A new approach to risk stratification using fetal MRI to predict outcomes in congenital diaphragmatic hernia: the preliminary retrospective single institutional study
Introduction
Congenital diaphragmatic hernia (CDH) is associated with a wide range of morbidity and mortality. Patients with mild physiologic consequences of CDH almost always survive, while those with the severest consequences have significant mortality. Pre- and post-natal management of this latter group remains challenging and sometimes controversial. In these cases, mortality rate was reported as high as 70% to 80% (1,2).
There has been significant interest in identifying prognostic factors, pre- and postnatal, which could assist in optimizing treatment algorithms. Identification of prenatal prognostic factors is considered particularly important as a potential guide for both prenatal intervention and postnatal management (1-3).
Among proposed factors, the lung to head ratio (LHR) and liver herniation estimation by prenatal sonography have both been widely evaluated. However, the significance of these findings varies between examiners and centers, and even in experienced hands appears to be highly subjective (2).
Recently, prenatal magnetic resonance imaging (MRI), which can provide more objective cross-sectional quantification, has been evaluated as a potential method for identifying prognostic factors (4-6). Although total lung volume has been demonstrated to correlate with prognosis, adjustment of these calculations by gestational age or fetal body weight is not always feasible or available. Considering the difficulty of measurement of lung volumes with MRI, a morphologic study may be preferable (7). Liver herniation into the chest and the position of the stomach have been evaluated as alternative prognostic factors (8). However, the detection of these are not always possible, and it can be difficult to assign a degree of abnormality to these observations.
Lung to liver signal intensity ratio (LLSIR) has been found to be related to lung maturity in T2-weighted MRI, and appears to be associated with prognosis in CDH patients (9,10). Therefore, we propose consideration of a novel stratification system, using LLSIR in fetal MRI, to improve the simplicity and, preferably accuracy of prediction for postnatal prognosis in patients with CDH.
Methods
Patients
With IRB approval from our hospital (R29-11), a retrospective chart review was conducted. Between September 2009 and February 2016, 31 patients underwent prenatal evaluation for CDH, with fetal MRI performed at our institution. Among these, six patients were excluded, due to other anomalies such as severe congenital heart disease, chromosomal aberrations, or poor image quality due to motion artifact precluding analysis. In total, 25 patients met study inclusion criteria.
The stratification algorithm
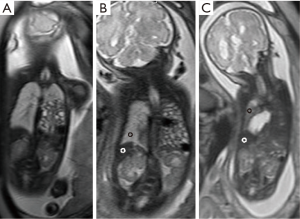
Stratification was performed utilizing coronal sections of T2-weighted MRI studies obtained after 29 weeks of gestation. LLSIR was calculated by the study radiologist (Y Sugioka) as reported by Oka et al. (11). We placed one region of interest (ROI) within the homogenous portion of the liver and the contralateral lung on the same section. LLSIR findings were graded as follows: Grade I, ipsilateral lung at the apex was detectable; Grade II, ipsilateral lung at the apex was undetectable and LLSIR was ≥2.0; Grade III, ipsilateral lung at the apex was undetectable and LLSIR was <2.0 (Figures 1,2). The primary outcome was death, and the secondary outcomes included gestational age, birth weight, Apgar scores at 1 and 5 minutes, severity [usage of inhaled nitric oxide (iNO) alone or with extracorporeal membrane oxygenation (ECMO) defined as severe], and need of a synthetic patch for closure. In addition, we examined the association between liver herniation and survival, severity and need of patch closure in this cohort.
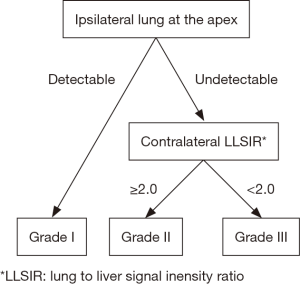
Statistical analysis was performed using chi square test (categorical variables) and Kruskal-Wallis test (continuous variables) to compare the distribution of valuables among three grades. Continuous values were expressed as median (range). In addition, logistic regression models were conducted to calculate odds ratios (ORs) and 95% confidence intervals (CIs) of each grade for death and patch closure. Fisher’s exact test was performed to analyze the association between the presence of liver herniation and survival, severity and the requirement of patch closure. All analyses were two-tailed and were conducted using JMP® 13.2
Results
Among 25 patients, 15 were Grade I, 6 were Grade II, and 4 were Grade III (Table 1). Median weeks of gestation at MRI were 33 weeks (range, 27–38 weeks) in Grade I, 33.5 weeks (range, 30–37 weeks) in Grade II and 31 weeks (range, 29–33 weeks) in Grade III (P=0.19). All Grade I patients survived, whereas 2 (33.3%) died in Grade II and 3 (75.0%) died before 2 days of life in Grade III groups (P=0.003). Median gestational age and birth weight were 37 weeks (range, 37–39 weeks), 2,932 g (range, 2,378–3,502 g) in Grade I, 37 weeks (range, 37–38 weeks), 2,570 g (range, 2,466–3,018 g) in Grade II and 33 weeks (range, 32–37 weeks), 2,129 g (range, 1,652–2,510 g) in Grade III (P=0.01, 0.007, respectively). Median Apgar scores at 1 and 5 minutes were 3 (range, 2–8), 6 (range, 3–9) in Grande I, 2 (range, 1–3), 4.5 (range, 4–5) in Grade II, and 3 (range, 1–3), 3.5 (range, 1–4) in Grade III (P=0.09, 0.03, respectively). iNO was used for 4 (26.7%) patients in Grade I, all patients in Grade II and 1 in Grade III (P=0.007). ECMO was used in three patients in Grade II, 2 of whom survived and 1 who died. A synthetic patch closure was required for 1 (6.7%) patient in Grade I, for all 4 (100.0%) patients who survived in Grade II, and for 1 (100.0%) patient who survived in Grade III (P<0.001).
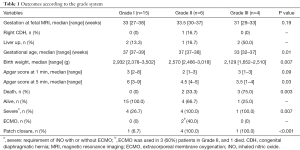
Full table
Compared to the Grade I and II patients, those in the Grade III group displayed significantly higher mortality (OR: 28.5; 95% CI, 1.9–420.5). Patients in Grade II and III (ipsilateral lung at the apex was undetectable) were more likely to require patch closure as compared to Grade I (OR: 414,238,332; 95% CI, 0–∞) (Table 2).

Full table
Liver herniation was noted in five patients in this cohort. Although survival was significantly different between the presence (liver up) and absence (liver down) of liver herniation in fetal MRI (P=0.04), there was no significant difference between liver herniation and either severity (P=1.00) nor the need of patch closure (P=0.52) (Table 3)
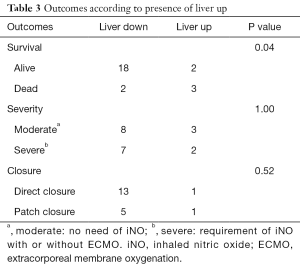
Full table
Discussion
Our proposed stratification algorithm is based on the lung volume and maturity, as characterized by fetal MRI. Total lung volume has been previously demonstrated to contribute significant prognostic information (12,13). Recently, observed to expected (o/e) total lung volume in fetal MRI has been considered a factor which predicts postnatal survival (14). However, the measurement of total lung volume and correction with gestational age may not always be feasible or available. We found that patients with higher Grades tended to lower gestational age, birth weight, and Apgar score at 5 minutes. Ipsilateral lung detected at the apex (Grade I) resulted in sufficient total lung volume for not only survival, but also appears to predict relatively mild persistent pulmonary hypertension. In our series, ipsilateral lung was less definitively detected at mediastinum in one patient in Grade II (data not shown), which was thought to be more compressed than at the apex.
Although the prognostic factors in CDH were generally related to the hypoplastic lung and LLSIR has been shown to correlate with lung maturity (15,16), LLSIR was also shown to be the potential prognostic factor for CDH. We hypothesized that when ipsilateral lung was not detected at the apex, contralateral lung maturity provided important prognostic information. We selected LLSIR values greater than 2.0 as indicative of maturity (11) in this study, and validated this as predictive of outcome, suggesting that this value reflects contralateral lung maturation. When values were less than 2.0, contralateral lung maturation was insufficient to support survival (Grade III), whereas contralateral lung maturation was more likely to allow survival (Grade II), although with a requirement for intensive management using iNO or ECMO.
Furthermore, it was revealed that all cases graded I and II with undetectable ipsilateral lung at the apex required a synthetic patch for closure. To our knowledge, there has been no previous identification of anatomic features on prenatal imaging which have been reported to be associated with patch closure in the English literature.
o/e-LHR and liver herniation estimated by prenatal ultrasound sonography have been widely accepted as prognostic factors for survival of patients with CDH. As o/e LHR measured by ultrasonography was highly subjective and competency and reliability were achieved only by experienced operators (17), the data were available in only ten cases of this cohort. Although, some reports showed o/e-LHR was correlated with not only survival but also disease severity (18,19). o/e-LHR was deemed not to be a good predictor for ECMO utilization and other factors related to severity (14,20,21). In our series, o/e-LHR in two patients with severe CDH who required ECMO were both more than 50% (data not shown).
Whether liver herniation is a prognostic factor for survival and severity is also controversial. Ruano et al. reported that liver herniation was not correlated to the mortality or the need for ECMO (22). Although a meta-analysis revealed that liver herniation was associated with poorer prognosis, the definition of liver herniation varied (23), which is difficult to use in clinical practice. In our series, although liver herniation regardless of proportion was significantly associated with mortality, there were no significant differences between liver herniation and either severity nor need of patch closure. This data could provide information optimizing operative planning for such infants.
The most advantageous feature in this stratification system compared to other prenatal prognostic factors is that image-defined stratification with the calculation of LLSIR is relatively easy on MRI images without any specific techniques nor the need of experienced examiners.
There are several limitations in our study. First, this was a retrospective single institution study, with small number of patients. Second, LLSIR was calculated by a single radiologist who was blinded to clinical outcomes, limiting the ability to demonstrate reproducibility of this metric. Third, we used MRI studies obtained after 29 weeks of gestation. However, it is possible that LLSIR changes dynamically during fetal development as lung maturity progresses, and thus the optimal timing of MRI for the grading system needs to be clarified.
Conclusions
Despite the above limitations, our pilot data suggests that this image-based stratification system, based on anatomic features and tissue characteristics detected by MRI, may provide additional useful information for patients with CDH. We propose that further investigation with a prospective multi-institutional study is warranted to validate this stratification system. The use of LLSIR in fetal MRI may add prognostic information regarding postnatal survival, disease severity, and the requirement for patch closure in CDH.
Acknowledgements
We thank Dr. Jessica Kandel and Dr. Charles Stolar for help in writing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board (IRB) of our hospital (R29-11).
References
- Daodu O, Brindle ME. Predicting outcomes in congenital diaphragmatic hernia. Semin Pediatr Surg 2017;26:136-9. [Crossref] [PubMed]
- Coughlin MA, Werner NL, Gajarski R, et al. Prenatally diagnosed severe CDH: mortality and morbidity remain high. J Pediatr Surg 2016;51:1091-5. [Crossref] [PubMed]
- Losty PD. Congenital diaphragmatic hernia: where and what is the evidence? Semin Pediatr Surg 2014;23:278-82. [Crossref] [PubMed]
- Victoria T, Danzer E, Adzick NS. Use of ultrasound and MRI for evaluation of lung volumes in fetuses with isolated left congenital diaphragmatic hernia. Semin Pediatr Surg 2013;22:30-6. [Crossref] [PubMed]
- Kastenholz KE, Weis M, Hagelstein C, et al. Correlation of Observed-to-Expected MRI Fetal Lung Volume and Ultrasound Lung-to-Head Ratio at Different Gestational Times in Fetuses With Congenital Diaphragmatic Hernia. AJR Am J Roentgenol 2016;206:856-66. [Crossref] [PubMed]
- Werner NL, Coughlin M, Kunisaki SM, et al. Prenatal and postnatal markers of severity in congenital diaphragmatic hernia have similar prognostic ability. Prenat Diagn 2016;36:107-11. [Crossref] [PubMed]
- Hattori T, Hayakawa M, Ito M, et al. The relationship between three signs of fetal magnetic resonance imaging and severity of congenital diaphragmatic hernia. J Perinatol 2017;37:265-9. [Crossref] [PubMed]
- Kitano Y, Okuyama H, Saito M, et al. Re-evaluation of stomach position as a simple prognostic factor in fetal left congenital diaphragmatic hernia: a multicenter survey in Japan. Ultrasound Obstet Gynecol 2011;37:277-82. [Crossref] [PubMed]
- Nishie A, Tajima T, Asayama Y, et al. MR prediction of postnatal outcomes in left-sided congenital diaphragmatic hernia using right lung signal intensity: comparison with that using right lung volume. J Magn Reson Imaging 2009;30:112-20. [Crossref] [PubMed]
- Sebastia C, Gomez O, Salvador R, et al. Prognostic usefulness of derived T2-weighted fetal magnetic resonance imaging measurements in congenital diaphragmatic hernia. Radiologia 2015;57:239-47. [PubMed]
- Oka Y, Rahman M, Sasakura C, et al. Prenatal diagnosis of fetal respiratory function: evaluation of fetal lung maturity using lung-to-liver signal intensity ratio at magnetic resonance imaging. Prenat Diagn 2014;34:1289-94. [Crossref] [PubMed]
- Paek BW, Coakley FV, Lu Y, et al. Congenital diaphragmatic hernia: prenatal evaluation with MR lung volumetry--preliminary experience. Radiology 2001;220:63-7. [Crossref] [PubMed]
- Barnewolt CE, Kunisaki SM, Fauza DO, et al. Percent predicted lung volumes as measured on fetal magnetic resonance imaging: a useful biometric parameter for risk stratification in congenital diaphragmatic hernia. J Pediatr Surg 2007;42:193-7. [Crossref] [PubMed]
- Akinkuotu AC, Cruz SM, Abbas PI, et al. Risk-stratification of severity for infants with CDH: Prenatal versus postnatal predictors of outcome. J Pediatr Surg 2016;51:44-8. [Crossref] [PubMed]
- Brewerton LJ, Chari RS, Liang Y, et al. Fetal lung-to-liver signal intensity ratio at MR imaging: development of a normal scale and possible role in predicting pulmonary hypoplasia in utero. Radiology 2005;235:1005-10. [Crossref] [PubMed]
- Moshiri M, Mannelli L, Richardson ML, et al. Fetal lung maturity assessment with MRI fetal lung-to-liver signal-intensity ratio. AJR Am J Roentgenol 2013;201:1386-90. [Crossref] [PubMed]
- Cruz-Martinez R, Figueras F, Moreno-Alvarez O, et al. Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2010;36:32-6. [Crossref] [PubMed]
- Wong M, Reyes J, Lapidus-Krol E, et al. Pulmonary hypertension in congenital diaphragmatic hernia patients: Prognostic markers and long-term outcomes. J Pediatr Surg 2018;53:918-24. [Crossref] [PubMed]
- Shah N, Chowdhary S, Kaul A. Role of Ultrasound-Based Prenatal Prediction of Pulmonary Function in Congenital Diaphragmatic Hernia: Does It Have Prognostic Significance Postnatally? J Obstet Gynaecol India 2017;67:33-6. [Crossref] [PubMed]
- Alfaraj MA, Shah PS, Bohn D, et al. Congenital diaphragmatic hernia: lung-to-head ratio and lung volume for prediction of outcome. Am J Obstet Gynecol 2011;205:43.e1-8. [Crossref] [PubMed]
- Bruns AS, Lau PE, Dhillon GS, et al. Predictive value of oxygenation index for outcomes in left-sided congenital diaphragmatic hernia. J Pediatr Surg 2018;53:1675-80. [Crossref] [PubMed]
- Ruano R, Lazar DA, Cass DL, et al. Fetal lung volume and quantification of liver herniation by magnetic resonance imaging in isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2014;43:662-9. [Crossref] [PubMed]
- Mullassery D, Ba'ath ME, Jesudason EC, et al. Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2010;35:609-14. [Crossref] [PubMed]


