mexico, and has been maintained in our laboratory for 24 years. The epimastigote stage was cultured in RPMI medium at 28 °C and the trypomastigote stage was maintained in infected CD-1 mice.
Parasite culture
Metacyclic trypomastigotes were obtained from contents of the anal glands of triatoma (after manual compression), that had previously been fed on pigeons2828. Deane MP, Lenzi HL, Jansen AM. Double development cycle of Trypanosoma cruzi in the opossum. Parasitol Today. 1986;2:146-7.. Metacyclic trypomastigotes were inoculated intraperitoneally into three CD-1 female mice. Fifteen days post-inoculation, the mice were anesthetized to obtain whole blood that was then aliquoted. This blood sample was divided and partly inoculated into Novy-MacNeal-Nicolle medium (N.N.N) and the other part into RPMI-1640. In these media, the trypomastigote-stage transforms into epimastigotes. These were grown at 28 °C in RPMI medium (Life Technologies, Gaithersburg, MD, USA) containing 10 U penicillin per milliliter, 25 µg streptomycin per milliliter, 25 mM HEPES buffer (Life Technologies, Gaithersburg, MD. USA), 2 mM L-glutamine (Sigma, St. Louis, MO, USA) and 10% heat-inactivated fetal bovine serum (Life Technologies, Gaithersburg, MD.USA)2929. Fernández-Presas AM, Tato P, Becker I, Solano S, Copitin N, Berzunza M, et al. Specific antibodies induce apoptosis in Trypanosoma cruzi epimastigotes. Parasitol Res. 2010;106:1327-37.. Parasites were harvested after 5-8 days of culture (log growth phase) according to previously published methods3030. Powell MR, Kuhn RE. Measurement of cytolytic antibody in experimental Chagas’ disease using a terminal radiolabelling procedure. J Parasitol. 1980;66:399-406.. The bloodstream trypomastigote stage was obtained from blood of infected mice. These bloodstream trypomastigotes (10 × 106) were used to infect monolayers of Vero cells during 48 h. The bloodstream trypomastigotes penetrate into VERO cells, where they transform into amastigotes, which divide and transform into tissue cultured-derived trypomastigotes, lysing Vero cells after 5 days post-infection. Tissue culture-derived trypomastigotes were obtained from the culture supernatant and were processed for electron microscopy.
Regarding the amastigotes, three CD-1 female mice (4 weeks old, 20 ± 2 g) were injected intraperitoneally with 1 × 106T. cruzi -bloostream trypomastigotes. After twenty days post-infection, mice were sacrified under anesthesia and the heart muscle tissues were recovered, cut into pieces and washed three times in PBS pH 7.2 at 4 °C, and then processed for electron microscopy in order to observe T.cruzi amastigotes nests.
The guidelines for Laboratory Animal Care established by the ethical committee of the School of Medicine at the National Autonomous University of Mexico were strictly followed.
Electron microscopy study
In total, 5 x 106 epimastigotes in the log growth phase, amastigotes from mouse heart and trypomastigotes from Vero cells were processed for electron microscopy. The specimens were washed three times in fresh medium at 4 °C, rinsed in 0.15 M cacodylate buffer and fixed in Karnovsky´s solution for 1 h at room temperature3131. Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of osmolality for use in electron microscopy. J Cell Biol. 1965;27:1A-149A.. They were then transferred into 0.1 M cacodylate buffer, postfixed in 1% of osmium tetroxide, dehydrated and embedded in Poly/Bed 812/ DMP30 (Polysciences, Inc, Valley Road Warrington, PA 18976). Ultrathin sections were obtained with a diamond knife on a Reichert Ultracut S microtome, collected on copper grids and stained first with 5% uranyl acetate in water and then with 0.25% lead citrate in 0.1M NaOH3232. Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208-12.. The sections were photographed using a Jeol JEM 1200 EXII transmission electron microscope.
RESULTS
We studied the ultrastructure of Trypanosoma cruzi epimastigotes grown in culture (Figures 1 and 2). The plasma membrane, subpellicular microtubules, Golgi cisternae, mitochondria, kinetoplast, nucleus, and flagellus of the epimastigotes were intact. Additionally, reservosomes (an acidic organelle that accumulates large amounts of cruzipain, the major cysteine proteinase) were found in Trypanosoma cruzi epimastigotes (Figure 1). The parasites showed vacuoles of different sizes in addition to acidocalcisomes (acidic Ca2+-storage vacuoles) (Figure 2). The parasites also showed some lipid bodies (Figure 1). Trypanosomes were surrounded by a unit membrane showing a characteristic homogenous 3-layered structure, that was approximately 7-9 nm in thickness (Figures 1 and 2). This plasma membrane covered the whole body surface of the parasite, including the pocket from which the flagellum originates and the flagellum itself. The single flagellum of the parasite originated from the basal body, close to the kinetoplast. It emerged from a deep invagination in the plasma membrane, termed the flagellar pocket, which was located anterior to the nucleus in the pro- and epimastigote stages) (Figure 1). All trypanosomatids show an array of microtubules underlying the plasma membrane. The kinetoplast was observed to run parallel to the subpellicular microtubules. The Golgi apparatus (Figure 1) was located near the base of the flagellar pocket and was surrounded by numerous smooth and coated vesicles (Figures 1 and 2). The endoplasmic reticulum (ER), consisting of a nuclear envelope and a connected system of cisternal or tubular membranes was found closely associated with the plasma membrane. The parasite displayed a spherical nucleus, and irregular patches of coarsely granular condensed chromatin were found beneath the nuclear envelope (Figure 2).
Transmission electron micrograph of T.cruzi epimastigotes showing normal morphology of the kinetoplast (K), plasma membrane (PM), subpellicular microtubules (MT), mitochondria (M), Golgi apparatus (GA), basal body (Bb), flagellar pocket (FP), flagellum (F), lipid bodies (LP), endoplasmic reticulum (ER) and reservosomes (R)
Transmission electron micrograph of T. cruzi epimastigotes cultured in RPMI medium. The longitudinal section shows a well preserved nucleus (N), kinetoplast (K), subpellicular microtubules (MT), Golgi apparatus (GA), mitochondria (M), plasma membrane (PM), endoplasmic reticulum (ER) and acidocalcisomes (A)
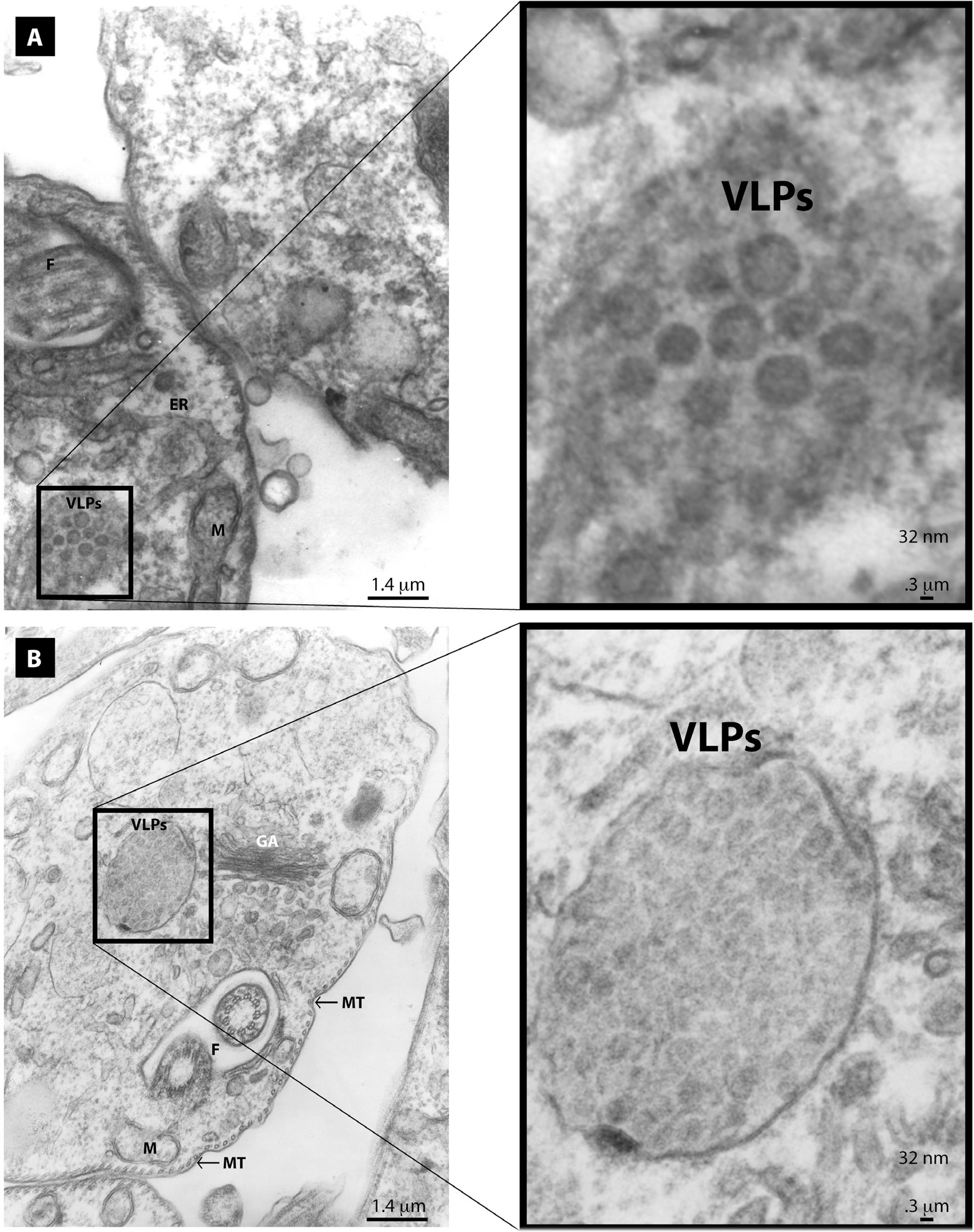
Through electron microscopy we also observed two classes of clases VLPs in the cytoplasm of T. cruzi epimastigotes (Figure 3a and 3b) and (Figure 4a and 4b). The larger VLPs (48 nm) comprised enveloped spherical or oblong electrodense particles. These clusters were observed close to the Golgi apparatus, partially surrounded by a membrane. They had the appearance of having being expelled in a burst-like manner from distended Golgi cisternae (Figure 3b). They were also evidenced as smaller clusters at a distance from the Golgi apparatus (Figure 3a). We also observed smaller electrodense VLPs (32 nm) that were non-enveloped spherical particles. These non-enveloped VLPs were observed as paracrystalline clusters in distended Golgi cisternae (Figure 4b) or as smaller clusters at a distance from the ER or Golgi (Figure 4a). VLPs were only observed in the cytoplasm of few parasites (about 1.9% of the of the T. cruzi epimastigotes analyzed), whereas no parasites were found to harbor both types of VLPs. Based on these findings, the two morphologically distinct VLPs observed suggest that the parasites were infected with different viruses. Furthermore, viral infections seem to be a rare event, only present in a small fraction of the parasites. With these findings, we were also interested in analyzing the possible presence of VLPs in the other T. cruzi life stages. It is noteworthy that we only found VLPs in epimastigotes with no evidence of virus-like particles in trypomastigotes or amastigotes, despite intensive analysis (Figure 5a and 5b).
Electron micrographs of T. cruzi epimastigotes grown in RPMI medium showing 48 nm VLPs: a) a small cluster of VLPs at a distance from the Golgi apparatus (GA); b) a large cluster of VLPs localized close to the GA. Also shown are the nucleus (N); nucleolus (n), endoplasmic reticulum (ER), flagellum (F) and mitochondria (M). Image was used with the permission of: Fernández-Presas A.M, Tay-Zavala J, Becker-Fauser I, Robert-Guerrero L, Willms, K. Ultrastructural damage of Trypanosoma cruzi epimastigotes exposed to decomplemented immune sera. Parasitol Res. 2001:87:619-25. © SpringerNature
Electron micrographs of T. cruzi epimastigotes grown in RPMI medium showing 32 nm VLPs: a) small clusters of VLPs are found at a distance from the endoplasmic reticulum (ER); b) large clusters of VLPs are localized close to the Golgi apparatus (GA). Also shown are the flagellum (F), microtubules (MT), and mitochondria (M)
Electron micrographs of morphological stages of Trypanosoma cruzi: a) the trypomastigote form exhibits typical morphology with the presence of a rugose surface. No VLPs were observed in the cytoplasm. Flagellum (F), undulating membrane (UM), Golgi apparatus (GA), mitochondria (M) and nucleus (N); b) amastigote nests within an infected murine myocardium. No VLPs were found. Note the seven amastigotes/ epimastigotes within myocytes. Nucleus (N), nucleolus (n), kinetoplast (K), muscle fibers (MF)
DISCUSSION
We show herein the probable infection of T. cruzi epimastigotes with two types of viruses, both enveloped (48 nm) and non-enveloped (32 nm). Viruses had not been identified in flagellates until 1986 and to date only members of the Totiviridae family are known to infect flagellates2727. Hartley MA, Ronet C, Zangger H, Beverley SM, Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol. 2012;2:99.,3333. Wang AL, Wang CC. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986;21:269-76.
34. Wang AL, Wang CC. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A. 1986;83:7956-60.-3535. Wang AL, Wang CC. Viruses of the protozoa. Annu Rev Microbiol. 1991;45:251-63.. Furthermore, flagellates have been shown to only harbor dsRNA viruses of the Totiviridae family that are vertically passed between individual hosts. Primitive bacterial and eukaryotic species have been shown to harbor dsRNA, and based on RNA polymerase sequence analysis it has been suggested that dsRNA viruses gave rise to +RNA viruses. In some fungal totivirus infections, only intracellular transmission has been demonstrated, suggesting that the virions probably lack the machinery for cell transmission3636. Ghabrial SA, Nibert ML. Victorivirus, a new genus of fungal viruses in the family Totiviridae. Arch Virol. 2009;154:373-9.. Single-stranded RNA viruses have been suspected to infect flagellates for some decades; however, no definitive proof exists so far.
The Flaviviridae family seems to be evolutionarily old, based on RNA-dependent RNA polymerase sequence analysis. The flaviviruses replicate in mammalian and arthropod hosts. They are closely related to the following three families of viruses that exclusively infect plants: Luteoviridae, Potyviridae, and Tobamoviridae3737. Bruenn JA. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991;19:217-26.. Among the flaviviruses, progeny viruses are thought to assembled by budding to the ER and passing through the Golgi apparatus to be released on the cell surface.
It is noteworthy that we observed two types of viral particles including VLPs (32 nm) that seem different from the enveloped VLPs (48 nm) and are thus unlikely to be related. Based on morphology, size and cytoplasmic localization, we cannot exclude the possibility that the 32 nm VLPs observed in T. cruzi epimastigotes belong to the Totiviridae family, the only virus family that has been demonstrated to infect protozoans.
In this study, we observed enveloped VLPs in the cytoplasm of T. cruzi epimastigotes for the first time. These particles were localized close to the Golgi apparatus in all cases, either as large clusters partially surrounded by membrane, as if they originated from disrupted Golgi cisternae, or in smaller clusters nearby. These observations are suggestive of an assembly pathway with budding to an intracellular membrane compartment. Given the rarity of finding VLPs (1.9%), we cannot exclude the possibility that accumulation of VLPs at the Golgi apparatus is rare or aberrant, considering that the particles are in transit and do not normally accumulate at this stage. Considering the three major enveloped arbovirus families, Bunyaviridae, Flaviviridae and Togaviridae, the 48 nm VLPs are only in the size range of flaviviruses. This family is one of the most versatile virus families, because they can affect both arthropod vectors and mammalian hosts. However, the finding of flaviviruses in protists is unprecedented. Further studies will be needed to determine if -the observed VLPs are indeed viruses of the Flaviviridae family.
It is not clear how T. cruzi have acquired these VLPs in natural conditions, either from the reduviid vector or from the mammalian host. It is also possible that VLPs were acquired in laboratory conditions. We speculate that one of the possible mechanisms of parasite infection in laboratory conditions could have occurred during the feeding of triatomas infected with T. cruzi on pigeons harboring Flaviviridae. Pigeons have been reported to become infected by Flaviviridae family members3838. Liu P, Lu H, Li S, Wu Y, Gao GF, Su J. Duck egg drop syndrome virus: an emerging Tembusu-related flavivirus in China. Sci China Life Sci. 2013;56:701-10.. Thus, T. cruzi parasites living within the triatomas could acquire the viruses after feeding on pigeon blood infected with the flavivirus. It is unlikely that T. cruzi epimastigotes could have acquired VLPs from triatomas infected with a Flaviviridae family member because, to the best of our knowledge, triatomas have not been shown to be infected by either of these viruses. Triatomas have been shown to harbor viruses such as Triatoma virus (TrV) that consist of a mixed population having RNA (full) or lacking RNA (empty). These were described as non-enveloped +ssRNA viruses belonging to the insect virus Dicistroviridae family3939. Agirre J, Aloria K, Arizmendi JM, Iloro I, Elortza F, Sanchez-Eugenia R, et al. Capsid protein identification and analysis of mature Triatoma virus (TrV) virions and naturally occurring empty particles. Virology. 2011;409:91-101.. The analysis of their virus capsids and smaller particles showed that the protein composition of different TrVs might be heterogeneous4040. Estrozi LF, Neumann E, Squires G, Rozas-Dennis G, Costabel M, Rey FA, et al. Phasing of the Triatoma virus diffraction data using a cryo-electron microscopy reconstruction. Virology. 2008;375:85-93.. Yet, it is also possible that the epimastigotes exposed to the Triatoma virus (TrV) present in the intestinal cells of the vector could be the source of the 32 nm VLPs observed in this study.
We have no explanation as to why VLPs were only detected in the epimastigote stage. Possibly the lack of expression in the other stages of the life cycle could be related to immune pressure exerted by the mammalian host on the parasite stages found in these hosts. Thus, trypomastigotes or amastigotes infected with viral-like particles could possibly be eliminated by the cytotoxic immune response of the mammalian host, such as that enacted by NK cells, that recognize viral particles and eliminate the trypomastigotes or amastigotes containing VLPs. The fact that epimastigotes possibly harbor VLPs could have biological implications for the disease severity, because an epimastigote-like stage has been described in infected heart-smears of mice4141. Hoare CA. The trypanosomes of mammals: a zoological monograph. Oxford: Blackwell Scientific Publications; 1972.
42. Guttreridge WE, Rogerson G W. Biochemical aspects of the biology of Trypanosoma cruzi. In: Lumsden WH, Evans DA, editors. Biology of the Kinetoplastida. Michigan: Academic Press; 1979. v.2, p. 619-52
43. Mehlhorn H, editor. Parasitology in focus : facts and trends. Berlin: Springer; 1988.
44. Andrews NW, Robbins ES, Ley V, Hong KS, Nussenzweig V. Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi. J Exp Med. 1988;167:300-14.
45. Faucher JF, Baltz T, Petry KG. Detection of an “epimastigote-like” intracellular stage of Trypanosoma cruzi. Parasitol Res. 1995;81:441-3.-4646. Fernandez-Presas AM, Zavala JT, Fauser IB, Merchant MT, Guerrero LR, Willms K. Ultrastructural damage of Trypanosoma cruzi epimastigotes exposed to decomplemented immune sera. Parasitol Res. 2001;87:619-25.. Yet, a more detailed investigation of VLPs in the trypomastigote and amastigote stages will be fundamental to better understand their biological implications on the severity of T. cruzi infection/ disease.
In contrast to T. cruzi, other trypanosomatids have been shown to become infected by members of the Totiviridae family. This is the case for Leishmania guyanensis, that has been shown to become infected with (LRV1), a member of the Totiviridae family. The infection of this parasite by the virus elicits a strong pro-inflammatory immune response via toll-like receptor 3, and the resulting inflammatory cascade leads to an enhanced virulence and resistance to anti-Leishmania drugs2727. Hartley MA, Ronet C, Zangger H, Beverley SM, Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol. 2012;2:99.. Leishmania RNA virus (LRV) infection of L. guyanensis has been related to a destructive hyperinflammatory response and elevated parasitemia in infected patients4747. Zangger H, Ronet C, Desponds C, Kuhlmann FM, Robinson J, Hartley MA, et al. Detection of Leishmania RNA virus in Leishmania parasites. PLoS Negl Trop Dis. 2013;7:e2006.. Unlike Leishmania RNA virus infections that lead to disease exacerbation, in T. cruzi, no enveloped viral-like particles have yet been reported.
Future studies are needed to clarify this observation; yet at this point it is probably important to show the first evidence of possible enveloped viral-like particles in T. cruzi epimastigotes. While we do not know the biological and clinical significance of the presence of a putative totivirus in T. cruzi epimastigotes, further studies are warranted to analyze whether these VLPs possibly affect the virulence of the parasite. Isolation and characterization of these enveloped VLPs will help to determine their possible role in the disease physiopathology. The microscopic evidence of VLPs is a novel finding in T. cruzi epimastigotes. The biological implication of this finding remains to be established.
ACKNOWLEDGMENTS
We are grateful to Dra. Maria Lucia Taylor da Cunha e Mello for reviewing the manuscript and for her careful reading and suggestions that greatly improved the manuscript, and we are also grateful to Dr. Rodolfo Paredes Díaz and Omar Agni García for their technical assistance.
REFERENCES
-
1World Health Organization. La enfermedad de Chagas (tripanosomiasis americana). Nota descriptiva N°340. [cited 2016 Dec 15]. Available from: http://www.who.int/mediacentre/factsheets/fs340/es/index.html
» http://www.who.int/mediacentre/factsheets/fs340/es/index.html -
2Rassi A Jr, Rassi A, Marcondes de Rezende J. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am. 2012;26:275-91.
-
3Motta MC, de Souza W, Thiry M. Immunocytochemical detection of DNA and RNA in endosymbiont-bearing trypanosomatids. FEMS Microbiol Lett. 2003;221:17-23.
-
4Elias MC, da Cunha JP, de Faria FP, Mortara RA, Freymuller E, Schenkman S. Morphological events during the Trypanosoma cruzi cell cycle. Protist. 2007;158:147-57.
-
5Schuster FL. Intranuclear virus-like bodies in the amoeboflagellate Naegleria gruberi. J Protozool. 1969;16:724-7.
-
6Diamond LS, Mattern CF, Bartgis IL. Viruses of Entamoeba histolytica. I. Identification of transmissible virus-like agents. J Virol. 1972;9:326-41.
-
7Samso A, Lenstra R, Le Bras J, Galibert F. Isolation of virus-like particles of in vitro cultures of Plasmodium falciparum. C R Acad Sci III. 1988;307:317-22.
-
8Molyneux DH. Virus-like particles in Leishmania parasites. Nature. 1974;249:588-9.
-
9Croft SL, Molyneux DH. Studies on the ultrastructure, virus-like particles and infectivity of Leishmania hertigi. Ann Trop Med Parasitol. 1979;73:213-26.
-
10Croft SL, Chance ML, Gardener PJ. Ultrastructural and biochemical characterization of stocks of Endotrypanum. Ann Trop Med Parasitol. 1980;74:585-9.
-
11Chang KP. Ultrastructure of symbiotic bacteria in normal and antibiotic-treated Blastocrithidia culicis and Crithidia oncopelti. J Protozool. 1974;21:699-707.
-
12Lewis JW, Ball SJ. Electron microscope study of the epimastigotes of a fish trypanosome, Trypanosoma cobitis, in culture. Ann Trop Med Parasitol. 1981;75:533-8.
-
13Molyneux DH, Heywood P. Evidence for the incorporation of virus-like particles into Trypanosoma. Z Parasitenkd. 1984;70:553-6.
-
14Brun R. Ultrastructure and life cycle of Herpetomonas muscarum, “Herpetomonas mirabilis” and Crithidia luciliae in Chrysomyia chloropyga. Acta Trop. 1974;32:219-90.
-
15Molyneux DH, Jenni L. Mechanoreceptors, feeding behaviour and trypanosome transmission in Glossina. Trans R Soc Trop Med Hyg. 1981;75:160-3.
-
16Jenni L. Virus-like particles in a strain of G. morsitans centralis, Machado 1970. Trans R Soc Trop Med Hyg. 1973;67:295.
-
17Jenni L, Steiger R. Viruslike particles of Glossina fuscipes fuscipes Newst. 1910. Acta Trop. 1974;31:177-80.
-
18Jaenson TG. Virus-like rods associated with salivary gland hyperplasia in tsetse, Glossina pallidipes. Trans R Soc Trop Med Hyg. 1978;72:234-8.
-
19Otieno LH, Kokwaro ED, Chimtawi M, Onyango P. Prevalence of enlarged salivary glands in wild populations of Glossina pallidipes in Kenya, with a note on the ultrastructure of the affected organ. J Invertebr Pathol 1980;36:113-8.
-
20Molyneux DH. Trypanosma (Megatrypanum) melophagium: modes of attachment of parasites to mid-gut, hindgut and rectum of the sheep ked, Melophagus ovinus. Acta Trop. 1975;32:65-74.
-
21Tesh RB, Modi GB. Growth and transovarial transmission of Chandipura virus (Rhabdoviridae: Vesiculovirus) in Phlebotomus papatasi. Am J Trop Med Hyg. 1983;32:621-3.
-
22Endris RG, Tesh RB, Young DG. Transovarial transmission of Rio Grande virus (Bunyaviridae: Phlebovirus) by the sand fly, Lutzomyia anthophora. Am J Trop Med Hyg. 1983;32:862-4.
-
23Mahy BW, Van Regenmortel MH, editors. Encyclopedia of virology. 3rd ed. Amsterdam: Academic; 2008.
-
24Tarr PI, Aline RF Jr, Smiley BL, Scholler J, Keithly J, Stuart K. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci U S A. 1988;85:9572-5.
-
25Weeks R, Aline RF Jr, Myler PJ, Stuart K. LRV1 viral particles in Leishmania guyanensis contain double-stranded or single-stranded RNA. J Virol. 1992;66:1389-93.
-
26Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Schutz F, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331:775-8.
-
27Hartley MA, Ronet C, Zangger H, Beverley SM, Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol. 2012;2:99.
-
28Deane MP, Lenzi HL, Jansen AM. Double development cycle of Trypanosoma cruzi in the opossum. Parasitol Today. 1986;2:146-7.
-
29Fernández-Presas AM, Tato P, Becker I, Solano S, Copitin N, Berzunza M, et al. Specific antibodies induce apoptosis in Trypanosoma cruzi epimastigotes. Parasitol Res. 2010;106:1327-37.
-
30Powell MR, Kuhn RE. Measurement of cytolytic antibody in experimental Chagas’ disease using a terminal radiolabelling procedure. J Parasitol. 1980;66:399-406.
-
31Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of osmolality for use in electron microscopy. J Cell Biol. 1965;27:1A-149A.
-
32Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208-12.
-
33Wang AL, Wang CC. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986;21:269-76.
-
34Wang AL, Wang CC. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A. 1986;83:7956-60.
-
35Wang AL, Wang CC. Viruses of the protozoa. Annu Rev Microbiol. 1991;45:251-63.
-
36Ghabrial SA, Nibert ML. Victorivirus, a new genus of fungal viruses in the family Totiviridae. Arch Virol. 2009;154:373-9.
-
37Bruenn JA. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991;19:217-26.
-
38Liu P, Lu H, Li S, Wu Y, Gao GF, Su J. Duck egg drop syndrome virus: an emerging Tembusu-related flavivirus in China. Sci China Life Sci. 2013;56:701-10.
-
39Agirre J, Aloria K, Arizmendi JM, Iloro I, Elortza F, Sanchez-Eugenia R, et al. Capsid protein identification and analysis of mature Triatoma virus (TrV) virions and naturally occurring empty particles. Virology. 2011;409:91-101.
-
40Estrozi LF, Neumann E, Squires G, Rozas-Dennis G, Costabel M, Rey FA, et al. Phasing of the Triatoma virus diffraction data using a cryo-electron microscopy reconstruction. Virology. 2008;375:85-93.
-
41Hoare CA. The trypanosomes of mammals: a zoological monograph. Oxford: Blackwell Scientific Publications; 1972.
-
42Guttreridge WE, Rogerson G W. Biochemical aspects of the biology of Trypanosoma cruzi. In: Lumsden WH, Evans DA, editors. Biology of the Kinetoplastida. Michigan: Academic Press; 1979. v.2, p. 619-52
-
43Mehlhorn H, editor. Parasitology in focus : facts and trends. Berlin: Springer; 1988.
-
44Andrews NW, Robbins ES, Ley V, Hong KS, Nussenzweig V. Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi. J Exp Med. 1988;167:300-14.
-
45Faucher JF, Baltz T, Petry KG. Detection of an “epimastigote-like” intracellular stage of Trypanosoma cruzi. Parasitol Res. 1995;81:441-3.
-
46Fernandez-Presas AM, Zavala JT, Fauser IB, Merchant MT, Guerrero LR, Willms K. Ultrastructural damage of Trypanosoma cruzi epimastigotes exposed to decomplemented immune sera. Parasitol Res. 2001;87:619-25.
-
47Zangger H, Ronet C, Desponds C, Kuhlmann FM, Robinson J, Hartley MA, et al. Detection of Leishmania RNA virus in Leishmania parasites. PLoS Negl Trop Dis. 2013;7:e2006.
-
FUNDINGThis work was supported by grants IN-215315 DGAPA-UNAM and was funded by the Departamento de Microbiología y Parasitología, Facultad de Medicina, Universidad Nacional Autónoma de México.
Publication Dates
-
Publication in this collection
2017
History
-
Received
28 Oct 2016 -
Accepted
22 Feb 2017