Developmental delay and behavioral disorders in 59 HIV-exposed uninfected infants
Introduction
Antiretroviral therapy (ART) in HIV pregnant women has led to a dramatic decrease in the rate of HIV mother-to-child transmission, with almost no transmission when viral load is suppressed at delivery and mothers do not breastfeed (1). However, this benefit is counterbalanced with possible adverse drug effects related to in utero and neonatal exposure to antiretroviral (ARV) drugs: premature delivery, birth defects, short-term reversible biological abnormalities (anemia, neutropenia, hyperlactatemia) or persistent clinical and biological abnormalities (mitochondrial toxicity) (2-4). In high-income countries, guidelines recommend treating pregnant women with triple-combination ART and to increase the number of HIV infected women receiving therapy before conception; so fetuses are more and more exposed to ARV during gestation. Data about ART exposure are discordant: studies in Pediatric HIV/AIDS Cohort Study (PHACS) found an association with in utero atazanavir (ATV) exposure (a preferred ARV for HIV pregnant women) and late language emergence (5-7); other studies found no evidence of developmental delay in HIV-exposed uninfected infants (HEU) (8).
Methods
In the outpatient department of Infectious Diseases, we have a dedicated team (infectiologist and pediatrician) who follows all the pregnant HIV infected women and their HEU infants, until the age of 24 months. According to French national guidelines, there is no more follow-up after this period.
Some parents spontaneously notified behavioral problems with their children and in October 2013, we decided to send a standardized letter to all the mothers who delivered over a 10-year period (from 01/01/2003 to 31/12/2012) in our hospital.
This letter included four questions: does your child present with growth retardation? Does your child present cognitive/delayed development (academic difficulties)? Does your child have behavioral problems (difficulties in relationships with other children or adults)? Does your child present cancer or other symptoms?
Data concerning mothers and children were extracted from electronic medical records: ARV exposure, gestational age, mode of delivery, demographic factors.
Lactate (LA) measurements were performed at regular intervals (1, 3, 6, 12 and 24 months) in routine follow-up and hyperlactatemia was defined by two determinations >2.5 mmol/L. Mothers/children characteristics were compared using chi-square tests.
Results
During this 10-year period, the number of pregnancies has gradually increased from 10 in 2003 to 30 in 2012 and 133 women delivered 167 infants (four sets of twins). No child was breastfed; all were followed until the age of 2 years.
We received 54 letters concerning 55 children, a 33% response rate. For four additional children, abnormalities were orally reported from parents to the pediatrician or the infectiologist and added to the database.
ART during pregnancy (Table 1) was generally consistent with French guidelines: 2 nucleoside reverse transcriptase inhibitors (NRTIs) + boosted protease inhibitor (PI/r) in 63% of patients and 2 NRTIs + nevirapine (NVP) for 25%. Among this cohort of 59 children [median age (IQR): 5 years (22 months–11 years 7 months)], 56% had had in utero zidovudine (ZDV) exposure and 39% abacavir (ABC) exposure; rate of tenofovir (TDF) exposure was low (only 7% of the cohort). In utero exposure to 3 or more NRTIs was reported in four children (6.8%), associated with boosted PI in three [because of high viral load (5.5 log10 c/mL) in two patients and high rate of NRTIs and non-nucleoside reverse transcriptase inhibitors (NNRTIs) resistance mutations in the third patient]. Most of children (81%) had had per partum ZDV exposure. The rate of premature birth was 6.8%. Seventeen (29%) lived in a monoparental family and 59% had one or two parents from sub-Saharan Africa. Significant hyperlactatemia was measured in 26 patients.
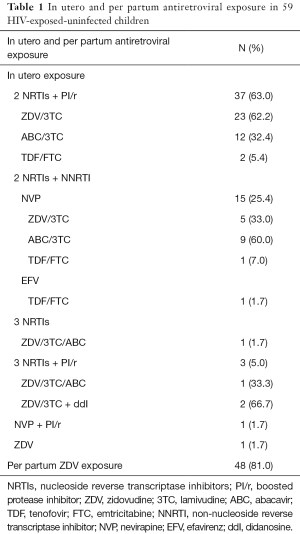
Full table
Abnormalities (defined as positive parental response to one or more item of the questionnaire) were notified in ten children (Table 2): three children had behavioral disorders; four had cognitive/developmental delay together with behavioral disorders; one had cognitive/developmental delay associated with height and weight growth retardation. Two children had had neoplasia (medullar pilocytic astrocytoma at the age of 3 years and myofibroblastic proliferation at the age of 3 months).

Full table
Among these ten children, eight had had in utero ZDV exposure and nine per partum ZDV exposure. Three children had been exposed to 3 NRTIs [ZDV + lamivudine (3TC) + didanosine (ddI), n=2; ZDV + 3TC + ABC, n=1]. Four children lived in a one-parent family and four had one or two parents from sub-Saharan Africa.
We compared the children with no abnormalities reported by parents to those with (Table 3): in this cohort of 59 children, with 5.8 years average follow-up (range, 1.8–11.7 years), we didn’t find a significant association between cancer, cognitive/developmental delay and/or behavioral disorders (n=10) and in utero (P=0.16) or per partum ZDV exposure (P=0.99); however, exposure to 3 NRTIs was significantly more frequent in infants with clinical abnormality (P=0.01). Median LA levels or significant episode of hyperlactatemia (P=0.49) were not different between the two groups.

Full table
To live in a one-parent family or to have parents from sub-Saharan Africa was not associated with symptoms. Clinical abnormality(ies) in infants was significantly associated with preterm birth (P=0.01).
Discussion
The significant findings of our study were (I) the lack of association between clinical abnormalities and prenatal or postnatal exposure to ZDV; (II) the higher frequency of prenatal exposure to 3 NRTIs in the group with clinical abnormalities; (III) the lack of correlation with hyperlactatemia in the group with abnormalities.
Some studies report a higher rate of preterm delivery in HIV + women (9) but the rate of premature birth in our study was 6.8%, in a similar range as that reported for European countries (10). Not surprisingly we found an association between preterm birth and clinical abnormalities: severe prematurity increases the risk of cerebral events, such as hypoxic brain injury and cerebral hemorrhage, which will affect future neuro-developmental potential.
All NRTIs have affinity with human DNA, a genotoxic potential. The inhibitory effect of ZDV on both HIV reverse transcriptase and DNA polymerase γ, an essential protein for mitochondrial DNA replication, is well known (2,3); the impact of the other NRTIs and other classes of ARV is less clear. Mitochondrial dysfunction due to ARV exposure before, during, or after birth is a broader toxicity that could have a continuing influence on childhood development; so, it is not surprising to find in our study a significant association between antenatal exposure to multiple NRTIs and developmental delay and/or behavioral disorders; however PACTG 219/219C found no overall association between ARV exposure in utero and neurologic development at 2 years of age in 1,037 HEU (9). Data from follow-up of PACTG 076 infants through age 6 years did not indicate any differences in growth parameters between infants who were exposed to the ZDV regimen and those who received placebo, and no malignancies were noted (11). The French Perinatal Cohort found a significant association of first-trimester ZDV exposure with congenital heart defects (12); this association has not been reported in the Antiretroviral Pregnancy Registry (13); in our study, no congenital abnormality was identified while 56% of children were exposed to AZT but it is likely that ARV therapy was introduced rather in the second trimester of pregnancy according to the French guidelines at this period [2003–2012]. The French Perinatal Cohort and PACTG 219/219C found no increase in early childhood cancer associated with ART exposure in HIV-exposed uninfected children (9,12). Malee found higher rates of mental health problems among HEU than HIV infected infants, not linked to in utero exposure to ART but in the caregiver-child relationship and the caregiving environment (14). Alimenti found that altered development and behavior in 39 HEU aged from 18 to 36 months was not linked to ART but with maternal substance use (15). In our study, we didn’t find any association between factors like living in a one parent family or to have a parent from Sub-Saharan Africa and no child was exposed to maternal substance use.
There are many limitations to our data: the small sample size of the cohort, the lack of matched control group and objectives measures to assess abnormalities and the declarative character of disorders but our results are consistent with results of other studies.
Conclusions
Increasing numbers of uninfected children will have prolonged in utero exposure to multiple ARV agents used for treatment of their mother’s HIV infection. Further studies are needed to detect and evaluate the type and rate of abnormalities, using longer follow-up period, more patients, matched group and more objective measures, Long-term follow-up into adulthood should continue because of the theoretical concerns regarding the potential for carcinogenicity of NRTIs.
Acknowledgements
The authors would like to thank all the parents responding to the letter.
Footnote
Conflicts of Interest: V Reliquet has received travel grants from Merck-Sharp and Dohme and honoraria from Gilead Sciences and Janssen-Cilag. F Raffi received research funding or honoraria from or consulted for Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Ferrer, Gilead Sciences, GlaxoSmith Kline, Janssen-Cilag, Merck-Sharp and Dohme, Pfizer, Splicos, ViiV Healthcare. C Brunet-Cartier received honoraria from Janssen-Cilag. E Launay has no conflicts of interest to declare.
Informed Consent: Every parent gave informed consent.
References
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Accessed 17 February, 2015. Available online: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf
- Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS 2003;17:1769-85. [Crossref] [PubMed]
- Noguera A, Fortuny C, Muñoz-Almagro C, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics 2004;114:e598-603. [Crossref] [PubMed]
- Mofenson LM, Watts DH. Safety of pediatric HIV elimination: the growing population of HIV- and antiretroviral-exposed but uninfected infants. PLoS Med 2014;11:e1001636. [Crossref] [PubMed]
- Sirois PA, Huo Y, Williams PL, et al. Safety of perinatal exposure to antiretroviral medications: developmental outcomes in infants. Pediatr Infect Dis J 2013;32:648-55. [Crossref] [PubMed]
- Rice ML, Zeldow B, Siberry GK, et al. Evaluation of risk for late language emergence after in utero antiretroviral drug exposure in HIV-exposed uninfected infants. Pediatr Infect Dis J 2013;32:e406-13. [Crossref] [PubMed]
- Himes SK, Huo Y, Siberry GK, et al. Meconium Atazanavir Concentrations and Early Language Outcomes in HIV-Exposed Uninfected Infants With Prenatal Atazanavir Exposure. J Acquir Immune Defic Syndr 2015;69:178-86. [Crossref] [PubMed]
- Williams PL, Marino M, Malee K, et al. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics 2010;125:e250-60. [Crossref] [PubMed]
- Heidari S, Mofenson L, Cotton MF, et al. Antiretroviral drugs for preventing mother-to-child transmission of HIV: a review of potential effects on HIV-exposed but uninfected children. J Acquir Immune Defic Syndr 2011;57:290-6. [Crossref] [PubMed]
- Zeitlin J, Szamotulska K, Drewniak N, et al. Preterm birth time trends in Europe: a study of 19 countries. BJOG 2013;120:1356-65. [Crossref] [PubMed]
- Hanson IC, Antonelli TA, Sperling RS, et al. Lack of tumors in infants with perinatal HIV-1 exposure and fetal/neonatal exposure to zidovudine. J Acquir Immune Defic Syndr Hum Retrovirol 1999;20:463-7. [Crossref] [PubMed]
- Sibiude J, Mandelbrot L, Blanche S, et al. Association between prenatal exposure to antiretroviral therapy and birth defects: an analysis of the French perinatal cohort study (ANRS CO1/CO11). PLoS Med 2014;11:e1001635. [Crossref] [PubMed]
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989 through 31 July 2015. Wilmington, NC: Registry Coordinating Center, 2015. Accessed 17 February, 2015. Available online: http://apregistry.com/forms/interim_report.pdf
- Malee KM, Tassiopoulos K, Huo Y, et al. Mental health functioning among children and adolescents with perinatal HIV infection and perinatal HIV exposure. AIDS Care 2011;23:1533-44. [Crossref] [PubMed]
- Alimenti A, Forbes JC, Oberlander TF, et al. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics 2006;118:e1139-45. [Crossref] [PubMed]


