Current status of the treatment of infrarenal abdominal aortic aneurysms
Introduction
Aortic aneurysms are the 13th leading cause of death in the United States and approximately 4,500 deaths each year are secondary to abdominal aortic aneurysm (AAA) rupture. An additional 1,400 deaths occur as a result of the 45,000 procedures performed to prevent rupture (1,2). The standardized death rate from ruptured AAAs in patients over the age of 45 is 5.6 per 100,000 individuals (3).
Aneurysms can occur along the entire length of the aorta, with the infrarenal location being the most common (4). The standard definition for an infrarenal AAA is a transverse aortic diameter ≥3.0 cm. Other studies have used a definition of 1.5 to 2.0 times the normal adjacent aortic diameter. Risk factors associated with increased infrarenal aortic diameter include male gender, age, smoking, hypertension, and family history (5,6). The approximate incidence of infrarenal AAAs in patients over the age of 65 is 1.7% in women and 5% in men (7). The goal of this review is to summarize the current management of infrarenal AAAs.
Screening
Targeted ultrasound screening has been shown to be an effective and economical means of preventing AAA rupture and reducing aneurysm-related mortality (8,9). Both the Society for Vascular Surgery (SVS) and the United States Preventative Services Task Force (USPSTF) recommend that one-time AAA screening by ultrasonography is offered to men age 65 years or older, particularly those with a smoking history and/or a family history of AAAs (10). Screening in women is controversial, but can be considered in women aged 65 years or older who have smoked or have a family history of AAA (11).
Imaging
Several imaging modalities exist to both diagnose and monitor AAAs.
Ultrasound
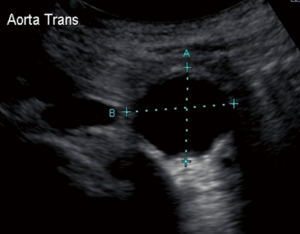
The most frequently used screening examination is abdominal sonography. Ultrasound remains an inexpensive and minimally invasive means of confirming suspected AAAs and following small AAAs (12). Ultrasound measurements are more accurate in the anteroposterior (AP) than in the transverse dimensions and this modality has an interobserver variability of 5 or less in 84% of patients (13) (Figure 1). Limitations such as obese body habitus and bowel gas can compromise the accuracy of this tool. When compared to computed tomography (CT), ultrasound tends to underestimate the diameter of AAAs by 2 mm in the AP dimension (14); accuracy decreases as the size of the aorta increases. Thus, while ultrasound is a useful screening and monitoring tool for small AAAs, CT angiography (CTA) is the preferred modality for preoperative planning and monitoring of larger AAAs.
CTA
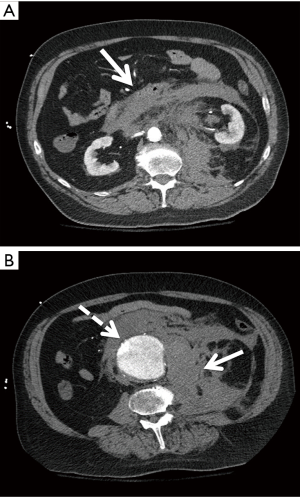
Due to financial and radiation considerations, CTA is not a suitable screening tool, but it remains the standard for pre-operative planning in AAA repair. It is a rapid and reliable modality for aortic imaging with an interobserver variability of less than 5 mm in 91% of studies (15). Additional methods such as standardized protocols, calipers, and magnification can decrease interobserver variability to within 2 mm in 90% of cases (14). Unlike sonography, CTA can detect ruptured/leaking AAAs (Figure 2). This modality comes at a nonzero risk to the patient and requires radiation doses as well as iodinated contrast.
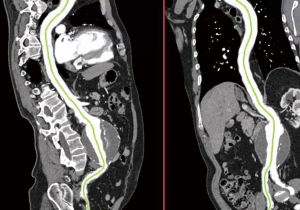
The aortic diameter can be overestimated with CTA if oblique cuts of the aorta are obtained secondary to vessel tortuosity. For this reason, CTA is often combined with three-dimensional (3D) reconstruction. Post-processing programs are able to use data obtained from CTA to reconstruct a 3D model so that centerline measurements via orthogonal planes are possible (Figure 3). This enables more accurate aortic diameter measurements and helps in preoperative planning, particularly for endovascular stent graft repairs (16). For infrarenal AAAs, curved planar reformats are helpful in determining the axial length of the aneurysm neck (distance from the lowermost renal artery to the beginning of the aneurysm) as well as neck angulation and condition (17). Noting the length, tortuosity, and condition of the iliac arteries is important; in 5–46% of cases, aneurysmal disease extends into the iliac system (18). When reviewing a CTA of the aorta for preoperative planning, one should take note of the number and location of the renal arteries as well as the presence of a retroaortic left renal vein (19). The mesenteric vessels should also be reviewed, ensuring a communication between the middle colic and superior left colic arteries, as the inferior mesenteric artery is usually sacrificed or occluded with infrarenal AAA repair. Knowing the location of the renal and mesenteric vessels as well as the character of the suprarenal aorta is useful in determining either a clamp location or landing zones for open and endovascular repair, respectively.
Magnetic resonance angiography (MRA)
MRA, like CTA, has the benefit of being able to visualize the entire aorta including its branch vessels. Unlike CTA, it does not expose the patient to radiation nor is iodinated contrast dye required. This can be more appealing for patients with a contrast allergy or renal insufficiency (20). However, long acquisition times, limited availability, and high costs have made MRA both less practical and common when it comes to aortic imaging. Additionally, MRA cannot be used in patients with metallic implants, nor is it well tolerated in patients with claustrophobia. There is also risk of nephrogenic systemic fibrosis due to gadolinium exposure in patients with glomerular filtration rates <30 mL/min (21). In patients with kidney disease, non-gadolinium contrast agents exist; blood pool contrast agents, such as ferumoxytol, can be used to enhance MRA to provide good imaging quality (22). Additionally, some studies have found that non-contrast magnetic resonance imaging (MRI), when paired with electrocardiographic- and cardiac-gated techniques, have similar image quality compared to contrast-enhanced MRA images of the aorta (23). Time-resolved 3D phase-contrast MRI with three-directional velocity encoding, also known as 4D flow MRI, has recently emerged as a tool to more comprehensively evaluate abdominal circulation. 4D flow MRI may allow for more accurate aortic modeling in the future due to its ability to simultaneously evaluate vascular anatomy and hemodynamics (24).
MRA is comparable to CTA with respect to accuracy of AAA measurements (3), but it visualizes calcified plaque poorly, with half the spatial resolution of CTA. For these reasons, MRA has played a secondary role in AAA imaging and vascular surgeons are less accustomed to using MRA images for operative endovascular interventions (25).
Expansion and rupture
The likelihood of rupture depends on several factors such as aneurysm size, expansion rate, aneurysm morphology, and gender (26).
Size
A well-established relationship between AAA size and rupture has been documented with studies as early as the 1960s demonstrating marked survival improvement after operative repair of AAAs (27). Size remains one of the strongest predictors of rupture with risk significantly increasing at diameters of 5.5 cm or greater. In 2003, the Joint Council of the American Association for Vascular Surgery and SVS estimated annual rupture risk based on aortic diameter; aneurysms smaller than 4.0 cm have a 0% annual risk of rupture compared with 3–15% in aneurysms 5.0 to 5.9 cm. Over 7.0 cm, the risk of rupture dramatically increases to 20–50% each year (28). For comparison, the 5-year cumulative rupture rate for AAAs larger than 5.0 cm is 25–40%, compared to 1–7% for aneurysms with diameters of 4.0–5.0 cm (29,30).
Expansion rate
Expansion rate is another important risk factor for aneurysm rupture (31). AAAs that expand 0.5 cm or more over 6 months or 1.0 cm or more over 1 year are at high risk for rupture (32). In the elective repair of small AAAs, it is this criterion that is often used.
Additional factors
Continued smoking and uncontrolled hypertension lead to a higher risk of aneurysm rupture (28). Additionally, clinical opinion holds that saccular aneurysms are at greater risk for rupture than diffuse fusiform aneurysms (3). Diabetes and peripheral vascular disease appear to have protective qualities with respect to rupture risk.
Indication for repair
When deciding between observation and surgical repair of an infrarenal aortic aneurysm, one must take several factors into account including risk of rupture, patient life expectancy, and operative risk (33). Appropriate patient selection and timing of intervention are essential. In patients who need emergency surgery for aortic aneurysm rupture, the mortality is 50% among the patients who reach the hospital, compared to 1% to 5% for elective AAA repair (34).
The 2009 SVS Guidelines recommend treatment of symptomatic AAAs, regardless of size, due to high risk of rupture. Fusiform AAAs greater than 5.4 cm in diameter should be electively repaired in healthy patients. In young, healthy patients, especially women, there may be a benefit to early repair for aneurysms 5.0 to 5.4 cm (11). Review of four randomized controlled trials, including the Aneurysms Detection and Management Trial (ADAM), the UK Small Aneurysm Trial (UKSAT), the Positive Impact of Endovascular Options for Treating Aneurysms Early Trial (PIVOTAL), and the Comparison of Surveillance vs. Aortic Endografting for Small Aneurysm Repair Trial (CESAR) all separately found that there were no long- or short-term benefits of repairing small aneurysms (4.0 to 5.5 cm) early (35).
For small aneurysms that are being observed, patients should receive appropriate management of cardiovascular risk factors such as hypertension, hyperlipidemia, and diabetes. Smoking cessation counseling should be provided and screening of family members is recommended. The 2009 SVS Guidelines also recommend that a statin and angiotensin-converting enzyme (ACE) inhibitor should be initiated (11). While two randomized trials have shown preoperative statins use improves cardiac morbidity and mortality within 30 days of vascular surgery (36,37), there are currently no randomized prospective studies related to aneurysms and statins. The AARDVARK trial found ACE inhibitors had no significant difference on small AAA growth rates (38), thus the benefit of statins and ACE inhibitors in the management of AAAs appears to be in reducing cardiovascular morbidity and mortality.
Infrarenal aortic aneurysm repair
Current treatment options for the repair of infrarenal aortic aneurysms are open surgical repair (OSR) and endovascular aneurysm repair (EVAR), which involves the insertion of a graft into the lumen of the aorta to exclude the aneurysm sac. Currently, EVAR is the primary treatment method for the repair of infrarenal AAAs due to improved morbidity and mortality results when compared to OSR (39).
EVAR
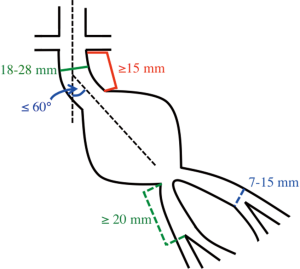
In contemporary practice, many infrarenal aortic aneurysms with favorable neck anatomy are treated with EVAR. Of the commercially available endografts in the United States, the Instructions-for-Use (IFU) criteria include a proximal neck maximum diameter of 28 mm and a minimum axial neck length of 15 mm. Additionally, it is recommended the neck is less than 60 degrees angulation and without excessive calcium or atherothrombotic disease. For the distal landing zones, the iliac arteries should be 13 to 15 mm in maximum diameter with a minimum length of 20 mm to the external-internal iliac artery bifurcation (Figure 4). The graft is delivered via the femoral arteries, which are accessed either via cutdown with direct exposure of the vessels or percutaneously with preclosure of the vessel access site using a suture mediated closure device such as the ProGlide (Abbott Vascular, Santa Clara, CA, USA). The less tortuous iliac artery is used for the delivery of the main body graft. Short-term success rates of EVAR are favorable ranging from 83% to 95% (40,41). EVAR is less invasive when compared to OSR and 30-day all-cause mortality rates are significantly lower with EVAR compared to OSR (1.6% vs. 4.8%) (42). Despite this short-term benefit, studies have failed to show the long-term benefit of EVAR over OSR after 2 years. EVAR patients additionally have shorter recovery times and hospital stays. While the majority of studies have found EVAR to be more expensive than OSR, the data is variable on cost-effectiveness (43).
OSR
Renal insufficiency, chronic obstructive pulmonary disease, cardiac disease, increasing age, and female gender have all been found to be independent predictors of mortality after open repair (44,45). Per the 2005 ACC/AHA guidelines, OSR should be performed in patients at low or average risk for complications (32). For patients that are deemed appropriate for OSR, the approach is via either the transabdominal (TA) or retroperitoneal (RP) route. There are conflicting views and data on whether there are physiologic benefits to a RP approach over TA, such as reductions in fluid losses, cardiac stress, and severity in ileus (46,47). RP does, however, tend to be the preferred approach in patients with hostile abdomens and extensive scarring, either the result of prior surgeries or radiation (Figure 5). The location of the proximal clamp site is determined preoperatively by reviewing CTA imaging. The decision of where to clamp is based on the presence of aortic calcification or mural thrombus, as well as the location of the start of the aneurysm to the lowest renal artery. For infrarenal aortic aneurysms, clamping below the renal is usually a viable option. In terms of graft selection, straight tube grafts tend to be preferred over bifurcated grafts due to less blood loss and shorter operative times. Many surgeons would agree that since many infrarenal aortic aneurysms with favorable neck anatomy are treated with EVAR, the complexity of OSR has increased over the past decade. OSR, when compared with EVAR, is associated with longer hospital stays, higher transfusion rates, greater use of intensive care resources, and higher 30-day mortality rates (48).
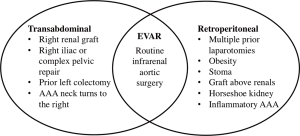
Surveillance following infrarenal AAA repair
EVAR
Patients who have undergone endovascular stent graft repair of infrarenal AAAs need lifelong imaging surveillance. Post-operative imaging aids in identifying complications such as stent migration, persistent sac expansion, endoleaks, and stent fracture (11) (Figure 6). While the risk of endoleak decreases with each negative annual scan, endoleaks have been identified as late as 7 years post-operatively (49). The imaging modality of choice is multidetector CTA with pre-contrast, arterial, and delayed phasing (50). This enables one to distinguish between calcifications and endoleaks, with the delayed phase being helpful in identifying slow-flow endoleaks. Using CTA, the sac size, stent graft durability, and location of the graft with respect to the renal arteries can all be assessed. Based on early FDA-sponsored EVAR trials, surveillance CTs have been recommended at 1, 6, and 12 months post-operatively (51). However, more recent data suggests that omitting the 6-month scan if the 1-month CTA demonstrates no evidence of endoleak is safe (52). The SVS practice guidelines endorse this method, recommending CTA at 1 and 12 months. CTA surveillance is not without limitations and has the disadvantage of repeated radiation exposure as well as added costs. Additionally, beam-hardening artifacts from aortic wall calcification or stent graft material can obscure smaller endoleaks (17). There have been reports of MRA being more sensitive in the detection of endoleaks (53), but CTA remains the post-EVAR surveillance modality of choice (Figure 7). Radiographs can be used to evaluate stent graft position and integrity, but have limited utility outside of these parameters. Sonography has limited and variable sensitivity in detecting endoleaks (54), but can be considered in combination with non-contrast CT in patients with renal insufficiency and normal scans within the first post-operative year. New ultrasound contrast agents have increased the sensitivity of ultrasound to detect endoleaks following EVAR (55).
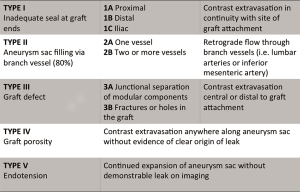
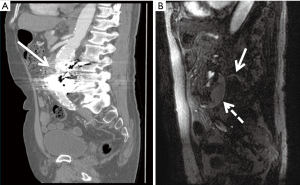
OSR
Unlike EVAR, OSR is not associated with risk of persistent sac enlargement. However, para-anastomotic aneurysm formation or graft infection can occur at rates of 1%, 5%, and 20% at years 5, 10, and 15, respectively. For this reason, the 2009 SVS Guidelines recommend follow-up CTA imaging at 5-year intervals after OSR, or more frequently if there is reason for clinical concern. Sonography is also a reasonable means for surveillance in this population to ensure there is no progressive aortic dilatation.
Conclusions
Abdominal aortic aneurysms remain one of the leading causes of morbidity and mortality in patients over the age of 65. Despite increased evidence supporting the utility of screening for AAAs in high risk patient populations, the most common way that these are detected is incidentally while undergoing an ultrasound, radiography of the back or abdomen, CT scan, or MRI for the evaluation of another problem. While CTA with 3D reconstruction remains the standard modality for pre-operative imaging, case planning, and postoperative surveillance, ultrasound is being increasingly used for post-operative surveillance in patients with stable aneurysm sac sizes and good anatomy. Endovascular repair has become the preferred therapy for the management of infrarenal AAAs and accounts for up to 80% of repairs in some institutions due to decreased perioperative morbidity and mortality as well as faster initial recovery times. However, concerns about the long-term durability of EVAR and the need for repeat intervention even after 8–10 years mandates lifelong surveillance in these patients. This fact also reiterates the importance of considering open repair in younger patients with low cardiac, pulmonary, and renal risk factors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Aggarwal S, Malik D. Clinical impact of USPSTF screening recommendations for abdominal aortic aneurysm: analysis of Nationwide Inpatient Sample data. Int J Cardiol 2015;195:77-8. [Crossref] [PubMed]
- McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg 2007;45:891-9. [Crossref] [PubMed]
- Holt P, Thompson MM. Abdominal aortic aneurysm, evaluation and decision making. In: Cronenwett JL, Johnston KW. editors. Rutherford’s vascular surgery. 8th ed. Philadelphia (PA): Elsevier Saunders, 2014:1999-2023.
- Kuivaniemi H, Elmore JR. Opportunities in abdominal aortic aneurysm research: epidemiology, genetics, and pathophysiology. Ann Vasc Surg 2012;26:862-70. [Crossref] [PubMed]
- Lederle FA, Johnson GR, Wilson SE, et al. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J Vasc Surg 1997;26:595-601. [Crossref] [PubMed]
- Lederle FA, Johnson GR, Wilson SE. Abdominal aortic aneurysm in women. J Vasc Surg 2001;34:122-6. [Crossref] [PubMed]
- Scott RA, Ashton HA, Kay DN. Abdominal aortic aneurysm in 4237 screened patients: prevalence, development and management over 6 years. Br J Surg 1991;78:1122-5. [Crossref] [PubMed]
- Thompson SG, Asthon HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 2012;99:1649-56. [Crossref] [PubMed]
- Søgaard R, Laustsen J, Lindholt JS. Cost effectiveness of abdominal aortic aneurysm screening and rescreening in men in a modern context: evaluation of a hypothetical cohort using a decision analytical model. BMJ 2012;345:e4276. [Crossref] [PubMed]
- LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:281-90. [Crossref] [PubMed]
- Chaikof EL, Brewster DC, Dalman RL, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009;50:S2-49. [Crossref] [PubMed]
- Kostun ZW, Malik RK. Screening for abdominal aortic aneurysms. Clin Imaging 2016;40:321-4. [Crossref] [PubMed]
- Jaakkola P, Hippeläinen M, Farin P, et al. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg 1996;12:230-7. [Crossref] [PubMed]
- Lederle FA, Wilson SE, Johnson GR, et al. Variability in measurement of abdominal aortic aneurysms. Abdominal Aortic Aneurysm Detection and Management Veterans Administration Cooperative Study Group. J Vasc Surg 1995;21:945-52. [Crossref] [PubMed]
- Chervu A, Clagett GP, Valentine RJ, et al. Role of physical examination in detection of abdominal aortic aneurysms. Surgery 1995;117:454-7. [Crossref] [PubMed]
- Lee WA. Endovascular abdominal aortic aneurysm sizing and case planning using the TeraRecon Aquarius workstation. Vasc Endovascular Surg 2007;41:61-7. [Crossref] [PubMed]
- Budovec JJ, Pollema M, Grogan M. Update on multidetector computed tomography angiography of the abdominal aorta. Radiol Clin North Am 2010;48:283-309. [Crossref] [PubMed]
- Olsen PS, Schroeder T, Agerskov K, et al. Surgery for abdominal aortic aneurysms. A survey of 656 patients. J Cardiovasc Surg (Torino) 1991;32:636-42. [PubMed]
- Karkos CD, Bruce IA, Thomson GJ, et al. Retroaortic left renal vein and its implications in abdominal aortic surgery. Ann Vasc Surg 2001;15:703-8. [Crossref] [PubMed]
- Bush RL, Lin PH, Bianco CC, et al. Endovascular aortic aneurysm repair in patients with renal dysfunction or severe contrast allergy: utility of imaging modalities without iodinated contrast. Ann Vasc Surg 2002;16:537-44. [Crossref] [PubMed]
- Wiginton CD, Kelly B, Oto A, et al. Gadolinium-based contrast exposure, nephrogenic systemic fibrosis, and gadolinium detection in tissue. AJR Am J Roentgenol 2008;190:1060-8. [Crossref] [PubMed]
- Ruangwattanapaisarn N, Hsiao A, Vasanawala S. Ferumoxytol as an off-label contrast agent in body 3-T MR angiography: a pilot study in children. Pediatr Radiol 2015;45:831-9. [Crossref] [PubMed]
- Srichai MB, Kim S, Axel L, et al. Non-Gadolinium-Enhanced 3-Dimensional Magnetic Resonance Angiography for the Evaluation of Thoracic Aortic Disease: A Preliminary Experience. Tex Heart Inst J 2010;37:58-65. [PubMed]
- Roldán-Alzate A, Francois CJ, Wieben O, et al. Emerging Applications of Abdominal 4D Flow MRI. AJR Am J Roentgenol 2016;207:58-66. [Crossref] [PubMed]
- Lau C, Feldman DN, Girardi LN, et al. Imaging for surveillance and operative management for endovascular aortic aneurysm repairs. J Thorac Dis 2017;9:S309-16. [Crossref] [PubMed]
- Aggarwal S, Qamar A, Sharma V, et al. Abdominal aortic aneurysm: A comprehensive review. Exp Clin Cardiol 2011;16:11-5. [PubMed]
- Szilagyi DE, Smith RF, DeRusso FJ, et al. Contribution of abdominal aortic aneurysmectomy to prolongation of life. Ann Surg 1966;164:678-99. [Crossref] [PubMed]
- Brewster DC, Cronenwett JL, Hallett JW Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003;37:1106-17. [Crossref] [PubMed]
- Nevitt MP, Ballard DJ, Hallett JW Jr. Prognosis of abdominal aortic aneurysms. A population-based study. N Engl J Med 1989;321:1009-14. [Crossref] [PubMed]
- Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet 1998;352:1649-55. [Crossref] [PubMed]
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: Effect of size and beta-adrenergic blockade. J Vasc Surg 1994;19:727-31. [Crossref] [PubMed]
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463-654. [Crossref] [PubMed]
- Katz DA, Littenberg B, Cronenwett JL. Management of small abdominal aortic aneurysms. Early surgery vs watchful waiting. JAMA 1992;268:2678-86. [Crossref] [PubMed]
- Schermerhorn M. A. 66-year-old man with an abdominal aortic aneurysm: review of screening and treatment. JAMA 2009;302:2015-22. [Crossref] [PubMed]
- Filardo G, Powell JT, Martinez MA, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev 2012;3:CD001835. [PubMed]
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004;39:967-75. [Crossref] [PubMed]
- Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med 2009;361:980-9. [Crossref] [PubMed]
- Kiru G, Bicknell C, Falaschetti E, et al. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomised placebo-controlled trial (AARDVARK). Health Technol Assess 2016;20:1-180. [Crossref] [PubMed]
- Schermerhorn ML, O'Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008;358:464-74. [Crossref] [PubMed]
- Blum U, Voshage G, Lammer J, et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N Engl J Med 1997;336:13-20. [Crossref] [PubMed]
- Elkouri S, Gloviczki P, McKusick MA, et al. Endovascular repair of abdominal aortic aneurysms: Initial experience with 100 consecutive patients. Mayo Clin Proc 2003;78:1234-42. [Crossref] [PubMed]
- Lederle FA, Kane RL, MacDonald R, et al. Systematic review: Repair of unruptured abdominal aortic aneurysm. Ann Intern Med 2007;146:735-41. [Crossref] [PubMed]
- van Bochove CA, Burgers LT, Vahl AC, et al. Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm. J Vasc Surg 2016;63:827-38.e2. [Crossref] [PubMed]
- Tang TY, Walsh SR, Fanshawe TR, et al. Comparison of risk-scoring methods in predicting the immediate outcome after elective open abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2007;34:505-13. [Crossref] [PubMed]
- Grant SW, Grayson AD, Mitchell DC, et al. Evaluation of five risk prediction models for elective abdominal aortic aneurysm repair using the UK National Vascular Database. Br J Surg 2012;99:673-9. [Crossref] [PubMed]
- Cambria RP, Brewster DC, Abbott WM, et al. Transperitoneal versus retroperitoneal approach for aortic reconstruction: a randomized prospective study. J Vasc Surg 1990;11:314-24. [Crossref] [PubMed]
- Sicard GA, Reilly JM, Rubin BG, et al. Transabdominal versus retroperitoneal incision for abdominal aortic surgery: report of a prospective randomized trial. J Vasc Surg 1995;21:174-81. [Crossref] [PubMed]
- Eliason JL, Upchurch GR. Endovascular abdominal aortic aneurysm repair. Circulation 2008;117:1738-44. [Crossref] [PubMed]
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004;239:800-5. [Crossref] [PubMed]
- Sharma P, Kyriakides C. Surveillance of patients post-endovascular aneurysm repair. Postgrad Med J 2007;83:750-3. [Crossref] [PubMed]
- Chaikof EL, Brewster DC, Dalman RL, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg 2009;50:880-96. [Crossref] [PubMed]
- Sternbergh WC 3rd, Greenberg RK, Chuter TA, et al. Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg 2008;48:278-4. [Crossref] [PubMed]
- van der Laan MJ, Bakker CJ, Blankensteijn JD, et al. Dynamic CE-MRA for endoleak classification after endovascular aneurysm repair. Eur J Vasc Endovasc Surg 2006;31:130-5. [Crossref] [PubMed]
- AbuRahma AF, Welch CA, Mullins BB, et al. Computed tomography versus color duplex ultrasound for surveillance of abdominal aortic stent-grafts. J Endovasc Ther 2005;12:568-73. [Crossref] [PubMed]
- Abraha I, Luchetta ML, De Florio R, et al. Ultrasonography for endoleak detection after endoluminal abdominal aortic aneurysm repair. Cochrane Database Syst Rev 2017;6:CD010296. [PubMed]


