
Serological biomarkers for acute mesenteric ischemia
Introduction
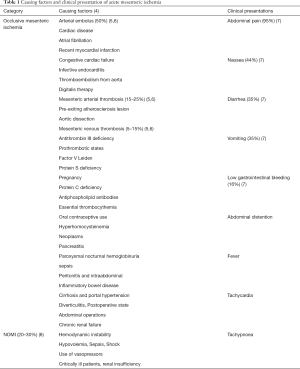
Acute mesenteric ischemia (AMI) is a rare but still remains a major challenge in diagnosis and treatment of most abdominal emergency, which caused by insufficient oxygen delivery or utilization to fill the metabolic needs of the visceral organs. Two main pathophysiological mechanisms may lead to mesenteric ischemia: acute thromboembolic occlusion in the arteries or veins of the gastrointestinal tract, or non-occlusive mesenteric ischemia (NOMI) reduced blood flow from cardiac insufficiency, shock states, major surgery, increased intra-abdominal pressure, trauma, atrial fibrillation, renal insufficiency, and sepsis (1-3) (Table 1). Though there are still no specific diagnostic biomarkers for AMI, occlusive mesenteric ischemia is quite easier to diagnosis with high specific computed tomography angiography (CTA). However, it is being tough to obtain the definitive diagnosis of NOMI, which compromises 20–30% of all cases of AMI (8), in clinical practice there has neither specific makers nor radiology test, especially in early stage. In this review, we provide an overview of the etiology of AMI, review the current research situation and future of biomarker research,aim to find the most promising biomarkers.
Full table
Mesenteric circulation and pathophysiology of AMI
The splanchnic circulation encompasses macro- and micro- vascular perfusion.
The macrovascular consist of three main arteries, including celiac artery (CA), superior mesenteric artery (SMA) (9), and inferior mesenteric artery (IMA) (10), and numerous collaterals. Normally at a state of rest, the splanchnic circulation accepts approximately 25% cardiac outputs while in a postprandial state demand an additional 10%. The CA, SMA and IMA have a diameter of 6, 7 and 1 mm respectively, thus, an IMA occlusion would reduce the total mesenteric vessel surface by only 4%, whereas stenosis of CA and SMA would reduce this by70% and 87%, respectively (1). So, it is widely considered the SMA is the most important of the mesenteric arteries in occlusive mesenteric ischemia.
The microvascular perfusion is including larger arteries on the serosal side, large networks of vessels in the outer layers (submucosal, muscular and serosal layers) and a central arteriole with surrounding venules. With a high metabolic demand, the mucosal layer receives more than two-thirds of the bowel wall blood flow (11,12). The countercurrent organization of the villus is capable of effectively autoregulation of the blood flow and maintains a constant level of oxygen uptake. To maintain circulatory homeostasis with enough oxygen levels in the circumstance of considerable vary mesenteric blood flow, oxygen exchange depends on the capacity of the villi to increase extraction and recruit additional capillary beds.
In circumstances of malperfusion or shock, arterial shunting would happen because of the prolonged circulation transit time across the villi (13). With prolonged ischemic insult or reperfusion injury, the countercurrent exchange intensifies injury to the villus-crypt axis, resulting in cellular dysfunction and cell death occurring initially at the mucosal villous tip. If malperfusion persist for longer periods, resulting in the degenerate or slough of mucosal barrier is beginning. Accompanying this process is intravascular hemoconcentration, leukocyte plugging, vasomotor dysfunction, and capillary narrowing, all of which lead to endothelial swelling and microvascular thrombosis, then followed by increased intestinal permeability, bacterial translocation, bacterial overgrowth due to infection, mesenteric infarction.
Due to these pathological processes of mucosal layer, it is obvious that the villus, the outermost layers are notably sensitive to ischemic damage. Consequently, ischemia damage starts from the mucosa and extends towards the serosa. In contrast, ischemic damage to the muscular and serosal layers is a late event in severe ischemia. However, intestinal mucosal ischemic injury is often not serious and reversible, but transmural injury often leads to inflammation, necrosis, sepsis and multiple organ failure (5,14). And it takes about 4 h to mucosal ischemic damage become critical, and cause transmural damage and necrosis (15,16). So mucosal layer should be the focus of early diagnostic test for immediate therapy for early ischemia to reach a successful outcome (1,17,18).
Clinical features and diagnosis of AMI
The clinical symptoms often associated with AMI including mild but sudden pain (median of 24 h duration) mostly out of proportion to physical examination, diarrhea, low gastrointestinal bleeding, abdominal distention with vomiting, nausea, fever, tachycardia, and tachypnoea, are not specific enough to differentiate AMI with other abdominal diseases (7,19). In a Swedish study, acute abdominal pain is the most common diagnoses in 2,222 patients, based on computed tomography (CT), were nonspecific abdominal pain (44%), and only 11 patients (0.5%) had AMI (20). At present, the diagnosis of AMI in clinical mostly achieved by a high degree of clinical suspicion after ruling out the other acute abdominal diseases and a prompt confirmation by an abdominal CTA, with a 95% to 100% accuracy (21), up to 95% specificity and sensitivity (22), CTA has become the most appropriate and recommended method of imaging for the diagnostic test, especially for the acute thromboembolic occlusion, in which the blockade of mesenteric blood flow is quite clear and much easy to make the diagnosis. The laboratory tests still mostly rely on the conventional non-specific biological markers of thrombosis, hypoxia inflammation, infection and some other parameters for laboratory assessment of AMI, include evaluation of white blood cell count, physiologic acid-base status (increased anion gap, pH, base excess), alanine aminotransferase, aspartate aminotransferase, lactic acidosis, alkaline phosphatase, and amylase levels, however, all of them are not specific enough to diagnose ischemia or sensitivity enough to rule out the disease. Besides, these inflammation laboratory markers increasing or decreasing significantly, mostly may indicate the progression of the disease to bowel necrosis (23).
The importance of early diagnosis of AMI
The diagnosis of AMI is often challenging in acute abdominal pain patients, and diagnostic uncertainty may ultimately require surgical explorations for an accurate assessment of the bowel. The severity of ischemia depends on the affected vessel, the extent of collateral-vessel blood flow and the time of duration. When observed the clinical signs of AMI, such as peritonitis, at physical examination indicate a strong probability of irreversible intestinal ischemia with bowel necrosis (24). In other word, delayed diagnosis leads to intestinal necrosis and even multiple organ failure. In a large multicenter study of 780 ICU patients with AMI, the overall mortality rate was 58% (25). The same data was also observed by others that the mortality of AMI ranged from 60% to 80% (23,26-28). The increase of the mortality is mostly because of the delay in diagnosis and treatment (5).
The main challenging risk of mesenteric ischemia is transmural infarction, which is mostly irreversible, leading to intestinal wall perforation, sepsis and death (29,30). Early diagnosis and timely intervention are therefore key factors to improving the clinical outcomes of patients with AMI. Now, surgical management is the most common treatment for most of the AMI being diagnosed in the late stage (22), which require prompt surgery to resect nonviable intestine (31). However, the ischemia is potentially fully reversible if the mesenteric arterial revascularization, a specific management of AMI, is done in the early period of AMI, when there is no sign of transmural infarction (5,6,32,33). Also, in the initial stages of NOMI when bowel wall ischemia is partial, surgical treatment is not indicated (34). However, many laboratory indexes were tested for their values to early diagnosis of mesenteric ischemia, unfortunately, most of the studied biomarkers appeared when the AMI developed to the late stage, such as Lactic acidosis (10,35).
Besides, even high-tech diagnostic equipment, such as computerized tomographic angiography (CTA), can sometimes miss acute occlusive intestinal ischemia, radiological findings are often less specific (2,36-38). Due to the lack of specific diagnostic sign, scanning test or biomarker, it still remains a challenge to the selection of patients requiring CTA evaluation in the early stage of AMI. Also, misdiagnosed cases may occur in CTA examination (30,38). In intensive care unit, most of the critically ill patients suffered from sepsis, shock or use of vasoconstrictive medication eventually induce NOMI, and duo to the patients under mechanical ventilation or not easy to move because of the severe condition, they may not able to do CTA examination.
As a whole, it’s emphasizing the importance of early and reliable diagnosis. So, there is a great need for a plasma biomarker, which would be best if its tissue specific, metabolic stable from intestine to peripheral blood with high specificity and sensitivity to AMI.
Most promising biomarkers
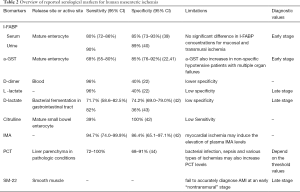
In the past decades, there are several most promising biomarkers, including Intestinal fatty acid binding protein (I-FABP), a-glutathione S-transferase (a-GST), D-dimer, L- and D-lactate, citrulline, ischemia modified albumin (10), procalcitonin (PCT), being studied for diagnosis of intestinal ischemia (Table 2). These makers are in relation with the intestinal mucosal layer, including the gut barrier dysfunction, the villi injury and the enterocyte mass, so they may the best candidate markers for early diagnosis of AMI.
Full table
I-FABP
I-FABP is a most studied plasma marker, released by mature enterocytes—situated at the tips of the intestinal mucosal villi—upon intestinal ischemia, has high value to diagnose mucosal damage with high tissue-specificity (7,45,46). I-FABP is a 15-kDa soluble protein, rapidly released into the blood circulation upon mucosal damage and is cleared via the urine, allowing both serum and urine available to test it (47). In physiological conditions, I-FABP is present in very small amounts in peripheral circulation, but levels rise rapidly after enterocyte necrosis and inflammation (48). A recent meta-analysis on the accuracy of circulating I-FABP for the diagnosis of AMI showing that a pooled sensitivity of 80% (95% CI: 72–86%) for serum I-FABP, a pooled specificity of 85% (95% CI: 73–93%), and an area under the ROC curve of 86% (95% CI: 83–89%) in the diagnosis of AMI (39). Another study in The Netherlands showed quite high sensitivity and specificity of 90% and 89%, respectively, for urinary I-FABP in detecting early mesenteric ischemia (40,43). However, one recent study reported that there is no significant difference in I-FABP concentrations for mucosal and transmural ischemia (15).
a-GST
a-GST is a detoxifying enzyme, involved in the detoxification and conjugation of endo and xenobiotics into glutathione, which is also released by mature enterocytes on intestinal mucosa and liver, and has potential value to diagnose early AMI (41,45,46,49). In these two analyses reported by Cudnik et al. (22) and Evennett et al. (41) showed that a-GST has a pooled sensitivity and specificity of 68% (95% CI: 55–80%) and of 85% (95% CI: 76–92%), respectively. However, in non-specific hypotensive patients with multiple organ failures, a-GST also increases (43).
D-dimer
D-dimer is a fibrin degradation product (or FDP), a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. D-dimers are usually increased either in arterial or venous occlusive forms, as well as in other confounding inflammatory and infectious diseases, including other causes of acute abdominal complaints (22,50,51), so it has high sensitivity for being early marker, but has low specificity. Cudnik et al. (22) reviewed pooled data from five studies estimating the diagnostic value of D-dimer as a biomarker for AMI. It showed a pooled high sensitivity of 96% and a quite lower specificity of 40%. So, it is accuracy raised doubt to predict early AMI (52).
L- and D-lactate
L-lactate is a ubiquitous product of glycolysis in the context of anaerobia. So many factors may result in increased serum lactate levels, thus can’t effectively differentiate intestinal ischemia from the other etiologies of abdominal emergencies or intensive care diseases (40,53,54). In a meta-analysis in 2013 on a total of 1,970 patients, Cudnik et al. (22) showed that L-lactate has a good pooled sensitivity of 0.96, but low specificity of 0.40 to be used as diagnostic markers.
D-lactate, the stereoisomer of L-lactate, is the product of bacterial fermentation in the gastrointestinal tract. The elevation of D-lactate levels in the circulation associated with intestinal ischemia, increased intestinal permeability, bacterial translocation or bacterial overgrowth due to infection (9) and mesenteric infarction (55). In recent meta-analysis showed that pooled sensitivity and specificity for D-lactate is 71.7% (95% CI: 58.6–82.5%) and 74.2% (95% CI: 69.0–79.0%), respectively (42), may reflecting its high value to become potential diagnostic tool for AMI. However, most pooled research reports good sensitivities of 82%, but lower specificities of 36% (43). Furthermore, most findings discovered that elevation of L-lactate and D-lactate levels mostly occur in the late stage of AMI, especially when initiated extensive transmural necrosis, anaerobic metabolism (56-60). With lower specificity, L-lactate and D-lactate are may not the potential candidate biomarkers to use for early diagnostic marker of AMI (52).
Citrulline
Citrulline is a non-proteinogenic amino acid synthesized from glutamine in the mitochondria of mature small bowel enterocytes. Citrulline is also a key intermediate in the urea cycle, so gut synthesis and renal elimination are the two main influencing factors to its plasmatic level. High plasmatic citrulline concentrations may cause by acute renal failure by decreasing renal clearance and citrulline transformation into arginine (61), while low plasmatic citrulline concentrations may see in short bowel conditions. Nevertheless, Citrulline is may a promising marker with high reported specificity (100%), in a meta-analysis which conducted only one study, although lower sensitivity (39%), and shown to be a reliable functional marker of enterocyte mass with short circulating half-life of 3–4 h (62-64).
Ischemia modified albumin
Ischemia modified albumin (10) is human serum albumin, which has a binding site at the N-terminus for metal ions, such as cobalt, and incapable of binding cobalt due to ischemia by alterations in this binding site (65). In recent meta-analysis, it showed that pooled sensitivity and specificity for IMA was 94.7% and 86.4%, respectively (42). Another two studies also showed significant higher serum IMA levels in AMI (66,67). Noted that myocardial ischemia may induce the elevation of plasma IMA levels (68).
PCT
PCT is a precursor of calcitonin and released by the C cells of the thyroid in healthy subjects, while in pathologic conditions it is known as the product of liver parenchyma while being stimulated by trauma, bacterial endotoxins, TNF-α and IL-6 or cardiogenic shock (69-71). Recent a systematic review by Cosse et al. (44) on five clinical studies with a total of 659 patients show a high sensitivity of 0.72–1.00 and specificity of 0.68–0.91 to diagnose AMI, however, it also mentioned by author that its diagnostic value in AMI may be affected by the presence of a bacterial infection, sepsis and various types of ischemias. So, it is used for the diagnosis of AMI might be limited by its low specificity.
Current research situation and future of biomarker research
AMI is a life-threatening condition that requires emergency treatment and so must be diagnosed as soon as possible. However, it is still tough to obtain a definitive early diagnosis, because current available clinical, radiological, and laboratory tests are not good enough to diagnose early, reversible stage mesenteric ischemia. Above those promising biomarkers showed high specificity and sensitivity with good tissue specific, metabolic stable from intestine to peripheral blood features. However, much of the studies about these makers finished with small patient populations, and it also exist heterogeneous among these populations. Besides, there is need for further research with large patient population to specify threshold values and accuracy standards for different aetiological forms. So, at present, none of these markers being perfect enough to be used solely. Besides there is still no available test or tool can differentiate a focal transmural infarction from an extensive nontransmural ischemia. Though Schellekens et al. (15), reported SM22, a smooth muscle biomarker, which concentration was significantly elevated in transmural intestinal ischemia, it also can’t be able to differentiate it (72).
As ischemia starts from the mucosa and progresses to the serosa, a mucosa-derived marker would be most useful for early diagnosis (52). However, duo to the intestinal truck lack of specific tissue different from other tissues and organs, so it still remains challenging to find ideal biomarker. So, it may be good way to study the mechanism of AMI on the molecule levels. In our recent study, we find miR-21 may regulate intestinal epithelial tight junction permeability and expression is unregulated during intestinal barrier dysfunction induced by Intestinal ischemia-reperfusion injury (73,74).
And studying the combination outcome of several biomarkers rather than the use of a single marker with properly powered analysis, which may reflect different types and stages of mesenteric ischemia, is probably a better way to go.
Acknowledgments
Funding: This project was supported by grant from the National Natural Science Foundation of China (no.81801943) and the Science and Technology Commission of Shanghai Municipality (no. 18411970200).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- van Noord D, Kolkman JJ. Functional testing in the diagnosis of chronic mesenteric ischemia. Best Pract Res Clin Gastroenterol 2017;31:59-68. [Crossref] [PubMed]
- Bourcier S, Oudjit A, Goudard G, et al. Diagnosis of non-occlusive acute mesenteric ischemia in the intensive care unit. Ann Intensive Care 2016;6:112. [Crossref] [PubMed]
- Vokurka J, Olejnik J, Jedlicka V, et al. Acute mesenteric ischemia. Hepatogastroenterology 2008;55:1349-52. [PubMed]
- Savlania A, Tripathi RK. Acute mesenteric ischemia: current multidisciplinary approach. J Cardiovasc Surg (Torino) 2017;58:339-50. [PubMed]
- Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med 2016;374:959-68. [Crossref] [PubMed]
- Acosta S. Mesenteric ischemia. Curr Opin Crit Care 2015;21:171-8. [Crossref] [PubMed]
- Park WM, Gloviczki P, Cherry KJ Jr, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg 2002;35:445-52. [Crossref] [PubMed]
- Trompeter M, Brazda T, Remy CT, et al. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol 2002;12:1179-87. [Crossref] [PubMed]
- Smith SM, Eng RH, Buccini F. Use of D-lactic acid measurements in the diagnosis of bacterial infections. J Infect Dis 1986;154:658-64. [Crossref] [PubMed]
- Tilsed JV, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg 2016;42:253-70. [Crossref] [PubMed]
- Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res 2000;93:182-96. [Crossref] [PubMed]
- Kalder J, Ajah D, Keschenau P, et al. Microcirculatory perfusion shift in the gut wall layers induced by extracorporeal circulation. J Vasc Surg 2015;61:497-503. [Crossref] [PubMed]
- Powell A, Armstrong P. Plasma biomarkers for early diagnosis of acute intestinal ischemia. Semin Vasc Surg 2014;27:170-5. [Crossref] [PubMed]
- Schellekens DH, Grootjans J, Dello SA, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol 2014;48:253-60. [Crossref] [PubMed]
- Schellekens DHSM, Reisinger KW, Lenaerts K, et al. SM22 a Plasma Biomarker for Human Transmural Intestinal Ischemia. Ann Surg 2018;268:120-6. [Crossref] [PubMed]
- Kanda T, Nakatomi Y, Ishikawa H, et al. Intestinal fatty acid-binding protein as a sensitive marker of intestinal ischemia. Dig Dis Sci 1992;37:1362-7. [Crossref] [PubMed]
- Inderbitzi R, Wagner HE, Seiler C, et al. Acute mesenteric ischaemia. Eur J Surg 1992;158:123-6. [PubMed]
- Bradbury AW, Brittenden J, McBride K, et al. Mesenteric ischaemia: a multidisciplinary approach. Br J Surg 1995;82:1446-59. [Crossref] [PubMed]
- Theodoropoulou A, Koutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol 2008;14:7302-8. [Crossref] [PubMed]
- Strömberg C, Johansson G, Adolfsson A. Acute abdominal pain: diagnostic impact of immediate CT scanning. World J Surg 2007;31:2347-54; discussion 2355-8. [Crossref] [PubMed]
- Hagspiel KD, Flors L, Hanley M, et al. Computed tomography angiography and magnetic resonance angiography imaging of the mesenteric vasculature. Tech Vasc Interv Radiol 2015;18:2-13. [Crossref] [PubMed]
- Cudnik MT, Darbha S, Jones J, et al. The diagnosis of acute mesenteric ischemia: A systematic review and meta-analysis. Acad Emerg Med 2013;20:1087-100. [Crossref] [PubMed]
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010;256:93-101. [Crossref] [PubMed]
- Bala M, Kashuk J, Moore EE, et al. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg 2017;12:38. [Crossref] [PubMed]
- Leone M, Bechis C, Baumstarck K, et al. Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases. Intensive Care Med 2015;41:667-76. [Crossref] [PubMed]
- Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg 2011;396:13-29. [Crossref] [PubMed]
- Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg 2010;23:4-8. [Crossref] [PubMed]
- Acosta-Merida MA, Marchena-Gomez J, Hemmersbach-Miller M, et al. Identification of risk factors for perioperative mortality in acute mesenteric ischemia. World J Surg 2006;30:1579-85. [Crossref] [PubMed]
- American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology 2000;118:951-3. [Crossref] [PubMed]
- Kozuch PL, Brandt LJ. Review article: diagnosis and management of mesenteric ischaemia with an emphasis on pharmacotherapy. Aliment Pharmacol Ther 2005;21:201-15. [Crossref] [PubMed]
- Acosta S, Bjorck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014;101:e100-8. [Crossref] [PubMed]
- Nuzzo A, Corcos O. Reversible Acute Mesenteric Ischemia. N Engl J Med 2016;375:e31. [Crossref] [PubMed]
- Corcos O, Castier Y, Sibert A, et al. Effects of a multimodal management strategy for acute mesenteric ischemia on survival and intestinal failure. Clin Gastroenterol Hepatol 2013;11:158-65.e2. [Crossref] [PubMed]
- Meilahn JE, Morris JB, Ceppa EP, et al. Effect of prolonged selective intramesenteric arterial vasodilator therapy on intestinal viability after acute segmental mesenteric vascular occlusion. Ann Surg 2001;234:107-15. [Crossref] [PubMed]
- Glenister KM, Corke CF. Infarcted intestine: a diagnostic void. ANZ J Surg 2004;74:260-5. [Crossref] [PubMed]
- Kärkkäinen JM, Acosta S. Acute mesenteric ischemia (part I) - Incidence, etiologies, and how to improve early diagnosis. Best Pract Res Clin Gastroenterol 2017;31:15-25. [Crossref] [PubMed]
- Piton G, Manzon C, Monnet E, et al. Plasma citrulline kinetics and prognostic value in critically ill patients. Intensive Care Med 2010;36:702-6. [Crossref] [PubMed]
- Angelelli G, Scardapane A, Memeo M, et al. Acute bowel ischemia: CT findings. Eur J Radiol 2004;50:37-47. [Crossref] [PubMed]
- Sun DL, Cen YY, Li SM, et al. Accuracy of the serum intestinal fatty-acid-binding protein for diagnosis of acute intestinal ischemia: a meta-analysis. Sci Rep 2016;6:34371. [Crossref] [PubMed]
- Thuijls G, van Wijck K, Grootjans J, et al. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg 2011;253:303-8. [Crossref] [PubMed]
- Evennett NJ, Petrov MS, Mittal A, et al. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg 2009;33:1374-83. [Crossref] [PubMed]
- Treskes N, Persoon AM, van Zanten ARH. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systematic review and meta-analysis. Intern Emerg Med 2017;12:821-36. [Crossref] [PubMed]
- van den Heijkant TC, Aerts BA, Teijink JA, et al. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol 2013;19:1338-41. [Crossref] [PubMed]
- Cosse C, Sabbagh C, Kamel S, et al. Procalcitonin and intestinal ischemia: a review of the literature. World J Gastroenterol 2014;20:17773-8. [Crossref] [PubMed]
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996;110:339-43. [Crossref] [PubMed]
- Delaney CP, O'Neill S, Manning F, et al. Plasma concentrations of glutathione S-transferase isoenzyme are raised in patients with intestinal ischaemia. Br J Surg 1999;86:1349-53. [Crossref] [PubMed]
- Derikx JP, Luyer MD, Heineman E, et al. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol 2010;16:5272-9. [Crossref] [PubMed]
- Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care 2016;22:152-60. [PubMed]
- van de Poll MC, Derikx JP, Buurman WA, et al. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg 2007;31:2033-8. [Crossref] [PubMed]
- Yang S, Fan X, Ding W, et al. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore) 2014;93:e270. [Crossref] [PubMed]
- Yang K, Wang W, Zhang WH, et al. The Combination of D-Dimer and Peritoneal Irritation Signs as a Potential Indicator to Exclude the Diagnosis of Intestinal Necrosis. Medicine (Baltimore) 2015;94:e1564. [Crossref] [PubMed]
- Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis 2012;33:355-61. [Crossref] [PubMed]
- Acosta S, Nilsson TK, Malina J, et al. L-lactate after embolization of the superior mesenteric artery. J Surg Res 2007;143:320-8. [Crossref] [PubMed]
- Chiu YH, Huang MK, How CK, et al. D-dimer in patients with suspected acute mesenteric ischemia. Am J Emerg Med 2009;27:975-9. [Crossref] [PubMed]
- Poeze M, Froon AH, Greve JW, et al. D-lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg 1998;85:1221-4. [Crossref] [PubMed]
- Nuzzo A, Maggiori L, Ronot M, et al. Predictive Factors of Intestinal Necrosis in Acute Mesenteric Ischemia: Prospective Study from an Intestinal Stroke Center. Am J Gastroenterol 2017;112:597-605. [Crossref] [PubMed]
- Ritz JP, Germer CT, Buhr HJ. Prognostic factors for mesenteric infarction: multivariate analysis of 187 patients with regard to patient age. Ann Vasc Surg 2005;19:328-34. [Crossref] [PubMed]
- Arthurs ZM, Titus J, Bannazadeh M, et al. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg 2011;53:698-704; discussion 704-5. [Crossref] [PubMed]
- Collange O, Tamion F, Chanel S, et al. D-lactate is not a reliable marker of gut ischemia-reperfusion in a rat model of supraceliac aortic clamping. Crit Care Med 2006;34:1415-9. [Crossref] [PubMed]
- Guzmán-de la Garza FJ, Ibarra-Hernández JM, Cordero-Pérez P, et al. Temporal relationship of serum markers and tissue damage during acute intestinal ischemia/reperfusion. Clinics (Sao Paulo) 2013;68:1034-8. [Crossref] [PubMed]
- Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 2008;27:328-39. [Crossref] [PubMed]
- Crenn P, Coudray-Lucas C, Thuillier F, et al. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000;119:1496-505. [Crossref] [PubMed]
- Gondolesi G, Fishbein T, Chehade M, et al. Serum citrulline is a potential marker for rejection of intestinal allografts. Transplant Proc 2002;34:918-20. [Crossref] [PubMed]
- Ruiz P, Tryphonopoulos P, Island E, et al. Citrulline evaluation in bowel transplantation. Transplant Proc 2010;42:54-6. [Crossref] [PubMed]
- Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med 2000;19:311-5. [Crossref] [PubMed]
- Gunduz A, Turedi S, Mentese A, et al. Ischemia-modified albumin in the diagnosis of acute mesenteric ischemia: a preliminary study. Am J Emerg Med 2008;26:202-5. [Crossref] [PubMed]
- Polk JD, Rael LT, Craun ML, et al. Clinical utility of the cobalt-albumin binding assay in the diagnosis of intestinal ischemia. J Trauma 2008;64:42-5. [Crossref] [PubMed]
- Sinha MK, Gaze DC, Tippins JR, et al. Ischemia modified albumin is a sensitive marker of myocardial ischemia after percutaneous coronary intervention. Circulation 2003;107:2403-5. [Crossref] [PubMed]
- Lavrentieva A, Kontakiotis T, Lazaridis L, et al. Inflammatory markers in patients with severe burn injury. What is the best indicator of sepsis? Burns 2007;33:189-94. [Crossref] [PubMed]
- Nanda N, Juthani-Mehta M. Novel biomarkers for the diagnosis of urinary tract infection-a systematic review. Biomark Insights 2009;4:111-21. [Crossref] [PubMed]
- Becker KL, Nylen ES, White JC, et al. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004;89:1512-25. [Crossref] [PubMed]
- Nuzzo A, Ronot M, Maggiori L, et al. Early Acute Mesenteric Ischemia: Many Rivers to Cross. Ann Surg 2018;268:e41. [Crossref] [PubMed]
- Zhang L, Shen J, Cheng J, et al. MicroRNA-21 regulates intestinal epithelial tight junction permeability. Cell Biochem Funct 2015;33:235-40. [Crossref] [PubMed]
- Zhang L, Zhang F, He D-K, et al. MicroRNA-21 is upregulated during intestinal barrier dysfunction induced by ischemia reperfusion. The Kaohsiung Journal of Medical Sciences 2018;34:556-63. [Crossref] [PubMed]


