Safety of selective internal radiation therapy (SIRT) with yttrium-90 microspheres combined with systemic anticancer agents: expert consensus
Introduction
Selective internal radiation therapy (SIRT) with resin or glass microspheres labelled with the β-emitter yttrium-90 (Y-90) enables targeted delivery of radiation to inoperable primary or metastatic liver tumors (1-6). Most frequently used for the management of hepatocellular carcinoma (HCC) (5) and liver metastasis of colorectal cancer (mCRC) (7), SIRT has also been used to treat the primary liver tumor intrahepatic cholangiocarcinoma (ICC) (8), and liver metastasis of many other tumor types including neuroendocrine tumors (NETs) (9), breast cancer (10,11) and uveal melanoma (12,13).
In HCC, SIRT has been effective in treating intermediate [Barcelona Clinic Liver Cancer (BCLC) stage B] and advanced stages (BCLC stage C), with or without portal vein thrombosis (PVT). In mCRC, SIRT has been used at all stages of treatment including as salvage therapy in treatment-refractory disease (14,15), in second-line settings (whether the switch occurred due to first-line toxicity or first-line treatment failure) (16,17), and in first-line induction and maintenance settings (7,18). Likewise, in other tumor types, there is evidence that SIRT can be given at various stages (10,19-25).
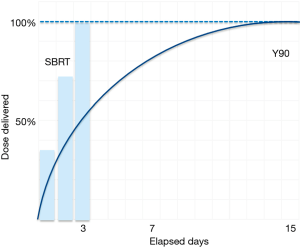
Whereas stereotactic body radiotherapy delivers high doses of external radiation, SIRT provides a continuous dose of radiation inside the liver over a 14-day period ( Figure 1). Since the Y-90-loaded microspheres preferentially localize in tumor arterial vasculature, very high radiation doses are delivered to tumors while maintaining tolerable radiation doses to normal hepatic tissue.
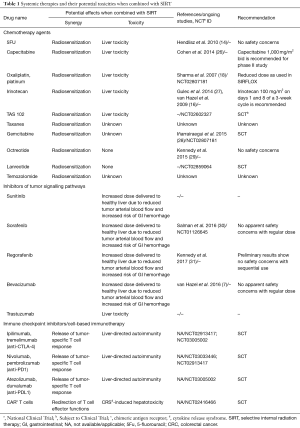
SIRT is administered to patients who have typically received systemic anticancer therapies. Subsets of patients will also receive these agents, concurrent with, or soon after the SIRT administration, all of which may interact with SIRT (Table 1).
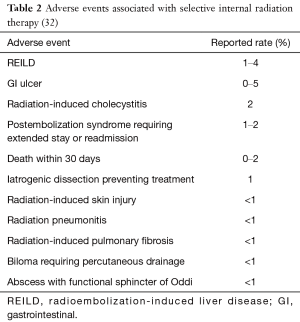
Full table
These include desirable radiation-sensitizing effects, along with the potential for adding to or intensifying expected SIRT-related adverse events (AEs) (Table 2). However, there are few large-scale, randomized clinical trials of SIRT that allow evidence-based guidance.
The SIRFLOX trial is the only large-scale randomized trial published to date of SIRT plus chemotherapy. It assessed the efficacy and safety of SIRT with 90 resin microspheres when combined with first-line mFOLFOX chemotherapy [5-fluorouracil (5FU), leucovorin and oxaliplatin] vs. mFOLFOX alone in patients with liver-only or liver-dominant mCRC (bevacizumab was also allowed at the discretion of the investigators) (7). The rationale for combining SIRT with these chemotherapeutic agents is partly based on the expectation that there would be a synergistic antitumor activity from combining fluoropyrimidine, oxaliplatin and radiation therapy (RT). The addition of 5FU/leucovorin to preoperative radiation improves local control in rectal cancer (33-35), but may also increase the rate of severe acute toxicity (33). The addition of oxaliplatin to a combination of RT and 5FU in patients undergoing surgery for rectal cancer also improved the pathologic complete response (pCR) rate from 13% to 17% (P=0.038) (36). The feasibility of combining SIRT with first-line 5FU/leucovorin-based chemotherapy or FOLFOX chemotherapy for mCRC has been demonstrated in small-scale studies (18,27,37,38), but a dose-limiting toxicity of grade 3/4 neutropenia led to modifying the oxaliplatin dose from 85 to 60 mg/m2 for the first three cycles of FOLFOX if SIRT was administered with cycle 1 (on day 3 or 4). Even with this modification to FOLFOX in the SIRT arm in SIRFLOX, neutropenia, febrile neutropenia, thrombocytopenia, fatigue and abdominal pain occurred at a significantly greater frequency in the arm receiving SIRT, but at a frequency and severity that was expected and manageable (7). No patient had a gastric/duodenal ulcer in the mFOLFOX arm but nine (3.7%) had this AE in the SIRT arm. Radiation hepatitis occurred in two (0.8%) patients receiving SIRT and was managed with low-molecular-weight heparin, diuretics and corticosteroids, and hepatic failure occurred in 3 (1.2%) patients receiving SIRT (7). However, the influence of the oxaliplatin dose on the occurrence of these SIRT-related AEs is difficult to prove.
These published data have been corroborated by the FOXFIRE-SIRFLOX-FOXFIRE Global combined analysis of 1,103 patients presented in abstract form (39). Despite improving objective response rate and liver-specific progression, the addition of SIRT had no impact on overall survival (OS) and increased the frequency of grade 3–5 AEs (39). Therefore, this combination in first-line treatment of metastatic colorectal cancer cannot be recommended by the expert panel.
A recent systematic review provided further guidance on the optimal approach to managing some of the most important complications of SIRT, and this guidance is central to managing these complications when they occur in the combination settings discussed here (40).
We met to reach consensus on combinations of systemic therapies and SIRT that are more likely to be encountered in current clinical practice than the first-line treatment of mCRC assessed in the FOXFIRE-SIRFLOX-FOXFIRE Global trials. We sought to investigate safety and practical considerations, grade the evidence, and provide guidance on the key considerations in management through a series of clinical scenarios (Table 2). Given the increased use of SIRT in multiple clinical settings, we developed this paper to provide clinical experience and consensus guidelines for its use with chemotherapy.
Methods
An international panel of experts, with extensive experience of using SIRT, from a range of disciplines, was convened in January 2017 to discuss a set of pre-formulated topics. Extensive literature searches were conducted on each topic: SIRT and cytotoxics; SIRT and biologics; SIRT and tyrosine kinase inhibitors (TKIs); SIRT and mechanistic target of rapamycin (mTOR) inhibitors; SIRT and immunotherapy; and SIRT and peptide receptor radionuclide therapy (PRRT). In light of the evidence, the panel developed relevant clinical questions/scenarios to discuss the evidence and provide advice on best practice. These were discussed and modified until consensus was reached. If no consensus was achieved on a specific point, the alternative views of the group are described in this paper. An adapted version of the ‘Infectious Diseases Society of America-United States Public Health Service Grading System’ was used to define the level of evidence (LoE) and strength of each recommendation (41).
Recommendations
Clinical question 1: integration of SIRT pre-progression, after at least 4 months of first-line chemotherapy as a potential conversion or consolidation approach for mCRC
In a patient with the colorectal primary adenocarcinoma in place, large-volume and liver-dominant metastasis, who has received approximately 4 months of first-line chemotherapy and has had a response but is unlikely to be a candidate for resection with continued chemotherapy, and is exhibiting chemotherapy-induced neuropathy, what are the issues when considering SIRT?
Rationale and recommendations
First-line chemotherapy strategies have evolved rapidly and the concept of ‘lines’ of therapy for mCRC often merges into a continuum of care. A change of either the backbone (irinotecan versus oxaliplatin) or the biological agent [vascular endothelial growth factor (VEGF) inhibitor vs. epidermal growth factor receptor (EGFR) inhibitor], is used here as the distinction between changing from a first-line to a second-line strategy.
The maximum response of mCRC to first-line chemotherapy is usually evident within 3–6 months of starting first-line chemotherapy (42), and if patients are not candidates for liver resection at this stage, further chemotherapy is unlikely to convert them to resectability. However, in one study of 1,104 patients with initially unresectable liver metastases of CRC, 12.5% became eligible for surgery, with 23% of these patients having undergone two or three lines of chemotherapy prior to resection (LoE V; grade C) (43). Following failure of first-line chemotherapy for mCRC, the addition of an anti-EGFR agent to second-line folinic acid, fluorouracil and irinotecan hydrochloride (FOLFIRI) in patients with wild-type (WT) KRAS tumors led to an improvement in the response rate from 10% to 35% (44). Patients with liver dominant 5FU-refractory disease receiving SIRT combined with irinotecan also had an improved response rate of 48% compared with 5–16% with irinotecan alone (16). However, administering additional cycles of combination chemotherapy beyond 4 months may result in decreased tolerance and increased toxicity.
The integration of SIRT following an initial ‘induction therapy’ phase may allow for a ‘treatment holiday’ from chemotherapy, or may have an additive or synergistic effect following radiosensitizing chemotherapy. Patients who have had an initial response to first-line chemotherapy but insufficient to make them candidates for resection, and who have chemotherapy-induced AEs, are often referred to a specialist center for SIRT. A preliminary report of 23 patients with liver dominant mCRC who received Y-90 resin microspheres 12–26 weeks (median 21 weeks) after starting first-line chemotherapy (oxaliplatin, 100%; 5FU, 8.7%; or capecitabine, 91.3%; plus cetuximab, 60.9%; irinotecan, 21.7%; or bevacizumab, 8.7%) resulted in partial response or stable disease in 91.3% of cases, resection in 13%, and no grade 3/4 AEs (45). Neuropathy is a common AE of oxaliplatin, (46) and it may lead to oxaliplatin withdrawal. In non-progressing cases, a maintenance approach (or de-escalation) such as with capecitabine plus bevacizumab may be recommended.
An ongoing trial (SIR-step; clinicaltrials.gov/ct2/show/NCT01895257) aims to recruit 162 patients with stable mCRC after 3–6 months’ induction chemotherapy with FOLFOX, FOLFIRI or XELOX (with or without bevacizumab) who will be randomized to receive either maintenance chemotherapy or maintenance chemotherapy plus SIRT with Y-90 resin microspheres.
Bevacizumab or other VEGF inhibitors may lead to ‘pruning’ from vasoconstriction and/or fragility within the hepatic vasculature. This may result in vascular dissection of the hepatic arteries, which can prevent safe and/or effective delivery of subsequent of concomitant SIRT (Figure 2). In a worst-case scenario, injury to the fragile vessels can result in a pseudoaneurysm. Therefore, if bevacizumab is already used in the first-line regimen, these issues must be considered before performing SIRT. Bevacizumab has a half-life of approximately 20 days (47); therefore, it is recommended to stop bevacizumab 4–6 weeks before the SIRT procedure to reduce the potential issues of vascular pruning/dissection, and restart bevacizumab after the patient has recovered from the procedure. The optimal timing of SIRT administration could potentially be at the end of a 3–4-week chemotherapy treatment cycle, thus avoiding potentially deleterious treatment interruptions. In SIRFLOX, where SIRT was integrated early into first-line chemotherapy, bevacizumab was withheld until at least cycle 4, and there was no evidence of any clinical detriment on progression-free survival (PFS) (7). This finding in the early first-line setting may give us confidence that delaying the introduction or re-introduction of bevacizumab will not have a negative effect in later settings.
The RAS/BRAF mutation status of the patient, and tumor location, appear to have prognostic value and predict the response to EGFR inhibitors and thus guide treatment decisions. At this stage, these biomarkers do not clearly inform the likely response to SIRT, due to lack of data.
Capecitabine, as a prodrug of 5FU, is a radiosensitizer. Based on limited data from studies, including a formal phase I study (26) and user experience, the consensus group concluded that capecitabine is safe to combine with SIRT in a similar way to 5FU. As capecitabine dosing is frequently prolonged (e.g., 2 out of 3 weeks), the guidance from this consensus in the first-line setting is similar to the salvage setting because SIRT appears to be safely administered in the third or fourth week (at the end of a treatment cycle).
Clinical question 2: using SIRT with second-line chemotherapy in mCRC
In a patient with the primary tumor in place and liver-dominant metastasis, who has progressed after receiving first-line chemotherapy and is initiating second-line chemotherapy with FOLFIRI, is it safe to add SIRT?
Rationale and recommendations
As with the first clinical question, referrals for SIRT in the second-line setting are a frequent occurrence in clinical practice.
In a large cohort study of 606 patients with mCRC receiving SIRT, 206 received SIRT in the second-line or later setting after 5FU and oxaliplatin-based chemotherapy. Overall, AE profiles were similar irrespective of the number of previous chemotherapy lines (48). Grade 3 or greater GI ulcer was observed in 1.7% of patients and radioembolization-induced liver disease (REILD) was observed in 0.5%, but the relative safety of different chemotherapy/SIRT combinations was not assessed.
Other smaller scale studies assessed SIRT in various second-line settings. For example, in 25 patients with mCRC who were refractory to 5FU-based first-line chemotherapy (8 patients had failed subsequent lines of chemotherapy also), SIRT (during the first cycle) was combined with second-line irinotecan (at three different doses on days 1 and 8 of a 3-week cycle) (16). The safety profile of the combination was similar to that of irinotecan alone, with fewer myelosuppression AEs than previously observed with SIRT plus FOLFOX. A phase II study assessed the use of SIRT after oxaliplatin-based first-line chemotherapy and before second-line irinotecan-based chemotherapy, but limited data were released (49). Similar findings were reported in 72 patients who had received mostly oxaliplatin and 5FU-based chemotherapy (one or more lines), and SIRT with Y-90 glass microspheres (3).
In clinical practice, a single whole liver treatment of SIRT or sequential lobar treatment via two sessions of SIRT 4–8 weeks apart may be delivered. Therefore, to manage the timing of, for example, second-line chemotherapy and/or bevacizumab administration, it is necessary to map out a 4–8-week treatment plan. If bevacizumab is to be added, similar principles as discussed in question 1 apply. In Europe, the use of biologics in the second-line setting is less frequent than in the USA, and FOLFIRI is often the preferred approach. Concurrent FOLFIRI and SIRT is considered a feasible approach; however, the timing of SIRT relative to second-line chemotherapy (at the start, in the middle of the chemotherapy course, or after all cycles of second-line chemotherapy have been administered) may depend on various factors such as the aim of treatment, patient fitness, and whether the metastasis is liver-dominant. Data are lacking for concomitant SIRT and FOLFIRI administration but giving SIRT on day 3 or 4 of the first cycle may be feasible. The use of SIRT is less likely in the situation of non-liver-dominant disease. In a cohort study of 260 patients, it was found that REILD only occurred in patients who had cirrhosis or who had been exposed to prolonged chemotherapy (50). The type of chemotherapeutic agent did not impact on the incidence of REILD. Among non-cirrhotic patients in this study, exposure to chemotherapy in the 2 months following SIRT was an independent predictor of REILD [odds ratio (OR) =6.46; 95% CI, 1.60–25.91].
Other VEGF inhibitors or EGFR inhibitors may also be used in this setting. EGFR inhibitors are radiosensitizers, which may provide a rationale for their use with SIRT but also may increase the risk of radiation toxicity to normal liver parenchyma (LoE V). Anecdotal clinical experience suggests there are fewer issues regarding vascular injury during catheterization with these agents before SIRT than there are with bevacizumab, and that administration of SIRT at 2 weeks after the last dose of cetuximab or panitumumab is feasible. However, owing to the theoretical issue of EGF receptor saturation, these agents may not be cleared from hepatocytes as rapidly as expected from plasma half-life (51). High-quality data are lacking to support any recommendation, and the combination of EGFR inhibitors and SIRT must be carefully considered on a case-by-case basis.
Clinical question 3: salvage settings in mCRC
In a patient who has progressive chemo-refractory mCRC and SIRT is being initiated, what are the considerations with respect to other treatments?
Rationale and recommendations
In chemorefractory disease, combining SIRT with radiosensitizing chemotherapy may increase PFS compared with chemotherapy alone. While acute toxicity associated with SIRT may be transient and manageable in this setting, the impact of REILD or GI ulcers on the patient need to be considered.
One phase III trial assessed SIRT with Y-90 resin microspheres plus 5FU compared with 5FU alone in 44 liver-limited mCRC patients refractory to previous chemotherapy. Time to liver progression and time to tumor progression were increased significantly with the addition of SIRT (LoE II; grade B) (14). There was no impact on OS, possibly due to control patients being allowed to cross over to SIRT at progression and the small sample size. There were fewer grade 3/4 toxicities in the patients given SIRT who also received a reduced 5FU dose (225 instead of 300 mg/m2) in the first cycle. Patients in this study may have failed multiple lines of chemotherapy (LoE I; grade A). Rather than 5FU infusion, some physicians may prefer to prescribe oral capecitabine.
Capecitabine is an important treatment for GI cancers that is not endorsed by the National Comprehensive Cancer Network (NCCN) but is used in some community practices as a sole agent in later lines of chemotherapy for mCRC. If considering SIRT, it is important to decide whether SIRT is feasible with capecitabine or whether this agent should be withdrawn. Capecitabine is a radiosensitizer that has been combined with SIRT in two recent phase I studies. In 24 patients (17 with mCRC) a maximum tolerated dose of capecitabine (given for 14 days every 21 days) was not reached when combined with SIRT with Y-90 resin microspheres (given on day 2 of the first cycle) (26). The authors suggested that a capecitabine dose of 1,000 mg/m2 bid be used in phase II studies. The combination of SIRT and capecitabine was well tolerated, with toxicities approximating those seen with either modality administered as monotherapy. Partial response or stable disease was observed in 87.5% of patients (LoE III; grade B) (26). In 16 patients (9 with mCRC) given full-dose capecitabine (during days 1–14 of a 21-day cycle), SIRT with Y-90 glass microspheres was given at days 1–7 of the second cycle (52). A maximum tolerated dose for SIRT was not reached when doses up to 170 Gy were assessed (LoE III; grade C). There are no specific safety concerns regarding the co-administration of SIRT and capecitabine, if doses do not exceed those used in the trials described here. In the absence of more robust data, some experts practise stopping all medication, regardless of the type of agent, for 2–4 weeks prior to SIRT.
Although several trials have demonstrated favorable outcomes with SIRT as monotherapy and together with concomitant chemotherapy in chemorefractory mCRC, the impact of adding chemotherapy to SIRT on survival in this setting has not been established. Some of the most encouraging results come from the combination of SIRT + 5FU, which improved the median time to liver progression compared with 5FU alone in patients with liver-limited chemorefractory mCRC (5.5 vs. 2.1 months, respectively; P=0.003) (14). In a recent retrospective study of 27 patients with 5FU-refractory disease, SIRT with concurrent chemotherapy (5FU, capecitabine, irinotecan or FOLFOX) was associated with increased disease control rates (84% vs. 14% with SIRT alone; P=0.001), as well as prolonged liver PFS (176 vs. 91 days with SIRT alone; P=0.0009) (53). The median OS was numerically superior in the SIRT plus chemotherapy group (212 vs. 154 days with SIRT alone); but did not reach significance (P=0.1023). Toxicity profiles were similar between the SIRT and the SIRT plus chemotherapy arms. However, liver-only patients in the salvage setting are uncommon and chemotherapy may not be needed as a radiosensitizer per se, but can be added to try to control extrahepatic disease progression.
After failure on first- and second-line chemotherapy, cetuximab can produce favorable outcomes in RAS WT patients (Figure 3). With regard to sequencing cetuximab doses and SIRT, experts suggest waiting 2–4 weeks between the last dose of this EGFR inhibitor and the SIRT procedure. There are few data on how to sequence SIRT with regorafenib (being addressed in a formal phase I study) or TAS-102. The latter can cause prolonged myelosuppression and as such we have to be mindful of a potential interaction with SIRT.
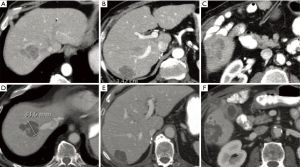
Patients who are clinically unsuitable for SIRT in the salvage setting include those with extensive uncontrolled extrahepatic disease or abnormal liver function tests (54).
Clinical question 4: advanced HCC receiving sorafenib
In a patient who has advanced HCC (Child-Pugh A/B), is it safe to combine SIRT with other therapies?
Rationale and recommendations
This type of patient constitutes the most frequent indication for SIRT in HCC, and combining SIRT with the TKI sorafenib may improve outcomes. Antiangiogenic agents have been shown to normalize the vessels and allow them to deliver oxygen more efficiently to tumors (55), which may increase sensitivity to tumor-delivered radiation (56). However, a recent study in cell lines suggested no synergistic activity between sorafenib and Y-90 (57). Despite this, the possibility of using SIRT in patients already receiving sorafenib is encountered in clinical practice (Figure 4).
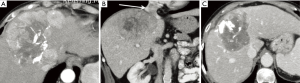
For patients with advanced HCC, sorafenib is the current standard of care. Although there are limited data on the effect of combining SIRT with sorafenib, the safety profile does appear acceptable. A study of 20 patients with HCC (Child Pugh ≤B8, no vascular invasion) compared SIRT with Y-90 glass microspheres alone versus the combination of SIRT and sorafenib (treated with sorafenib 400 mg bid for 14 days before SIRT, with the intent to bridge to transplantation). The combination arm was associated with more peri-operative biliary complications than with SIRT alone (LoE III) (58). The ongoing SORAMIC study compares SIRT with Y-90 resin microspheres followed 3 days later with oral sorafenib (200 mg bid for 1 week and then 400 mg bid) vs. sorafenib alone with a matching dosing schedule in patients with Child-Pugh ≤B7 BCLC A–C class HCC. Initial safety results of 40 patients suggested similar AE profiles in both arms (LoE II) (59). A phase II study assessed the safety and efficacy of SIRT followed by the initiation of sorafenib therapy (400 mg bid) 14 days later in 29 patients with inoperable HCC (BCLC stage B or C; 69% Child-Pugh A) (60). The response rate was promising (7% complete response; 18% partial response; 54% stable disease), and the AE profile was similar to sorafenib monotherapy (the most frequent grade 3 or higher AE was hand-foot syndrome; grade ≥3 hyperbilirubinemia was also observed in three patients) (LoE III) (60). In a recent study of patients with unresectable BCLC A-C class HCC (82.5% were Child-Pugh A; >50% BCLC C), sorafenib 400 mg bid was administered for 6–8 weeks before SIRT with Y-90 glass microspheres. Sorafenib treatment was not interrupted and was continued after the SIRT procedure (30). The addition of SIRT did not appear to affect the expected toxicity profile from sorafenib (LoE III).
There are no specific recommendations on the optimal timing of SIRT relative to sorafenib initiation. As with bevacizumab, there is a theoretical risk of damage to the large blood vessels, and there are anecdotal reports of pruning with the use of sorafenib in clinical practice. However, as sorafenib is a twice-daily oral therapy with a short half-life, such events can be managed with cessation of treatment for up to a week before the SIRT procedure. The cirrhotic liver appears to tolerate SIRT and the combination with sorafenib well (59). For low burden disease, the interventional radiologist should attempt to treat the smallest required volume of the liver. Additionally, maximal tumor vascularity will result in the greatest volume of Y-90 microspheres being delivered to the tumor. Intra-arterial therapy + sorafenib is frequently performed for HCC with limited metastatic disease. A formal study focusing on consolidation therapy after several months of sorafenib to assess the impact on mortality, has not been performed. Regorafenib is now approved as a second-line treatment option after sorafenib failure, but there are few data on regorafenib and SIRT.
Clinical question 5: advanced HCC with lobar but not main PVT
How do outcomes with SIRT compare with standard of care sorafenib in patients with advanced HCC?
Rationale and recommendations
Although sorafenib is generally recommended for the management of HCC with PVT, it provides only minor improvements in OS and low response rates in this group of patients (61,62). Given the tolerability of SIRT in patients with compensated liver disease and the lack of an embolic effect, SIRT is used in many clinical situations for advanced HCC (BCLC stage C) (Figure 5). One potential advantage of this approach is keeping sorafenib as a second-line therapy when extrahepatic disease occurs.
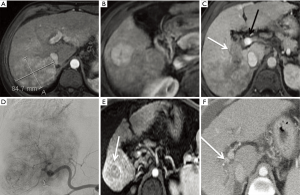
In a review of treatment regimens selected for 1,717 HCC patients in Korea, 608 BCLC stage C patients were identified (63). Only 36 patients (4.3%) received sorafenib. When time to progression and OS are compared directly, outcomes with sorafenib and SIRT for advanced HCC were similar. More recently, the initial report from the randomized SARAH trial also suggested similar efficacy of SIRT and sorafenib (median OS 8.0 and 9.9 months in the SIRT and sorafenib groups, respectively, P=0.179), but with patients receiving SIRT having a better quality of life and experiencing fewer AEs than patients receiving sorafenib (82 and 111 treatment-related serious AEs, respectively). Objective response rate was 19.0% and 11.6% (P=0.042) with SIRT and sorafenib, respectively (64). SIRveNIB, with a similar design and patient eligibility to SARAH but conducted in South-East Asia, reported a very similar result (65).
When considering locoregional therapies in the setting of PVT, performance status and level of venous occlusion are important considerations. Patients who are Child-Pugh A with an ECOG performance status of 0 and receiving intra-arterial therapy have superior survival to those with Child-Pugh B/C disease or any ECOG limitations (66). There is also a difference in outcomes when SIRT is used in patients with segmental/lobar PVT versus main PVT. Kulik et al. reported toxicities and outcomes in 108 patients with no PVT (n=71), segmental/lobar PVT (n=25) and main PVT (n=12) (67). Median survival for the three groups was 467, 304, and 134 days, respectively. Similar dosimetry was used for all patients. In general, SIRT was well tolerated with limited liver toxicity. There were no differences in the level of PVT and changes in hepatic encephalopathy or total bilirubin. New ascites was an AE significantly more common with main PVT (55%) compared with no PVT (15%) or segmental/lobar PVT (5%). In 41 patients with HCC and PVT (68) the injected activity of SIRT was adjusted upward when there was increased tumor-to-normal-liver uptake on mapping scintigraphy. Patients with high tumor-to-normal liver ratios receiving 205 Gy or more had superior outcomes compared with patients who received a dose of less than 205 Gy. OS was 12 months with main PVT and 22 months with segmental/lobar PVT. The ability to deliver doses up to 205 Gy was limited in some cases by the non-tumor liver dose or hepatopulmonary shunting. HCC and portal vein invasion are both associated with increased pulmonary shunting (69). HCC had a median and upper value lung shunt fraction of 11.7% and 39.4%, respectively, while PVT averaged 15.8%, with a maximal value of 31.2%. In the setting of HCC with PVT, the mean lung shunt fraction was 16.6%, with a maximum of 31%.
Clinical question 6: HCC or metastatic disease patient receiving immunotherapy
What is the rationale for combining SIRT and immunotherapy in a patient with HCC or metastatic disease such as colorectal adenocarcinoma and unresectable liver metastases, who has failed standard chemotherapy?
Rationale and recommendations
Radiation treatment has remarkable immunomodulatory effects (70). Promotion and suppression of tumor-specific immunity have been described in association with radiotherapy, and depend on whether the elicited inflammation is acute or chronic. Acute inflammation is a potent stimulator of anti-tumor immunity through a variety of mechanisms, including activation of tumor-associated dendritic cells (71), expression of cancer-testis (CT) and MHC class I antigens on the tumor surface (72), and tumor infiltration by CD8+ T cells (73). Promotion of anti-tumor immunity in the context of acute inflammation is critically dependent upon transient complement activation involving local production of C3a and C5a anaphylatoxins (74). Indeed, blocking complement activation via administration of glucocorticoids drastically reduces radiotherapy-mediated anti-tumor immune effects (74). In contrast to acute inflammation, chronic inflammation is known to promote development of a tumor-permissive, tolerogenic microenvironment (75), involving immune suppressor cells (76,77) and, potentially, alternatively (M2) activated macrophages, immune suppressive cytokines and checkpoint upregulation.
Different therapeutic radiation modalities may trigger distinct inflammatory responses. A 14-day long, continuous intrahepatic radiation delivery by SIRT may elicit a prevalently suppressive, chronic inflammation. In addition, continuous exposure to radiation may exert deleterious effects on rapidly proliferating, radiosensitive T cells infiltrating the tumor. Not surprisingly, SIRT resulted in an early (24 h), profound (>50%) and prolonged (up to 30 months) lymphopenia in a study enrolling 92 HCC patients, and delayed recovery of T and B lymphocytes was associated with a poor prognosis (78). Moreover, increased neutrophil-to-lymphocyte (NTL) and platelet-to-lymphocyte (PTL) ratios were observed in patient receiving SIRT, and found to be associated with worse clinical outcome (79).
The use of SIRT in combination with immunotherapy has only recently begun to be investigated. Ongoing clinical trials are exploring SIRT in combination with CAR-T hepatic artery infusion for carcinoembryonic antigen (CEA)-expressing liver metastases (ClinicalTrials.gov ID: NCT02416466), with nivolumab in a phase II single-center recruiting Asian patients with HCC (ClinicalTrials.gov ID: NCT03033446), with concurrent ipilimumab and nivolumab in uveal melanoma with liver metastases (ClinicalTrials.gov ID: NCT02913417), and with durvalumab and tremelimumab in patients with liver-predominant mCRC and microsatellite-stable disease (ClinicalTrials.gov ID: NCT03005002).
Therefore, it is not possible to formulate evidence-based recommendations on SIRT-immunotherapy combinatorial approaches. Immunotherapy could counter immune suppression due to chronic inflammation and continuous radiation. Hence, adoptive transfer of autologous (including CAR-engineered) lymphocytes, checkpoint blockade inhibitors and tumor-associated antigen targeting might result in improved clinical outcomes. Delivery of concurrent stereotactic, hypofractionated high-dose irradiation may synergize these effects by skewing radiation-induced injury towards acute inflammation.
Other common questions
SIRT follow-up recommendations
The scheduled follow-up visit following SIRT should be 2 weeks after the procedure, with most teams performing additional evaluations at 4 or 6 weeks, and again at 12 weeks post-SIRT. At these visits, a physical examination, complete blood count, and liver function tests are a minimum to inform on safety of ongoing treatments. Data from a variety of experiences demonstrate optimal radiological response to treatment at 12 weeks post SIRT.
SIRT and surgery
SIRT may downsize tumors to allow resection (80-84), or be a bridge to liver transplantation and for down-staging HCC before transplantation (85-87). In addition, SIRT can produce concomitant hypertrophy in the contralateral lobe (88), a phenomenon that occurs in approximately 26% to 47% of patients after between 44 days and 9 months (88). Although not the primary objective of the SIRT procedure, contralateral hypertrophy represents a potential benefit for patients who subsequently undergo hepatic resection. However, it is important to consider the feasibility and safety of surgery after SIRT, and to determine the impact of concomitant chemotherapy. A preliminary study of 13 patients who underwent SIRT followed by lobectomy found the approach to be an effective method to induce hypertrophy of the future liver remnant (FLR) prior to resection (89). The median time to resection in this study was 86 days (30–210 days); and with PVE the median time to resection was 37 days (21–84 days) (90). The P4S study may help formulate guidance on the practicalities and safety of surgery after SIRT (91). In this study of 100 patients undergoing liver surgery after receiving SIRT, mortality and complication rates appeared acceptable given the risk profile of the recruited patients (91). Resection after SIRT appears to be safe even for major resections, and in cirrhotic and non-cirrhotic patients. Generally, preserving the future liver remnant from radiation is recommended for patients with borderline resectability. However, recommendations on timing and other considerations are not possible at this time.
Treatment combinations in other tumors
SIRT can be used for other solid tumors, and it is important to consider the therapeutic agents that such patients have received, the total duration of systemic agent use, agents they are currently receiving, and the possible interactions these agents may have with SIRT (Table 1).
ICC
Several prospective and retrospective studies have assessed SIRT in ICC (all second- or later-line), which demonstrate median OS of between 9.3 and 22 months (19,92-100). A pooled analysis of 12 studies, with a total of 298 patients with unresectable ICC treated with SIRT, demonstrated a median OS of 15.5 months and treatment response rate of 28% (101). However, these studies provide little guidance on possible interactions with other therapies. A common chemotherapy for ICC is the radiosensitizer gemcitabine and possible interactions between this agent and SIRT need to be considered (102). The radiosensitizing effects of gemcitabine can last several weeks, and so it may be prudent to stop gemcitabine therapy 4–8 weeks before SIRT [if there is a risk of tumor progression during this period, second-line chemotherapy (e.g., with FOLFOX) can be started during this phase)]. After SIRT, a delay of a minimum of 2 weeks before initiating gemcitabine is advisable until further data emerge. In the ongoing SIRCCA trial, systemic cisplatin-gemcitabine chemotherapy is being administered 14–16 days after SIRT (ClinicalTrials.gov ID: NCT02807181).
Pancreatic (exocrine) cancer
A prospective phase II study of SIRT and systemic chemotherapy in liver-dominant metastatic pancreatic adenocarcinoma concluded that the approach appeared to be effective, and is likely to be of most benefit in those patients with a resected primary tumor and liver-only disease (24). Importantly, patients who received gemcitabine at least 8 weeks after the SIRT procedure had a similar rate of AEs to the expected rates with gemcitabine alone. There was no evidence of REILD, which suggests that it is acceptable to treat patients with gemcitabine after SIRT.
Metastatic breast cancer
The data on SIRT use in liver metastases from breast cancer are from both prospective and retrospective series from single centers. Although these studies all showed positive results with SIRT in this setting, none reported on the combination of SIRT with other therapies (10,103-108). Experienced SIRT teams regularly treating breast cancer metastases advise caution in total activity delivered in those patients with several years of chemotherapy exposure. Hepatic tolerance to SIRT is sometimes lower in this situation than in mCRC. Unpublished modification of standard approaches among these teams includes sequential lobar SIRT, empiric reduction of total activity by 25%, and consideration of concurrent use of ursodiol and methylprednisolone as is recommended in HCC patients at high-risk for REILD.
NETs
The NCCN, North American Neuroendocrine Tumor Society (NANETS) and European Neuroendocrine Tumor Society (ENETS) guidelines all recommend embolization for symptomatic or progressive neuroendocrine liver metastases refractory to somatostatin receptor blockade, without preference among the embolization techniques (109-111). Several studies have investigated the use of SIRT for NETs as first-line in treatment-refractory disease or as salvage therapy (20,21,112-115). These studies include concurrent use of somatostatin analogs, and no specific issues have been reported with this combination. A systematic analysis of 12 series encompassing 423 patients found widely variable objective, clinical and biochemical response rates (116). The few series with long-term follow-up reported 2- and 5-year survival ranging from 57% to 69% and from 45% to 55%, respectively. There appears to be little difference in the relative benefits of SIRT and other embolization techniques (117), but the quality and strength of the data do not allow firm conclusions (29). Nevertheless, SIRT may cause fewer side-effects and requires fewer treatments than chemoembolization techniques. Nearly all the patients in the studies reviewed were concurrently receiving octreotide or other somatostatin analog at the time of SIRT.
SIRT has been used in patients with NET receiving 5FU (20,113,118). For example, SIRT has been given with a 7-day systemic infusion of 5FU in 34 patients with progressive unresectable NET liver metastasis (118). As this combination is used widely in mCRC, the same safety considerations apply. A commonly applied systemic approach to metastatic NETs is an oral combination regimen of capecitabine and temozolomide (CapTem), which alone has a 60% objective response rate for well-differentiated NETs (119). A feasibility and safety study of integrated CapTem and SIRT delivered on day 7 of the 14-day cycle, demonstrated no worse than additive toxicities, with better than expected disease control in the liver, suggesting a synergy justifying expansion into a phase II trial (120).
The imminent introduction of PRRT in the USA raises questions about both sequencing and tolerance of systemic and liver-directed radiotherapies. Limited retrospective data demonstrated no significant toxicity when giving SIRT in NET patients who had received PRRT, suggesting that radiation exposure by PRRT may not cause significant toxicity in subsequent SIRT (121). Conversely, patients who undergo PRRT following prior liver-directed therapies derive less benefit, while sustaining higher rates of liver, renal and marrow toxicities (122). Another concern is the growing recognition of late radiation-induced hepatotoxicity following SIRT in long-lived NET patients, particularly when other therapeutic options include potentially hepatotoxic agents (123,124).
The targeted agents everolimus (mTOR inhibitor) and sunitinib (a receptor TKI) are both approved for NETs based on improved PFS in controlled trials. There is no data suggesting harm or benefit in combining these biological agents with SIRT.
Retreatment with SIRT
When SIRT has been effective and either new tumor appears in the previously treated liver or persistent hepatic tumor is increasing, a second (or more) application(s) of SIRT is a reasonable consideration. It is beyond the scope of this paper to discuss the wealth of supportive data showing safety and efficacy of subsequent treatments of SIRT; however, the timing of a second SIRT should be considered. Provided the patient is otherwise eligible for SIRT using standard criteria, a separation of 90 days between SIRT treatments is typically recommended. This is due to the risk of REILD, which can have a latency of up to 90 days.
If the original treatment was to only one hepatic lobe, and the new SIRT is to the previously untreated hepatic lobe, no delay is required, as this would not be considered ‘retreatment’.
Conclusions
- SIRT appears to be safely integrated into the pathways of the standard of care for most tumor types, however level 1 evidence is lacking except for first-line use in mCRC;
- While precautions need to be taken with certain drugs such as VEGF inhibitors and gemcitabine, in general, there are no reported specific toxicities indicating a need to withhold other agents in order to perform SIRT;
- Data on combining SIRT with 5FU or with FOLFOX (in large phase III trials) are reassuring but caution is still required when combining SIRT with other chemotherapy regimens in the salvage setting;
- Continuous tumor radiation delivered by SIRT may have immunosuppressive effects. Combining immune checkpoint inhibition or T cell therapy with SIRT could potentially result in improved clinical outcomes, a hypothesis being tested by ongoing clinical trials. Targeted radiosurgery of an index lesion in combination with SIRT may induce immune stimulation.
Acknowledgements
Funding for the consensus meeting was provided by Sirtex Medical. Editorial assistance was provided by Martin Gilmour, PhD (ESP Bioscience) funded by Sirtex Medical. We also thank Professor George Fisher (Stanford University School of Medicine, Division of Oncology, Stanford, California, USA) for his expert opinion and contribution to the initial phase of discussions.
Footnote
Conflicts of Interest: The institution of A Kennedy has received funding for a clinical trial for A Kennedy’s participation in an advisory board meeting from Sirtex Medical; no compensation has been received personally from Sirtex Medical by AKennedy. DB Brown has been a consultant for Bard Medical, participated in a Speaker’s Bureau for Boston Scientific, acted as consultant for BTG, and received research support from Sirtex. J Marshall has received honoraria from Caris (part time CMO), Genentech, Amgen, Bayer, Celgene, and Taiho for lectures, and advisory boards. J Marshall has received research grants from Genentech, Amgen, Bayer, Celgene, and Taiho. H Wasan reports grants, personal fees, non-financial support as well as other uncompensated work for Sirtex Medical, Celgene, Merck KGA, Sanofi, Servier, and Eli Lilly. M Fakih has received compensation for consulting and for educational activities from Sirtex. P Gibbs has received honoraria from Sirtex for advisory boards and lectures, honoraria from Roche, Amgen, Merck, Servier, and Bayer for advisory boards. B Sangro has received lecture and consulting fees from Sirtex Medical and consulting fees from BTG. MC Soulen has received research grants from Guerbet LLC, BTG international, acts as a consultant for Guerbet LLC, Merit Medical, Terumo Medical, Bayer, and is a proctor for Sirtex. RA Sharma has received research funding, consulting fees and honoraria from Sirtex Medical and BTG. The other authors have no conflicts of interest to report.
References
- Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin Nucl Med 2010;40:105-21. [Crossref] [PubMed]
- Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol 2012;23:96-105. [Crossref] [PubMed]
- Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 2009;115:1849-58. [Crossref] [PubMed]
- Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247:1029-35. [Crossref] [PubMed]
- Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78. [Crossref] [PubMed]
- Seidensticker R, Denecke T, Kraus P, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol 2012;35:1066-73. [Crossref] [PubMed]
- van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus Selective Internal Radiation Therapy in patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1723-31. [Crossref] [PubMed]
- Mosconi C, Gramenzi A, Ascanio S, et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: a survival, efficacy and safety study. Br J Cancer 2016;115:297-302. [Crossref] [PubMed]
- Devcic Z, Rosenberg J, Braat AJ, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med 2014;55:1404-10. [Crossref] [PubMed]
- Saxena A, Kapoor J, Meteling B, et al. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol 2014;21:1296-303. [Crossref] [PubMed]
- Gordon AC, Salem R, Lewandowski RJ. Yttrium-90 radioembolization for breast cancer liver metastases. J Vasc Interv Radiol 2016;27:1316-9. [Crossref] [PubMed]
- Gonsalves CF, Eschelman DJ, Sullivan KL, et al. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol 2011;196:468-73. [Crossref] [PubMed]
- Kennedy AS, Nutting C, Jakobs T, et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest 2009;27:682-90. [Crossref] [PubMed]
- Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. [Crossref] [PubMed]
- Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65:412-25. [Crossref] [PubMed]
- van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol 2009;27:4089-95. [Crossref] [PubMed]
- Lim L, Gibbs P, Yip D, et al. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer 2005;5:132. [Crossref] [PubMed]
- Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 2007;25:1099-106. [Crossref] [PubMed]
- Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. [Crossref] [PubMed]
- Saxena A, Chua TC, Bester L, et al. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg 2010;251:910-6. [Crossref] [PubMed]
- Paprottka PM, Hoffmann RT, Haug A, et al. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc Intervent Radiol 2012;35:334-42. [Crossref] [PubMed]
- Klingenstein A, Haug AR, Zech CJ, et al. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovasc Intervent Radiol 2013;36:158-65. [Crossref] [PubMed]
- Eldredge-Hindy H, Ohri N, Anne PR, et al. Yttrium-90 microsphere brachytherapy for liver metastases from uveal melanoma: Clinical outcomes and the predictive value of fluorodeoxyglucose positron emission tomography. Am J Clin Oncol 2016;39:189-95. [Crossref] [PubMed]
- Gibbs P, Do C, Lipton L, et al. Phase II trial of selective internal radiation therapy and systemic chemotherapy for liver-predominant metastases from pancreatic adenocarcinoma. BMC Cancer 2015;15:802. [Crossref] [PubMed]
- Cao C, Yan TD, Morris DL, et al. Radioembolization with yttrium-90 microspheres for pancreatic cancer liver metastases: results from a pilot study. Tumori 2010;96:955-8. [PubMed]
- Cohen SJ, Konski AA, Putnam S, et al. Phase I study of capecitabine combined with radioembolization using yttrium-90 resin microspheres (SIR-Spheres) in patients with advanced cancer. Br J Cancer 2014;111:265-71. [Crossref] [PubMed]
- Gulec SA, Pennington K, Wheeler J, et al. Yttrium-90 microsphere-selective internal radiation therapy with chemotherapy (chemo-SIRT) for colorectal cancer liver metastases: an in vivo double-arm-controlled phase II trial. Am J Clin Oncol 2013;36:455-60. [Crossref] [PubMed]
- Iñarrairaegui M, Alejandra A, Chopitea A, et al., editors. Radioembolization for the treatment of intrahepatic cholangiocarcinoma. ILCA Annual Conference; 2015; Paris, France.
- Kennedy A, Bester L, Salem R, et al. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford) 2015;17:29-37. [Crossref] [PubMed]
- Salman A, Simoneau E, Hassanain M, et al. Combined sorafenib and yttrium-90 radioembolization for the treatment of advanced hepatocellular carcinoma. Curr Oncol 2016;23:e472-e80. [Crossref] [PubMed]
- Kennedy AS, Shipley D, Blakely JL, et al. Safety analysis of regorafenib prior to selective internal radiation therapy (SIRT) using 90Y resin microspheres for the treatment (tx) of patients (pts) with refractory metastatic colorectal cancer (mCRC) with liver metastases (mets). J Clin Oncol 2017;35:abstr e15040.
- Padia SA, Lewandowski RJ, Johnson GE, et al. Radioembolization of Hepatic Malignancies: Background, Quality Improvement Guidelines, and Future Directions. J Vasc Interv Radiol 2017;28:1-15. [Crossref] [PubMed]
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620-5. [Crossref] [PubMed]
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. [Crossref] [PubMed]
- Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26:3687-94. [Crossref] [PubMed]
- Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012;13:679-87. [Crossref] [PubMed]
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. [Crossref] [PubMed]
- Kosmider S, Tan TH, Yip D, et al. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol 2011;22:780-6. [Crossref] [PubMed]
- Sharma R, Wasan HS, Van Hazel GA, et al. Overall survival analysis of the FOXFIRE prospective randomized studies of first-line selective internal radiotherapy (SIRT) in patients with liver metastases from colorectal cancer. J Clin Oncol 2017;35:A3507.
- Sangro B, Martinez-Urbistondo D, Bester L, et al. Prevention and treatment of complications of selective internal radiation therapy: Expert guidance and systematic review. Hepatology 2017;66:969-82. [Crossref] [PubMed]
- Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 2001;33:139-44. [Crossref] [PubMed]
- Xu RH, Shen L, Li J, et al. Expert consensus on maintenance treatment for metastatic colorectal cancer in China. Chin J Cancer 2016;35:13. [Crossref] [PubMed]
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57; discussion 657-8. [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [Crossref] [PubMed]
- Sangro B, Chopitea A, Rodriguez J, et al. Radioembolization using Y90-labeled resin microspheres (Y90-RE) as consolidation treatment after first-line chemotherapy (CxT) for liver metastases from colorectal cancer (CRC). ASCO Gastrointestinal Cancers Symposium 2010:Abstract 250.
- Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother 2005;39:128-35. [Crossref] [PubMed]
- Lu JF, Bruno R, Eppler S, et al. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 2008;62:779-86. [Crossref] [PubMed]
- Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol 2015;6:134-42. [PubMed]
- Narsinh K, Newton I, Kikolski S, et al. Phase II study of yttrium-90 resin microspheres in treatment of colorectal adenocarcinoma metastatic to the liver after failure of first-line oxaliplatin-based chemotherapy:preliminary. J Vasc Intervent Radiol 2014;25:S103. [Crossref]
- Gil-Alzugaray B, Chopitea A, Inarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013;57:1078-87. [Crossref] [PubMed]
- Milenic DE, Wong KJ, Baidoo KE, et al. Cetuximab: preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm 2008;23:619-31. [Crossref] [PubMed]
- Hickey R, Mulcahy MF, Lewandowski RJ, et al. Chemoradiation of hepatic malignancies: prospective, phase 1 study of full-dose capecitabine with escalating doses of yttrium-90 radioembolization. Int J Radiat Oncol Biol Phys 2014;88:1025-31. [Crossref] [PubMed]
- Cho M, Kessler J, Park JJ, et al. A single institute retrospective trial of concurrent chemotherapy with SIR-Spheres® versus SIR-Spheres® alone in chemotherapy-resistant colorectal cancer liver metastases. J Gastrointest Oncol 2017;8:608-13. [Crossref] [PubMed]
- Kennedy AS, Ball D, Cohen SJ, et al. Baseline hemoglobin and liver function predict tolerability and overall survival of patients receiving radioembolization for chemotherapy-refractory metastatic colorectal cancer. J Gastrointest Oncol 2017;8:70-80. [Crossref] [PubMed]
- Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004;6:553-63. [PubMed]
- Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist 2007;12:465-77. [Crossref] [PubMed]
- Edeline J, Coulouarn C, Crouzet L, et al. Gemcitabine and oxaliplatin, but not sorafenib or paclitaxel, have a synergistic effect with yttrium-90 in reducing hepatocellular carcinoma and cholangiocarcinoma cell line viability. J Vasc Interv Radiol 2015;26:1874-78.e2. [Crossref] [PubMed]
- Kulik L, Vouche M, Koppe S, et al. Prospective randomized pilot study of Y90+/-sorafenib as bridge to transplantation in hepatocellular carcinoma. J Hepatol 2014;61:309-17. [Crossref] [PubMed]
- Ricke J, Bulla K, Kolligs F, et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int 2015;35:620-6. [Crossref] [PubMed]
- Chow PK, Poon DY, Khin MW, et al. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS One 2014;9:e90909. [Crossref] [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [Crossref] [PubMed]
- Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer 2012;48:1452-65. [Crossref] [PubMed]
- Kim BK, Kim SU, Park JY, et al. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naive patients with hepatocellular carcinoma. Liver Int 2012;32:1120-7. [Crossref] [PubMed]
- Vilgrain V, Bouattour M, Sibert A, et al., editors. SARAH: a randomised controlled trial comparing efficacy and safety of selective internal radiation therapy (with yttrium-90 microspheres) and sorafenib in patients with locally advanced hepatocellular carcinoma. The International Liver Congress; 2017 April 19-23; Amsterdam.
- Chow PHW, Gandhi M. Asia-Pacific Hepatocellular Carcinoma Trials Group. Phase III multi-centre open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: The SIRveNIB study. J Clin Oncol 2017;35:A4002.
- Choi JW, Kim HC, Lee JH, et al. Transarterial chemoembolization of hepatocellular carcinoma with segmental portal vein tumour thrombus. Eur Radiol 2017;27:1448-58. [Crossref] [PubMed]
- Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [Crossref] [PubMed]
- Garin E, Rolland Y, Edeline J, et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med 2015;56:339-46. [Crossref] [PubMed]
- Powerski MJ, Erxleben C, Scheurig-Munkler C, et al. Hepatopulmonary shunting in patients with primary and secondary liver tumors scheduled for radioembolization. Eur J Radiol 2015;84:201-7. [Crossref] [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [Crossref] [PubMed]
- Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012;189:558-66. [Crossref] [PubMed]
- Sharma A, Bode B, Wenger RH, et al. gamma-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One 2011;6:e28217. [Crossref] [PubMed]
- Sharma A, Bode B, Studer G, et al. Radiotherapy of human sarcoma promotes an intratumoral immune effector signature. Clin Cancer Res 2013;19:4843-53. [Crossref] [PubMed]
- Surace L, Lysenko V, Fontana AO, et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity 2015;42:767-77. [Crossref] [PubMed]
- Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759-71. [Crossref] [PubMed]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009;182:4499-506. [Crossref] [PubMed]
- Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 2011;186:4388-95. [Crossref] [PubMed]
- Carr BI, Metes DM. Peripheral blood lymphocyte depletion after hepatic arterial 90Yttrium microsphere therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;82:1179-84. [Crossref] [PubMed]
- D'Emic N, Engelman A, Molitoris J, et al. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients treated with selective internal radiation therapy. J Gastrointest Oncol 2016;7:269-77. [PubMed]
- Ahmadzadehfar H, Meyer C, Ezziddin S, et al. Hepatic volume changes induced by radioembolization with 90Y resin microspheres. A single-centre study. Eur J Nucl Med Mol Imaging 2013;40:80-90. [Crossref] [PubMed]
- Chua TC, Bester L, Akther J, et al. Successful right hepatectomy after four treatments of yttrium-90 microspheres (SIR-Spheres) and concomitant FOLFOX as bridging therapy to resection of colorectal liver metastases. Anticancer Res 2010;30:3005-7. [PubMed]
- Fernández-Ros N, Silva N, Bilbao JI, et al. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB (Oxford) 2014;16:243-9. [Crossref] [PubMed]
- Gaba RC, Lewandowski RJ, Kulik LM, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol 2009;16:1587-96. [Crossref] [PubMed]
- Gulec SA, Pennington K, Hall M, et al. Preoperative Y-90 microsphere selective internal radiation treatment for tumor downsizing and future liver remnant recruitment: a novel approach to improving the safety of major hepatic resections. World J Surg Oncol 2009;7:6. [Crossref] [PubMed]
- Iñarrairaegui M, Pardo F, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol 2012;38:594-601. [Crossref] [PubMed]
- Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8. [Crossref] [PubMed]
- Tohme S, Sukato D, Chen HW, et al. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol 2013;24:1632-8. [Crossref] [PubMed]
- Teo JY, Allen JC Jr, Ng DC, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford) 2016;18:7-12. [Crossref] [PubMed]
- Lewandowski RJ, Donahue L, Chokechanachaisakul A, et al. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol 2016;114:99-105. [Crossref] [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Pardo F, Sangro B, Lee RC, et al. The Post-SIR-Spheres Surgery Study (P4S): retrospective analysis of safety following hepatic resection or transplantation in patients previously treated with selective internal radiation therapy (SIRT) with yttrium-90 resin microspheres. Ann Surg Oncol 2017;24:2465-73. [Crossref] [PubMed]
- Coldwell D. Treatment of unresectable nodular cholangiocarcinoma with yttrium-90 microspheres. World Congress on Gastrointestinal Cancer, Ann Oncol 2006;17:vi56 Abstract P-102.
- Khanna V, Dhanasekaran R, Barron B, et al. Yttrium-90 radioembolization (SIR-Spheres) for cholangiocarcinoma: Preliminary study. J Vasc Intervent Radiol 2009;26:S116-S117. [Crossref]
- Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. [Crossref] [PubMed]
- Hoffmann RT, Paprottka PM, Schon A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. [Crossref] [PubMed]
- Gaba RC, Bui JT, Knuttinen MG, et al. Re: radiation lobectomy-a minimally invasive treatment model for liver cancer. J Vasc Interv Radiol 2009;20:1394-6; author reply 6. [Crossref] [PubMed]
- Camacho JC, Kokabi N, Xing M, et al. Modified response evaluation criteria in solid tumors and European Association for The Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol 2014;25:256-65. [Crossref] [PubMed]
- Haug AR, Heinemann V, Bruns CJ, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging 2011;38:1037-45. [Crossref] [PubMed]
- Soydal C, Kucuk ON, Bilgic S, et al. Radioembolization with (90)Y resin microspheres for intrahepatic cholangiocellular carcinoma: prognostic factors. Ann Nucl Med 2016;30:29-34. [Crossref] [PubMed]
- Filippi L, Pelle G, Cianni R, et al. Change in total lesion glycolysis and clinical outcome after (90)Y radioembolization in intrahepatic cholangiocarcinoma. Nucl Med Biol 2015;42:59-64. [Crossref] [PubMed]
- Al-Adra DP, Gill RS, Axford SJ, et al. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol 2015;41:120-7. [Crossref] [PubMed]
- de Lange SM, van Groeningen CJ, Meijer OW, et al. Gemcitabine-radiotherapy in patients with locally advanced pancreatic cancer. Eur J Cancer 2002;38:1212-7. [Crossref] [PubMed]
- Coldwell DM, Kennedy AS, Nutting CW. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys 2007;69:800-4. [Crossref] [PubMed]
- Haug AR, Tiega Donfack BP, Trumm C, et al. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med 2012;53:371-7. [Crossref] [PubMed]
- Cianni R, Pelle G, Notarianni E, et al. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol 2013;23:182-9. [Crossref] [PubMed]
- Pieper CC, Meyer C, Wilhelm KE, et al. Yttrium-90 Radioembolization of Advanced, Unresectable Breast Cancer Liver Metastases-A Single-Center Experience. J Vasc Interv Radiol 2016;27:1305-15. [Crossref] [PubMed]
- Fendler WP, Lechner H, Todica A, et al. Safety, Efficacy, and Prognostic Factors After Radioembolization of Hepatic Metastases from Breast Cancer: A Large Single-Center Experience in 81 Patients. J Nucl Med 2016;57:517-23. [Crossref] [PubMed]
- Gordon AC, Gradishar WJ, Kaklamani VG, et al. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy. J Vasc Interv Radiol 2014;25:1523-32, 1532.e1-2.
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine Tumors. 2014;Version 2.
- Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735-52. [Crossref] [PubMed]
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76. [Crossref] [PubMed]
- Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31:271-9. [Crossref] [PubMed]
- Cao CQ, Yan TD, Bester L, et al. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg 2010;97:537-43. [Crossref] [PubMed]
- Ceelen F, Theisen D, de Albeniz XG, et al. Towards new response criteria in neuroendocrine tumors: which changes in MRI parameters are associated with longer progression-free survival after radioembolization of liver metastases? J Magn Reson Imaging 2015;41:361-8. [Crossref] [PubMed]
- Barbier CE, Garske-Román U, Sandström M, et al. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur J Nucl Med Mol Imaging 2016;43:1425-31. [Crossref] [PubMed]
- Yang TX, Chua TC, Morris DL. Radioembolization and chemoembolization for unresectable neuroendocrine liver metastases - a systematic review. Surg Oncol 2012;21:299-308. [Crossref] [PubMed]
- Chen JX, Rose S, White SB, et al. Embolotherapy for Neuroendocrine Tumor Liver Metastases: Prognostic Factors for Hepatic Progression-Free Survival and Overall Survival. Cardiovasc Intervent Radiol 2017;40:69-80. [Crossref] [PubMed]
- King J, Quinn R, Glenn DM, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer 2008;113:921-9. [Crossref] [PubMed]
- Cives M, Ghayouri M, Morse B, et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016;23:759-67. [Crossref] [PubMed]
- Soulen MC, Deitrick G, van Houten D, et al. Feasibility of combining capecitabine and temozolomide with yttrium 90 radioembolization (CapTemY90) for intermediate grade neuroendocrine tumors. JVIR Posters and Exhibits 2014;25:S159. [Crossref]
- Ezziddin S, Meyer C, Kahancova S, et al. 90Y Radioembolization after radiation exposure from peptide receptor radionuclide therapy. J Nucl Med 2012;53:1663-9. [Crossref] [PubMed]
- Riff BP, Yang YX, Soulen MC, et al. Peptide Receptor Radionuclide Therapy-Induced Hepatotoxicity in Patients With Metastatic Neuroendocrine Tumors. Clin Nucl Med 2015;40:845-50. [Crossref] [PubMed]
- Barraza J, Currie C, Nadolski G, et al. Delayed hepatotoxicity of Y-90 radioembolization. JVIR Scientific Session 2017;28:S163-S4.
- Su Y, Mackey R, Desai K, et al. Long-term hepatotoxicity of Y90 radioembolization for treatment of metastatic neuroendocrine tumor to the liver. JVIR Scientific Session 2017;28:S163.