Association between Opioid Receptor mu 1 (OPRM1) Gene Polymorphisms and Tobacco and Alcohol Consumption in a Spanish Population
DOI:
https://doi.org/10.17305/bjbms.2015.243Keywords:
Tobacco, Alcohol, Drug abuse, Genetic polymorphismAbstract
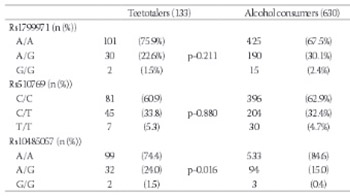
Evidence gained from animals and humans suggests that the encephalic opioid system might be involved in the development of drug addiction through its role in reward. Our aim is to assess the influence of genetic variations in the opioid receptor mu 1 on alcohol and tobacco consumption in a Spanish population. 763 unrelated individuals (465 women, 298 men) aged 18-85 years were recruited between October 2011 and April 2012. Participants were requested to answer a 35-item questionnaire on tobacco and alcohol consumption, as well as to complete the AUDIT and Fagerström tests. Individuals were genotyped for three polymorphisms in the opioid receptor mu 1 (OPRM1) gene, using a TaqMan® protocol. In males, the rs10485057 polymorphism was associated with total pure ethanol intake and with the risk of being an alcohol consumer. Also, this polymorphism was significantly associated with higher Fagerström scores. Rs1799971 had a different influence on adaptive and maladaptive patterns of alcohol use. Despite the limited sample size, our study might enrich current knowledge on patterns of alcohol use, because it encompasses both extreme and adaptive phenotypes, providing thus a wider perspective on this subject.
Downloads
References
Mague SD, Blendy JA. OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 2010;108(3):172–182. DOI: http://dx.doi.org/10.1016/j.drugalcdep.2009.12.016
Loh el W, Fann CS, Chang YT, Chang CJ, Cheng AT. Endogenous opioid receptor genes and alcohol dependence among Taiwanese Han. Alcohol Clin Exp Res 2004;28(1):15–19. DOI: http://dx.doi.org/10.1097/01.ALC.0000106303.41755.B8
Moles A, Kiefer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science 2004;304(5679):1983-1986. DOI: http://dx.doi.org/10.1126/science.1095943
Walters CL, Cleck JN, Kuo YC, Blendy JA .Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 2005;46(6):933–943. DOI: http://dx.doi.org/10.1016/j.neuron.2005.05.005
Wewers ME, Dhatt RK, Snively TA, Tejwani GA .The effect of chronic administration of nicotine on antinociception, opioid receptor binding and metenkelphalin levels in rats. Brain Res 1999;822(1-2):107–113. DOI: http://dx.doi.org/10.1016/S0006-8993(99)01095-1
Boyadjieva NI, Sarka, DK. The secretory response of hypothalamic betaendorphin neurons to acute and chronic nicotine treatments and following nicotine withdrawal. Life Sci 1997;61(6):59-66. DOI: http://dx.doi.org/10.1016/S0024-3205(97)00444-X
Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, et al. Correlation of Stable Elevations in Striatal μ-Opioid Receptor Availability in Detoxified Alcoholic Patients With Alcohol Craving: A Positron Emission Tomography Study Using Carbon 11–Labeled Carfentanil. Arch Gen Psychiatry 2005;62(9):57-64. DOI: http://dx.doi.org/10.1001/archpsyc.62.1.57
Soyka M, Rosner S. Opioid antagonists for pharmacological treatment of alcohol dependence – a critical review. Curr Drug Abuse Rev 2008;1(3):280–291. DOI: http://dx.doi.org/10.2174/1874473710801030280
Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu-opioid receptor. J Neurochem 2007;103(1):77–87.
Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu-opioid receptor (OPRM1) caused by variant A118G. J Biol Chem 2005;280(38):32618–32624. DOI: http://dx.doi.org/10.1074/jbc.M504942200
Weerts EM, Macul ME, Kuwabara H, Yang X, Xu X, Dannals RF et al. Influence of OPRM1 Asn40Asp variant (A118G) on [11C] carfentanil binding potential: preliminary findings in human subjects. Int J Neuropsychopharmacol 2013;8(1):1-7.
Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA 1998;95(16):9608–9613. DOI: http://dx.doi.org/10.1073/pnas.95.16.9608
Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res 2011;35(1):166–174. DOI: http://dx.doi.org/10.1111/j.1530-0277.2010.01333.x
Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem 2004;89(3):553–560. DOI: http://dx.doi.org/10.1111/j.1471-4159.2004.02340.x
van der Zwaluw CS, van denWildenberg E, Wiers RW, Franke B, Buitelaar J, Scholte RH, et al. Polymorphisms in the mu-opioid receptor gene (OPRM1) and the implications for alcohol dependence in humans. Pharmacogenomics 2007;8(10):1427–1436. DOI: http://dx.doi.org/10.2217/14622416.8.10.1427
Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend 2006; 83(3):262–268. DOI: http://dx.doi.org/10.1016/j.drugalcdep.2005.11.024
Nishizawa D, Han W, Hasegawa J, Ishida T, Numata Y, Sato T, Kawai A, et al. Association of mu-opioid receptor gene polymorphism A118G with alcohol dependence in a Japanese population. Neuropsychobiology 2006;5(3)3:137-41.
van den Wildenberg E, Wiers RW, Dessers J, Janssen RG, Lambrichs EH, Smeets HJ, et al. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res 2007;31(1):1–10. DOI: http://dx.doi.org/10.1111/j.1530-0277.2006.00258.x
Pratt WM, Davidson D. Role of the HPA axis and the A118G polymorphism of the mu-opioid receptor in stress-induced drinking behavior. Alcohol 2009;44(4):358–365. DOI: http://dx.doi.org/10.1093/alcalc/agp007
Miranda R Jr, Reynolds E, Ray L, Justus A, Knopik VS, McGeary J, et al. Preliminary Evidence for a Gene-Environment Interaction in Predicting Alcohol Use Disorders in Adolescents. Alcohol Clin Exp Res 2013;37(2):325-31. DOI: http://dx.doi.org/10.1111/j.1530-0277.2012.01897.x
Pieters S, van Der Vorst H, Burk WJ, Schoenmakers TM, van der Willenberg E, Smeets HJ, et al. The effect of the OPRM1 and DRD4 polymorphisms on the relation between attentional bias and alcohol use in adolescence and young adulthood. Dev Cogn Neurosci 2011;1(4):591-599. DOI: http://dx.doi.org/10.1016/j.dcn.2011.07.008
Koller G, Zill P, Rujescu D, Ridinger M, Pogarell O, Fehr C, et al. Possible association between OPRM1 genetic variance at the 118 locus and alcohol dependence in a large treatment sample: relationship to alcohol dependence symptoms. Alcohol Clin Exp Res 2012;36(7):1230-1236. DOI: http://dx.doi.org/10.1111/j.1530-0277.2011.01714.x
Chen D, Liu L, Xiao Y, Peng Y, Yang C, Wang Z. Ethnic-specific meta-analyses of association between the OPRM1 A118G polymorphism and alcohol dependence among Asians and Caucasians. Drug Alcohol Depend 2012;123(1-3):1-6. DOI: http://dx.doi.org/10.1016/j.drugalcdep.2011.10.012
Munafò MR, Johnstone EC, Aveyard P, Marteau T. Lack of Association of OPRM1 Genotype and Smoking Cessation. Nicotine Tob Res 2013;15(3):739-44. DOI: http://dx.doi.org/10.1093/ntr/nts174
Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, et al. The functional mu-opioid receptor (OPRMI) Asn 40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J 2004;4(3):184–93. DOI: http://dx.doi.org/10.1038/sj.tpj.6500238
Corella D, Sáiz C, Guillén M, Portolés O, Mulet F, González JI, et al. Association of TaqIB polymorphism in the cholesteryl ester transfer protein gene with plasma lipid levels in a healthy Spanish population Atherosclerosis 2000;152(2):367-76. DOI: http://dx.doi.org/10.1016/S0021-9150(99)00477-3
Cuevas-Badenes J, Sanchís-Fortea M. Tratado de alcohología. Madrid: DuPont Pharma; 2000.
Saunders JB, Aasland OG, Babor TF, DeLaFuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addiction 1993;88(6):791–804. DOI: http://dx.doi.org/10.1111/j.1360-0443.1993.tb02093.x
Ehlers CL, Lind PA, Wilhelmsen KC. Association between single nucleotide polymorphisms in the mu-opioid receptor gene (OPRM1) and self-reported responses to alcohol in American Indians. BMC Med Genet 2008;9:35. DOI: http://dx.doi.org/10.1186/1471-2350-9-35
Chamorro AJ, Marcos M, Mirón-Canelo JA, Pastor I, González-Sarmiento R, Laso FJ. Association of µ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addic Biol 2012;17(3):505-512. DOI: http://dx.doi.org/10.1111/j.1369-1600.2012.00442.x
Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No Gene Is an Island: The Flip-Flop Phenomenon. Am J Hum Genet 2007;80(3): 531–538. DOI: http://dx.doi.org/10.1086/512133
Kim DK, Hwang CK, Wagley Y, Law PY, Wei LN, Loh HH. P38 Mitogen-activated protein kinase and PI3-kinase are involved in up-regulation of mu-opioid receptor transcription induced by cycloheximide. J Neurochem 2011;116(6):1077–1087. DOI: http://dx.doi.org/10.1111/j.1471-4159.2010.07163.x
Kraus J, Lehmann L, Börner C, Höllt V. Epigenetic mechanisms involved in the induction of the mu-opioid receptor gene in Jurkat T-cells in response to interleukin-4. Mol Immunol 2010;48(1-3):257–263. DOI: http://dx.doi.org/10.1016/j.molimm.2010.08.002
Verde Z, Santiago C, Rodríguez González-Moro JM, de Lucas Ramos P, López Martín S, Bandrés F, et al. 'Smoking genes': a genetic association study. PLoS One 2011;6(10):e26668. DOI: http://dx.doi.org/10.1371/journal.pone.0026668