THP-1 cells as a model for human monocytes
In mammals, circulating monocytes provide effective protection against infection with Gram-negative bacteria. Once challenged with lipopolysaccharide (LPS), a macromolecular structure of the cell wall of Gram-negative bacteria, peripheral monocytes migrate to lymph nodes, differentiate into dendritic cells, and present antigen to CD4+ and CD8+ T cells with high efficacy, thereby initiating adaptive immunity against Gram-negative bacteria (1). In addition, circulating monocytes have the potential to differentiate into tissue macrophages, thereby contributing to the pool of embryonically derived macrophages and providing help in the phagocytosis of invading pathogens (2).
THP-1 designates a spontaneously immortalized monocyte-like cell line, derived from the peripheral blood of a childhood case of acute monocytic leukemia (M5 subtype) (3). THP-1 cells, including their genetically engineered derivatives, represent valuable tools for investigating monocyte structure and function in both health and disease. A recent article by Hu and colleagues (4), published in the May 2016 (No. 9) issue of Annals of Translational Medicine, illustrates the potential of this cell line for basic and applied research, including its use for gene expression microarray analysis. In their report, Hu and colleagues describe the gene expression pattern of THP-1 cells exposed to heat-inactivated Candida albicans. The data are truly impressive, despite the fact, that the results have not yet been validated by reverse transcription (RT) polymerase chain reaction (PCR) or Western blot analysis, as mentioned by the authors.
However, it is important to emphasize the limitations of the THP-1 cell line as a model for primary monocytes and point out important differences between these immortalized cells and their physiological counterparts, i.e., human peripheral blood monocytes, which they are thought to represent. The use of cultured THP-1 cells in vitro as a model for primary human monocytes ex vivo exemplifies the basic concept of translational research. Unfortunately, the obvious approximation that characterizes this type of translational research, i.e., the use of cultured, immortalized cells as a proxy for primary cells, often receives marginal or no consideration in the discussion section of research reports. Moreover, the term monocyte is occasionally used synonymously for cultured monocyte-derived cells, such as THP-1 cells, as well as for primary blood-derived monocytes. The use of definite terminology (monocyte designating the primary cell and monocyte-like designating the monocyte-derived immortalized cell) would substantially enhance the quality of studies in the field of monocyte physiology, as it would encourage investigators to more systematically address the similarities and differences between primary and immortalized cells.
For many years, we have conducted studies by using both THP-1 cells and primary human peripheral blood monocytes obtained from venous whole blood of healthy volunteers. Using the in vitro (THP-1 cells) and ex vivo (primary monocytes) approach side-by-side allowed us to make informed judgments on the usefulness of THP-1 cells as a model for primary monocytes.
For example, monocytes, when compared with THP-1 cells, are far more responsive to LPS. The remarkable LPS responsiveness of human peripheral blood monocytes results mainly from the high expression levels of CD14, a cell surface-localized glycosylphosphatidylinositol-anchored monocyte differentiation antigen. CD14, together with toll-like receptor 4 (TLR4), a signal transducing protein and member of the TLR family, and myeloid differentiation factor 2 (MD2), a secreted protein that associates with TLR4, forms a highly sensitive LPS signaling complex (CD14-TLR4-MD2) (5-8).
As opposed to primary monocytes, THP-1 cells express low levels of CD14 (9). Therefore, concerning LPS responses, THP-1 cells appear to be a poor model for primary monocytes. Stably transfecting THP-1 cells with human CD14 (10,11) and selecting the transfectants for high-level expression of CD14 results in more pronounced LPS responses of the CD14-transfected THP-1 cells (9).
We compared the production of the proinflammatory cytokine interleukin-8 (IL-8) in cultured CD14-transfected THP-1 cells, cultured mock-transfected THP-1 cells, and primary human monocytes after stimulation with Escherichia coli O111:B4 LPS (Sigma-Aldrich, St. Louis, Missouri, USA). THP-1-rsv transfectants (mock transfectants) contain the expression vector pRc/RSV, which carries a neomycin resistance gene for selection with the aminoglycoside and ribosomal inhibitor G418 (Geneticin, Life Technologies, Carlsbad, California, USA) (12).
THP-1-rsv-CD14 transfectants carry the pRc/RSV vector with wild-type human CD14 cDNA cloned into the XbaI restriction site (10,11). Expression of CD14 in this system is controlled by the Rous sarcoma virus (RSV) long terminal repeat promoter. Stably transfected cells were kept in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, St. Louis, Missouri, USA), supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
After addition of saline (0 ng/mL LPS) or LPS (1,000, 100, 10 ng/mL), cell culture aliquots were either placed on ice immediately (Figure 1A) or incubated for 2 h (Figure 1B) or 4 h (Figure 1C) at 37 °C. Supernatants were assayed for secreted IL-8 by using the OptEIA enzyme-linked immunosorbent assay technology as previously described (9). As shown in Figure 1, IL-8 production appears to be tightly dependent on CD-14 expression. After 4 h of incubation with 10 ng/mL LPS, THP-1-rsv-CD14 transfectants, but not THP-1-rsv transfectants, produced significant amounts of IL-8 (Figure 1C).
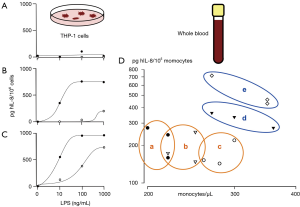
LPS-induced IL-8 production in primary human monocytes was assessed as described earlier (13). Briefly, human whole blood was obtained from five healthy volunteers by collecting venous blood from cubital veins into acid-citrate-dextrose (ACD) Vacutainers (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA). Venous blood was diluted with an equal volume of RPMI 1640 medium immediately after blood sampling. After the addition of either saline or LPS (10 ng/mL), aliquots were incubated for 4 h at 37 °C and supernatants assayed for secreted IL-8 as described above (Figure 1D).
The experiment showed that, despite CD14 overexpression, IL-8 production in THP-1 transfectants, stimulated with 10 ng/mL LPS (Figure 1C), remains roughly one order of magnitude lower when compared with equal numbers of primary human monocytes stimulated with 10 ng/mL LPS ex vivo (Figure 1D). With this limitation in mind, it could be argued that THP-1 cells, stably transfected with wild-type CD14, represent a valid model for the investigation of LPS effects in monocytes.
Our experiments showed, however, that CD14-transfected THP-1 cells are not an entirely robust model for investigating LPS effects in monocytes. For instance, human cationic antimicrobial protein (CAP37) enhances LPS-induced IL-8 responses in monocytes by 3- to 4-fold, presumably by acting as an LPS-binding protein that also binds to monocytic surfaces (13). Interestingly, such enhancement is not observed in CD14-transfected THP-1 cells. Moreover, in THP-1-rsv-CD14 transfectants, LPS-induced IL-8 responses are diminished by 1- to 2-fold in the presence of CAP37 (data not shown). Thus far, no immortalized cell line has been identified that binds CAP37 with similar avidity to that of human monocytes (Hans Flodgaard, Denmark, personal communication). Therefore, in a THP-1 cell system, CAP37 appears to function as an LPS scavenger, trapping LPS in the culture medium rather than transferring LPS to the cell surface. Diverging LPS-induced IL-8 responses between THP-1 cells and human monocytes are also observed in the presence of heparin, a naturally occurring glycosaminoglycan that has been widely used to treat and prevent venous thromboembolism.
Although heparin enhances LPS-induced IL-8 release by 3- to 5-fold in human primary monocytes (14), no such enhancement is observed in THP-1-rsv-CD14 transfectants. In fact, we observed a marginal enhancement of such responses only after preincubating CD14-transfected THP-1 cells with heparin for 24 h, followed by exposure to 1 μg/mL LPS for 4 h at 37 °C (data not shown). Contrary to these observations, synthetic poly-L-histidines appear to inhibit LPS-induced IL-8 responses, at neutral pH, with similar potency in both THP-1-rsv-CD14 transfectants and primary human monocytes (15).
The study by Hu and colleagues (4) uses THP-1 cells, i.e., cells with a stable genetic background and with a reproducible treatment response which defines these cells as an apparently ideal monocyte proxy for screening purposes. However, the examples of CAP37 and heparin show that the biological effects of some compounds are only revealed in ex vivo systems consisting of primary cells, an observation that may well extend to THP-1 cells exposed to heat-inactivated Candida albicans.
Acknowledgements
The authors thank the many volunteers who participated in their studies. The authors thank Barbara Every, ELS, of BioMedical Editor, for English language editing.
Funding: The work was funded in part by the Zurich State Government and the University of Zurich (account number 34080310 on behalf of Herbert Bosshart).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sallusto F, Lanzavecchia A. Monocytes join the dendritic cell family. Cell 2010;143:339-40. [Crossref] [PubMed]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014;41:21-35. [Crossref] [PubMed]
- Tsuchiya S, Yamabe M, Yamaguchi Y, et al. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 1980;26:171-6. [Crossref] [PubMed]
- Hu ZD, Wei TT, Tang QQ, et al. Gene expression profile of THP-1 cells treated with heat-killed Candida albicans. Ann Transl Med 2016;4:170. [Crossref] [PubMed]
- Kim JI, Lee CJ, Jin MS, et al. Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J Biol Chem 2005;280:11347-51. [Crossref] [PubMed]
- Ohto U, Fukase K, Miyake K, et al. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science 2007;316:1632-4. [Crossref] [PubMed]
- Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007;130:906-17. [Crossref] [PubMed]
- Park BS, Song DH, Kim HM, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009;458:1191-5. [Crossref] [PubMed]
- Bosshart H, Heinzelmann M. Lipopolysaccharide-mediated cell activation without rapid mobilization of cytosolic free calcium. Mol Immunol 2004;41:1023-8. [Crossref] [PubMed]
- Lee JD, Kato K, Tobias PS, et al. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med 1992;175:1697-705. [Crossref] [PubMed]
- Lee JD, Kravchenko V, Kirkland TN, et al. Glycosyl-phosphatidylinositol-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc Natl Acad Sci U S A 1993;90:9930-4. [Crossref] [PubMed]
- Pugin J, Kravchenko VV, Lee JD, et al. Cell activation mediated by glycosylphosphatidylinositol-anchored or transmembrane forms of CD14. Infect Immun 1998;66:1174-80. [PubMed]
- Bosshart H, Heinzelmann M. Arginine-rich cationic polypeptides amplify lipopolysaccharide-induced monocyte activation. Infect Immun 2002;70:6904-10. [Crossref] [PubMed]
- Heinzelmann M, Bosshart H. Heparin binds to lipopolysaccharide (LPS)-binding protein, facilitates the transfer of LPS to CD14, and enhances LPS-induced activation of peripheral blood monocytes. J Immunol 2005;174:2280-7. [Crossref] [PubMed]
- Bosshart H, Heinzelmann M. Endotoxin-neutralizing effects of histidine-rich peptides. FEBS Lett 2003;553:135-40. [Crossref] [PubMed]


