Clinical Experience with Cerebrospinal Fluid Aβ42, Total and Phosphorylated Tau in the Evaluation of 1,016 Individuals for Suspected Dementia
Abstract
Background:
Elevated total tau (tTau), 181-phosphorylated phosphorylated tau (pTau), and low amyloid-β42 (Aβ42) in cerebrospinal fluid (CSF) represent a diagnostic biomarker for Alzheimer’s disease (AD).
Objective:
The goal was to determine the overall accuracy of CSF Aβ42, tTau, pTau, and the Aβ42/total tau index (ATI) in a non-research, clinical setting for the diagnosis of AD.
Methods:
From medical records in 1,016 patients that had CSF studies for dementia over a 12-year period (2005 to 2017), we calculated the sensitivity and specificity of CSF Aβ42, tTau, and pTau and the ATI in relation to the final clinical diagnosis.
Results:
Compared with non-demented patients and patients with other dementias or mild cognitive impairment (MCI), the sensitivity and specificity of the recommended ATI and pTau cut-offs (ATI < 1.0 and pTau >61 pg/ml) for the diagnosis of AD were 0.88 and 0.72, respectively. Similar results were obtained comparing AD with non-demented patients only (0.88, 0.82) and AD with other types of dementia (0.81, 0.77). A subgroup of patients with presumed normal pressure hydrocephalus (n = 154) were biopsied at the time of shunt placement. Using the pathological manifestations of AD as the standard, the sensitivity was 0.83 while the specificity was 0.72.
Conclusions:
In a non-research setting, CSF biomarkers for AD showed a high sensitivity in accordance with previous studies, but modest specificity differentiating AD from other types of dementia or MCI. This study of unselected patients provides a valid and realistic assessment of the diagnostic accuracy of these CSF biomarkers in clinical practice.
INTRODUCTION
Diagnostic accuracy in the clinical diagnosis of Alzheimer’s disease (AD) is important to differentiate it from conditions with similar manifestations, take advantage of novel therapeutic agents, monitor disease progression, and end-of-life planning. While autopsy remains the “gold standard” for a definitive diagnosis of AD, elevated levels of total tau (tTau), 181-phosphorylated phosphorylated tau (pTau), and decreasing levels of amyloid-β42 (Aβ42) in antemortem lumbar [1, 2] cerebrospinal fluid (CSF) have been associated with AD and correlated with postmortem amyloid plaque load [1]. In additional, a strong relationship exists between in vivo amyloid plaque load assessed with Pittsburgh Compound (PIB)-PET or Florbetapir for amyloid and 18FFDNP for both tangles and plaques and CSF Aβ42 levels [3–5]. Therefore, inclusion of these CSF biomarkers in the clinical evaluation of patients suspected of having AD would aid in diagnostic accuracy. A meta-analysis including data from 231 studies for 11,341 patients with AD and 7,086 controls reported significant differences in CSF Aβ42, tTau, and pTau when comparing patients with AD to healthy controls [6, 7]. However, a subsequent Cochrane review in 2017 [8] concluded that sensitivity and specificity of CSF biomarkers “have limited clinical value” because of methodological differences across the studies including the: “sources of recruitment, participant sampling, index test methodology and inadequate blinding.”
To provide a realistic and unbiased evaluation of these CSF biomarkers in a non-research setting, we assessed retrospective data from a large cohort of patients attending an academic medical center to sensitivity, specificity, and area under the curve of CSF Aβ42, tTau, pTau, and the Aβ/tTau ratio (ATI). We hypothesized that such analyses from this large patient group at a single site might provide a more homogenous and accurate assessment of the accuracy of these biomarkers in the clinical diagnosis of AD.
MATERIALS AND METHODS
Participants
The results from 1,137 CSF samples were ascertained from the medical records of outpatients and hospitalized patients at the New York Presbyterian Hospital-Columbia University Irving Medical Center between 2005 and 2017. We excluded patients with dementia of uncertain etiology or whose diagnosis was not completely documented (n = 121). This analysis then focused on the remaining 1,016 (89.3%) for this study, including 264 (26%) with a pretest diagnosis of probable AD; 53 (5%) with mild cognitive impairment (MCI); 65 (6.3%) with dementia with Lewy bodies (DLB); 53 (5%) with frontotemporal dementia (FTLD, including patients with semantic dementia, progressive non-fluent aphasia and behavioral type frontotemporal dementia); 31 (3%) with vascular dementia (VaD); 21 (2%) with progressive supranuclear palsy (PSP); 14 (0.9%) with corticobasal degeneration (CBD); 218 (21.4%) with normal pressure hydrocephalus (NPH); and 30 (3%) with Creutzfeldt-Jacob disease (CJD). In addition, results from lumbar puncture were obtained from 37 (3.6%) with a nonspecific psychiatric disorders (PSY) and 230 (22.6%) with either subjective memory complaints (SMC) or no memory complaints but with altered mental status at time of admission. These 267 patients were considered as non-demented patient group (N = 267; 26.2%). Finally, 97 (8.67%) of the patients died during the study period with 13 (13.4%) undergoing autopsy.
Clinical diagnoses were made by several different neurologists not involved in the current analysis using published diagnostic criteria: National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for AD and MCI [9]; consensus criteria frontotemporal lobar degeneration for FTLD [10]; McKeith criteria for DLB [11]; National Institute of Neurological Disorders and Stroke (NINDS)–Association Internationale pour la Recherche en l’Enseignement en Neurosciences for VaD [12]; criteria of Boeve for CBD [13]; NINDS–Society for Progressive Supranuclear Palsy criteria for PSP [14]; referred criteria for CJD [15].
Patients who were evaluated for NPH had ventriculomegaly with some combination of Hakim’s triad (gait disorder, incontinence, and cognitive decline), usually with gait disorder predominance. The majority of patients with suspected NPH underwent lumbar drainage trial prior to ventriculoperitoneal shunt (VPS) placement. Patients with NPH were included in this study only if they underwent VPS with neuropathological assessment of the cortical brain biopsy obtained at the time of shunt placement (N = 154). Though a biopsy provided only a small amount of tissue, we used the neuropathological manifestations found in AD as the gold standard for sensitivity and specificity analyses in these cases.
CSF analysis
Lumbar puncture was performed by neurology residents or the treating neurologist, after informed consent to use such laboratory results for research purposes was obtained. CSF aliquots were collected in polypropylene tubes and caps under standardized conditions. After centrifuged at 1000 g/min for 10 min, 0.5 mL aliquots were collected and stored at –80°C within 2 h. The New York Presbyterian Hospital shipped all such samples to the commercial laboratory where the CSF samples were analyzed using ADmark® ELISA kit (https://www.athenadiagnostics.com/view-full-catalog/a/admark-reg;-alzheimer-s-evaluation and https://www.mayomedicallaboratories.com/testcatalog/Clinical+and+Interpretive/91925).
CSF concentrations of Aβ42, t-Tau, and p-Tau were measured and the ATI calculated. ADmark® essay results were reported as associated with AD according to CSF biomarkers pattern using ATI < 1.0 and pTau > 61 pg/ml as thresholds in both laboratories. Thus, for all main analyses ATI < 1 and pTau > 61 pg/ml were used as the threshold of choice.
CSF analysis in patients with NPH
CSF data was available from 218 patients with suspected NPH who subsequently underwent VPS. During the procedure, neuropathological specimens from the frontal lobe were also harvested for pathological assessment. We restricted our analyses to 154 (70.6%) samples with both CSF and neuropathological data available. After hematoxylin and eosin stained sections were submitted to preliminary analysis, immunohistochemistry for neuritic plaques and neurofibrillary tangles was performed. Neuropathological diagnosis of AD was attempted when criteria were met, according to NIA-AA guidelines [16], although sufficient material for diagnosis was not always available from the biopsy.
Statistical analysis
Direct measures of CSF Aβ42, t-Tau, and p-Tau levels and the ATI were compared across diagnostic groups (i.e., AD group versus non-demented patients and across other diagnostic groups compared to AD) using the Kruskal-Wallis test, followed by Mann-Whitney test with Monte Carlo method. We used the ATI (Aβ42/T-tau Index) computed as:
The calculation of sensitivity and specificity across the clinical subgroups (AD versus all other patients, AD versus non-demented patients, AD versus other types of dementia, AD versus NPH) was performed and converted into receiving operating characteristic (ROC) analyses. We also measured sensitivity and specificity of combined CSF biomarkers by computing the area under the curve (AUC) using predictions from a logistic regression model that included other measures as predictors (e.g., ATI + pTau).
Accuracy as determined by AUC was defined as 1.0–0.90 excellent; 0.90–0.80 good; 0.80–0.70 fair; 0.70–0.60 poor; and 0.60–0.50 failure. We applied validated threshold from literature for each CSF biomarkers: 500 pg/ml [18, 19] for Aβ42; 350 pg/mL for tTau [20, 21]; 61 pg/ml for pTau [22].
Validated thresholds in literature for ATI levels indicated that ATI≤0.8 (ATI0.8) was strongly associated with AD, while ATI≥1.2 (ATI1.2) was less robustly associated with AD and ultimately ATI = 1 (ATI1.0) could be considered as an effective threshold to discriminate demented versus non-demented patients [17, 22]. Therefore, we tested each of these cut-offs in terms of sensitivity and specificity. The significance threshold for all analyses was set to p < 0.05. Analyses were performed using SPSS v.24 [23]. Amos (Version 24.0). Chicago: IBM SPSS) and R version 3.3.3 (R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/), package “pROC” [24].
RESULTS
Demographics
A statistically significant difference in mean age and sex was found comparing probable AD versus non-demented patients and other types of dementia (p < 0.0001). However, there were no differences in years of education or mortality rates. Similar differences were found comparing sex, age, and education by diagnostic groups (all pairwise comparison with p-value < 0.0001, Table 1).
Table 1
Demographics and summary CSF biomarker data from patients in the analyses. Variables with * are means with standard deviation in parentheses
| Variables | Non-demented hospital patients | Other dementias | Probable Alzheimer’s disease | p values |
| N | 267 (26.28%) | 485 (50.10%) | 264 (25.98%) | |
| Women (%) | 52% | 41% | 55% | p = 0.001b |
| Age (y)* | 61.49 (15.34) | 72.49 (9.66) | 67.71 (10.37) | p < 0.0001a |
| Education (y)* | 16.9 (3.42) | 16.42 (3.79) | 15.44 (4.13) | p > 0.5a |
| Deaths % | 7.86% | 11.39% | 6.44% | p < 0.5b |
| Aβ42* | 505.40 (292.86) | 498.52 (250.02) | 376.36 (159.25) | p < 0.0001a |
| tTau* | 423.67 (930.19) | 628.52 (1461.53) | 594.03 (371.07) | p < 0.001a |
| pTau* | 41.23 (30.30) | 45.54 (24.52) | 82.47 (38.60) | p < 0.0001a |
| ATI* | 1.017 (0.67) | 0.89 (0.58) | 0.46 (0.24) | p < 0.0001a |
Non-demented hospital controls: subjective memory complaints and psychiatric disorders; Other dementias: mild cognitive impairment, dementia with Lewy bodies, frontotemporal lobar dementia, vascular dementia, progressive supranuclear palsy, corticobasal degeneration, normal pressure hydrocephalus, and Creutzfeldt-Jakob disease. aKruskall Wallis test was used for comparing means across continuous nonstandard distributed variables. bChi-square test was used for comparing means across dichotomized variables.
CSF biomarkers distribution
Statistically significant differences were found comparing Aβ42, tTau, pTau, and ATI in both AD versus other conditions overall, and in AD versus non-demented patients (all pairwise comparison with p-value < 0.0001, Table 1). We observed significant differences in the Aβ42, pTau, and ATI values distribution between AD and MCI, DLB, FTLD, PSP, SMC, and PSY (all pairwise comparison with p-value < 0.0001, Supplementary Table 1).
Sensitivity and specificity
Overall analyses for Aβ42, tTau, pTau, and ATI
For Aβ42 = 500 pg/ml (AUC = 0.622, SE = 0.017, 95% CI [0.588–0.656], p < 0.0001), sensitivity and specificity were 0.81 and 0.44; for tTau = 350 pg/ml (AUC = 0.751, SE = 0.015, 95% CI [0.722–0.781], p < 0.0001), sensitivity 0.77 and specificity 0.70. pTau = 61 pg/ml showed the best AUC (0.834, SE = 0.015, 95% CI [0.806–0.862], p < 0.0001), sensitivity 0.73 and specificity 0.82. ATI0.8, the recommended value (AUC = 0.732, SE = 0.015, 95% CI [0.703–0.761], p < 0.0001) was found to have a sensitivity of 0.90 and a specificity of 0.51. The sensitivity and specificity of ATI1 for AD versus all other diagnostic groups included in the cohort was found to be 0.97 and 0.42, respectively. For ATI1.2, the sensitivity was 0.98 and the specificity 0.32 (Fig. 1).
Combined analysis for ATI < 1 and pTau > 61 pg/ml (AUC = 0.8524, 95% CI [0.8288–0.8759], p < 0.0001) computed 0.88 sensitivity and 0.72 specificity as final results.
Fig.1
Receiver operation curve (ROC). Alzheimer’s disease compared to overall population of the cohort. Alzheimer’s disease (n = 264) was compared to the overall population of the cohort (n = 752). Aβ42 (red), tTau (green), pTau (blue), and ATI (black) CSF biomarker ROC curves are reported here. AUC analyses fully reported in the text.
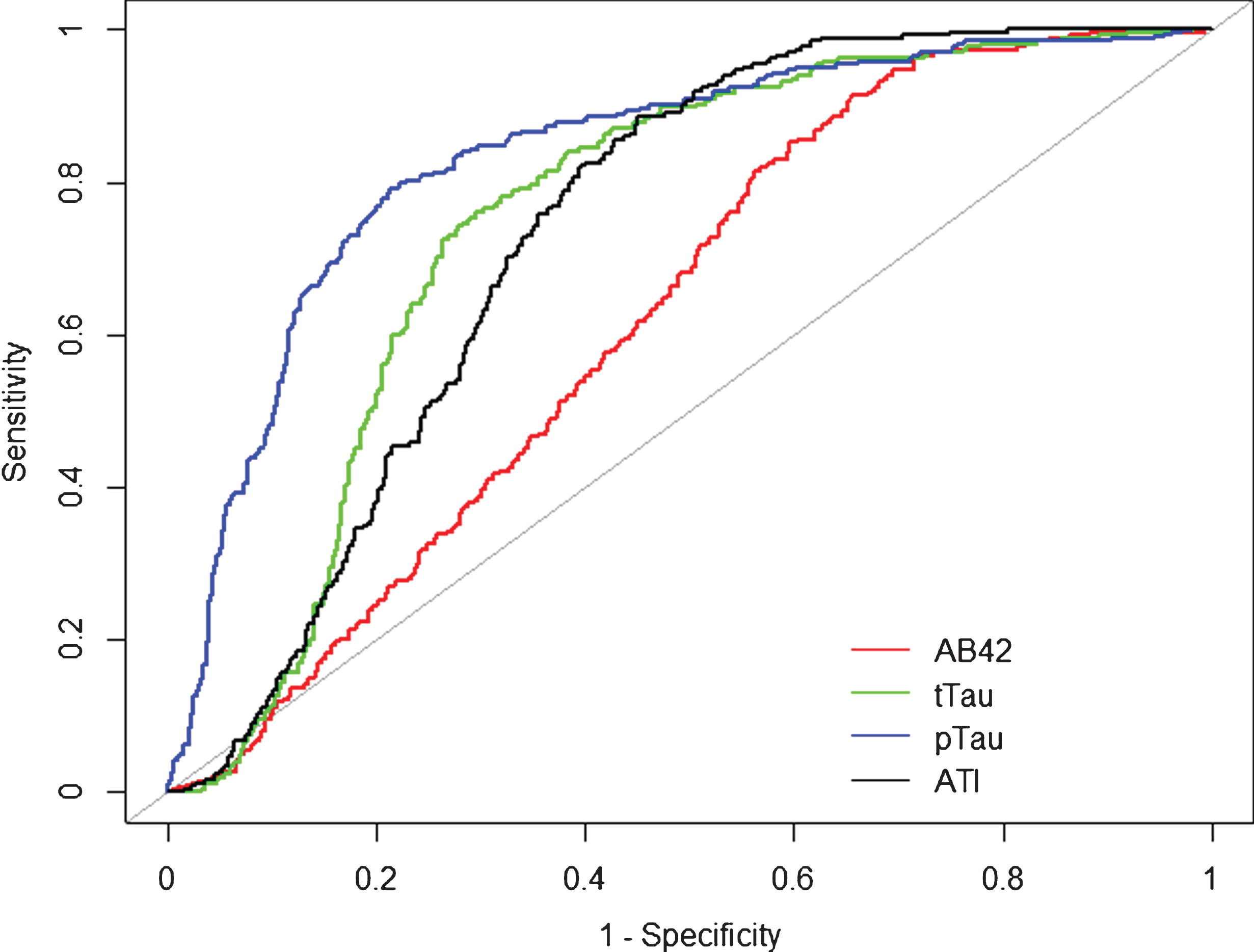
AD versus non-demented patients
For Aβ42 = 500 pg/ml (AUC = 0.616, SE = 0.025, 95% CI [0.567–0.665], p < 0.0001) sensitivity and specificity were 0.81 and 0.45; for tTau = 350 pg/ml (AUC = 0.811, SE = 0.020, 95% CI [0.772–0.851], p < 0.0001) sensitivity 0.77 and specificity 0.79. pTau = 61 pg/ml showed the best AUC (0.864, SE = 0.017, 95% CI [0.831–0.897], p < 0.0001), sensitivity 0.73 and specificity 0.87). ATI0.8 (AUC = 0.764, SE = 0.021,95% CI [0.723–0.806], p < 0.0001) was found having a sensitivity of 0.90 and a specificity of 0.56; ATI1 was found to be 0.97 and 0.43, respectively. for ATI1.2, the sensitivity was 0.99 and specificity of 0.46. Combined analysis for ATI < 1.0 and pTau > 61 pg/ml (AUC = 0.8922, 95% CI [0.8627–0.9216] p < 0.0001) computed 0.88 sensitivity and 0.82 specificity as final results.
AD versus other types of dementia
In (n = 749) patients with symptoms and signs of memory impairment after clinical and radiological investigation, 264 (35.3%) were diagnosed with probable AD while the remaining 485 were diagnosed with other types of dementia (FTLD, DLB, PSP, VaD, CBD, NPH, or CJD) or MCI at follow-up. The subset was investigated with each of the three biomarkers and ATI was calculated.
For Aβ42 = 500 pg/ml (AUC = 0.641, SE = 0.020, 95% CI 0.603–0.680, p < 0.0001), sensitivity and specificity were 0.81 and 0.54; for tTau = 350 pg/ml (AUC = 0.724, SE = 0.018, 95% CI 0.688–0.760, p < 0.0001), sensitivity 0.77 and specificity 0.34. pTau = 61 pg/ml showed the best AUC (0.830, SE = 0.016, 95% CI [0.799–0.861], p < 0.0001), sensitivity 0.73 and specificity 0.20. ATI0.8 (AUC = 0.729, SE = 0.017, 95% CI [0.694–0.763], p < 0.0001) was found having a sensitivity of 0.90 and a specificity of 0.51; for ATI1 sensitivity and specificity were 0.97 and 0.60, respectively while for ATI1.2, we observed a sensitivity of 0.99 and a specificity of 0.69.
Combined analysis for ATI < 1 and pTau > 61 pg/ml (AUC = 0.8487, 95% CI [0.8209–0.8764, p < 0.0001) computed 0.81 sensitivity and 0.77 specificity as final results.
CSF biomarkers performance in differentiating AD versus each type of dementia was examined and results were summarized in the Supplementary Table 2.
Normal pressure hydrocephalus with biopsy for AD pathology
For Aβ42 = 500 pg/ml (AUC = 0.767, SE = 0.048, 95% CI 0.673–0.860], p < 0.0001), sensitivity and specificity were 0.93 and 0.44; tTau and pTau showed AUC values < 0.5 with asymptotic significance values >0.1 and further measurement were omitted. ATI0.8 (AUC = 0.688, SE = 0.052, 95% CI [0.587; 0.790], p < 0.002) was found having a sensitivity of 0.83 and a specificity of 0.57. The sensitivity and specificity of ATI1 was found to be 0.87 and 0.45, respectively. Employing ATI1.2 as our threshold sensitivity of 0.93 and a specificity of 0.33 were found. Combined analysis for ATI < 1 and pTau > 61 pg/ml (AUC = 0.7847, 95%CI [0.6898–0.8797, p < 0.0001) computed 0.83 sensitivity and 0.72 specificity as final results.
Re-analyses of CSF biomarkers to define thresholds
We conducted additional analyses by attempting to compute the best CSF thresholds based on the data obtained from this group of patients that best discriminated between groups. In the overall analyses rthresholds for CSF biomarkers in identifying AD versus non-demented patients and all other diagnostic groups were: Aβ42 = 565.7 pg/ml (AUC = 0.62">0.6898–0.8797, p < 0.0001) computed 0.83 sensitivity and 0.72 specificity as final results.
Re-analyses of CSF biomarkers to define thresholds
We conducted additional analyses by attempting to compute the best CSF thresholds based on the data obtained from this group of patients that best discriminated between groups. In the overall analyses, thresholds for CSF biomarkers in identifying AD versus non-demented patients and all other diagnostic groups were: Aβ42 = 565.7 pg/ml (AUC = 0.62, 95% CI 0.5867–0.6546], p < 0.0001, sensitivity 0.91 and specificity 0.34), tTau = 357 pg/ml (AUC = 0.75, 95% CI [0.7216–0.7813], p < 0.0001, sensitivity 0.77 and specificity 0.70), pTau = 57.6 pg/ml (AUC = 0.83, 95% CI [0.8063–0.8619], p < 0.0001, sensitivity 0.79 and specificity 0.79) and ATI = 0.72 (AUC = 0.73, 95% CI [0.7029–0.7608], p < 0.0001, sensitivity 0.88 and specificity 0.55).
The tests were repeated to compare AD with non-demented patients: Aβ42 = 641.50 pg/ml (AUC = 0.61, 95% CI [0.5600–0.6600], p < 0.0001, sensitivity 0.96 and specificity 0.30), tTau = 356.10 pg/ml (AUC = 0.81, 95% CI [0.7700–0.8500], p < 0.0001, sensitivity 0.77 and specificity 0.79), pTau = 51.10 pg/ml (AUC = 0.86, 95% CI [0.8300–0.9000], p < 0.0001, sensitivity 0.84 and specificity 0.79), and ATI = 0.83 (AUC = 0.76, 95% CI [0.7200–0.8000], p < 0.0001, sensitivity 0.92 and specificity 0.56). Comparing AD to other types of dementia the analysis showed: Aβ42 = 641.40 pg/ml (AUC = 0.67, 95% CI [0.6200–0.7300], p < 0.0001, sensitivity 0.96 and specificity 0.37); tTau = 388.30 pg/ml (AUC = 0.81, 95% CI [0.7700–0.8500], p < 0.0001, sensitivity 0.74 and specificity 0.82); pTau = 55.90 pg/ml (AUC = 0.85, 95% CI [0.8200–0.8900], p < 0.0001, sensitivity 0.80 and specificity 0.80); and ATI = 0.73 (AUC = 0.81, 95% CI [0.7700–0.8600], p < 0.0001, sensitivity 0.88 and specificity 0.62).
Finally, CSF biomarkers were tested on a subgroup of patients with NPH who cortical biopsy with neuropathological evaluation after ventriculoperitoneal shunting procedure: Aβ42 = 468.15 pg/ml (AUC = 0.78, 95% CI [0.6878–0.8745], p < 0.0001, sensitivity 0.93 and specificity 0.54), tTau = 299.2 pg/ml (AUC = 0.52, 95% CI [0.4085–0.6413], p < 0.0001, sensitivity 0.48 and specificity 0.61), pTau = 55.90 pg/ml (AUC = 0.85, 95% CI [0.8200–0.8900], p < 0.0001, sensitivity 0.80 and specificity 0.80), and ATI = 0.63 (AUC = 0.69, 95% CI [0.5906–0.7952], p < 0.0001, sensitivity 0.72 and specificity 0.70).
DISCUSSION
The results reported here provide an unbiased assessment of CSF biomarkers in evaluation of patients suspected of having AD in a non-research, clinical setting. These results indicate that individually CSF biomarkers Aβ42, tTau, pTau, and the computed ATI, tested at recommended thresholds provide excellent sensitivity, but moderate to low specificity for clinically diagnosed AD compared to patients with other diseases or and with other forms of dementias in routine practice. Based on the AUC, the level of pTau was found to provide the best overall accuracy of any single CSF biomarker, regardless of the comparison group.
While, the use of these CSF biomarkers is recommended for the diagnosis of AD, they can be helpful in situations where the diagnosis is uncertain and AD is one of the diagnoses considered in the differential diagnosis of a patient. We assumed that when these CSF biomarkers were used in patients with diagnoses other than AD, the physician was attempting to exclude AD as a diagnosis. Certainly, these CSF biomarkers are best used when distinguishing AD from other forms of dementia.
Most published studies have been in research settings that compared AD to healthy controls [25, 26], but this does not reflect what is generally done in clinical practice. Similarly, validity of these CSF biomarkers has been established previously using data from patients sampled during life and subsequently undergoing autopsy at the time of death [27–29].The approach in the current study differs from most previous studies for number of total patients for whom diagnoses and CSF measures were obtained and a single center. Struyfs et al. [30] for example, reported higher sensitivity and specificity versus healthy control group rather than comparing these measures to differentiate AD from other conditions, as Johansson et al. [31] did, reporting comparable findings in a cohort of 60 patients. The Alzheimer’s Biomarkers Standardization Initiative (ABSI) [32] suggested that the use of CSF biomarkers should be considered in all patients referred for memory complaints or admitted to hospitals for cognitive impairment and complex differential diagnoses of dementia. In addition, younger patients with early-onset dementia, MCI, or atypical clinical signs should be taken into account [32]. Though previous studies had reported the sensitivity and specificity of these CSF biomarker in the diagnosis of AD compared with healthy controls, or patients with MCI or depression [28], we considered the alternative approach used here, other forms of dementia, a less biased and more appropriate to assess validity of these CSF biomarkers. The main difference in our report compared to those in literature [33–35] is the reduction in specificity that is likely explained by including patients with other dementing disorders (FTLD, DLB, and VaD). What we address in this study is a measurement of CSF biomarker accuracy as a diagnostic in a clinical practice setting, assessing sensitivity and specificity in the differential diagnosis of AD versus other types of dementia and NPH.
The highest sensitivity and specificity in this subset of patients compared to the previous studies [33–35] was achieved when we used ATI = 1 (sensitivity of 0.96 and a specificity of 0.60). pTau > 61 showed a sensitivity of 0.78 and a specificity of 0.83, but the highest accuracy as measured by the AUC. In each of these analyses, specificity was lower than reported in a number of previous studies [36–38]. In the subset of patients with NPH, we further tested the ability of CSF biomarkers to identify and correctly classify AD pathology. However, the sensitivity and specificity were similar to that found in the clinical diagnosis of AD. This reinforced our conclusion that CSF biomarkers have the highest degree of specificity only when comparing patients with dementia to healthy controls. The specificity decreases if tested in a group of patients that represent a typical patients in memory clinics and hospital settings [39].
The results obtained here for pTau indicated that this individual measure was by far the most accurate for clinically diagnosed AD as measured by AUC. This is consistent with what reported by Koopman et al. [40] in an autopsy-based study which assessed a specificity of 0.60 for pTau in differentiating AD versus other conditions. However, the recommended combination of ATI < 1.0 and pTau > 61 pg/ml consistently showed the highest accuracy measured by AUC ranging from 0.78 to 0.89. The AUC was lowest among patients undergoing VPS for NPH and brain biopsy, those with compared to those without AD pathology, and highest among patients with AD compared with non-demented hospital controls. Using the data collected here to define the most optimal score for each biomarker did not improve sensitivity, specificity or accuracy over the recommended combination of ATI < 1.0 and pTau > 61 pg/ml. Thus, the results here indicate that the recommended combination of ATI < 1.0 and pTau > 61 pg/ml provides the best sensitivity, specificity, and overall accuracy for a clinical diagnosis of AD. However, in terms of overall accuracy based on AUC using this combination of threshold would considered this CSF biomarker analysis as “good”. Improvement in specificity would be required to move the overall accuracy to “excellent”.
The study here has several strengths including sample size, unbiased data collection from a single non-research clinical site of typical patients, the two national laboratories involved (using the same immunoassay kit), and confirmation of our findings in a subset with neuropathological information.
There are limitations of this study including the reliance on the biopsy-based diagnoses was limited making it difficult to assess complete neuropathological criteria. We did not attempt to compare the accuracy of these CSF biomarkers with imaging biomarkers, such as measure of white matter hyperintensities and regional atrophy or fluorodeoxyglucose or amyloid positron emission tomography, because these were not systematically obtained over the time period.
A clinically reliable and valid biomarker should provide a sensitivity and specificity close to 80–90%. In this study, we found that the results of previous studies may have overestimated CSF biomarkers specificity by the frequent comparison to healthy controls. Whereas in this unbiased case series we found the sensitivity to be fairly consistent (0.8 to 0.9 or better), the specificity varied from 0.72 overall, to 0.82 and only when compared to healthy controls. Our findings suggest the specificity of CSF biomarkers in differentiating between AD and other type of dementias is adequate for clinical decision when the recommend combination of ATI < 1.0 and pTau > 61 pg/ml is used. All other measures, with the exception of pTau, lacked the accuracy for contributing to the diagnostic evaluation.
The use of CSF biomarkers in the diagnosis of patients meeting the clinical criteria listed by Alzheimer’s Biomarkers Standardization Initiative needs to be the state of the art in identifying AD; the results presented here indicate that further work needs to be done to improve the specificity and overall accuracy.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institute on Aging P50AG08702.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0548r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: 10.3233/JAD-180548.
REFERENCES
[1] | Strozyk D , Blennow K , White LR , Launer LJ ((2003) ) CSF Aβ42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60: , 652–656. |
[2] | Tapiola T , Alafuzoff I , Herukka S , Parkkinen L , Hartikainen P , Soininen H , Pirttilä T ((2009) ) Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66: , 382–389. |
[3] | Fagan AM , Mintun MA , Mach RH , Lee SY , Dence CS , Shah AR , LaRossa GN , Spinner ML , Klunk WE , Mathis CA , DeKosky ST , Morris JC , Holtzman DM ((2006) ) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42in humans. Ann Neurol 59: , 512–519. |
[4] | Tolboom N , van der Flier WM , Yaqub M , Boellaard R , Verwey NA , Blankenstein MA , Windhorst AD , Scheltens P , Lammertsma AA , van Berckel BNM ((2009) ) Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med 50: , 1464–1470. |
[5] | Mattsson N , Insel PS , Landau S , Jagust W , Donohue M , Shaw LM , Trojanowski JQ , Zetterberg H , Blennow K , Weiner M ((2014) ) Diagnostic accuracy of CSF Aβ42 and florbetapir PET for Alzheimer’s disease. Ann Clin Transl Neurol 1: , 534–543. |
[6] | Öst M , Nylén K , Csajbok L , Öhrfelt AO , Tullberg M , Wikkelsö C , Nellgård P , Rosengren L , Blennow K , Nellgård B ((2006) ) Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 67: , 1600–1604. |
[7] | ((2017) ) Diagnostic biomarkers for Alzheimer’s disease: A regulatory view. Lancet Neurol 16: , 580–581. |
[8] | Ritchie C , Smailagic N , Noel-Storr AH , Ukoumunne O , Ladds EC , Martin S ((2017) ) CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 3: , CD010803. |
[9] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–939. |
[10] | Neary D , Snowden JS , Gustafson L , Passant U , Stuss D , Black S , Freedman M , Kertesz A , Robert PH , Albert M , Boone K , Miller BL , Cummings J , Benson DF ((1998) ) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51: , 1546–1554. |
[11] | Mckeith IG ((2006) ) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 66: , 1455; author reply 1455. |
[12] | Román GC , Tatemichi TK , Erkinjuntti T , Cummings JL , Masdeu JC , Garcia JH , Amaducci L , Orgogozo JM , Brun A , Hofman A ((1993) ) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: , 250–260. |
[13] | Boeve BF , Lang AE , Litvan I ((2003) ) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54: (Suppl 5), S15–19. |
[14] | Litvan I , Agid Y , Calne D , Campbell G , Dubois B , Duvoisin RC , Goetz CG , Golbe LI , Grafman J , Growdon JH , Hallett M , Jankovic J , Quinn NP , Tolosa E , Zee DS ((1996) ) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP International Workshop. Neurology 47: , 1–9. |
[15] | Zerr I , Kallenberg K , Summers DM , Romero C , Taratuto A , Heinemann U , Breithaupt M , Varges D , Meissner B , Ladogana A , Schuur M , Haik S , Collins SJ , Jansen GH , Stokin GB , Pimentel J , Hewer E , Collie D , Smith P , Roberts H , Brandel JP , Van Duijn C , Pocchiari M , Begue C , Cras P , Will RG , Sanchez-Juan P ((2009) ) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132: , 2659–2668. |
[16] | Hyman BT , Phelps CH , Beach TG , Bigio EH , Cairns NJ , Carrillo MC , Dickson DW , Duyckaerts C , Frosch MP , Masliah E , Mirra SS , Nelson PT , Schneider JA , Thal DR , Thies B , Trojanowski JQ , Vinters HV , Montine TJ ((2012) ) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8: , 1–13. |
[17] | Formaglio M , Costes N , Seguin J , Tholance Y , Le Bars D , Roullet-Solignac I , Mercier B , Krolak-Salmon P , Vighetto A ((2011) ) In vivo demonstration of amyloid burden in posterior cortical atrophy: A case series with PET and CSF findings. J Neurol 258: , 1841–1851. |
[18] | Sjögren M , Vanderstichele H , Agren H , Zachrisson O , Edsbagge M , Wikkelsø C , Skoog I , Wallin A , Wahlund LO , Marcusson J , Nägga K , Andreasen N , Davidsson P , Vanmechelen E , Blennow K ((2001) ) Tau and Abeta42 in cerebrospinal fluid from healthy adults 21-93 years of age: Establishment of reference values. Clin Chem 47: , 1776–1781. |
[19] | Zetterberg H , Wahlund L-O , Blennow K ((2003) ) Cerebrospinal fluid markers for prediction of Alzheimer’s disease. Neurosci Lett 352: , 67–69. |
[20] | Hansson O , Zetterberg H , Buchhave P , Londos E , Blennow K , Minthon L ((2006) ) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol 5: , 228–234. |
[21] | Buchhave P , Minthon L , Zetterberg H , Wallin AK , Blennow K , Hansson O ((2012) ) Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 69: , 98. |
[22] | Seguin J , Formaglio M , Perret-Liaudet A , Quadrio I , Tholance Y , Rouaud O , Thomas-Anterion C , Croisile B , Mollion H , Moreaud O , Salzmann M , Dorey A , Bataillard M , Coste M-H , Vighetto A , Krolak-Salmon P ((2011) ) CSF biomarkers in posterior cortical atrophy. Neurology 76: , 1782–1788. |
[23] | IBM Corp. Released (2016) IBM SPSS Statistics for Windows, Version 24.0. 2016. |
[24] | Robin X , Turck N , Hainard A , Tiberti N , Lisacek F , Sanchez JC , Müller M ((2011) ) pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: , 77. |
[25] | Llorens F , Schmitz M , Knipper T , Schmidt C , Lange P , Fischer A , Hermann P , Zerr I ((2017) ) Cerebrospinal fluid biomarkers of Alzheimer’s disease show different but partially overlapping profile compared to vascular dementia. Front Aging Neurosci 9: , 289. |
[26] | Shaw LM , Vanderstichele H , Knapik-Czajka M , Clark CM , Aisen PS , Petersen RC , Blennow K , Soares H , Simon A , Lewczuk P , Dean R , Siemers E , Potter W , Lee VM-Y , Trojanowski JQ ((2009) ) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65: , 403–413. |
[27] | Engelborghs S , De Vreese K , Van de Casteele T , Vanderstichele H , Van Everbroeck B , Cras P , Martin J-J , Vanmechelen E , De Deyn PP ((2008) ) Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging 29: , 1143–1159. |
[28] | Ewers M , Mattsson N , Minthon L , Molinuevo JL , Antonell A , Popp J , Jessen F , Herukka S-K , Soininen H , Maetzler W , Leyhe T , Bürger K , Taniguchi M , Urakami K , Lista S , Dubois B , Blennow K , Hampel H ((2015) ) CSF biomarkers for the differential diagnosis of Alzheimer’s disease: A large-scale international multicenter study. Alzheimers Dement 11: , 1306–1315. |
[29] | Shaw LM , Vanderstichele H , Knapik-Czajka M , Clark CM , Aisen PS , Petersen RC , Blennow K , Soares H , Simon A , Lewczuk P , Dean R , Siemers E , Potter W , Lee VM-Y , Trojanowski JQ , Alzheimer’s Disease Neuroimaging Initiative ((2009) ) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65: , 403–413. |
[30] | Struyfs H , Molinuevo JL , Martin J-J , De Deyn PP , Engelborghs S ((2014) ) Validation of the AD-CSF-Index in autopsy-confirmed Alzheimer’s disease patients and healthy controls. J Alzheimers Dis 41: , 903–909. |
[31] | Johansson P , Mattsson N , Hansson O , Wallin A , Johansson J-O , Andreasson U , Zetterberg H , Blennow K , Svensson J ((2011) ) Cerebrospinal fluid biomarkers for Alzheimer’s disease: Diagnostic performance in a homogeneous mono-center population. J Alzheimers Dis 24: , 537–546. |
[32] | Molinuevo JL , Blennow K , Dubois B , Engelborghs S , Lewczuk P , Perret-Liaudet A , Teunissen CE , Parnetti L ((2014) ) The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 10: , 808–817. |
[33] | Neuropathology Group. Medical Research Council Cognitive Function and Aging Study ((2001) ) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 357: , 169–175. |
[34] | Bigio EH , Mishra M , Hatanpaa KJ , White CL , Johnson N , Rademaker A , Weitner BB , Deng H-X , Dubner SD , Weintraub S , Mesulam M , Mesulam M ((2010) ) TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol 120: , 43–54. |
[35] | Schneider JA , Arvanitakis Z , Bang W , Bennett DA ((2007) ) Mixed brain pathologies account for most dementia cases in community- dwelling older persons. Neurology 69: , 2197–2204. |
[36] | Visser PJ , Verhey F , Knol DL , Scheltens P , Wahlund L-O , Freund-Levi Y , Tsolaki M , Minthon L , Wallin ÅK , Hampel H , Bürger K , Pirttila T , Soininen H , Rikkert MO , Verbeek MM , Spiru L , Blennow K ((2009) ) Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol 8: , 619–627. |
[37] | Blennow K , Zetterberg H ((2013) ) The application of cerebrospinal fluid biomarkers in early diagnosis of Alzheimer disease. Med Clin North Am 97: , 369–376. |
[38] | Brunnström H , Rawshani N , Zetterberg H , Blennow K , Minthon L , Passant U , Englund E ((2010) ) Cerebrospinal fluid biomarker results in relation to neuropathological dementia diagnoses. Alzheimers Dement 6: , 104–109. |
[39] | Oi S ((2010) ) Hydrocephalus research update–controversies in definition and classification of hydrocephalus. Neurol Med Chir (Tokyo) 50: , 859–869. |
[40] | Koopman K , Le Bastard N , Martin JJ , Nagels G , De Deyn PP , Engelborghs S ((2009) ) Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau181P. Neurochem Int 55: , 214–218. |



