Body surface area: a novel predictor for conversion to thoracotomy in patients undergoing video-assisted thoracoscopic lung cancer lobectomy
Introduction
Rationale
Surgical therapy plays a critical role in the multidisciplinary treatments of lung cancer and is generally considered as the optimal therapeutic measure for early-stage non-small cell lung cancer (NSCLC) (1). Since the 1990s, video-assisted thoracoscopic surgery (VATS) has been dramatically developed and widely accepted in the modern surgical modality for early-stage NSCLC (2,3). This minimally invasive technique has more advantages offered to patients than traditional thoracotomy, including less postoperative pain and stress, preservation of pulmonary function, better cosmetic wounds, lower morbidity rate, shorter hospital stay and enhanced recovery with early return to normal life (3,4). However, these advantages will be hardly embodied once conversion to thoracotomy is required.
Conversion to thoracotomy occurs in up to 23% of the VATS patients (5). There is always a probability of intraoperative conversion in the VATS procedure, especially during a surgeon’s training period (6). The most recent high-volume institutional series have reported some common risk factors for conversion, such as the elder, male sex, fibro-calcified lymph nodes, dense pleural adhesion and deterioration of pulmonary function (7-9). Patients who suffer from conversion to thoracotomy are most likely to experience a prolonged operating time, increased blood loss, extra lung manipulation and risk of injury to adjacent tissues (7).
As a basic biometric unit, body surface area (BSA) is widely used to normalize the physiological variables and determine the appropriate drug dosages of cancer chemotherapy (10). The clinical significance of BSA has been investigated in laparoscopic colorectal surgery (LCRS), showing a high predictive value for conversion to laparotomy and longer laparoscopic operative time (11). However, its roles in surgical difficulty and post-VATS outcomes are still inadequately understood because only limited data regarding the effects of BSA in VATS has been reported (12).
Objectives
The primary purpose of our study was to demonstrate the predictive value of BSA for conversion to thoracotomy in patients undergoing VATS lobectomy for NSCLC. While our secondary objective was to evaluate its impact on post-VATS complications.
Methods
Protocols
This is a monocentric retrospective analysis based on the data from a prospectively-maintained database with their medical records in our institution. It was written in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (see Table S1) (13). Our regional ethics committee approved this study and waived the need for informed consent (ID: 2016-255).
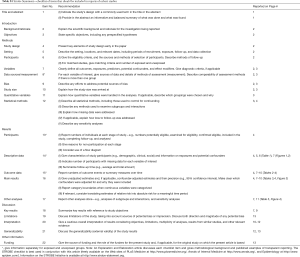
Full table
Participant selection
Settings
We reviewed the medical records of patients who underwent VATS lobectomy for NSCLC in our unit from March 2014 to August 2015. All available data for patient characteristics and surgical outcomes were collected for further analysis.
Eligibility criteria
First, the target disease was limited to primary NSCLC. Small cell lung cancer and metastatic carcinomas were excluded.
Second, single lobectomy operated by a VATS procedure was included in our study. Other anatomical resections, including bi-lobectomy, segmentectomy and pneumonectomy would not be considered.
Third, patients who could not finish our entire clinical pathways due to various reasons were excluded.
Fourth, patients with loss of accurate records on estimated variables were excluded.
Follow-up
The endpoints of our study were in-hospital outcomes, so the follow-up would be continued until 30 days after surgery or death in hospital.
Outcome data, measures and definitions
The following estimated variables and outcome data were recorded and defined.
Preoperative variables
Patient basic information included the age, gender, height, weight, body mass index (BMI), BSA and smoking history.
Taking account of potential ethnic differences between Chinese and Western peoples, we calculated the baseline BSA according to the following formula for contemporary Chinese subjects (14): BSA (m2) = [0.0061 × height (cm) + 0.0124 × weight (kg) – 0.0099].
Preoperative comorbidities included the chronic obstructive pulmonary disease (COPD), tuberculosis, preoperative respiratory infection (PRI), hypertension, diabetes mellitus, coronary heart disease, renal insufficiency, severe liver diseases, previous malignancy and steroid use. We defined PRI as the presence of one or more of the following infectious conditions: obstructive pneumonia, aspiration pneumonia, bronchiectasis, lung abscess and respiratory bacterial/fungal infections. Severe liver diseases involved the hepatitis B, hepatitis C, severe fatty liver, hepatocirrhosis and hepatic parasitic infections.
Combined treatment modalities would be discussed by a multi-disciplinary team meeting before surgery if necessary. Neoadjuvant induction therapy (NIT) was determined as a cisplatin/paclitaxel-combined chemo-radiotherapy based on the National Comprehensive Cancer Network Guidelines: China Editions.
Intraoperative variables
Intraoperative parameters included in the comparable analyses are presented as follows: tumor location, degrees of pleural adhesion, pleural invasion and pulmonary fissure completeness, and conversion to thoracotomy.
Pathological variables
We estimated the following major pathological parameters, including the differentiation degrees, tumor invasion, lymph node metastasis and TNM stage, which were defined in accordance with the Union for International Cancer Control seventh edition.
Primary and secondary outcomes
The primary outcome was conversion to thoracotomy, while the secondary outcomes were postoperative complications.
Overall morbidity was defined by the presence of any complication developed within 30 days after surgery or later during the same hospitalization, which was judged according to the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons joint definitions (15).
Grouping criterion
There was no concerted cut-off value addressing on the effects of BSA reported in previous studies. Thus, in order to report more clinically meaningful findings, we categorized BSA values using the grouping criterion proposed by Vaccaro et al. (11) in their LCRS study. The entire cohort of patients was divided into two groups by their median BSA value. Patients with BSA > median value were classified as the “large” group, while those with BSA ≤ median value were classified as the “non-large” group.
Surgical procedure and perioperative management
The single lobectomy with a systematic mediastinal lymphadenectomy was operated by a three-portal VATS procedure using the single-direction thoracoscopic technique as Liu et al. previously described (16). A stapler line reinforcement was also utilized on the bronchial stump.
All the enrolled patients were managed in compliance with a standardized clinical pathway, including comprehensive routine assessments, antibiotic prophylaxis, pulmonary rehabilitation and physiotherapy before surgery (17,18). All of them received intravenous patient-controlled analgesia for pain control. A chest tube was placed on the suction device (−20 cmH2O) at the end of surgery, and then, either alternated or removed the suction according to institutional policies. A chest X-ray was done on postoperative day 1 to stratify the degrees of lung expansion. Chest tube removal would be allowed when the pleural drainage remained <200 mL in 24 hours and the air leak cessation was detected in chest drainage unit.
Statistical analysis
We used the SPSS22.0 software to accomplish the following statistical analyses.
The continuous data were presented as the means with standard deviations (SDs), while the dichotomous data were expressed as the patient numbers with their percentages. We used the Pearson’s chi-squared test or Fisher’s exact test to compare the dichotomous variables, and the Student’s t-test to compare the mean values of continuous variables.
Subsequently, we established a multivariate logistic-regression model to identify the significant risk factors for conversion to thoracotomy. The median BSA value and other dichotomous variables with a univariate P value <0.05 were included into the logistic-regression model (19). According to what Vaccaro et al. (11) suggested, we would also include a BMI categorization (BMI ≥25.0 vs. <25.0 kg/m2) into the multivariate logistic-regression model to minimize the potential bias caused by the inextricable interaction between BMI and BSA, even though it had no statistical significance in the univariate analysis. Finally, odds ratio (OR) with the corresponding 95% confidence interval (CI) would be extrapolated.
The receiver operating characteristic (ROC) analysis was carried out to assess the discriminative power of BSA for predicting conversion risk. The area under curve (AUC) with its 95% CI would be finally calculated.
The statistical significance would be showed in both univariate and multivariate analysis when P value <0.05.
Results
Patient characteristics
During the study period, a total of 475 patients who underwent VATS lobectomy for NSCLC met the eligibility criteria and were enrolled in the present study. Their basic characteristics are presented in Table 1.
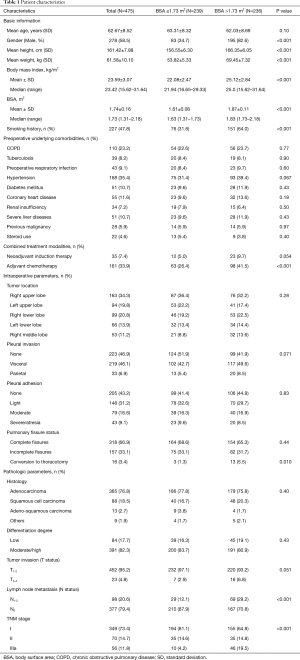
Full table
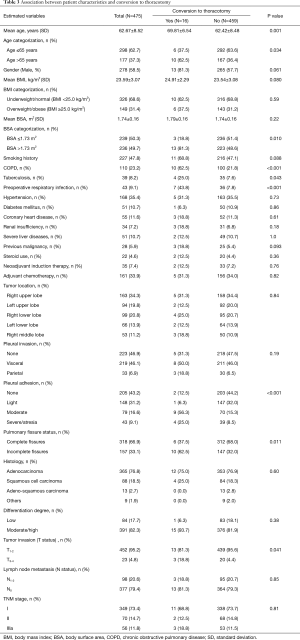
Our cohort included 278 male (ratio =58.5%) and 197 female patients (ratio =41.5%), with a mean age of 62.67±8.52 years (ranged 38–82 years). The mean BMI and BSA of the entire cohort were 23.59±3.07 kg/m2 (ranged 15.62–31.64 kg/m2) and 1.74±0.16 m2 (ranged 1.31–2.18 m2), respectively. Two hundred and twenty-seven patients were active smokers (ratio =47.8%), and 350 patients had preoperative underlying comorbidities (ratio =73.7%). Thirty-five patients underwent NIT before surgery (ratio =7.4%), while 161 patients were treated with adjuvant chemotherapy followed by surgery (ratio =33.9%).
The tumor located in right upper lobe (n=163) accounted for the largest proportion of all NSCLC cases, with a rate of 34.3%. The great majority of patients were diagnosed with stage I-II NSCLC (n=419, ratio =88.2%). Lung adenocarcinoma was diagnosed in 365 patients (ratio =76.8%), followed by squamous cell carcinoma in 88 patients (ratio =18.5%), adeno-squamous carcinoma in 13 patients (ratio =2.7%) and other subtypes of NSCLC in 9 patients (ratio =1.9%). Lymph node metastasis was confirmed by pathological criteria in 98 patients (ratio =20.6%).
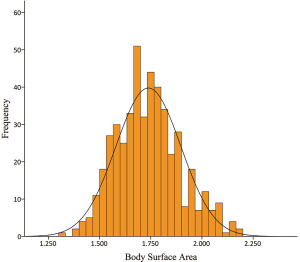
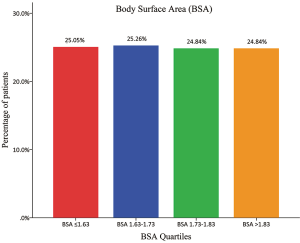
A frequency distribution histogram of BSA is shown in Figure 1. Besides, the patient percentages distributed in BSA quartiles are shown in Figure 2. We thus got a median BSA value of 1.73 m2 in our cohort for further grouping and comparisons.
Demographic differences between “large” patients and “non-large” patients
On the basis of information from Figure 2, there were 239 patients with BSA ≤1.73 m2 included in the “non-large” group and 236 patients with BSA >1.73 m2 included in the “large” group. The “non-large” patients and “large” patients differed in many baseline characteristics, as shown in Table 1. Compared with the “non-large” patients, the “large” patients had significantly higher means of height, weight, BMI and BSA, and significantly higher proportions of male sex, smoking history, adjuvant chemotherapy, lymph node metastasis and TNM stages. No significant difference was observed in the other variables between these two groups (Table 1).
Surgical outcomes
Conversion to thoracotomy
There were 16 patients converted to thoracotomy, with an overall conversion rate of 3.4%. The reasons for conversion are presented in Table 2.

Full table
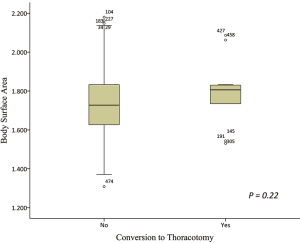
Thirteen patients in the “large” group converted to thoracotomy, while another three converted patients belong to the “non-large” group. The “large” patients had a significantly higher conversion rate than the “non-large” patients (P=0.010; Table 1). However, these was no significant difference in the mean BSA between converted patients and completely VATS patients (1.79±0.16 vs. 1.74±0.16 m2; P=0.22; Table 3; Figure 3).
Full table
The outcome data derived from the univariate analyses on the relationships between patient characteristics and conversion to thoracotomy are shown in Table 3. The univariate analysis showed eight perioperative categorical factors, including the age >65 years, BSA >1.73 m2, COPD, tuberculosis, PRI, dense pleural adhesion, incomplete pulmonary fissures and advanced tumor invasion, were significantly associated with the risk of conversion. There was no difference in the BMI categorization between converted patients and completely VATS patients (P=0.59; Table 3).
Overall morbidity
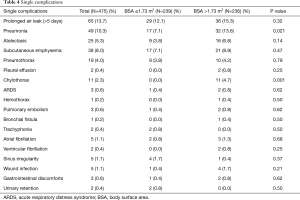
There were 241 postoperative complications occurred in 135 patients, with an overall morbidity rate of 28.4%. The overall complication rate in the “large” group was higher than that in the “non-large” group but without statistical significance (76 cases, 32.2% vs. 59 cases, 24.7%; P=0.069).
The development of any individual complication was shown in Table 4. The “large” patients had significantly higher rates of pneumonia (P=0.021) and chylothorax (P=0.001) than the “non-large” patients. No statistical difference was observed in the other complications between these two groups (Table 4).
Full table
ROC analysis on prediction of BSA for conversion to thoracotomy
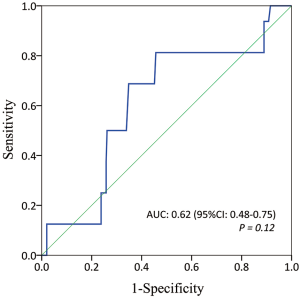
The ROC analysis showed an AUC of 0.62 (95% CI: 0.48–0.75; P=0.12) for BSA to predict conversion to thoracotomy (Figure 4). On the basis of ROC curve, we found that the median BSA of the entire cohort (1.73 m2) was exactly the optimal cut-off value, with the maximum joint sensitivity of 81.3% and specificity of 54.3%, for the prediction of conversion to thoracotomy.
Multivariate analysis on predictors for conversion to thoracotomy
Our multivariate logistic-regression model included the BSA >1.73 m2, BMI categorization (BMI ≥25.0 vs. <25 kg/m2) and another seven significant dichotomous parameters in the univariate analysis, as shown in Table 3. Finally, age >65 years (OR: 4.28; 95% CI: 1.14–16.09; P=0.031), BSA >1.73 m2 (OR: 7.17; 95% CI: 1.23–41.71; P=0.028), COPD (OR: 4.77; 95% CI: 1.26–18.05; P=0.021), tuberculosis history (OR: 20.57; 95% CI: 3.77–112.37; P=0.001) and dense pleural adhesion (OR: 2.62; 95% CI: 1.29–5.34; P=0.008) were found to be strongly independent risk factors for conversion to thoracotomy (Table 5). Advanced tumor invasion was almost a significant predictor for conversion to thoracotomy but failed to reach statistical significance (OR: 5.47; 95% CI: 0.96–31.19; P=0.056).

Full table
Discussion
Key results
The present study examined the association between BSA and conversion to thoracotomy in patients undergoing VATS lobectomy for NSCLC. The main finding of our study was that the larger BSA could be an independent risk factor for conversion to thoracotomy after adjusting the influence from other clinicopathological parameters by multivariate logistic-regression analysis. In addition, the “large” patients had a higher overall morbidity rate than the “non-large” patients but without statistical significance. Only the incidences of pneumonia and chylothorax in the “large” patients were significantly higher than those in the “non-large” patients.
Interpretations
The overall morbidity rate in our study was 28.4%, which were within the confines of complication rate ranged 24.9–36.3% in recent large-scale registry trials (4,20). There was only 3.4% of our patient cohort forced into conversion to thoracotomy, which compared favorably with the conversion rates of 6.2–23.0% in the literature (7-9). The possible reason might be that we enrolled the surgical patients in a later period of our learning curve. Conversion rate was supposed to be dropped with the increase in VATS experiences (9). The most common cause of conversion in our study was the presence of severe pleural/hilar adhesions, resulting in six converted patients. Uncontrolled bleeding due to pulmonary vessel injuries was the second cause of conversion, which was responsible for four converted patients. Besides, it was the most serious intraoperative complication in VATS procedure, as reported by most of the previous studies (7).
The highlight of the present study was to demonstrate a significant impact of BSA on conversion to thoracotomy for the first time. As a simple way to measure the body size, BSA has a much closer connection with height than BMI because of essential differences between their calculating formulas. Accordingly, BSA may be more related to the anthropometric measurements and potentially affect the conversion rate (11). Several calculating formulas of BSA have been proposed across different races so that we used an updated formula appropriate for contemporary Chinese peoples to extrapolate the BSA values (14). We explored the clinical significance of BSA by consulting the grouping criterion proposed by Vaccaro et al. (11) in LCRS. All of enrolled VATS patients were divided into the “large” group and the “non-large” group according to their median BSA value. Finally, we found that a larger BSA (>1.73 m2) was significantly associated with the risk of conversion after eliminating the confounding influence caused by other significant variables by multivariate analysis.
To our knowledge, there was only one retrospective analysis evaluating the effects of BSA on conversion to thoracotomy in 208 consecutive robotic-assisted lobectomies (12). The authors found that the patients with larger BSA had an obviously lower conversion rate than those with smaller BSA (P=0.030), although they did not conduct a multivariate analysis to further confirm the significance of BSA on serving as an independent risk factor. That was the biggest difference compared to what we analyzed in the present study. Our series showed a significantly higher conversion rate in the “large” patients compared to the “non-large” patients (P=0.010). Then, we further identified that BSA >1.73 m2 was a strongly independent predictor for conversion to thoracotomy in the multivariate logistic-regression model (OR: 7.17; P=0.028). Our results about the morbidity rates between two groups of BSA, showing no significant difference, were similar to what Velez Cubian et al. (12) reported in their cohort.
It will be understandable that smaller body size can lead to a limited access to the operative field in VATS lobectomy. Patients with the smaller BSA are considered to have the smaller pleural cavities, resulting in the limited visualization and instrument mobility during the VATS procedure (12). Therefore, small body habitus may have the potential to increase the surgical difficulty. However, our results, which were similar to what Vaccaro et al. (11) reported in their large-scale analysis of LCRS, seemed to be at variance with this explanation. There was no credible evidence to demonstrate this unexpected phenomenon. We speculated that the following three hypotheses might be considered for possible interpretations.
First of all, we found that most of the converted patients caused by difficultly dissected hilar structures and pleural adhesion due to the dense fibro-calcification belong to the “large” group (7/9, ratio =77.8%). Several preoperative factors that contributed to the presence of calcification and adhesion on the lung parenchyma, such as the smoking history and the receipt of NIT, were significantly more frequent in the “large” patients than those in the “non-large” patients. We hypothesized that the patients with a larger pleural cavity might have the larger area of severe pleural adhesion and fibro-calcification needing to be dissected and divided in VATS lobectomy, which might increase the probability of conversion and postoperative complications, although there was no evidence to confirm that until now.
Secondly, in our cohort, the “large” patients had a higher proportion of advanced-stage tumor invasion (T3-4) than the “non-large” patients, although this difference did not reach statistical significance (P=0.051). Moreover, advanced tumor invasion was almost predictive of conversion to thoracotomy in the final multivariate analysis (OR: 5.47; P=0.056). As a general rule, more advanced tumor stage usually means the huge tumors or bronchovascular bundle invasion around the hilum of the lung. On the one hand, when dealing with such complicated tumors and invisible hilar structures in the VATS lobectomy, thoracic surgeons will be forced to decide whether to convert to thoracotomy so that an en-bloc resection can be performed (7,21). On the other hand, most surgeons agree that the completely VATS lobectomy will be feasible and safe as long as the malignant lesion is located in the peripheral area of the lung and the hilar structures are clearly visible (21). Therefore, we suspected that more advanced stage lesions might possibly predispose the “large” patients to conversion to thoracotomy, although the multivariate logistic-regression analysis has minimized the confounding influence as much as possible.
Finally, prior studies indicated that lymph nodes could be an important etiology affecting the conversion of VATS to thoracotomy (8,22). In our study, lymph node metastasis was not associated with the conversion (P=0.85), but was significantly more frequent in the “large” patients (P<0.001). Mediastinal and hilar lymph nodes are generally located around important blood vessels and bronchia. Once they are affected by the inflammatory responses and oncological progression, enlarged lymph nodes can directly obscure the local anatomic structures and increase the surgical difficulty on endoscopic dissection and division of bronchovascular structures (23). Therefore, the high levels of lymph node interference and enlargement hinder the performance of lymphadenectomy, regardless of whether Chinese peoples are more prone to tuberculosis or other infectious diseases (21). When performing the lymph node dissections, forcible and sharp adhesiolysis may easily cause the vascular injury, and even the uncontrollable bleeding if the tears are on the pulmonary artery with its major branches (23). All of four converted patients caused by uncontrollable bleeding in our cohort were diagnosed with lymph node metastasis and belong to the “large” group. This result supported the above hypothesis as another one important explanation for the predictive value of BSA on conversion to thoracotomy.
Generalizability
The primary objective of introducing the VATS technique is to generalize this minimally invasive surgery in as many patients with operable lung cancer as possible. In the modern specialty of VATS lobectomy, the risk of conversion has been minimized by many teams of experienced thoracic surgeons in high-volume centers. Therefore, conversion to thoracotomy is an unplanned procedure but rather an exit when it is no longer possible to safely complete the VATS operation due to some unforeseeable events (7). According to our study results, it may be more sufficient to incorporate a BSA categorization into the assessment model to stratify the conversion risk and assist to design the best therapeutic strategy, although their BSA cannot be significantly modified by preoperative interventions.
Despite the multivariate data has showed the predictive value of BSA for conversion to thoracotomy, it may not qualify as crucial factor changing the mentality of an experienced thoracic surgeon when planning VATS lobectomy for NSCLC. However, for young surgeons, our results may help to select the participants in their early learning curve or in a teaching program of VATS techniques, in order to avoid unexpected conversion and train themselves more effectively.
Limitations
Several limitations in our study should be sufficiently acknowledged.
First, the present study is subject to the inherent limitations of any retrospective monocentric analysis. The retrospective design makes it difficult to control the selection bias. Although we have involved all the significant and correlated variables into the multivariate logistic-regression model to minimize their confounding effects, a case-matched analysis may be more reliable. In addition, our sample size is relatively small, resulting in a low analytical power.
Second, the median BSA value (1.73 m2), as well as the threshold value of BSA identified by ROC analysis on prediction of conversion, was used as the grouping criterion in our cohort, which might differ from others (12). Besides, our ROC analysis showed both slightly lower sensitivity (81.3%) and specificity (54.3%) for median value of BSA on prediction of conversion. That may attenuate the practical purpose of our findings in the clinical practices.
Third, the conversion rate can also depend on surgeon’s experience. However, it may be difficult to quantitatively analyze this artificial factor. This is another one important limitation that cannot be ignored.
Fourth, the calculating formula of BSA is developed among Chinese subjects (14). Thus, our findings should be judiciously considered in other ethnic populations.
Fifth, according to the objectives of this study, only VATS lobectomies were analyzed. Thus, our findings may not be generalized to sub-lobar resections.
Finally, complete laboratorial indexes were not available in all enrolled patients, so that we did not included them into the comparisons.
Conclusions
The present study demonstrates that BSA can be an excellent categorical predictor for conversion to thoracotomy in patients undergoing VATS lobectomy for NSCLC. No significant relationship was found between BSA categorization and overall morbidity. Our results suggest that BSA can be considered when informing patients about intraoperative risks and selecting cases in the early learning curve of VATS techniques. Large-scale and multi-institutional studies are expected to further confirm and modify our findings in the future.
Acknowledgements
We sincerely thank the assistance of the staff in Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China. Special thanks to the English language polishing contributions from Mr. Stanley Crawford, from the Institution of Medical English, West China Medical Center, Sichuan University, Chengdu, China.
Funding: This study was supported by Foundation of Science and Technology support plan Department of Sichuan Province (2015SZ0158).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our regional ethics committee approved this study and waived the need for informed consent (ID: 2016-255).
References
- Li S, Wang Z, Huang J, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the 'obesity paradox' really exist? Eur J Cardiothorac Surg 2017;51:817-28. [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Jung HS, Kim HR, Choi SH, et al. Clinical feasibility and efficacy of video-assisted thoracic surgery (VATS) anatomical resection in patients with central lung cancer: a comparison with thoracotomy. J Thorac Dis 2015;7:1774-9. [PubMed]
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [Crossref] [PubMed]
- Reed MF, Lucia MW, Starnes SL, et al. Thoracoscopic lobectomy: introduction of a new technique into a thoracic surgery training program. J Thorac Cardiovasc Surg 2008;136:376-81. [Crossref] [PubMed]
- Byun CS, Lee S, Kim DJ, et al. Analysis of Unexpected Conversion to Thoracotomy During Thoracoscopic Lobectomy in Lung Cancer. Ann Thorac Surg 2015;100:968-73. [Crossref] [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 62.e1.
- Verbraecken J, Van de Heyning P, De Backer W, et al. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 2006;55:515-24. [Crossref] [PubMed]
- Vaccaro CA, Vaccarezza H, Rossi GL, et al. Body surface area: a new predictor factor for conversion and prolonged operative time in laparoscopic colorectal surgery. Dis Colon Rectum 2012;55:1153-9. [Crossref] [PubMed]
- Velez Cubian FO, Zhang WW, Rodriguez KL, et al. Effect of small body habitus on peri-operative outcomes after robotic-assisted pulmonary lobectomy: retrospective analysis of 208 consecutive cases. J Thorac Dis 2016;8:1245-9. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. [Crossref] [PubMed]
- Li S, Zhou K, Du H, et al. Body surface area is a novel predictor for surgical complications following video-assisted thoracoscopic surgery for lung adenocarcinoma: a retrospective cohort study. BMC Surg 2017;17:69. [Crossref] [PubMed]
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Lai Y, Huang J, Yang M, et al. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. J Surg Res 2017;209:30-6. [Crossref] [PubMed]
- Lai Y, Su J, Qiu P, et al. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: a randomized trial. Interact Cardiovasc Thorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Dominguez Almendros S, Benitez-Parejo N, Gonzalez-Ramirez AR. Logistic regression models. Allergol Immunopathol (Madr) 2011;39:295-305. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Li Y, Wang J, Yang F, et al. Indications for conversion of thoracoscopic to open thoracotomy in video-assisted thoracoscopic lobectomy. ANZ J Surg 2012;82:245-50. [Crossref] [PubMed]
- Lee HS, Jang HJ. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg 2012;24:131-41. [Crossref] [PubMed]
- Li Y, Wang J. Analysis of lymph node impact on conversion of complete thoracoscopic lobectomy to open thoracotomy. Thorac Cancer 2015;6:704-8. [Crossref] [PubMed]