IABP plus ECMO—Is one and one more than two?
Prognosis of patients with cardiogenic shock or after cardiac arrest is poor, with no drugs at hand to substantially lower high mortality. Therefore, all hope is pinned on percutaneous life-supporting devices like intra-aortic balloon pump (IABP), venoarterial extracorporeal membrane oxygenation (VA-ECMO) and others (1-4). The Extracorporeal Life Support (ECLS) Organization Registry International Report 2016 (5) contains information on 78,397 ECLS patients. In Germany, about 5,000 IABPs and 3,000 VA-ECMO systems have been implemented in 2014 (6). The bad news is that for none of these percutaneous devices a survival benefit has yet been documented in adequately sized randomized clinical trials (RCTs) (4,7). Such a RCT only exists for the use of IABP in patients with cardiogenic shock complicating myocardial infarction, with neutral effects on mortality and morbidity (8,9). This neutral finding is supported by the latest Cochrane review on IABP use in this patient group (10). A meta-analysis including a total of 100 patients in three small RCTs with the TandemHeart (n=2) and the Impella PL2.5 pump (n=1) did not see a survival benefit in comparison to the IABP, despite better hemodynamic effects (11). And for VA-ECMO, the best evidence is a meta-analysis, reporting for patients with return of spontaneous circulation (ROSC) after cardiac arrest (8 trials; 2,774 patients) an absolute increase of 30 days survival of 13% [95% confidence interval (CI) 6−20%; P<0.001; number needed to treat (NNT) 7.7] and a higher rate of favorable neurological outcome at 30 days (absolute risk difference 14%; 95% CI: 7−20%; P<0.0001; NNT 7.1) [(12), see also editorial (13)]. However, the evidence in the same meta-analysis is much weaker for the role of VA-ECMO in treating patients with cardiogenic shock, with much less patients included (5 cohort studies; 233 patients): VA-ECMO showed a 33% higher 30-day survival compared to IABP [95% CI: 14−52%; P<0.001; NNT 3], but no difference when compared to TandemHeart/Impella [−3%; 95%CI: −21% to 14%; P=0.70; NNH 33]. Admission SOFA scores <14, shockable rhythm and INR ≤2.4 might be prognostically favorable markers in cardiogenic shock and post-cardiac arrest patients treated with VA-ECMO (14).
All these devices are implemented to improve cardiac output, but all of them with different mechanisms of action. So what about a combination of two of them, if the solitary approach of each does not work satisfactorily? This approach—the combination of IABP and VA-ECMO—can be found in the nationwide Japanese Diagnosis Procedure Combination national inpatient database (15): the retrospective cohort study of 1,650 cardiogenic shock adult patients—out-of-hospital-cardiac arrest (OHCA)-patients excluded—with implemented VA-ECMO on admission (July 1, 2010, to March 31, 2013) were divided into the IABP/VA-ECMO-group (n=604) and the VA-ECMO-alone-group (n=1,064). Propensity score matching created matched cohort of 533 pairs. 28-day mortality and in-hospital mortality (Figure 1) were significantly lower in the IABP/VA-ECMO-group than in the VA-ECMO-alone-group (48.4% vs. 58.2%; P=0.001 and 55.9% vs. 64.5%; P=0.004), with a significant difference in survival (HR, 0.74; 95% CI: 0.63−0.86; P<0.001). The proportion of patients weaned from VA-ECMO (Figure 1) was significantly higher in the IABP/VA-ECMO-group than in the VA-ECMO-alone-group (82.6% vs. 73.4%; P<0,001). The authors (15) concluded that in this national inpatient database, IABP combined with VA-ECMO was associated with reduced mortality and successful weaning from VA-ECMO. They also concluded, of course, that randomized controlled studies are required to confirm the mortality-reducing effect of the combination of IABP and VA-ECMO.
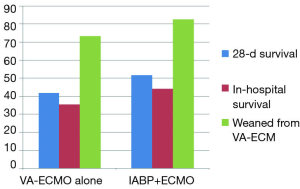
Though the Extracorporeal Life Support Organization Guidelines for Adult Cardiac Failure recommend that IABP should be applied to cardiogenic shock patients under VA-ECMO (16), in literature this topic is discussed controversially [see references in (15)]. With the data from this registry analysis (15), the arguments are now somewhat more in favor of this combination, but no more! It would be the wrong way to now recommend application of this combination in clinical routine. A lot of missing links need to be be clarified!
First, to better understand why this device combination should work, we should have a look into the different mechanisms of action of IABP and VA-ECMO:
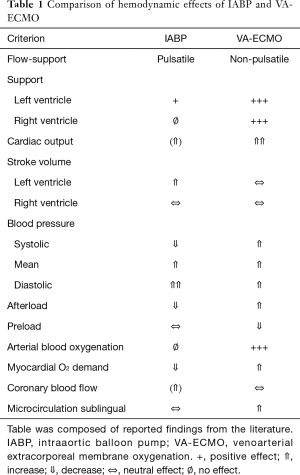
With the percutaneous IABP in place in the descending thoracic aorta, inflation of the balloon in diastole and active deflation in systole induce higher diastolic perfusion pressures in the coronary arteries and unload the diseased heart by reducing left ventricular afterload during systole. Volume shifting by the balloon of 40 mL per beat increases left ventricular stroke volume and cardiac output by up to 1 L/min or about 15−30%, with the best effects seen at very low cardiac output (4). In summary, the hemodynamic effects of the IABP can be characterized (Table 1) as:
Full table
- A pulsatile support;
- An increase in stroke volume and cardiac output;
- An increase in systemic blood pressure in the presence of a markedly increased diastolic pressure in the upper part of the body and a reduced systolic blood pressure (17);
- An increased coronary blood flow in open coronary arteries (18);
- A reduction in left ventricular preload, left ventricular end-diastolic pressure, and pulmonary capillary wedge pressure;
- A decrease in left ventricular wall tension, wall stress and LV myocardial oxygen demand/consumption (19);
- An improved reperfusion after thrombolysis in STEMI patients;
- No improvement of sublingual microcirculatory flow (20,21).
However, in severe coronary artery stenosis or acute coronary syndrome, more findings argue against (18,22,23) than in favor (24) of a clinically relevant increase in coronary blood flow after IABP insertion beyond critical stenoses, despite an increase in coronary perfusion pressure.
The percutaneous VA-ECMO system is a modified heart-lung-machine (4). It consists of a centrifugal pump, a heat exchanger, and a membrane oxygenator. Venous desaturated blood is aspirated from the conduit of the inferior caval vein and the right atrium into a centrifugal pump through a long steel wire-reinforced cannula inserted via the femoral vein. The pump outflow is directed towards a membrane oxygenator and is guided via an outflow cannula into the descending aorta via the (mostly contralateral) femoral artery. In summary the hemodynamic effects of the VA-ECMO can be summarized (Table 1) as:
- A non-pulsatile support;
- A substantial haemodynamic support of both ventricles with a considerable increase in cardiac output;
- A reduction in left ventricular preload;
- An increase in left ventricular afterload, thereby increasing oxygen demand and impeding myocardial protection (25) and triggering left ventricular distention in 10−60% of patients (26);
- An improvement in sublingual microcirculatory flow (27), which might be even intensified by the combination of VA-ECMO with IABP (28).
Comparing the hemodynamic effects of IABP to those of VA-ECMO (Table 1), one can see that—from a mechanistic point of view—IABP could neutralize some of the unwanted effects of VA-ECMO: the afterload increase by afterload reduction and the rise in myocardial O2 demand by lowering myocardial O2. Further, IABP might add some positive effects—like a possible increase in coronary perfusion—not inherent in the VA-ECMO mechanism. However, at the moment all this is merely hypothetical.
Second, for a better understanding we need to measure all those parameters of macro- and microcirculation in a controlled study with patients first treated with VA-ECMO alone and then in combination with IABP. This is mandatory for a better pathophysiological explanation. Such a scientific approach could be feasible with a relatively low number of patients, already proved for the IABP by the randomized IABP-SHOCK study having included 40 patients (29,30).
Third, if we should achieve convincing hemodynamic results, then we can go on and think about a RCT with the endpoint “survival”. Such a RCT is feasible as well: the IABP-SHOCK II trial (8) with 600 patients – exclusively included in a single country (Germany)—was the proof of principle for the missing positive effect of IABP in patients with cardiogenic shock complicating myocardial infarction.
Following the line of the IABP-Shock trial and the IABP-SHOCK II trial: can we await a “VA-ECMO + IABP − SHOCK”-trial?
Acknowledgements
The authors gratefully acknowledge stimulating discussions with members of the TEMPHUS project team FKZ: I3GW0034B (J. Schröder, T. Otto and L. Thieme).
Footnote
Conflicts of Interest: S. Nuding and K. Werdan are engaged in the TEMPHUS project about temporary mechanical support. The TEMPHUS project (FKZ: I3GW0034B) about temporary mechanical support is sponsored by the German Ministry of Education and Research (BMBF).
References
- Thiele H, Ohman EM, Desch S., et al. Management of cardiogenic shock. Eur Heart J 2015;36:1223-30. [Crossref] [PubMed]
- Trost JC, Hillis LD. Intra-aortic balloon counterpulsation. Am J Cardiol 2006;97:1391-8. [Crossref] [PubMed]
- Ventetuolo CE, Muratore CS. Extracorporeal life support in critically ill adults. Am J Respir Crit Care Med 2014;190:497-508. [Crossref] [PubMed]
- Werdan K, Gielen S, Ebelt H, et al. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156-67. [Crossref] [PubMed]
- Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60-67. [Crossref] [PubMed]
- Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889-96. [Crossref] [PubMed]
- Prondzinsky R, Werdan K. Extracorporeal life support during cardiac arrest and ardiogenic shock – how good is the evidence really? Ann Transl Med 2017;5:58. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638-45. [Crossref] [PubMed]
- Unverzagt S, Buerke M, de Waha A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database of Systematic Reviews 2015.3. [PubMed]
- Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J 2009;30:2102-8. [Crossref] [PubMed]
- Ouweneel DM, Schotborgh JV, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med 2016;42:1922-34. [Crossref] [PubMed]
- Aneman A, Macdonald P. Arteriovenous extracorporeal membrane oxygenation for cardiac arrest/cardiogenic shock. Intensive Care Med 2017;43:116-8. [Crossref] [PubMed]
- de Chambrun MP, Bréchot N, Lebreton G, et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock post-cardiac arrest. Intensive Care Med 2016;42:1999-2007. [Crossref] [PubMed]
- Aso S, Matsui H, Fushimi K, et al. The Effect of Intraaortic Balloon Pumping Under Venoarterial Extracorporeal Membrane Oxygenation on Mortality of Cardiogenic Patients: An Analysis Using a Nationwide Inpatient Database. Crit Care Med 2016;44:1974-9. [Crossref] [PubMed]
- Extracorporeal Life Support Organization: ELSO Guidelines for Adult Cardiac Failure v1.3. Michigan, USA, Extracorporeal Life Support Organization, 2015. Available online: http://www-elso.org/Resource/Guidelines.aspx
- Santa-Cruz RA, Cohen MG, Ohman EM. Aortic counterpulsation: a review of the hemodynamic effects and indications for use. Catheter Cardiovasc Interv 2006;67:68-77. [Crossref] [PubMed]
- Kern MJ, Aguirre FV, Tatineni S, et al. Enhanced coronary blood flow velocity during intraaortic balloon counterpulsation in critically ill patients. J Am Coll Cardiol 1993;21:359-68. [Crossref] [PubMed]
- Williams DO, Korr KS, Gewirtz H, et al. The effect of intraaortic balloon counterpulsation on regional myocardial blood flow and oxygen consumption in the presence of coronary artery stenosis in patients with unstable angina. Circulation 1982;66:593-7. [Crossref] [PubMed]
- den Uil CA, Lagrand WK, van der Ent M, et al. The effects of intra-aortic balloon pump support on macrocirculation and tissue microcirculation in patients with cardiogenic shock. Cardiology 2009;114:42-6. [Crossref] [PubMed]
- Jung C, Fuernau G, de Waha S, et al. Intraaortic balloon counterpulsation and microcirculation in cardiogenic shock complicating myocardial infarction: an IABP-SHOCK II substudy. Clin Res Cardiol 2015;104:679-87. [Crossref] [PubMed]
- Kern MJ, Aguirre F, Bach R, et al. Augmentation of coronary blood flow by intra-aortic balloon pumping in patients after coronary angioplasty. Circulation 1993;87:500-11. [Crossref] [PubMed]
- Kimura A, Toyota E, Lu S, et al. Effects of intraaortic balloon pumping on septal arterial blood flow velocity waveform during severe left main coronary artery stenosis. J Am Coll Cardiol 1996;27:810-6. [Crossref] [PubMed]
- Fuchs RM, Brin KP, Brinker JA, et al. Augmentation of regional coronary blood flow by intra-aortic balloon counterpulsation in patients with unstable angina. Circulation 1983;68:117-23. [Crossref] [PubMed]
- Kawashima D, Gojo S, Nishimura T, et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J 2011;57:169-76. [Crossref] [PubMed]
- Rupprecht L, Flörchinger B, Schopka S, et al. Cardiac decompression on extracorporeal life support: a review and discussion of the literature. ASAIO J 2013;59:547-53. [Crossref] [PubMed]
- Jung C, Ferrari M, Gradinger R, et al. Evaluation of the microcirculation during extracorporeal membrane-oxygenation. Clin Hemorheol Microcirc 2008;40:311-4. [PubMed]
- Jung C, Lauten A, Roediger C, et al. In vivo evaluation of tissue microflow under combined therapy with extracorporeal life support and intra-aortic balloon counterpulsation. Anaesth Intensive Care 2009;37:833-5. [PubMed]
- Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med 2010;38:152-60. [Crossref] [PubMed]
- Prondzinsky R, Unverzagt S, Russ M, et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial. Shock 2012;37:378-84. [Crossref] [PubMed]


