
Diagnosis of hypogonadism in patients treated with low energy shock wave therapy for erectile dysfunction: a narrative review
Introduction
An erection is a complex neurovascular process. Obtaining an erection is determined by a relatively rapid flow of blood into the corpora cavernosa, which leads to tumescence of the corpora cavernosa and activation of the veno-occlusive mechanism. The critical property allowing for such a significant increase in blood flow is rooted in the capacity for relaxation of the small cavernous arteries (helicine arteries), which enables them to delete by about 80%. This significantly surpasses the abilities of other vessels, which can only delete by about 15% (1). We present the following article on the role of testosterone measurement diagnosing hypogonadism during low energy shock wave therapy for erectile dysfunction (ED) in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/tau-20-796).
Importance of testosterone for sexual performance
Testosterone also has an impact on an erection. It increases penile rigidity and prolongs erectile response. It stimulates libido, arousal, orgasm and sexual satisfaction. The concentration of testosterone is associated with nocturnal penile tumescence, which serves as a specific exercise to maintain the fitness of erectile tissue. The lack of nocturnal penile tumescence results in an increased production of endothelin 1 (a strong vasoconstrictor), apoptosis, as well as long-term hypoxia, which leads to the loss of smooth muscles, an increase in collagen synthesis, and to fibrosis within the corpora cavernosa (2). Next, testosterone participates in the maturation of penile tissue by promoting the commitment of mesenchymal pluripotent cells into myogenic lineage and inhibiting their differentiation into adipogenic lineage (3). Moreover, testosterone stimulates perineal muscles anatomically connected to the corpora cavernosa. This helps creating and maintain a higher pressure than this in large arteries and securing proper function of the veno-occlusive mechanism.
The mechanism of action of testosterone is linked to vascular endothelium, where it regulates the production of nitric oxide through the stimulation of nitric oxide synthase (NOS) and the formation of cyclic guanosine monophosphate (cGMP). In the case of a low testosterone concentration, abnormal relaxation of smooth muscles and corpora cavernosa occurs. An opposite, catabolic role of testosterone regulates the expression and activity of phosphodiesterase-5 (PDE5) and hydrolytic enzymes engaged in the degradation of cGMP (4).
Deterioration of the overall and sexual condition of men with age was for the first time linked to testosterone by Heller and Meyers in 1944. In men, a decrease in plasma concentration is constant and amounts to about 1.4% per year (5). Unlike other endocrine diseases defined by abnormal hormone concentrations, androgen insufficiency requires specific symptoms to occur, with sexual disorders being the most important. The European Male Aging Study conducted examinations on 3,369 men aged between 40–79 years and revealed that nine symptoms are associated with the concentration of total or free testosterone. They include three main disorders of sexual health (less morning erections, less sexual thoughts, and ED); three symptoms characteristic of physical fitness [limited of physical activity (e.g., running, ability to lift heavy objects, participation in endurance sports and inability to walk more than 1 km], and difficulty kneeling, bending and skewing; and three psychological symptoms (decrease in energy, pessimism and fatigue) (6). A higher risk of sexual health disorders and reduced physical fitness correlates with a testosterone concentration of 8–13 nmol/L (2.3–3.7 ng/mL) for total testosterone, and 160–280 pmol/L (46–81 pg/mL) for free testosterone. Based on the analysis of results of men seeking sexological help, Rastrelli et al. determined two thresholds—below 20 nmol/L the probability of the occurrence of sexual insufficiency rises, while above 38 nmol/L, this probability is almost equal to zero (7).
According to the European Association of Urology (EAU), plasma testosterone concentrations <8 nmol/L are considered abnormally low and require substitution. For higher concentrations, the relationship between circulating testosterone and sexual performance is very low. According to the British Society for Sexual Medicine, men with total testosterone that is consistently lower than 8 nmol/L (free testosterone <0.18 nmol/L) usually require treatment, while those with total testosterone of <12 nmol/L (free testosterone <0.225 nmol/L) may be offered a 6-month trial of testosterone replacement therapy. According to the American Urological Association, a testosterone concentration should be checked in all men with ED in order to determine if testosterone deficiency, defined as a total testosterone level of <300 ng/dL along with hypogonadism symptoms, occurs. Additionally, men with ED and testosterone deficiency, who are considering treatment for ED with PDE5 inhibitors, should be informed that these drugs may be more effective if combined with testosterone therapy. However, many authors identify higher testosterone thresholds with libido loss and a decrease in sexual arousal (above 15 nmol/L). Rastrelli et al. use a threshold of 20 nmol/L, which is twice as high as that stated in official recommendations (7). Additionally, an increased concentration of luteinising hormone (LH) with a concentration of testosterone of <15 nmol/L may suggest late-onset hypogonadism (LOH). The term referring to the individual norm of testosterone concentration is returning and is defined as a typical individual value that guarantees well-being for a particular man. Sensitivity to testosterone varies from person to person; the degree of the decrease in plasma testosterone concentration is a better predictor of LOH then the current total and bioavailable testosterone (8). A probable drop in testosterone receptor sensitivity in the central nervous system can explain both a reduction in sexual arousal of ageing men and the need to increase the dose of testosterone during the treatment of hypogonadism.
Low energy shock-wave therapy (LESWT) for ED
One of the treatments of ED that attracts great attention is LESWT. This method, despite the lack of precise recommendations due to limited data on its long-term safety and effectiveness, was included in 2013 and maintained in the newest issue of EAU guidelines. However, evidence on its efficacy in the treatment of ED is growing. ED mainly affects men over 50 years of age and is associated with vascular disorders. Many reports have documented that LESWT stimulates and activates the release of angiogenic factors that promote tissue neovascularisation, and as a result improve blood supply. Young and Dyson observed higher dynamics of formation of new blood vessels exposed to ultrasound during the early stage of healing of skin lesions in adult rats (9). The study by Nishida et al. on a porcine model of chronic myocardial ischemia showed a significant improvement in regional blood flow through myocardium treated with shock wave therapy for four weeks (9 spots; 200 shots per spot, 0.09 mJ/mm2) in comparison to the control group without treatment (10). Nishida et al. reported that exposure to shock waves leads to a significant overexpression of the mRNA of strong angiogenesis ligands, e.g., vascular endothelial growth factor (VEGF) along with its Flt-1 receptor (VEGFR-1) and protein expression in human umbilical vein endothelial cells (HUVECs) in vitro, as well as the production of VEGF in the ischemic myocardium in animal models in vivo (10). Exposure of tissues from a healthy and ischaemic myocardium to LESWT in vitro promotes proliferation and differentiation of endothelial cells, an increase in the number of mature endothelial cells and those engaged in angiogenesis, as well as an increase in the number of primary cardiomyocytes and smooth muscle cells. More obvious effects were obtained for cells taken from a healthy heart than from an ischemic heart (10). Moreover, two different mechanisms promoting the production of NO during exposure to shock waves were described; enzymatic, based on the increased expression of endothelial NOS, and non-enzymatic, requiring the presence of L-arginine and hydrogen peroxide molecules.
Targeted literature review
We conducted a targeted literature review of studies which exclusively examined the efficacy of LESWT in various group of patients or compared LESWT to sham protocol in the treatment of ED. We used combinations of the following key words: erectile dysfunction and low energy shock wave treatment. No specific timeframe, geographical scope or language restrictions were applied. The search in PubMed and Embase via Ovid was run on in January 2020. Our review showed that the majority of studies on the efficacy of this method did not provide any information on the current testosterone concentration, or only excluded patients with hormonal deprivation of hormonal disorders. However, all those patients suffered from a key symptom of hypogonadism, which is ED. Only 8 of 25 studies examined and showed values of testosterone concentrations (Table 1). Interestingly, testosterone levels were more often provided for cohorts treated with PDE5i likely due to the recommendation to check testosterone status when pharmacotherapy is not effective. Therefore, the question arises of which symptoms of hormonal disorders were used as exclusion criteria from these trials. A significant percentage of patients included in these studies was burdened with cardiovascular disease (CVD) or CDV risk factors that intensify a physiological drop in the testosterone concentration in men (41). Only one of these analyses checked the relationship between the efficacy of LESWT and testosterone concentration; however, the study group was small (n=20) and heterogeneous. As a result, meta-analyses published to date may not show the full value of LESWT in the treatment of ED.
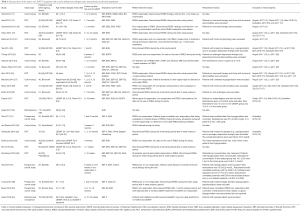
Full table
Taking into account the testosterone mechanism of action, the evidence of its benefit in patients with ED who are not-responders to PDE5 inhibitors, and the vascular mode of action of LESWT, we recommend including systematic, standardized diagnostics of hypogonadism and performing analysis on testosterone concentration and LESWT efficacy into further studies on this method in the treatment of ED.
Conclusions
In the light of the significant role testosterone plays in the process of an erection and the mechanism of LESWT action, it can be recommended to examine testosterone concentration and to diagnose hypogonadism during the qualification of patients to studies on LESWT efficacy. Moreover, the effectiveness of LESWT in relation to the current testosterone concentration should also be further investigated. Testosterone check and excluding hypogonadism is important before enrollment to studies on LESWT efficacy.
Acknowledgments
Funding: This research was carried out at Wroclaw Medical University within the projects no. SUB.A310.19.014 and SUB.A140.19.023, according to the records in the SIMPLE system.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-796
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-796). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaiser DR, Billups K, Mason C, et al. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol 2004;43:179-84. [Crossref] [PubMed]
- Moreland RB. Pathophysiology of erectile dysfunction: the contributions of trabecular structure to function and the role of functional antagonism. Int J Impot Res 2000;12 Suppl 4:S39-46. [Crossref] [PubMed]
- Singh R, Artaza JN, Taylor WE, et al. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003;144:5081-8. [Crossref] [PubMed]
- Zhang XH, Morelli A, Luconi M, et al. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol 2005;47:409-16; discussion 416. [Crossref] [PubMed]
- Belchetz PE, Barth JH, Kaufman JM. Biochemical endocrinology of the hypogonadal male. Ann Clin Biochem 2010;47:503-15. [Crossref] [PubMed]
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123-35. [Crossref] [PubMed]
- Rastrelli G, Corona G, Tarocchi M, et al. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest 2016;39:473-84. [Crossref] [PubMed]
- Holm AC, Fredrikson MG, Theodorsson E, et al. Change in testosterone concentrations over time is a better predictor than the actual concentrations for symptoms of late onset hypogonadism. Aging Male 2011;14:249-56. [Crossref] [PubMed]
- Young SR, Dyson M. Effect of therapeutic ultrasound on the healing of full-thickness excised skin lesions. Ultrasonics 1990;28:175-80. [Crossref] [PubMed]
- Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 2004;110:3055-61. [Crossref] [PubMed]
- Vardi Y, Appel B, Jacob G, et al. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol 2010;58:243-8. [Crossref] [PubMed]
- Vardi Y, Appel B, Kilchevsky A, et al. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol 2012;187:1769-75. [Crossref] [PubMed]
- Angulo JC, Arance I, de Las Heras MM, et al. Efficacy of low-intensity shock wave therapy for erectile dysfunction: A systematic review and meta-analysis. Actas Urol Esp 2017;41:479-90. [Crossref] [PubMed]
- Clavijo RI, Kohn TP, Kohn JR, et al. Effects of Low-Intensity Extracorporeal Shockwave Therapy on Erectile Dysfunction: A Systematic Review and Meta-Analysis. J Sex Med 2017;14:27-35. [Crossref] [PubMed]
- Man L, Li G. Low-intensity Extracorporeal Shock Wave Therapy for Erectile Dysfunction: A Systematic Review and Meta-analysis. Urology 2018;119:97-103. [Crossref] [PubMed]
- Sokolakis I, Hatzichristodoulou G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic review and meta-analysis of randomised controlled trials. Int J Impot Res 2019;31:177-94. [Crossref] [PubMed]
- Zou ZJ, Tang LY, Liu ZH, et al. Short-term efficacy and safety of low-intensity extracorporeal shock wave therapy in erectile dysfunction: a systematic review and meta-analysis. Int Braz J Urol 2017;43:805-21. [Crossref] [PubMed]
- Gruenwald I, Appel B, Vardi Y. Low-intensity extracorporeal shock wave therapy--a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med 2012;9:259-64. [Crossref] [PubMed]
- Olsen AB, Persiani M, Boie S, et al. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol 2015;49:329-33. [Crossref] [PubMed]
- Lu Z, Lin G, Reed-Maldonado A, et al. Low-intensity Extracorporeal Shock Wave Treatment Improves Erectile Function: A Systematic Review and Meta-analysis. Eur Urol 2017;71:223-33. [Crossref] [PubMed]
- Reisman Y, Hind A, Varaneckas A, et al. Initial experience with linear focused shockwave treatment for erectile dysfunction: a 6-month follow-up pilot study. Int J Impot Res 2015;27:108-12. [Crossref] [PubMed]
- Yee CH, Chan ES, Hou SS, et al. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol 2014;21:1041-5. [Crossref] [PubMed]
- Chung E, Cartmill R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: an Australian first open-label single-arm prospective clinical trial. BJU Int 2015;115 Suppl 5:46-9. [Crossref] [PubMed]
- Hisasue S, China T, Horiuchi A, et al. Impact of aging and comorbidity on the efficacy of low-intensity shock wave therapy for erectile dysfunction. Int J Urol 2016;23:80-4. [Crossref] [PubMed]
- Pelayo-Nieto M, Linden-Castro E, Alias-Melgar A, et al. Linear shock wave therapy in the treatment of erectile dysfunction. Actas Urol Esp 2015;39:456-9. [Crossref] [PubMed]
- Srini VS, Reddy RK, Shultz T, et al. Low intensity extracorporeal shockwave therapy for erectile dysfunction: a study in an Indian population. Can J Urol 2015;22:7614-22. [PubMed]
- Bechara A, Casabe A, De Bonis W, et al. Twelve-Month Efficacy and Safety of Low-Intensity Shockwave Therapy for Erectile Dysfunction in Patients Who Do Not Respond to Phosphodiesterase Type 5 Inhibitors. Sex Med 2016;4:e225-32. [Crossref] [PubMed]
- Kitrey ND, Gruenwald I, Appel B, et al. Penile Low Intensity Shock Wave Treatment is Able to Shift PDE5i Nonresponders to Responders: A Double-Blind, Sham Controlled Study. J Urol 2016;195:1550-5. [Crossref] [PubMed]
- Ruffo A, Capece M, Prezioso D, et al. Safety and efficacy of low intensity shockwave (LISW) treatment in patients with erectile dysfunction. Int Braz J Urol 2015;41:967-74. [Crossref] [PubMed]
- Ayala HAC, Cuartas JPS, Cleves DC. Impact on the Quality of Erections after Completing a Low-Intensity Extracorporeal Shock Wave Treatment Cycle on a Group of 710 Patients. Adv Urol 2017;2017:1843687. [Crossref] [PubMed]
- Tsai CC, Wang CJ, Lee YC, et al. Low-Intensity Extracorporeal Shockwave Therapy Can Improve Erectile Function in Patients Who Failed to Respond to Phosphodiesterase Type 5 Inhibitors. American Journal of Men's Health 2017;11:1781-90. [Crossref] [PubMed]
- De Oliveira PS, De Oliveira TR, Nunes A, et al. Low-intensity shock wave therapy for erectile dysfunction and the influence of disease duration. Arch Ital Urol Androl 2019;90:276-82. [Crossref] [PubMed]
- Yamaçake KGR, Carneiro F, Cury J, et al. Low-intensity shockwave therapy for erectile dysfunction in kidney transplant recipients. A prospective, randomized, double blinded, sham-controlled study with evaluation by penile Doppler ultrasonography. Int J Impot Res 2019;31:195-203. [Crossref] [PubMed]
- Eryilmaz R, Kaplan S, Aslan R, et al. Comparison of focused and unfocused ESWT in treatment of erectile dysfunction. Aging Male 2020;23:206-9. [Crossref] [PubMed]
- Kim KS, Jeong HC, Choi SW, et al. Electromagnetic Low-Intensity Extracorporeal Shock Wave Therapy in Patients with Erectile Dysfunction: A Sham-Controlled, Double-Blind, Randomized Prospective Study. World J Mens Health 2020;38:236-42. [Crossref] [PubMed]
- Sramkova T, Motil I, Jarkovsky J, et al. Erectile Dysfunction Treatment Using Focused Linear Low-Intensity Extracorporeal Shockwaves: Single-Blind, Sham-Controlled, Randomized Clinical Trial. Urol Int 2020;104:417-24. [Crossref] [PubMed]
- Vita R, Benvenga S, Giammusso B, et al. Determinants of Early Response to Low-Intensity Extracorporeal Shockwaves for the Treatment of Vasculogenic Erectile Dysfunction: An Open-Label, Prospective Study. J Clin Med 2019;8:1017. [Crossref] [PubMed]
- Costa P, Dias J, Gouveia R, et al. Low intensity extracorporeal shockwave therapy on erectile dysfunction—first results from a prospective study. AME Med J 2019;4:32. [Crossref]
- Musa ZS, El-Assmy A, Shokry AM, et al. Long-term effectiveness and predictors of success of low-intensity shockwave therapy in phosphodiesterase type 5 inhibitors non-responders. Arab J Urol 2019;18:54-8. [Crossref] [PubMed]
- Verze P, Capece M, Creta M, et al. Efficacy and safety of low-intensity shockwave therapy plus tadalafil 5 mg once daily in men with type 2 diabetes mellitus and erectile dysfunction: a matched-pair comparison study. Asian J Androl 2020;22:379-82. [Crossref] [PubMed]
- Kałka D, Gebala J, Smolinski R, et al. Low-energy Shock Wave Therapy-A Novel Treatment Option for Erectile Dysfunction in Men With Cardiovascular Disease. Urology 2017;109:19-26. [Crossref] [PubMed]


