
Livin is involved in TGF-β1-induced renal tubular epithelial-mesenchymal transition through lncRNA-ATB
Introduction
It is well-known that renal interstitial fibrosis is an important factor that contributes to end-stage renal failure (ESRD). Furthermore, epithelial-mesenchymal transition (EMT) is accepted as a crucial process of renal interstitial fibrosis. EMT is characterized by morphological changes to mesenchymal phenotypes, loss of epithelial markers, acquisition of interstitial markers, and enhanced invasive or transfer abilities (1,2). EMT can destroy the junction between tubular epithelial cells (TECs), increase the extracellular matrix secretion, and lead to the migration of epithelial cells (3). These morphological and functional changes cause TECs to become more mesenchymal, in addition to promoting and sustaining inflammation, fibrosis, and chronic tissue damage (4,5). Thus, investigating the regulators of EMT is important for managing renal fibrosis and will provide possible targeted therapies. Among the cytokines related to the EMT process, TGF-β1 is the most important and major inducer of EMT (6,7).
Livin/BIRC7 was first noticed in melanoma and identified as the seventh member of the inhibitor of apoptosis protein (IAP) family (8). Livin inhibits apoptosis by combining caspase 3, 7, 9 with its BIR structural domain and RING domain to control the cascading activation reaction. Livin has stronger anti-apoptotic effect because of its two subunits (α and β) compared with the other IAP members (9). Apart from its anti-apoptosis effects, studies have found that Livin is involved in the development, progression, and drug resistance of various human tumors through the activation of EMT (10,11). Thus, we followed a bold assumption that Livin might be involved in the EMT of human renal tubular cells and examined the regulatory mechanism in detail.
Long non-coding RNAs (lncRNA) are RNAs without a protein-coding capacity which are longer than 200 nucleotides (12). LncRNA activated by TGF-β are called lncRNA-ATB which has been found to promote EMT in colon cancer (13), renal cell carcinoma (14), and breast cancer (15). Our most definite knowledge concerning the mechanism of lncRNA-ATB involved in EMT is that lncRNA-ATB can act as a competing endogenous RNA (ceRNA) for miRNAs and interfere with their inhibition of EMT (16).
The purpose of this study is to verify our hypothesis that Livin is involved in regulating EMT. To achieve this aim, we constructed the EMT model in vivo and vitro and found that this resulted in high expression levels of Livin. As Livin is nearly undetectable in normal differentiated tissues, it can be an effective targeted gene therapy for renal fibrosis.
Methods
Animals and unilateral ureteral obstruction (UUO) model
Male Sprague Dawley rats (3–4 weeks, 100–150 g) were provided by (Beijing Vital River Laboratory Animal Technology Co., Ltd). A total of 16 rats were randomly divided into 2 groups (n=8 in sham group; n=8 in UUO group). Rats were housed on a constant 12 hours light-dark cycle and fed freely. After adaptive feeding for one week, the rats in UUO group were anesthetized by intraperitoneal injection of 10% chloral hydrate, the left ureter was ligated and cut near the renal hilum, then closed the abdominal cavity. In sham group, we just dissociated the left ureter, then closed the abdominal cavity. After continued feeding for 14 days, we took the blood samples for Scr and BUN tests. Then all the rats were euthanized, parts of renal tissues were fixed with 4% paraformaldehyde for immunohistochemistry and parts of renal tissues were stored in liquid nitrogen for qPCR.
Immunohistochemistry
2–3 µm sections were dewaxing and hydration treated. After blocked by goat serum for 30 min, the sections were incubated with anti-Livin at 1:100, anti-E-cadherin at 1:100, anti-α-SMA at 1:100, anti-Vimentin at 1:100 overnight. The kidney sections were then incubated with secondary antibodies for 20 min in dark room followed by Hematoxylin staining slightly. Images of the sections were captured with a Nikon DS-Ri2.
Cell culture and treatment
Human renal TEC line (HK2 cells) was purchased from the China Center for Type Culture Collection (CCTCC, Wuhan, China). Cells were cultured in DMEM-F12 medium (Hyclone), adding 10% FBS (Gibco, USA). When the cells reached about 70–80% confluence, they were starved in serum-free medium overnight, then stimulated with 0, 1, 2, 5, 10 ng/mL TGF-β1 (CST). Then the cells were cultured in an atmosphere of 5% CO2 at 37 °C for different time intervals (0, 24, 48, 72 h). We observed the morphology changes under the phase contrast microscope (OLYMPUS AX70, Japan).
Quantitative PCR
Total RNA (tissues or cells) was isolated by TRIzol reagent (Invitrogen/Life Technologies, USA) from cells, cDNA was synthesized by reverse transcription reagents (Promega, Beijing, China). According to the products manual, qPCR was performed using Roche LightCycler480 with SYBR-Green I Mix (Promega. Beijing, China). Each experiment was repeated for three times and the 2−ΔΔCt method was used to calculate the relative expression of RNAs. The sequences of primers were as follows: a-SMA, sense 5'-CGGGACATCAAGGAGAAACT-3', antisense 5'-CCATCAGGCAACTCGTAACTCT-3'. Vimentin (VIM), sense 5'-ACAGGCTTTAGCGAGTTATT-3', antisense 5'-AAGAGGCGAACGAGGG-3'. E-cadherin, sense 5'-CCGCCATCGCTTACA-3', antisense 5'-GGCACCTGACCCTTGTA-3'. Livin, sense 5'-CGCCGTGTCCATCGTCTTTGT-3', antisense 5'-ACACAGTCCAGAACAGGCAGAG-3'. LncRNA-ATB, sense 5'-ACAAGCTGTGCAGTCTCAGG-3', antisense 5'-CTAGGCCCAAAGACAATGGA-3'. 18s RNA, sense 5'-AATAGCCTTTGCCATCAC-3', antisense 5'-CGTTCCACCTCATCCTC-3'.
Western blot analysis
HK2 cells were lysed by RIPA lysis buffer (Beyotime, Shanghai, China) with protease inhibitors. Protein was extracted by centrifugation at 12,000 g for 10 min at 4 °C, and the concentration was measured by the BCA protein assay kit (Beyotime, Shanghai, China). Samples were boiled for 5 min, and equal amounts of protein were separated by 10% SDS-PAGE and transferred onto PVDF membranes. The membranes were incubated overnight at 4 °C with the indicated primary antibody Livin (CST, MA, USA); α-SMA (CST), 1:1,000; vimentin (CST), 1:1,000; E-cadherin (CST), 1:1,000. The membranes were washed by TBST buffer and incubated for 1 h at 37 °C with secondary antibodies (CST), 1:5,000. The density of bands was analyzed by Image J, and GAPDH (Wanleibio, China) was used as an endogenous reference in all WB processes.
Transwell assay
Cell migration ability was assessed by 24-well 8.0 µm Transwell chamber (Corning, NY, USA). The cells were cultured to 5×104 after being starved with serum-free medium for 24 h and were seeded into the upper chambers (about 2×104 each chamber). Meanwhile, serum-free medium was added to the upper chambers, and complete medium was added to the lower chambers. The chambers were placed in a 5% CO2 incubator at 37 °C for 24 h. After the cells were removed from the upper chamber, the migrated cells were fixed on the bottom surface of the membrane with 4% paraformaldehyde for 30 min. The cells were then stained with 0.1% crystal violet. Images of five random fields were taken by inverted microscopy (Olympus, Tokyo, Japan). Five random fields were chosen for cell counting, and the rates of cellular migration were normalized by that of the control group. Each migration experiment was conducted in triplicate.
Immunofluorescence
Cells were cultured on coverslips in 6-well plates. Initially, cells were fixed with 4% formaldehyde and permeabilized with 0.1% Triton X-100 for 30 min at room temperature. After fixation, cells were incubated with the primary antibodies at 4 °C overnight. After washing three times in PBS, the cells were incubated with a FITC-conjugated secondary antibody in a dark room for 30 min. Finally, cells were counterstained with DAPI to distinguish the cellular nuclei and visualized using a fluorescence microscope (Olympus, Tokyo, Japan).
Cell transfection
Small interfering RNAs that targeted Livin (si-Livin) were designed and synthesized by Sigma-Aldrich (Shanghai, China). A plasmid that was designed to overexpress the full-length lncRNA-ATB and small interfering RNAs that targeted lncRNA-ATB (si-ATB) were constructed by Genechem (Shanghai, China). HK2 cells were transfected by lipofectamine 3000 (Thermo Fisher Scientific, MA, USA) according to the manufacturer’s instructions. Cell transfection lasted for 24 h, and the effect of gene knockout and overexpression were verified by qPCR and/or WB. If necessary, the remaining transfected cells which were treated together had testing continued after verifying the silence effect.
Statistical analysis
Data were presented as mean ± SD. Difference between the two groups was analyzed using the Student’s t-test. All P values were two-tailed and were considered significant if P<0.05. Data were analyzed using SPSS 16.0 software package.
Results
Livin was involved in renal fibrosis in vivo
First, we built a renal fibrosis model by UUO. Compared with the sham group, the rats in the UUO group had higher levels of Scr and BUN (P<0.01; Table 1). Immunohistochemistry staining showed that in the sham group, E-cadherin was highly expressed in renal tubular epithelium while there was a significantly lower level of expression of E-cadherin in the UUO group. The expression of α-SMA and vimentin, which are interstitial markers, were lower in the sham group and higher in the UUO group. Meanwhile, the immunohistochemistry tests showed that Livin had low levels of expression in the sham group. However, in the UUO group, Livin was highly expressed in the cytoplasm of renal TECs and in part of the nuclei (Figure 1A). From qPCR results, we also found that the mRNA levels of α-SMA, vimentin and Livin were elevated (P<0.01). The mRNA level of E-cadherin was lower in the UUO group (P<0.05; Figure 1B).
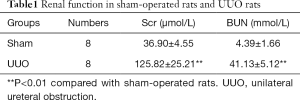
Full table
TGF-β1 induced EMT in HK2 cells
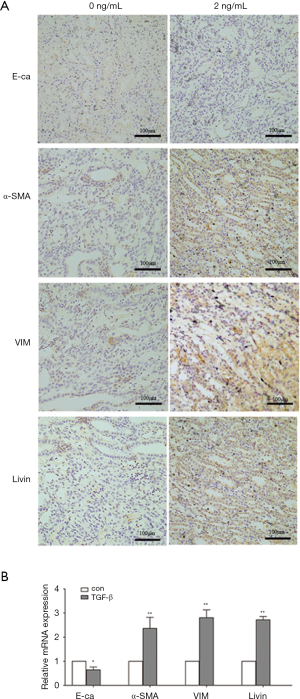
First, we used different concentrations of TGF-β1 (0, 1, 2, 5, 10 ng/mL) to induce EMT at different time intervals (0, 24, 48 and 72 h). TGF-β1-induced morphological changes were time and TGF-β1 concentration dependent to a certain degree. The control HK2 cells were epithelium-like; they were round or oval and connected closely. When EMT was induced by TGF-β1, HK2 cells become larger and elongated, disassociating from surrounding epithelial cells. We found that at 48 h, the 2 ng/mL group showed notable morphological changes (Figure 2A).
As shown in Figure 2B, the epithelial marker E-cadherin was downregulated while the mesenchymal markers, α-SMA and vimentin, were upregulated after treatment with 2 ng/mL TGF-β1. The relative mRNA expression of these markers followed similar trends as determined by qPCR (Figure 2C). Simultaneously, the immunofluorescence results were also in agreement with the above-mentioned findings. In the control group, E-cadherin was expressed in the membrane continuously while α-SMA and vimentin were expressed at low levels. After treatment with 2 ng/mL TGF-β1 for 48 h, E-cadherin expression was reduced while α-SMA and vimentin expression were increased (Figure 2D). The transwell assay showed that the migratory activity also increased in the TGF-β1-treated group (P<0.01; Figure 2E). These results suggested that the cells treated with TGF-β1 lost their epithelial characteristics and acquired mesenchymal cell properties. Taken together, these findings support the notion that TGF-β1 successfully induced EMT.
Livin is involved in EMT induced by TGF-β1 in HK2 cells
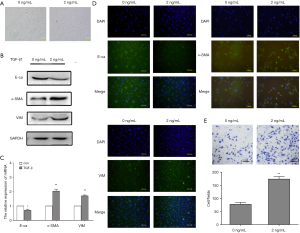
Furthermore, HK2 cells were treated with TGF-β1 at different concentrations for 48 h. Using these cells, we discovered that the protein expression of Livin was significantly increased in the 2 ng/mL TGF-β1 group (Figure 3A). Immunofluorescence results also showed that Livin was upregulated in the cytoplasm and nucleus after treatment with TGF-β1 (Figure 3B). The qPCR results showed that the mRNA expression in the 2 ng/mL TGF-β1 group was the highest (P<0.01; Figure 3C). Thus, we hypothesized that Livin might be involved in human renal epithelial EMT. To confirm this hypothesis, we chose 2 ng/mL TGF-β1 to treat HK2 cells for 48 h.
Silencing of Livin alleviated TGF-β1-induced EMT in HK2 cells
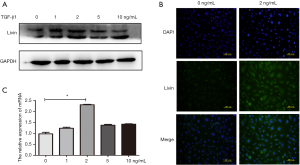
To further study the role of Livin in TGF-β1-induced EMT in HK2 cells, we designed a siRNA to knock out Livin. The efficiency of gene silencing was verified through qPCR and Western blot. We found that si-Livin1 was the most effective silencer and could block about 80% of the mRNA expression of Livin (Figure 4A). WB also showed that Livin was significantly suppressed by siRNA1 (Figure 4B). The control group (con) included HK2 cells transfected with control siRNA. Compared with the con + TGF-β1 group, E-cadherin protein in the si-Livin + TGF-β1 group was downregulated while α-SMA and vimentin were upregulated (Figure 4C). The mRNA levels of E-cadherin, α-SMA, and vimentin were consistent with the WB results (P<0.05; Figure 4D). Immunofluorescence results also proved that the si-Livin + TGF-β1 group showed higher E-cadherin protein expression and lower α-SMA and Vimentin protein expression (Figure 4E). The transwell assay showed that lower levels of Livin could weaken the migratory ability of HK2 cells, with a significant difference in the number of cells (P<0.01; Figure 4F). Generally speaking, the silencing of Livin could effectively suppress EMT induced by TGF-β1.
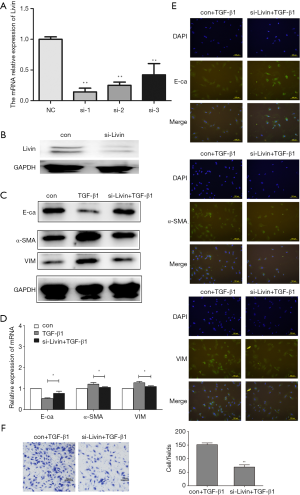
Livin stimulated TGF-β1-induced EMT by the regulation of lncRNA-ATB
As an important regulator of EMT, lncRNA-ATB was shown to promote tumor growth and metastasis. In our research, qPCR showed that the relative mRNA expression of lncRNA-ATB was significantly increased both in vivo and vitro, which indicated that lncRNA-ATB could be stimulated by TGF-β1 and in UUO model (Figure 5A,B). These results proved that lncRNA-ATB was involved in EMT in human renal epithelial cells.
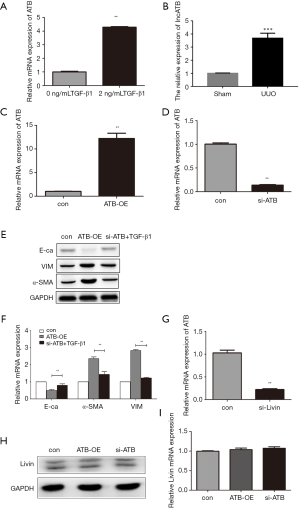
To further investigate the role of lncRNA-ATB in TGF-β1-induced EMT, we transfected a siRNA of lncRNA-ATB to silence its expression, and transfected plasmid GV219-ATB to overexpress lncRNA-ATB. The qPCR results confirmed that lncRNA-ATB was effectively depleted or overexpressed (Figure 5C,D). The cells were divided into three groups: cells transfected with the vector marked as the control group, the group with overexpression of lncRNA-ATB marked as ATB-OE and the si-ATB treated with TGF-β1 group. WB showed that in the ATB-OE group, E-cadherin was downregulated while α-SMA and vimentin were upregulated compared to the control group. Thus, we concluded that the overexpression of lncRNA-ATB could induce EMT in dependent of TGF-β1. Furthermore, we found that there were only slight differences in the protein levels of E-cadherin, α-SMA, and vimentin between the control group and si-ATB + TGF-β1 group (Figure 5E). This proved that the silencing of lncRNA-ATB could relieve EMT induced by TGF-β1 to a significant extent. The qPCR results also confirmed this conclusion at the transcriptional level (Figure 5F).
We found that Livin and lncRNA-ATB were all stimulators in the process of EMT in vitro, and thus, we wanted to examine the potential relationship between Livin and lncRNA-ATB in TGF-β1-induced EMT. When Livin was depleted, the expression of lncRNA-ATB was dramatically suppressed (Figure 5G), which suggested that the expression of lncRNA-ATB was regulated by Livin. We presumed that Livin might be the upstream regulator of lncRNA-ATB. Furthermore, we transfected siRNA and GV219-ATB to silence and overexpress lncRNA-ATB before measuring the level of expression of Livin. The expression of Livin was barely affected no matter lncRNA-ATB was overexpressed or depleted (Figure 5H,I). Consequently, we concluded that Livin stimulated TGF-β1-induced EMT by regulating lncRNA-ATB.
Discussion
Chronic kidney diseases (CKD) can be caused by many diseases, including urinary obstruction, transplants, immune disorder and inflammation (17). However, interstitial fibrosis is a common stage in CKD. Nowadays, the inner mechanisms that induce renal interstitial fibrosis are still focuses of intense researches and there are no specific therapies to cure renal fibrosis. Previous studies showed that EMT was important for renal fibrosis both in vivo and in vitro. However, by the fate tracing technique, it was demonstrated that epithelial cells could not directly convert into myofibroblasts (18). Thus, there were fierce debates on the roles of EMT in renal fibrogenesis. However, studies also show that EMT is important in renal fibrosis and inhibiting EMT can slow down renal fibrosis, in spite of epithelial cells are unable to convert into myofibroblast directly (5). For example, according to the study from Nieto, by inhibiting the Snail1-induced partial EMT in renal fibrosis, they reduced the inflammatory and fibrotic cytokines, such as TGF-β1, and prevent the generation of myofibroblasts and the development of fibrosis (19). As a result, the concept of “partial EMT” was proposed. EMT may relieve renal fibrosis in the following aspects. First, EMT can impact the function of TECs by reducing the expression of proteins with absorption and secretion activities. Second, EMT can cause cell-cycle arrest at the G2 phase, thus impairing the cells’ ability to repair any damage (20). Third, EMT aggravates interstitial fibrosis and cause immune recruitment and inflammation by modifying the epithelial secretome profile (20,21). Considering the above-mentioned knowledge, it is clear that EMT is a key factor in promoting and sustaining kidney inflammation, fibrosis, and chronic function injury.
The mechanisms of EMT in fibrosis are complex and remain unclear. Multiple signaling pathways are involved in the promotion and progression of EMT, such as TGF-β/Smads, MAPK, Wnt/β-catenin, Notch and Rho-like GTPase (7). There are various fibrotic growth factors that can induce EMT and the most important one is TGF-β1.
In vivo study, we built a renal fibrosis model by UUO surgery on rats. Immunohistochemical staining showed that E-cadherin was upregulated while α-SMA and vimentin were downregulated, which suggested that EMT occurs during the process of renal fibrosis. During our vitro experiment, we treated HK2 cells with TGF-β1. As a result of this treatment, HK2 cells underwent morphological changes, with epithelial markers being downregulated, interstitial markers being upregulated, and transferability improving. By observing the changes in the cell phenotype, we found that incubating HK2 cells in 2 ng/mL TGF-β1 for 48 h could induce obvious cell phenotype changes.
Livin is a member of the IAP family, which is also known as melanoma IAP or kidney IAP. Livin is accepted as a tumor-specific gene because it is rarely expressed in the healthy organs of adults and is specifically over-expressed in tumor cells. It has an N-terminal, conserved, repetitive BIR structural domain and/or a RING domain, which can combine with caspases 3 and 8. Through this combination, Livin contributes to the apoptosis, migration, and invasion of various cancers (22,23). Recent studies found that Livin regulated EMT by the activation of NF-κB signaling in colorectal cancer (10), AKT signaling in breast cancer (11) and p38/GSK3β pathway in breast cancer (24). In our study, we first revealed that Livin was involved in renal fibrosis and renal tubular EMT. In the UUO groups, Livin was upregulated in renal TECs which indicated that Livin was involved in the process of renal fibrosis in vivo. We also found that Livin had increased levels of expression during the process of TGF-β1-induced EMT in vitro, which proved that Livin was involved in TGF-β1-induced EMT. To further investigate the effect of Livin in EMT, we depleted Livin by siRNA and discovered that the silencing of Livin could stop EMT to a certain degree. Consequently, we concluded that Livin was involved in EMT and promoted this process.
Only about 2% of the human genome are protein-coding RNAs, and the majority of the transcriptional outputs of the mammalian genome are acknowledged to be non-coding genes (25). These non-coding RNAs were considered to be “transcriptional noise.” In recent decades, functional lncRNAs were found to be regulators of many types of diseases, especially cancers. Studies have shown that lncRNA can be considered as an important biological marker in diagnosing cancers, explaining the pathogenesis and mechanism of development of malignant tumors, and predicting the prognosis and the therapeutic effects in different cancers (14). Functional lncRNAs are now accepted as regulators in many types of tumors, occurring via various pathophysiological pathways, such as apoptosis, metastasis, and EMT (16,26,27). Recently, researchers found that lncRNAs also played important stimulatory roles in fibrosis diseases, such as pulmonary fibrosis (28) and hepatic fibrosis (29) by activating EMT. EMT-regulating lncRNAs are divided into promoting-EMT (pro-EMT) lncRNAs and antagonizing-EMT (anti-EMT) lncRNAs (16), and lncRNA-ATB is an important member of pro-EMT lncRNAs. As one of the identified mechanisms of lncRNA-ATB, it functions as a ceRNA for the miR-200 family, which can suppress EMT by impeding miR-200-EMT-associated targets genes, such as ZEB1, ZEB2, ZNF217 and TGF-2 (15,30,31). In our study, we found that lncRNA-ATB was highly expressed in UUO model and in TGF-β1-induced EMT, which proved that lncRNA-ATB was involved in human renal tubular EMT in vivo and vitro. We depleted lncRNA-ATB by siRNA, as a result, TGF-β1-induced EMT was obviously inhibited because EMT relative markers were similar to the control group, consequently, we concluded that the silencing of lncRNA-ATB could relieve TGF-β1-induced EMT. After this, we used GV219-ATB to overexpress lncRNA-ATB and we found that even without treatment with TGF-β1, E-cadherin was downregulated while α-SMA and vimentin were upregulated after transfection. This meant that lncRNA-ATB was a relatively independent stimulative factor in the process of EMT.
We learnt that lncRNA-ATB was an important stimulator in EMT. Combining this result with our previous findings, we concluded that Livin and lncRNA-ATB were all stimulators in TGF-β1-induced EMT. Then we studied the relationship between Livin and lncRNA-ATB. By silencing the expression of Livin, we found that lncRNA-ATB also decreased, which suggested that Livin might be the upstream regulator of lncRNA-ATB in EMT. To further verify this speculation, we transfected the HK2 cells to overexpress or silence lncRNA-ATB and we found that the expressions of Livin were nearly unchanged at both transcriptional and translational levels. Consequently, we concluded that Livin stimulated TGF-β1-induced EMT by the regulation of lncRNA-ATB in renal fibrosis.
Since TGF-β is accepted as a crucial factor in renal fibrosis, there are several strategies targeting TGF-β in renal fibrosis treatment. Voelker et al. found that LY2382770, which was the anti-TGF-β1 antibody could not slow progression of diabetic nephropathy (32). Vincenti et al. found that fresolimumab, a monoclonal anti-TGF-β antibody could slow the glomerular filtration rate decline in steroid-resistant focal segmental glomerulosclerosis with insignificant trend compared with the placebo group (33). Li et al. investigated that pirfenidone, an inhibitor of TGF-β at the transcription and translation levels, was able to attenuate EMT and fibrosis in vivo and in vitro through antagonizing the MAPK pathway (34). However, researches also showed that continuous suppression of TGF-β might lead to unacceptable toxicity which might affect wound healing, tissue repair, and anti-inflammatory actions because of its highly pleiotropic function (35). Consequently, precise targeted therapies are the focus of further researches. In our research, we found that Livin and lncRNA-ATB were the potential therapy targets which needed to be studied further.
In summary, we have demonstrated the crucial regulatory mechanism of Livin in human renal tubular EMT induced by TGF-β1. We find that Livin participates in renal fibrosis in rats and Livin promotes TGF-β1-induced human renal tubular EMT by regulating lncRNA-ATB. Consequently, the silencing of Livin and lncRNA-ATB might be effective therapeutic targets. Our research will be helpful for the treatment of renal fibrosis.
Acknowledgments
We thank the Department of Pediatrics. The First Hospital of China Medical University.
Funding: Our work was also supported by 2015 Chinese Medicine Industry Research Specialized “Chinese Medicine Treatment of Chronic Diseases Clinical Efficacy Study (1)” Children’s purpuric nephritis TCM treatment plan cohort study and chronic disease information management system (Grant Number: 201507001-03); Correlation research of FAM3C and renal interstitial fibrosis; Science and Technology Program of Shenyang City, Grant/Award Number: F16-206-9-44.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All applicable international, national, and institutional guidelines for the care and use of animals were followed. All the animal experiments were approved by the Animal Experiment Ethics Committee of China Medical University (IACUC-15052111). This article does not contain any studies with human participants performed by any of the authors. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Son H, Moon A. Epithelial-mesenchymal Transition and Cell Invasion. Toxicol Res 2010;26:245-52. [Crossref] [PubMed]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004;15:1-12. [Crossref] [PubMed]
- Hills CE, Squires PE. The role of TGF-beta and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev 2011;22:131-9. [PubMed]
- Huang S, Susztak K. Epithelial Plasticity versus EMT in Kidney Fibrosis. Trends Mol Med 2016;22:4-6. [Crossref] [PubMed]
- Lovisa S, Zeisberg M, Kalluri R. Partial Epithelial-to-Mesenchymal Transition and Other New Mechanisms of Kidney Fibrosis. Trends Endocrinol Metab 2016;27:681-95. [Crossref] [PubMed]
- Kim MK, Maeng YI, Sung WJ, et al. The differential expression of TGF-beta1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: an immunohistochemical study. Int J Clin Exp Pathol 2013;6:1747-58. [PubMed]
- Sutariya B, Jhonsa D, Saraf MN. TGF-β: the connecting link between nephropathy and fibrosis. Immunopharmacol Immunotoxicol 2016;38:39-49. [Crossref] [PubMed]
- Vucic D, Stennicke HR, Pisabarro MT, et al. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr Biol 2000;10:1359-66. [Crossref] [PubMed]
- de Almagro MC, Vucic D. The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy. Exp Oncol 2012;34:200-11. [PubMed]
- Ge Y, Cao X, Wang D, et al. Overexpression of Livin promotes migration and invasion of colorectal cancer cells by induction of epithelial-mesenchymal transition via NF-kappaB activation. Onco Targets Ther 2016;9:1011-21. [PubMed]
- Li F, Yin X, Luo X, et al. Livin promotes progression of breast cancer through induction of epithelial-mesenchymal transition and activation of AKT signaling. Cell Signal 2013;25:1413-22. [Crossref] [PubMed]
- Moran VA, Perera RJ, Khalil AM. Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucleic Acids Res 2012;40:6391-400. [Crossref] [PubMed]
- Iguchi T, Uchi R, Nambara S, et al. A long noncoding RNA, lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer. Anticancer Res 2015;35:1385-8. [PubMed]
- Xiong J, Liu Y, Jiang L, et al. High expression of long non-coding RNA lncRNA-ATB is correlated with metastases and promotes cell migration and invasion in renal cell carcinoma. Jpn J Clin Oncol 2016;46:378-84. [Crossref] [PubMed]
- Shi SJ, Wang LJ, Yu B, et al. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget 2015;6:11652-63. [Crossref] [PubMed]
- Heery R, Finn SP, Cuffe S, et al. Long Non-Coding RNAs: Key Regulators of Epithelial-Mesenchymal Transition, Tumour Drug Resistance and Cancer Stem Cells. Cancers (Basel) 2017;9. [Crossref] [PubMed]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011;7:684-96. [Crossref] [PubMed]
- Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 2010;176:85-97. [Crossref] [PubMed]
- Grande MT, Sanchez-Laorden B, Lopez-Blau C, et al. Erratum: Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 2016;22:217. [Crossref] [PubMed]
- Lovisa S, LeBleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 2015;21:998-1009. [Crossref] [PubMed]
- Grande MT, Sanchez-Laorden B, Lopez-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 2015;21:989-97. [Crossref] [PubMed]
- Kim SA, Yoon TM, Lee DH, et al. Livin enhances tumorigenesis by regulating the mitogen-activated protein kinase signaling pathway in human hypopharyngeal squamous cell carcinoma. Mol Med Rep 2016;14:515-20. [Crossref] [PubMed]
- Chen S, Ma P, Li B, et al. LncRNA CCAT1 inhibits cell apoptosis of renal cell carcinoma through up-regulation of Livin protein. Mol Cell Biochem 2017;434:135-42. [Crossref] [PubMed]
- Han Y, Zhang L, Wang W, et al. Livin promotes the progression and metastasis of breast cancer through the regulation of epithelialmesenchymal transition via the p38/GSK3beta pathway. Oncol Rep 2017;38:3574-82. [PubMed]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011;12:861-74. [Crossref] [PubMed]
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009;136:629-41. [Crossref] [PubMed]
- Eades G, Zhang YS, Li QL, et al. Long non-coding RNAs in stem cells and cancer. World J Clin Oncol 2014;5:134-41. [Crossref] [PubMed]
- Sun H, Chen J, Qian W, et al. Integrated long non-coding RNA analyses identify novel regulators of epithelial-mesenchymal transition in the mouse model of pulmonary fibrosis. J Cell Mol Med 2016;20:1234-46. [Crossref] [PubMed]
- Fu N, Zhao SX, Kong LB, et al. LncRNA-ATB/microRNA-200a/beta-catenin regulatory axis involved in the progression of HCV-related hepatic fibrosis. Gene 2017;618:1-7. [Crossref] [PubMed]
- Ma CC, Xiong Z, Zhu GN, et al. Long non-coding RNA ATB promotes glioma malignancy by negatively regulating miR-200a. J Exp Clin Cancer Res 2016;35:90. [Crossref] [PubMed]
- Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014;25:666-81. [Crossref] [PubMed]
- Voelker J, Berg PH, Sheetz M, et al. Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol 2017;28:953-62. [Crossref] [PubMed]
- Vincenti F, Fervenza FC, Campbell KN, et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep 2017;2:800-10. [Crossref] [PubMed]
- Li Z, Liu X, Wang B, et al. Pirfenidone suppresses MAPK signalling pathway to reverse epithelial-mesenchymal transition and renal fibrosis. Nephrology (Carlton) 2017;22:589-97. [Crossref] [PubMed]
- Isaka Y. Targeting TGF-β Signaling in Kidney Fibrosis. Int J Mol Sci 2018. [Crossref]


