APOEɛ4 Gene Dose and Sex Effects on Alzheimer’s Disease MRI Biomarkers in Older Adults with Mild Cognitive Impairment
Abstract
Background:
APOE ɛ4 and sex have been linked to increased risk for conversion to Alzheimer’s disease (AD). However, the relationship between APOE ɛ4 gene dose, sex, and AD biomarkers remains understudied.
Objective:
To investigate the effect of APOE ɛ4 dose on AD biomarkers in a sample of older adults with mild cognitive impairment (MCI), and to examine whether APOE ɛ4 dose modifies AD risk differently in MCI women and men.
Methods:
We examined cross-sectional AD biomarkers for participants with MCI (n = 930, 55–96 years old) from three large aging cohorts. Region of interest MRI volumes, global cognition, and episodic memory were analyzed by number of APOE ɛ4 alleles and stratified by sex.
Results:
Across all participants, number of APOE ɛ4 alleles was associated with smaller hippocampal and amygdala volumes and poorer cognition. When stratified by sex, women showed an APOE ɛ4 dose effect for bilateral hippocampal and left amygdala volumes and cognition. In contrast, men showed an APOE ɛ4 dose effect for hippocampal volumes with a trend in amygdala, but cognition did not differ between men with 1 and 2 APOE ɛ4 alleles. Women with 2 APOE ɛ4 alleles had poorer memory between 65–69 and poorer global cognition between 70–74 compared to men with 2 APOE ɛ4 alleles.
Conclusion:
APOE ɛ4 confers a dose effect on AD biomarkers in patients with MCI, and the number of APOE ɛ4 alleles has a greater detrimental impact in women than men, which may be specific to a critical time window.
INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia. Currently, there are no effective treatments for AD, creating a critical public health concern [1]. Because it is likely that effective therapies will require early intervention, understanding the role of AD risk factors, such as apolipoprotein E ɛ4 (APOE ɛ4) and sex, is critical for identifying individuals who are at greatest risk [2].
The greatest known genetic risk factor for late-onset sporadic AD is the APOE ɛ4 allele [3]. APOE ɛ4 is more prevalent in people with AD compared to cognitively normal individuals [4, 5], and extensive research has demonstrated that relative to APOE ɛ3, APOE ɛ4 significantly increases the risk of developing mild cognitive impairment (MCI) [6] and AD [7, 8]. One APOE ɛ4 allele increases risk by 2- to 3-fold, and 2 alleles increase risk by 10-fold [5, 9]. While a number of studies have shown that AD risk increases as the number of APOE ɛ4 alleles increases [5, 10], few have examined the effect of APOE ɛ4 dose on specific biomarkers for AD. Evidence for an APOE ɛ4 dose effect on cognitive and MRI biomarkers is needed.
Female sex is another major risk factor for AD [11]. Studies that have examined the interaction between APOE ɛ4 and sex suggest that women may be more adversely affected. Indeed, 1 APOE ɛ4 allele has been shown to increase lifetime AD risk in women, whereas 2 APOE ɛ4 alleles increased lifetime risk in men [5]. Similar APOE ɛ4 by sex effects have been observed for hippocampal volumes [12]. A recent meta-analysis of approximately 58,000 subjects reported a critical time window starting at 65 years old in which women with APOE ɛ4 are at greater risk of conversion than men with APOE ɛ4 [13]. Another recent paper examined the longitudinal differences between men and women and the effect of APOE ɛ4 status across the AD spectrum on hippocampal volume, cognition, and association with cerebrospinal fluid (CSF) biomarkers of tau and amyloid [14]. The findings from this study suggest pronounced sex differences exist within the MCI stage, motivating additional explicit examination of this phase of disease progression.
In the present study, we examined the effects of APOE ɛ4, sex, and their interaction on MRI brain volumes and cognition in a sample of older adults with MCI [15] via within-sex and between-sex analyses.
METHODS
Study design
De-identified and coded data from 930 participants with MCI between 55–96 years old were acquired with permission from three aging cohorts: The Alzheimer’s Disease Neuroimaging Initiative (ADNI), the National Alzheimer’s Coordinating Center (NACC), and the Australian Imaging, Biomarker & Lifestyle Study (AIBL). This study was approved by our local institutional review board as non-human subjects research. Subjects with a baseline diagnosis of MCI with a probable etiology of AD were included. APOE ɛ4 carriers who may have conferred protection from AD [16] were excluded from the analysis to include only those who were APOE ɛ3/ɛ3, APOE ɛ3/ɛ4, and APOE ɛ4/ɛ4. Participants with no available diagnosis, genetic information for APOE, imaging and cognitive data, less than 6 years of education, or possible co-enrollment across cohorts were excluded. Variables were harmonized across datasets. For example, education was binned into 4 groups to enable comparison with the AIBL dataset: 6 to 8 years, 9 to 12 years, 13 to 15 years, and 15 + years.
Datasets
Data from the ADNI (n = 723), NACC (n = 121), and AIBL (n = 86) cohorts were aggregated to create the final dataset for analysis (n = 930). ADNI was launched in 2003 as a public-private partnership with the primary goal of testing whether serial collection of imaging, biomarker, clinical, and neuropsychological data can be combined to measure the progression of MCI and early AD [17]. AIBL is an ADNI collaborative study established in the Australian cities Melbourne and Perth in 2006 in order to assemble a cohort of individuals who could be assessed at regular intervals for AD [18]. NACC was established by the National Institute on Aging in 1999 to support collaborative research in AD from participants at 34 past and present NIH-funded Alzheimer’s Disease Centers (ADCs) [19].
All three cohorts collect imaging, genetics, cognitive, and biological biomarker data, and evaluate enrolled participants approximately every 12–18 months with a comprehensive cognitive battery and clinical assessment. Diagnostic classification for study participants in the ADNI and AIBL cohorts are determined by a clinician or physician, and diagnoses are monitored by a clinical review committee to ensure uniform application of the diagnostic criteria across sites. NACC aggregates data from multiple ADCs, and diagnoses are made either by a physician or by a consensus committee, according to each ADC’s protocol. A detailed description of how MCI is determined for each cohort is included in the Supplemental Materials. ADNI, AIBL, and NACC data collected between August 2005 and October 2014 were included in this study.
Brain imaging and quality control
Baseline T1-weighted MPRAGE MRI images from both 1.5T and 3T scanners were downloaded with authorization from ADNI, AIBL, and NACC databases and processed using FreeSurfer software version 5.3.0 (http://surfer.nmr.mgh.harvard.edu, Boston, MA) [20]. Hippocampal volumes were selected a priori as the region of interest based on its relevance to cognition and AD. Exploratory MRI analyses included total gray matter brain volume and five additional brain regions in medial and lateral temporal lobes that have shown sex and APOE differences [51] that are affected in early AD [21–23]. All hippocampal output volumes were visually inspected and scored by two experienced raters using ITK-SNAP software version 3.4.0 (http://www.itksnap.org) for accuracy [24]. Segmentations failed if a substantial segmentation error was identified, defined as an unambiguous mislabeling of a substantial portion of the total volume. 131 (14.1%) subjects were excluded from the analysis for poor segmentation accuracy. Subjects who failed quality control or had more than 12 months between cognitive and MRI acquisition were excluded from hippocampal analyses but included in the cognition analyses.
Cognitive tests
Baseline scores from the Mini-Mental State Examination (MMSE) [25, 26] and Immediate Recall and Delayed Recall from the Wechsler Memory Scale - Revised (WMS-R) [27] Logical Memory tests were examined as measures of global cognition and episodic memory, respectively. These were the common cognitive tests available across the cohorts.
APOE ɛ4 gene dose
APOE ɛ4 dose was defined as the number of APOE ɛ4 alleles (0, 1, or 2) carried by a participant. An APOE ɛ4 dose effect was defined as a significant difference that corresponded with the number of APOE ɛ4 alleles, in which the effect was significant between 0 versus 1 allele, and 1 versus 2 alleles. By this definition, each APOE ɛ4 allele had a significant, measurable effect on the biomarker and thus may have increased AD risk.
Statistical analysis
Analysis of covariance (ANCOVA) was used to assess the effect of number of APOE ɛ4 alleles on MRI brain volumes and cognition using SPSS version 25. Main effects of APOE ɛ4 dose and sex in the entire sample was assessed, along with main effects of APOE ɛ4 dose when stratified by sex and 5-year age bins. Cohort, baseline age, and education were included as covariates. Total intracranial volume and scanner field strength were also included as covariates when analyzing brain volumes. Post-hoc pairwise comparisons were used to examine group differences based on the number of APOE ɛ4 alleles. Because of our a priori hypothesis that APOE ɛ4 effects may differ by sex, brain volumes, and cognition were examined separately for women and men. Post-hoc pairwise comparisons of brain volumes and cognition by APOE group were tested via within-sex and between-sex analyses. Additionally, APOE ɛ4 effects across the aging spectrum were examined at each 5-year period, separately for women and men, and predicted values for cognition were derived from models with factors for age, APOE ɛ4, cohort, and education. Sex and APOE ɛ4 dose-dependent effects are expected to be subtle, especially given the low prevalence of ɛ4/ɛ4 carriers, therefore results were not corrected for multiple comparisons.
RESULTS
Participant demographics
Demographic data for the study population by APOE ɛ4 genotype are summarized in Table 1. Participants were predominately white (84%). The ratio of women:men did not differ significantly by APOE ɛ4 group (p = 0.75). Average years of education did not differ by APOE ɛ4 group (p = 0.79); however, the average years of education was significantly higher for men than for women (p = 0.001). Age differed significantly by APOE ɛ4 genotype (p < 0.001). Participants with 1 APOE ɛ4 allele were younger than those with 0 APOE ɛ4 alleles (p = 0.026) and those with 2 APOE ɛ4 alleles were younger than those with 1 APOE ɛ4 allele (p = 0.001). Participant demographics are also shown separated by cohort in Supplementary Table 1.
Table 1
Sample Characteristics, Hippocampal Volume and Cognitive Measures stratified by Sex
| Women | Men | |||||
| ɛ3/ɛ3 n = 189 | ɛ3/ɛ4 n = 153 | ɛ4/ɛ4 n = 48 | ɛ3/ɛ3 n = 250 | ɛ3/ɛ4 n = 226 | ɛ4/ɛ4 n = 64 | |
| Age, range | 56–96 | 55–92 | 57–83 | 55–90 | 56–89 | 55–87 |
| Age, mean | 74.2 | 71.4 | 69.2 | 74.6 | 74.7 | 71.5 |
| Education, group * | 4.3 (0.9) | 4.1 (1.0) | 4.3 (0.9) | 4.4 (0.9) | 4.5 (0.8) | 4.5 (0.9) |
| Brain Structure, mm3 | ||||||
| Left Hippocampal Volume | 3238 (626) | 3151 (616) | 3018 (489) | 3411 (615) | 3275 (598) | 3172 (569) |
| Right Hippocampal Volume | 3371 (630) | 3254 (640) | 3117 (477) | 3548 (647) | 3403 (643) | 3373 (570) |
| Cognition | ||||||
| CDR Global | 0.49 (0.1) | 0.49 (0.1) | 0.5 (0) | 0.49 (0.09) | 0.5 (0.06) | 0.5 (0.09) |
| MMSE | 27.7 (2) | 27.2 (2.1) | 26.7 (2.2) | 27.5 (2) | 27.1 (2.1) | 27.05 (2.1) |
| WMS-R Immediate Recall | 8.8 (3.5) | 7.6 (3.4) | 6.9 (3.2) | 8.5 (3.5) | 7.8 (3.7) | 7.8 (3.2) |
| WMS-R Delayed Recall | 6 (3.4) | 4.4 (3.4) | 3.3 (3.3) | 5.9 (3.5) | 5.1 (3.6) | 5 (3.4) |
Data are presented as mean (standard deviation) unless otherwise specified. CDR Global, Global Clinical Dementia Rating Score; MMSE, Mini-Mental State Examination; WMS, Wechsler Memory Scale - Revised; ɛ3/ɛ3, two APOE ɛ3 alleles; ɛ3/ɛ4, one APOE ɛ4 allele; ɛ4/ɛ4, two APOE ɛ4 alleles. *Education groups are 1 = 7-8, 2 = 9–12, 3 = 13–15, 4 = 15+.
Biomarkers across all participants
Hippocampus
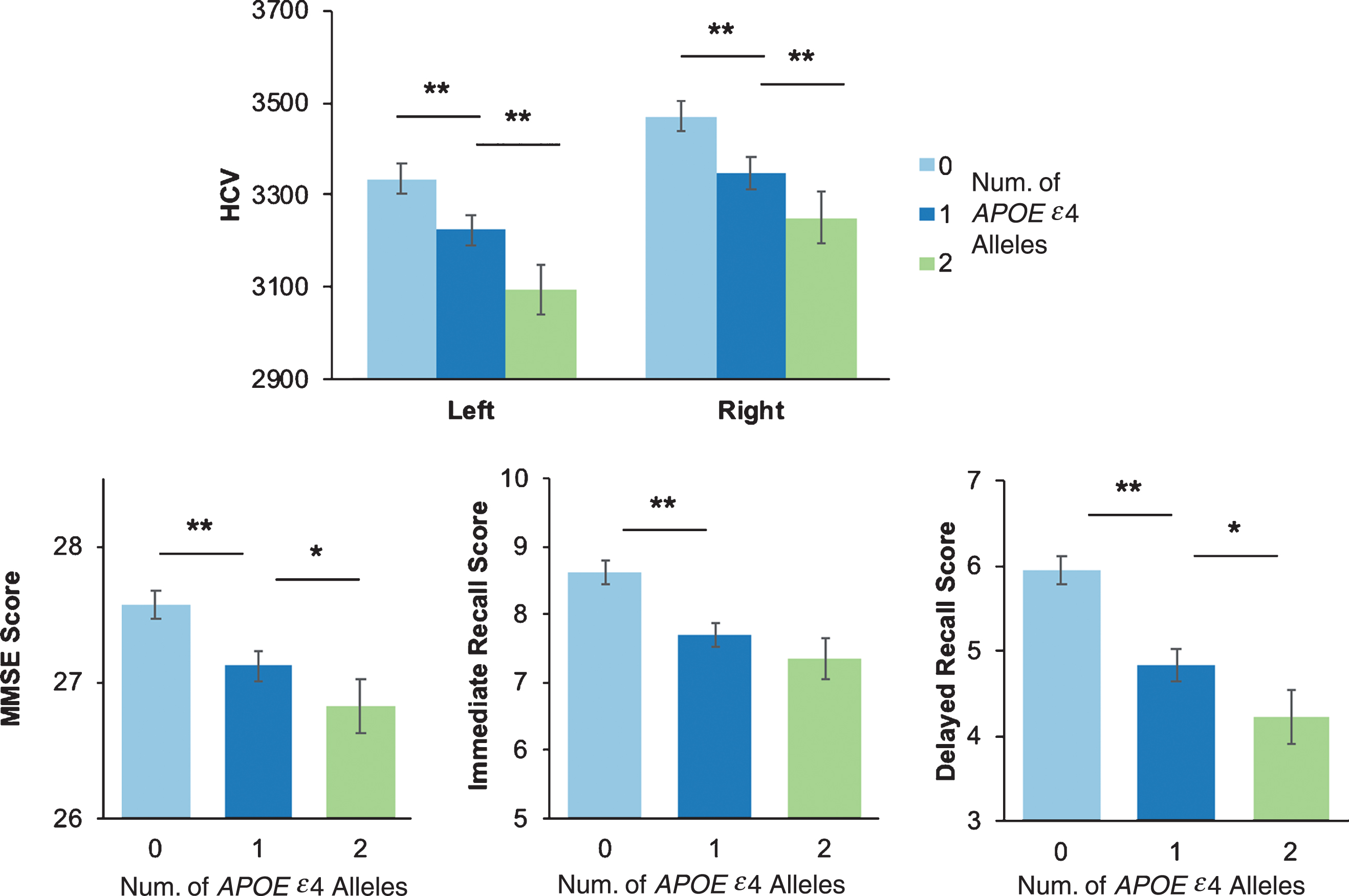
There was a main effect of APOE ɛ4 dose for left and right hippocampal volumes, our a priori ROI (left & right, p’s < 0.001). Post-hoc tests showed smaller left and right hippocampal volume with each APOE ɛ4 allele in a dose-dependent manner (0 > 1 > 2, all ps < 0.001) (Fig. 1).
Fig.1
Hippocampal volume and cognitive performance by number APOE ɛ4 alleles. A) Hippocampal volume (HCV) and cognitive performance on the Mini-Mental State Examination (MMSE) (B), Wechsler Memory Scale- Revised Immediate Recall (C) and Delayed Recall (D) stratified by number of APOE ɛ4 alleles. *p < 0.05; **p < 0.001.
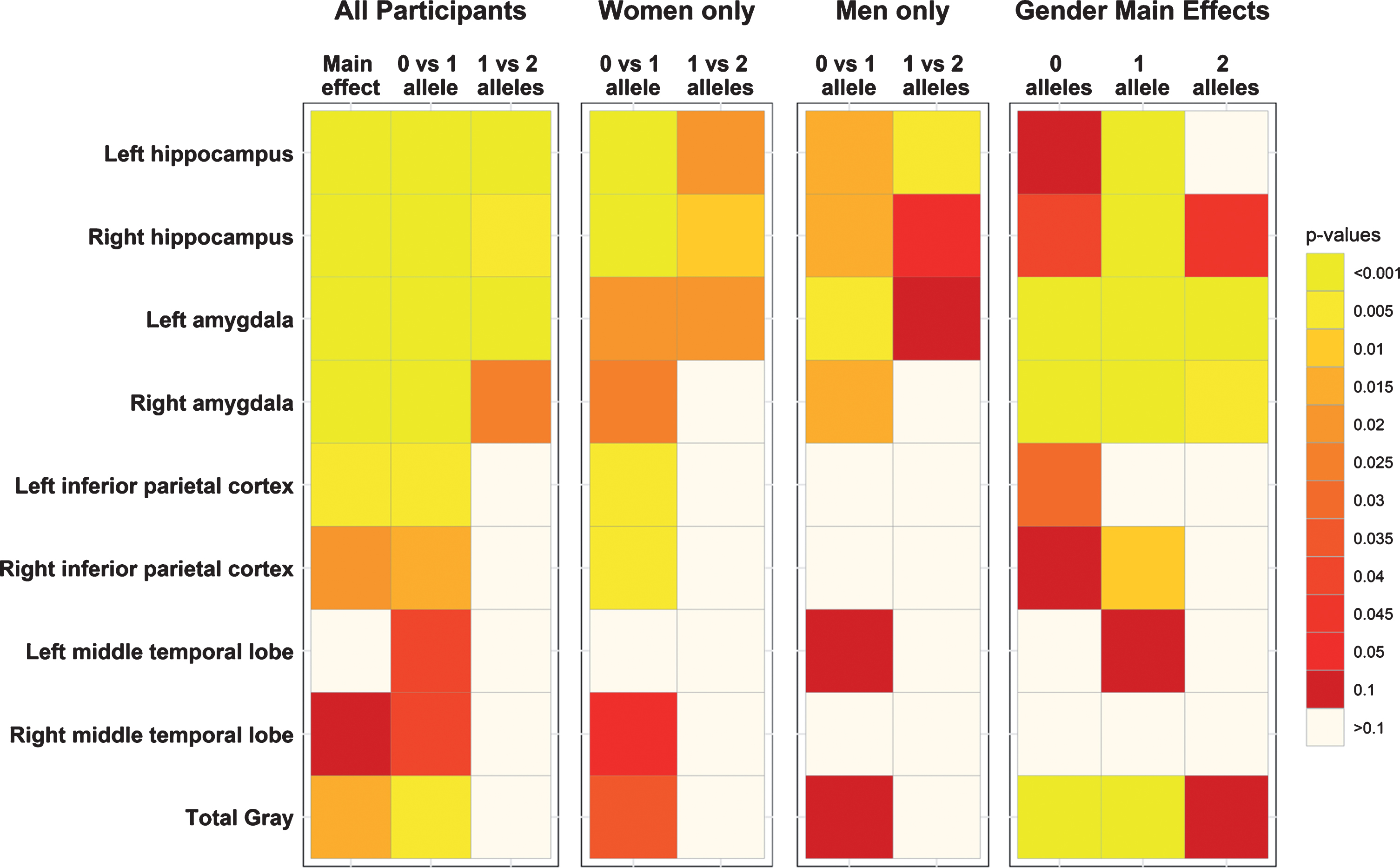
Among the exploratory MRI volumes, a main effect of APOE ɛ4 was also seen bilaterally in the amygdala (p < 0.0001) (Fig. 2, Supplementary Table 2). Post-hoc tests showed a dose-dependent response in the left (0 > 1 > 2, p≤0.001) and right (0 > 1 > 2, p < 0.05) hemispheres. No dose dependent differences were seen in the other brain regions (Fig. 2). Effect size, standard error (SE), and significance are reported in Supplementary Table 2 for all ROIs.
Fig.2
Significance heatmaps for brain regions of interest. Region of interest p-value heatmaps showing significant APOE ɛ4 effects for all participants, sex-stratified effects, and the main effects of sex by APOE ɛ4 allele.
Cognition
For cognition, there was a significant main effect of APOE ɛ4 dose on MMSE, Immediate Recall, and Delayed Recall across all participants (all ps < 0.001) (Fig. 1). Post-hoc comparisons showed that worse performance on MMSE and Delayed Recall was associated with each APOE ɛ4 allele in a dose-dependent manner (0 > 1 > 2). Participants with 1 APOE ɛ4 allele had significantly worse cognitive performance on all tests than non-carriers (all ps < 0.001), and participants with 2 APOE ɛ4 alleles had significantly worse performance than those with one allele on MMSE and Delayed Recall (all ps < 0.05).
APOE ɛ4 dose effects
Between-sex analyses
For age, between-sex analyses showed there was no difference between women and men with no APOE ɛ4 alleles (p = 0.57). However, women with 1 APOE ɛ4 allele were younger than men with 1 APOE ɛ4 allele (p < 0.001). Similarly, women with 2 APOE ɛ4 alleles were younger than men with 2 APOE ɛ4 alleles (p = 0.03).
For between-sex analyses of hippocampal volume, left hippocampal volumes were significantly smaller for women than men with 1 APOE ɛ4 allele, while right hippocampal volume was significantly smaller for women than men with 0, 1, or 2 APOE ɛ4 alleles (all ps < 0.05). Sex-differences were also observed in the left and right amygdala for women with 0, 1, and 2 APOE ɛ4 alleles relative to men (all ps < 0.002). No dose dependent effects (0 versus 1 versus 2) were observed in other ROIs (Fig. 2, Supplementary Table 2).
For cognition, delayed recall differed by sex, such that women with 2 APOE ɛ4 alleles had lower delayed recall scores than men with 2 APOE ɛ4 alleles (p = 0.005). A trend for the same pattern was observed for men and women with 1 APOE ɛ4 allele (p = 0.078) There were no sex-specific differences on MMSE or immediate recall.
Sex-stratified analyses
Sex-stratified analyses showed an APOE ɛ4 dose effect of age for women with 1 or 2 APOE ɛ4 alleles compared to women with 0 APOE ɛ4 alleles (all ps < 0.0001), but no age difference between women with 1 and 2 APOE ɛ4 alleles (p = 0.19). Men were only of a younger age if they had 2 alleles when compared to men with 1 APOE ɛ4 allele (p < 0.005).
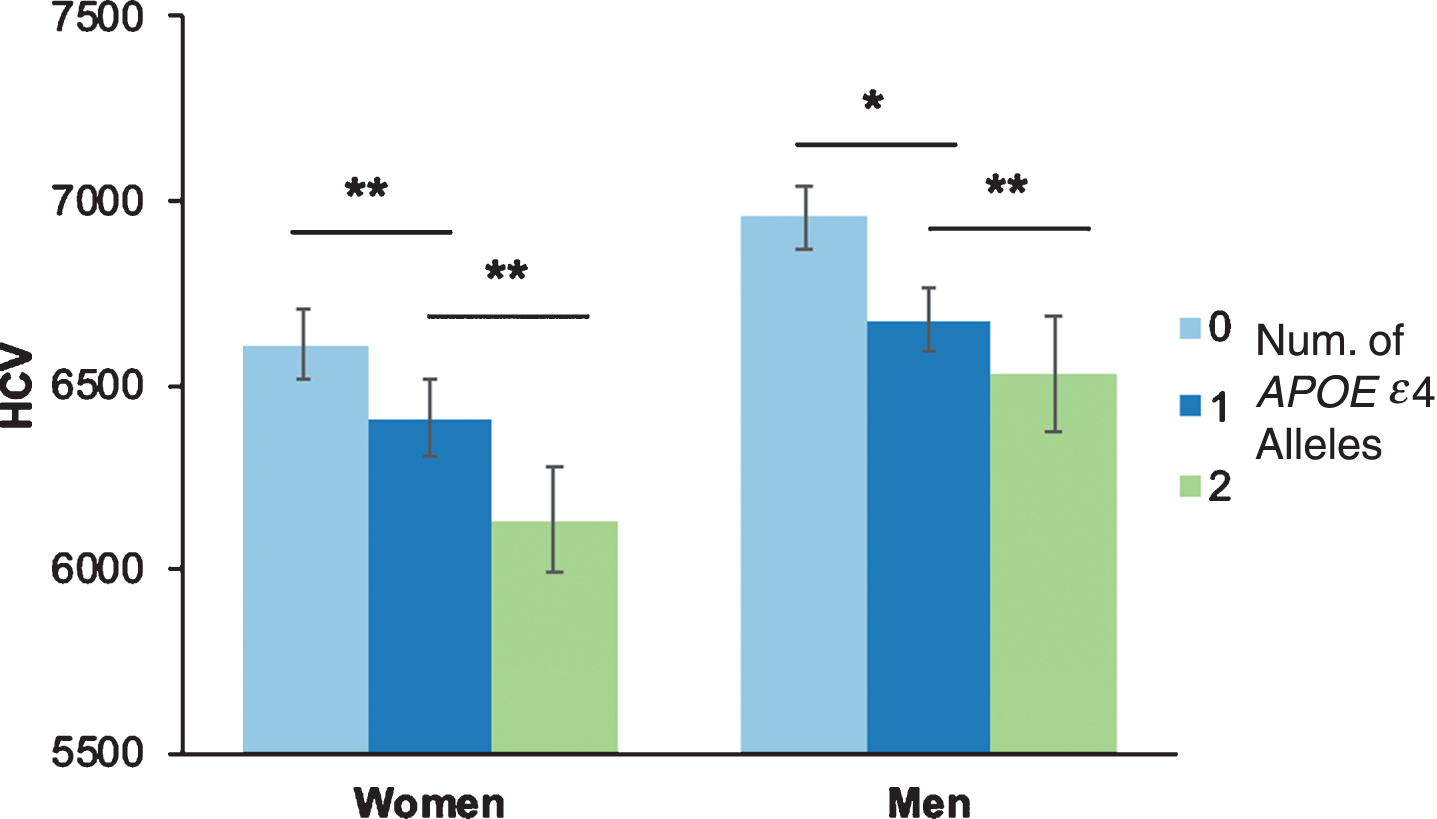
When stratified by sex, the APOE ɛ4 dose effect on hippocampal volumes remained significant for both women and men, separately (all ps≤0.05). No differences were observed between left and right hippocampal volumes, so analyses combined left and right hippocampi. In women, post-hoc analyses showed that those with 1 APOE ɛ4 allele had smaller hippocampal volumes than those with 0 APOE ɛ4 alleles, and those with 2 APOE ɛ4 alleles had smaller hippocampal volumes than those with 1 APOE ɛ4 allele (all ps < 0.05). Men showed a similar pattern, such that those with 1 APOE ɛ4 allele had smaller hippocampal volumes than those with 0 APOE ɛ4 alleles, and those with 2 APOE ɛ4 alleles had smaller hippocampal volumes than those with 1 APOE ɛ4 allele (all ps < 0.05) (Fig. 3). The above analyses were repeated using a residual normalization method for the left and right hippocampi against intracranial volume, and the significance of the results remained unchanged.
Fig.3
Hippocampal volumes by number of APOE ɛ4 alleles and sex. Hippocampal volume (HCV) stratified by number of APOE ɛ4 alleles and sex. Because there was no difference between left and right HCV, the figure depicts total hippocampal volume (combined left and right). *p < 0.05; **p < 0.001.
In women, an APOE ɛ4 dose dependent volumetric difference was observed in the left amygdala, such that women with 1 APOE ɛ4 allele had smaller volumes than women with 0 APOE ɛ4 alleles (p = 0.016) and women with 2 APOE ɛ4 alleles smaller than women with only 1 APOE ɛ4 allele (p = 0.019). For women with 1 APOE ɛ4 allele compared to women with 0 APOE ɛ4 alleles, smaller volumes were observed in the right amygdala, left and right inferior parietal cortex, the right middle temporal lobe, and in total brain volume (all ps≤0.05; Fig. 2; Supplementary Table 2). In men, the left and right amygdala were smaller for men with 1 APOE ɛ4 allele than men with 0 APOE ɛ4 alleles (all ps < 0.05; Fig. 2; Supplementary Table 2). No other APOE ɛ4 dose differences in the other brain regions were observed for men.
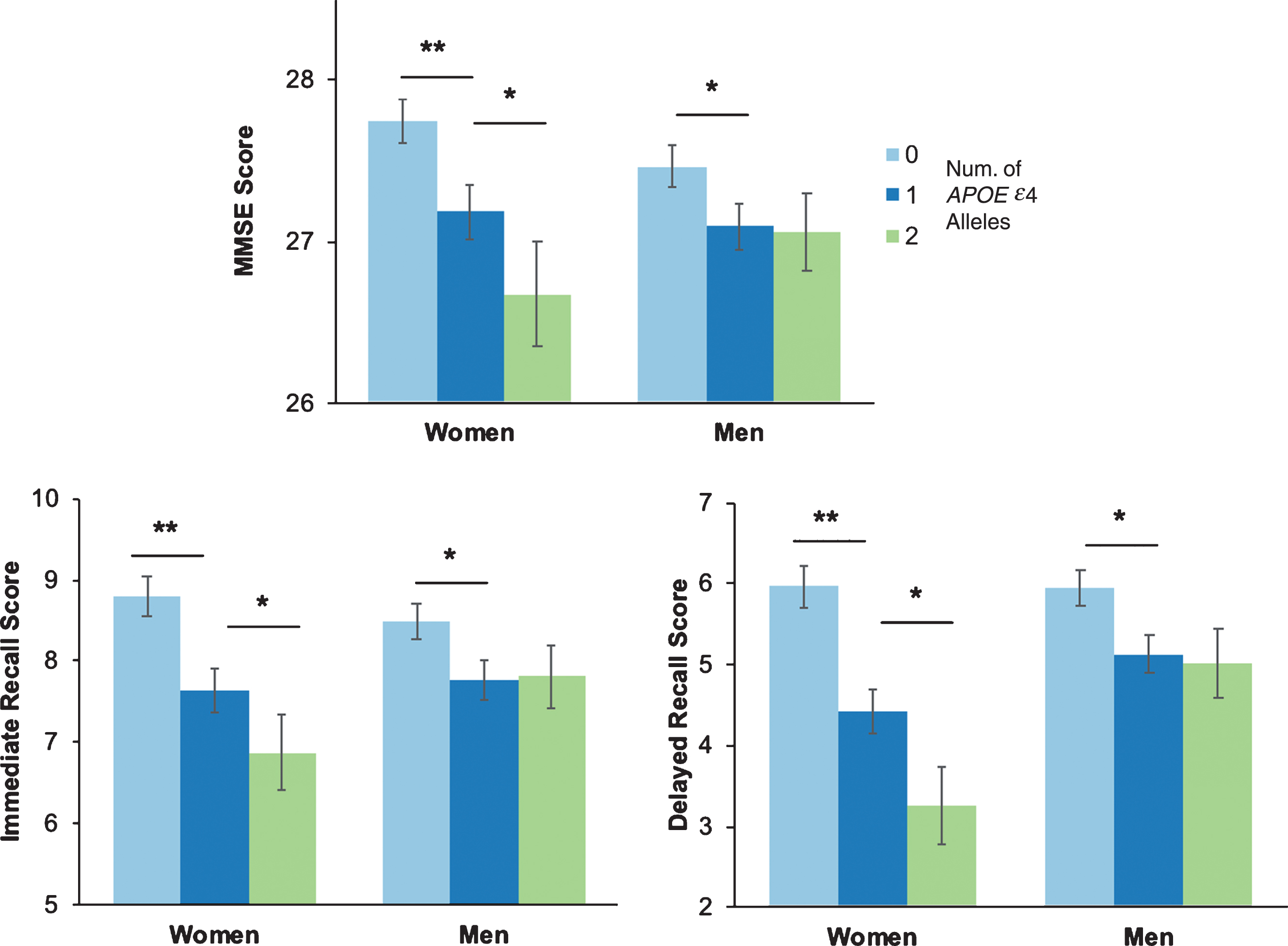
Sex-stratified analyses showed that women had a significant APOE ɛ4 dose effect, with lower scores with each APOE ɛ4 allele for all cognitive tests (all ps, p < 0.05). In contrast, men showed lower scores for those with 1 APOE ɛ4 allele compared to those with 0 APOE ɛ4 alleles for all cognitive tests (MMSE: p = 0.011, Immediate Recall: p = 0.015, Delayed Recall: p = 0.004); however, there was no difference in cognition between men with 1 or 2 APOE ɛ4 alleles (Fig. 4).
Fig.4
Cognitive performance by number of APOE ɛ4 alleles and sex. Cognitive performance on the Mini-Mental State Examination (MMSE) (A), Wechsler Memory Scale- Revised Immediate Recall (B) and Delayed Recall (C), stratified by APOE ɛ4 alleles and sex. *p < 0.05; **p < 0.001.
Age-specific effects
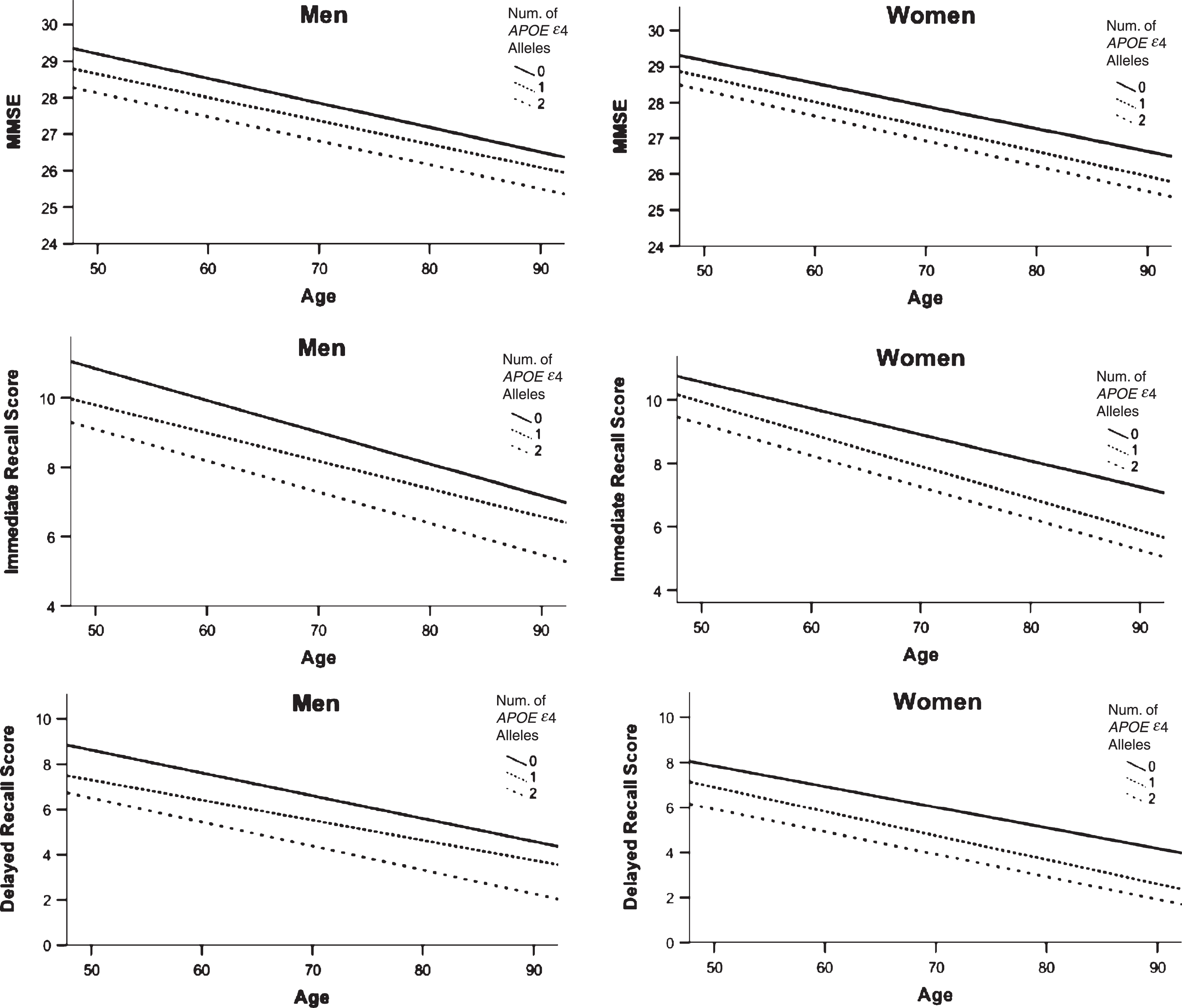
Based on our recent work demonstrating a relationship between APOE ɛ4 status, sex, and age [13], we examined the effect of age and APOE ɛ4 on our biomarkers. There was no significant age by APOE ɛ4 interaction on the AD biomarkers (all ps > 0.14). In age-stratified analyses of 5-year bins, there was a trend for an APOE ɛ4 by sex interaction on delayed memory in those 65–69 years of age (p = 0.09). Additionally, there was a trend for an APOE ɛ4 by sex interaction on the MMSE for those 70–74 years of age (p = 0.06). Post-hoc analyses in this age group showed that women with 2 APOE ɛ4 alleles performed worse than men with 2 APOE ɛ4 alleles on delayed memory (65–69 years, p = 0.047) and MMSE (70–74 years, p = 0.004) (Fig. 5). There were no significant differences at the other 5-year age bins.
Fig.5
Predicted values for cross-sectional data of Global Cognition and Episodic Memory by APOE ɛ4 allele and sex. Predicted values generated from cross-sectional data for global cognition (MMSE, A) and episodic memory (Logical Memory Immediate, B and Delayed Recall, C), separately for men and women. Lines represent intercept and slope of predicted values over the lifespan, grouped by number of APOE ɛ4 alleles. Women with 2 APOE ɛ4 alleles performed worse than men with 2 APOE ɛ4 alleles on delayed memory (65–69 years, p = 0.042) and MMSE (70–74 years, p = 0.002) at specific 5-year age bins.
DISCUSSION
Understanding the contribution of number of APOE ɛ4 alleles and how these effects are modified by sex and age is important for determining AD risk [28, 29]. In the present study, we showed an association between number of APOE ɛ4 alleles and the AD biomarkers for hippocampal and amygdala volume and cognition in older adults with MCI, providing insight into those at risk for AD. Importantly, we demonstrated that the effect of APOE ɛ4 dose on cognition differs by sex and preliminary, exploratory analyses suggest that cognitive differences for women compared to men exist between 65–69 for episodic memory and between 70–74 for global cognition. While APOE ɛ4 dose is associated with smaller hippocampal volume in both sexes, 2 APOE ɛ4 alleles may have a greater impact on cognition in women than in men.
Across all participants, those with 2 APOE ɛ4 alleles were significantly younger than those with 1 APOE ɛ4 allele who were younger than those with 0 APOE ɛ4 alleles, suggesting that APOE ɛ4 may shift risk of AD earlier. However, this effect was modified by sex, showing men with 2 APOE ɛ4 alleles were younger than men with 0 or 1 APOE ɛ4 allele, while women with 1 or 2 APOE ɛ4 alleles were significantly younger than women with 0 APOE ɛ4 alleles.
Previous research has demonstrated an APOE ɛ4 dose effect of increased AD risk and undesirable changes to a variety of AD biomarkers in participants with MCI. APOE ɛ4 carriers with MCI are at increased risk of converting to AD [30], have poorer cognitive function [12, 31, 32], smaller hippocampal volumes [12, 31, 33, 34], lower CSF amyloid- β, higher CSF hyperphosphorylated tau [35] and higher total tau [14]. Our study expands this work to examine how APOE ɛ4 dose and sex modify risk based on AD biomarkers.
Our general finding of a significant APOE ɛ4 dose effect on hippocampal and amygdala volumes in this sample of individuals with MCI suggests that each APOE ɛ4 allele has a measurable effect on regional brain structure. While not all studies have found this [36], our result is supported by previous research which found reduced hippocampal volume [33] and cognition [31] with each APOE ɛ4 allele and reduced amygdala volume for APOE ɛ4 carriers versus non-carriers [36, 37] in individuals with MCI. Furthermore, our results show that, while there was a significant APOE ɛ4 dose effect on hippocampal and amygdala volume for both men and women, the same was not true for cognition. In our analysis of cognitive performance, women had worse performance with each APOE ɛ4 allele, while men with 1 and 2 APOE ɛ4 alleles did not differ, suggesting a differential impact of APOE ɛ4 allele between the sexes on cognition. Importantly, at 1 and 2 APOE ɛ4 alleles performance on delayed memory was significantly worse for women than men, even though women were also significantly younger.
To date, the effects of APOE ɛ4 dose on brain structure and cognition, two important AD biomarkers, have not been demonstrated conclusively in women and men. The research predominantly informing current views on the interaction between APOE ɛ4 and sex reports differences in AD risk. These studies reported that APOE ɛ4 confers greater AD risk to women than men. Specifically, it has been reported in a number of studies that one copy of the ɛ4 allele is sufficient to increase AD risk in women, whereas two copies but not one, increase risk in men [5, 7, 9, 38, 39]. A similar finding was demonstrated by Fleisher et al., who reported an APOE ɛ4 by sex interaction in the hippocampal volume of MCI subjects [12]. Recent studies reported that women with higher amyloid burden were at greater risk of cognitive decline than men and that this effect was even stronger in women who were APOE ɛ4 carriers [14, 40]. Additionally, women who were APOE ɛ4 carriers had greater tau burden than men who were APOE ɛ4 carriers [14], especially in those who had significant amyloid burden [41]. New genetic targets associated with amyloid and tau pathology have been implicated in women’s risk for AD, suggesting that the sex differences may extend beyond APOE ɛ4 [42].
While previous research suggests that APOE ɛ4 relates to AD risk differently for women and men, a recent meta-analysis by our group reported that women and men had nearly the same risk of developing MCI or AD between 55–85 years of age [13]. However, a critical period of risk for APOE ɛ4 women to convert to MCI was found between 55–70 years of age whereas APOE ɛ4 women to convert to AD were at elevated risk between 65–75 years of age. This study, which aggregated data for nearly 58,000 participants, suggests that the sex-specific effect of APOE ɛ4 may not differ, but that sex differences are evident at critical time periods. Although preliminary and exploratory in nature, the trends in our current findings support this critical period in women with 2 APOE ɛ4 alleles compared to men with 2 APOE ɛ4 alleles between 65–69 years of age in delayed memory performance. Additionally, this same relationship was identified for global cognition, but for those 5 years older, between 70–74 years of age, positing a temporal relationship of differences in memory preceding differences in global cognition, although this cannot be directly assessed in our cross-sectional analysis.
Potential mechanistic explanations for the consistent sex and APOE ɛ4 differences may be related to hormonal changes experienced by women during menopause, which has cascading effects in the subsequent aging process. Higher levels of estradiol are associated with better cognitive performance [43] and estrogen has been shown to reduce vulnerability to cell death in the hippocampus in the presence of amyloid-β [44], which may be exacerbated in APOE ɛ4 carriers because of higher amyloid-β burden [45, 46].
A primary strength of our study is the direct, intentional analysis of sex differences in AD by utilizing large, retrospective datasets to examine subtle and understudied effects. Obtaining and harmonizing large datasets to increase the sample of participants with 2 APOE ɛ4 alleles was a necessary task to examine APOE ɛ4 dose effects stratified by sex and age, although the study was underpowered for multiple comparison correction likely due to subtle sex and APOE ɛ4 dose effects. However, there are limitations to conducting retrospective analyses. Many aging cohorts have only a limited number of cognitive tests available for analysis. There is some variability in how MCI is defined (see Supplementary Materials). Other risk factors that may differ between women and men, such as smoking, alcohol use, and cardiovascular disease [47], were not available in all the datasets and therefore, not included in our models. Other sex-specific considerations may also be contributing to this, such higher rates of mortality due to cardiovascular disease and stroke in men that might lead to a surviving population of older adult men who are healthier than their female counterparts [48]. Additionally, ascertainment bias poses several problems for population-based studies. For example, our cohort is enriched for APOE ɛ4 carriers beyond what is observed in the general population. Individuals with a family history of AD may be more likely to participate [49], and importantly, these studies may oversample healthier individuals due to the difficulty for sick participants to participate in follow up data collection [50]. Lastly, the combination of 1.5T and 3T MRI scans is less favorable than MRI scans from the same scanner and/or MRI field strength.
Our findings, taken together with previous literature, support the hypothesis that detrimental effects conferred by APOE ɛ4 are dose-dependent and sex-specific in the MCI stage. While the importance of APOE ɛ4 is key to understanding AD risk, sex as a biological factor may additionally modify level of risk at critical time periods.
ACKNOWLEDGMENTS
This project is supported by the National Institutes of Health (R01-AG054617, R01-AG046928, P41-EB015922, U54-EB020406), Big Data Discovery Science (BDDS), and the Global Alzheimer’s Association Interactive Network (GAAIN 14-244631).
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0859r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180859.
REFERENCES
[1] | Abacus ((1992) ) StatView, Abacus Concepts, Inc., Berkeley, CA. |
[2] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[3] | Coon KD , Myers AJ , Craig DW , Webster JA , Pearson JV , Lince DH , Zismann VL , Beach TG , Leung D , Bryden L , Halperin RF , Marlowe L , Kaleem M , Walker DG , Ravid R , Heward CB , Rogers J , Papassotiropoulos A , Reiman EM , Hardy J , Stephan DA ((2007) ) A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 68: , 613–618. |
[4] | Czech C , Forstl H , Hentschel F , Monning U , Besthorn C , Geiger-Kabisch C , Sattel H , Masters C , Beyreuther K ((1994) ) Apolipoprotein E-4 gene dose in clinically diagnosed Alzheimer’s disease: Prevalence, plasma cholesterol levels and cerebrovascular change. Eur Arch Psychiatry Clin Neurosci 243: , 291–292. |
[5] | Farrer LA , Cupples LA , Haines JL , Hyman B , Kukull WA , Mayeux R , Myers RH , Pericak-Vance MA , Risch N , van Duijn CM ((1997) ) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278: , 1349–1356. |
[6] | Boyle PA , Buchman AS , Wilson RS , Kelly JF , Bennett DA ((2010) ) The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology 34: , 43–49. |
[7] | Corder EH , Saunders AM , Strittmatter WJ , Schmechel DE , Gaskell PC , Small GW , Roses AD , Haines JL , Pericak-Vance MA ((1993) ) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261: , 921–923. |
[8] | Henderson AS , Easteal S , Jorm AF , Mackinnon AJ , Korten AE , Christensen H , Croft L , Jacomb PA ((1995) ) Apolipoprotein E allele epsilon 4, dementia, and cognitive decline in a population sample. Lancet 346: , 1387–1390. |
[9] | Payami H , Zareparsi S , Montee KR , Sexton GJ , Kaye JA , Bird TD , Yu CE , Wijsman EM , Heston LL , Litt M , Schellenberg GD ((1996) ) Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: A possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet 58: , 803–811. |
[10] | Payami H , Montee K , Kaye J ((1994) ) Evidence for familial factors that protect against dementia and outweigh the effect of increasing age. Am J Hum Genet 54: , 650–657. |
[11] | Riedel BC , Thompson PM , Brinton RD ((2016) ) Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol 160: , 134–147. |
[12] | Fleisher A , Grundman M , Jack CR Jr. , Petersen RC , Taylor C , Kim HT , Schiller DH , Bagwell V , Sencakova D , Weiner MF , DeCarli C , DeKosky ST , van Dyck CH , Thal LJ , Alzheimer’s Disease Cooperative S ((2005) ) Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol 62: , 953–957. |
[13] | Neu SC , Pa J , Kukull W , Beekly D , Kuzma A , Gangadharan P , Wang LS , Romero K , Arneric SP , Redolfi A , Orlandi D , Frisoni GB , Au R , Devine S , Auerbach S , Espinosa A , Boada M , Ruiz A , Johnson SC , Koscik R , Wang JJ , Hsu WC , Chen YL , Toga AW ((2017) ) Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol 74: , 1178–1189. |
[14] | Koran MEI , Wagener M , Hohman TJ , Alzheimer’s Neuroimaging Initiative ((2017) ) Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav 11: , 205–213. |
[15] | Petersen RC , Smith GE , Waring SC , Ivnik RJ , Tangalos EG , Kokmen E ((1999) ) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: , 303–308. |
[16] | Suri S , Heise V , Trachtenberg AJ , Mackay CE ((2013) ) The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neurosci Biobehav Rev 37: , 2878–2886. |
[17] | Petersen RC , Aisen PS , Beckett LA , Donohue MC , Gamst AC , Harvey DJ , Jack CR Jr , Jagust WJ , Shaw LM , Toga AW , Trojanowski JQ , Weiner MW ((2010) ) Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 74: , 201–209. |
[18] | Ellis KA , Bush AI , Darby D , De Fazio D , Foster J , Hudson P , Lautenschlager NT , Lenzo N , Martins RN , Maruff P , Masters C , Milner A , Pike K , Rowe C , Savage G , Szoeke C , Taddei K , Villemagne V , Woodward M , Ames D , AIBL Research Group ((2009) ) The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr 21: , 672–687. |
[19] | Morris JC , Weintraub S , Chui HC , Cummings J , Decarli C , Ferris S , Foster NL , Galasko D , Graff-Radford N , Peskind ER , Beekly D , Ramos EM , Kukull WA ((2006) ) The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 20: , 210–216. |
[20] | Sanchez-Benavides G , Gomez-Anson B , Sainz A , Vives Y , Delfino M , Pena-Casanova J ((2010) ) Manual validation of FreeSurfer’s automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer disease subjects. Psychiatry Res 181: , 219–225. |
[21] | Arriagada PV , Marzloff K , Hyman BT ((1992) ) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42: , 1681–1688. |
[22] | Arnold SE , Hyman BT , Flory J , Damasio AR , Van Hoesen GW ((1991) ) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients in Alzheimer’s disease. Cereb Cortex 1: , 103–116. |
[23] | Braak H , Braak E ((1997) ) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18: , 351–357. |
[24] | Yushkevich PA , Piven J , Hazlett HC , Smith RG , Ho S , Gee JC , Gerig G ((2006) ) User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31: , 1116–1128. |
[25] | Galasko D , Klauber MR , Hofstetter CR , Salmon DP , Lasker B , Thal LJ ((1990) ) The Mini-Mental State Examination in the early diagnosis of Alzheimer’s disease. Arch Neurol 47: , 49–52. |
[26] | Tombaugh TN , McIntyre NJ ((1992) ) The mini-mental state examination: A comprehensive review. J Am Geriatr Soc 40: , 922–935. |
[27] | Wechsler D , Stone CP ((1987) ) Wechsler memory scale-revised. Psychological Corporation. |
[28] | Nebel RA , Aggarwal NT , Barnes LL , Gallagher A , Goldstein JM , Kantarci K , Mallampalli MP , Mormino EC , Scott L , Yu WH , Maki PM , Mielke MM ((2018) ) Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement 14: , 1171–1183. |
[29] | Mazure CM , Swendsen J ((2016) ) Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol 15: , 451–452. |
[30] | Elias-Sonnenschein LS , Viechtbauer W , Ramakers IH , Verhey FR , Visser PJ ((2011) ) Predictive value of APOE-epsilon4 allele for progression from MCI to AD-type dementia: A meta-analysis. J Neurol Neurosurg Psychiatry 82: , 1149–1156. |
[31] | Farlow MR , He Y , Tekin S , Xu J , Lane R , Charles HC ((2004) ) Impact of APOE in mild cognitive impairment. Neurology 63: , 1898–1901. |
[32] | Ramakers IH , Visser PJ , Aalten P , Bekers O , Sleegers K , van Broeckhoven CL , Jolles J , Verhey FR ((2008) ) The association between APOE genotype and memory dysfunction in subjects with mild cognitive impairment is related to age and Alzheimer pathology. Dement Geriatr Cogn Disord 26: , 101–108. |
[33] | Hostage CA , Roy Choudhury K , Doraiswamy PM , Petrella JR , Alzheimer’s Disease Neuroimaging Initiative ((2013) ) Dissecting the gene dose-effects of the APOE epsilon4 and epsilon2 alleles on hippocampal volumes in aging and Alzheimer’s disease. PLoS One 8: , e54483. |
[34] | Manning EN , Barnes J , Cash DM , Bartlett JW , Leung KK , Ourselin S , Fox NC , Alzheimer’s Disease NeuroImaging Initiative ((2014) ) APOE epsilon4 is associated with disproportionate progressive hippocampal atrophy in AD. PLoS One 9: , e97608. |
[35] | Herukka SK , Helisalmi S , Hallikainen M , Tervo S , Soininen H , Pirttila T ((2007) ) CSF Abeta42, Tau and phosphorylated Tau, APOE epsilon4 allele and MCI type in progressive MCI. Neurobiol Aging 28: , 507–514. |
[36] | Liu Y , Paajanen T , Westman E , Wahlund LO , Simmons A , Tunnard C , Sobow T , Proitsi P , Powell J , Mecocci P , Tsolaki M , Vellas B , Muehlboeck S , Evans A , Spenger C , Lovestone S , Soininen H , AddNeuroMed Consortium ((2010) ) Effect of APOE epsilon4 allele on cortical thicknesses and volumes: The AddNeuroMed study. J Alzheimers Dis 21: , 947–966. |
[37] | Soldan A , Pettigrew C , Lu Y , Wang MC , Selnes O , Albert M , Brown T , Ratnanather JT , Younes L , Miller MI , BIOCARD Research Team ((2015) ) Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Hum Brain Mapp 36: , 2826–2841. |
[38] | Altmann A , Tian L , Henderson VW , Greicius MD , Alzheimer’s Disease Neuroimaging Initiative ((2014) ) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 75: , 563–573. |
[39] | Kim S , Kim MJ , Kim S , Kang HS , Lim SW , Myung W , Lee Y , Hong CH , Choi SH , Na DL , Seo SW , Ku BD , Kim SY , Kim SY , Jeong JH , Park SA , Carroll BJ , Kim DK ((2015) ) Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: A CREDOS study. Compr Psychiatry 62: , 114–122. |
[40] | Buckley RF , Mormino EC , Amariglio RE , Properzi MJ , Rabin JS , Lim YY , Papp KV , Jacobs HIL , Burnham S , Hanseeuw BJ , Dore V , Dobson A , Masters CL , Waller M , Rowe CC , Maruff P , Donohue MC , Rentz DM , Kirn D , Hedden T , Chhatwal J , Schultz AP , Johnson KA , Villemagne VL , Sperling RA ; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging, Biomarker and Lifestyle study of ageing; Harvard Aging Brain Study ((2018) ) Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement 14: , 1193–1203. |
[41] | Hohman TJ , Dumitrescu L , Barnes LL , Thambisetty M , Beecham G , Kunkle B , Gifford KA , Bush WS , Chibnik LB , Mukherjee S , De Jager PL , Kukull W , Crane PK , Resnick SM , Keene CD , Montine TJ , Schellenberg GD , Haines JL , Zetterberg H , Blennow K , Larson EB , Johnson SC , Albert M , Bennett DA , Schneider JA , Jefferson AL , Alzheimer’s Disease Genetics C, the Alzheimer’s Disease Neuroimaging Initiative ((2018) ) Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol 75: , 989–998. |
[42] | Deming Y , Dumitrescu L , Barnes LL , Thambisetty M , Kunkle B , Gifford KA , Bush WS , Chibnik LB , Mukherjee S , De Jager PL , Kukull W , Huentelman M , Crane PK , Resnick SM , Keene CD , Montine TJ , Schellenberg GD , Haines JL , Zetterberg H , Blennow K , Larson EB , Johnson SC , Albert M , Moghekar A , Del Aguila JL , Fernandez MV , Budde J , Hassenstab J , Fagan AM , Riemenschneider M , Petersen RC , Minthon L , Chao MJ , Van Deerlin VM , Lee VM , Shaw LM , Trojanowski JQ , Peskind ER , Li G , Davis LK , Sealock JM , Cox NJ , Alzheimer’s Disease Neuroimaging Initiative (ADNI); Alzheimer Disease Genetics Consortium (ADGC), Goate AM , Bennett DA , Schneider JA , Jefferson AL , Cruchaga C , Hohman TJ ((2018) ) Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta Neuropathol 136: , 857–872. |
[43] | Luine VN ((2014) ) Estradiol and cognitive function: Past, present and future. Horm Behav 66: , 602–618. |
[44] | Nilsen J , Chen S , Irwin RW , Iwamoto S , Brinton RD ((2006) ) Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci 7: , 74. |
[45] | Stephen TL , Cacciottolo M , Balu D , Morgan TE , LaDu MJ , Finch CE , Pike CJ ((2019) ) APOE genotype and sex affect microglial interactions with plaques in Alzheimer’s disease mice. Acta Neuropathol Commun 7: , 82. |
[46] | Lim YY , Mormino EC , Alzheimer’s Disease Neuroimaging Initiative ((2017) ) APOE genotype and early beta-amyloid accumulation in older adults without dementia. Neurology 89: , 1028–1034. |
[47] | Kivipelto M , Helkala EL , Laakso MP , Hanninen T , Hallikainen M , Alhainen K , Soininen H , Tuomilehto J , Nissinen A ((2001) ) Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ 322: , 1447–1451. |
[48] | Appelros P , Stegmayr B , Terent A ((2009) ) Sex differences in stroke epidemiology: A systematic review. Stroke 40: , 1082–1090. |
[49] | Hebert LE , Scherr PA , McCann JJ , Beckett LA , Evans DA ((2001) ) Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol 153: , 132–136. |
[50] | Lautenschlager NT , Cupples LA , Rao VS , Auerbach SA , Becker R , Burke J , Chui H , Duara R , Foley EJ , Glatt SL , Green RC , Jones R , Karlinsky H , Kukull WA , Kurz A , Larson EB , Martelli K , Sadovnick AD , Volicer L , Waring SC , Growdon JH , Farrer LA ((1996) ) Risk of dementia among relatives of Alzheimer’s disease patients in the MIRAGE study: What is in store for the oldest old? Neurology 46: , 641–650. |
[51] | Holland D , Desikan RS , Dale AM , and McEvoy LK , for the Alzheimer’s Disease Neuroimaging Initiative ((2013) ) Higher rates of decline for women and APOE ɛ4 carriers. Am J Neuroradiol 34: , 2287–2293. |



