Orthostatic Hypotension: An Important Risk Factor for Clinical Progression to Mild Cognitive Impairment or Dementia. The Amsterdam Dementia Cohort
Abstract
Background:
Orthostatic hypotension (OH) has been cross-sectionally and longitudinally related to dementia in the general population. Whether OH contributes to clinical progression to mild cognitive impairment (MCI) or dementia is less certain. Also, differences in risk of progression between patients with early OH (EOH) versus delayed and/or prolonged OH (DPOH) are unclear.
Objective:
Assess the prevalence of EOH and DPOH, investigate the longitudinal association between EOH and DPOH and either incident MCI or dementia.
Methods:
1,882 patients from the Amsterdam Dementia Cohort [64±8 years; 43% female; n = 500 with subjective cognitive decline (SCD), n = 341 MCI, n = 758 Alzheimer’s disease (AD), n = 49 vascular dementia (VaD), n = 146 frontotemporal dementia (FTD), n = 88 Lewy body dementia (DLB)]. Definition OH: systolic blood pressure (BP) drop≥20 mmHg and/or a diastolic BP drop≥10 mmHg at 1 and/or 3 minutes after standing. EOH: OH only at 1 minute, DPOH: OH at (1 and) 3 minutes.
Results:
Prevalence OH: 19% SCD, 28% MCI, 41% dementia. Compared to SCD, odds of having OH were highest in patients with VaD and DLB; ORs (95% CI) were 2.6 (1.4–4.7) and 5.1 (3.1–8.4), respectively. After a mean (SD) follow-up of 2.2 (1.4) years, 105 (22%) of SCD or MCI patients showed clinical progression. Compared to patients without OH, those with DPOH had an increased risk of progression; hazard ratio (95% CI) was 1.7 (1.1–2.7), and those with EOH did not; 0.8 (0.3–1.9).
Conclusion:
Compared to SCD, prevalence of OH was higher in MCI and highest in dementia, particularly in VaD and DLB. DPOH, more likely associated with autonomic dysfunction, is a risk factor for incident MCI or dementia.
INTRODUCTION
Orthostatic hypotension (OH) is highly prevalent in older adults affecting 10–28% of the older population [1, 2], and is associated with an increased risk of falls, cardiovascular events, mortality and poor cognition [3, 4]. OH is defined as a physiological inadequately compensated blood pressure (BP) drop after standing due to autonomic dysfunction decreased baroreceptor sensitivity, arterial stiffening, or reduced parasympathetic nervous system activity [5]. OH can be categorized into early OH occurring only at 1 minute after standing and delayed and/or prolonged OH occurring at (1 and) 3 minutes after standing [6]. In the general population, OH has proven to be more prevalent in older adults with mild cognitive impairment (MCI) and dementia [7, 8], and has been associated with incident MCI and incident dementia [9–12]. However, less is known about the link between OH, MCI, and dementia in a clinical setting (i.e., patients visiting a memory clinic). Also, no studies investigated differences in risk of cognitive decline or dementia between patients with early OH versus delayed and/or prolonged OH. While early OH is a brief drop in BP with a fast recovery not jeopardizing organ perfusion, delayed and/or prolonged OH is more likely to cause periods of organ hypoperfusion. Thus, we hypothesized that patients with delayed and/or prolonged OH are at a greater risk of cognitive decline or incident dementia than patients with early OH, as they are more likely to experience longer periods of cerebral hypoperfusion, and consequently ischemia, leading to brain damage and neurodegeneration.
For this study, we set out to assess the prevalence of OH and of early versus delayed and/or prolonged OH in a large group of patients with subjective cognitive decline (SCD), MCI, and different types of dementia from the Amsterdam dementia cohort [13]. Secondly, in initially non-demented individuals, we assessed the longitudinal association between these types of OH at baseline and clinical progression to MCI or dementia during follow-up.
MATERIALS AND METHODS
Design and study population
For this study, data from the Amsterdam Dementia Cohort were used [13]. We included subjects with SCD, MCI, or dementia at baseline who visited the memory clinic between 2005 and 2015 in whom a valid BP measurement was performed at baseline (n = 1,882). Each patient received a standardized multidisciplinary workup including a medical history taking, a neurological and physical examination, a cognitive assessment, and a brain MRI. 500 subjects had SCD, 341 had been diagnosed with MCI, 758 with AD, 49 with vascular dementia (VaD), 146 with frontotemporal dementia (FTD), and 88 with Lewy body dementia (DLB). The diagnosis MCI was made according to the Petersen criteria [14, 15]. For AD, all patients met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke - Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) as well as the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria [16, 17]. The FTD diagnosis was made according to the Neary criteria [18] and DLB according to the McKeith’s criteria [19, 20]. VaD was diagnosed according to the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignementen Neurosciences (NINDS-ARIEN) criteria [21] combined with the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DMS-IV) criteria. All diagnoses were made based upon all clinical data in a multidisciplinary consensus meeting with, i.e., experienced neurologists and neuropsychologists. Subjects with SCD were those visiting the memory clinic with cognitive complaints which could not be confirmed with clinical examination and/or neuropsychological test (i.e., criteria for MCI, dementia, or any other neurological or psychiatric disease that might cause cognitive decline were not met).
The cross-sectional analyses were performed in the total baseline sample (n = 1,882). For the longitudinal analyses, data on follow-up was available in 476 patients (n = 210 with SCD and n = 266 with MCI). Clinical progression was defined as progression from SCD to MCI or dementia or from MCI to dementia during follow-up. These diagnoses were made according to previously mentioned criteria in a multi-disciplinary meeting. The study was approved by the local Medical Ethical Committee, all patients gave written informed consent to use data collected for medical care for research purposes.
Blood pressure measurement, orthostatic hypotension
BP was measured once at baseline in supine position after lying down for at least 5 minutes, followed by a BP measurement 1 minute and 3 minutes after standing. OH was defined as having a≥20 mmHg drop in systolic BP and/or a≥10 mmHg drop in diastolic BP within 1 and/or 3 minutes after standing [6, 22]. Early OH, and delayed and/or prolonged OH were defined as having the previous mentioned systolic and diastolic BP drops only at 1 minute or at (1 and) 3 minutes after standing, respectively.
Medical history and drug use
Smoking status, presence of hypertension, hypercholesterolemia, and diabetes mellitus was based on self-reported medical history. Cardiovascular disease was defined as having coronary artery disease (angina pectoris, and/or having experienced an acute myocardial infarction), and/or having experienced a stroke or transient ischemic accident (TIA). Drug use was based on a patient’s most recent medication list and self-report. OH-inducing drugs were classified as use of antipsychotics, antidepressant agents, alpha blocking drugs, long-acting nitrates, and/or antihypertensive drugs [23, 24].
Statistical analyses
Baseline differences for the total population and according to OH status were assessed using t-tests or Kruskal-Wallis tests for continuous variables, and chi-square tests for categorical variables. Median (interquartile range, IQR) SBP and DBP drop at 1 and 3 minutes after standing were calculated for each diagnosis group.
For the cross-sectional analyses with the dichotomous outcome (no OH versus OH), logistic regression analyses were performed to assess the odds (odds ratio (OR), 95% confidence intervals (CI)) of having OH in patients with MCI, and different types of dementia compared to patients with SCD. For the cross-sectional analyses with the categorical outcome (no OH, early OH, delayed and/or prolonged OH), multinomial logistic regression analyses were performed to evaluate the odds of having early or delayed and/or prolonged OH in patients with MCI, and different types of dementia compared to having no OH at baseline. Subjects with SCD were set as the reference group. All analyses were adjusted for age and sex (model 1). Additional adjustments were made for OH-inducing drugs (antihypertensive, antidepressant, antipsychotic drugs, alpha-blockers, long-acting nitrates) (model 2), and presence of diabetes or cardiovascular disease (stroke/TIA, coronary artery disease) (model 3). For the longitudinal analyses, Cox-regression analyses were performed to assess whether OH at baseline, including early and delayed and/or prolonged OH separately, was associated with clinical progression. Patients without OH were used as comparator, and time to clinical progression (days) as outcome variable. To assess the possibility of selection bias, baseline characteristics in subjects included in the longitudinal analyses were compared to those not included in the longitudinal analyses. We adjusted all longitudinal analyses according to models 1, 2, and 3 of the cross-sectional analyses. To assess whether the relation between OH and MCI or dementia was (partially) driven by presence of hypertension and/or OH-inducing drugs, we repeated our analyses stratifying for 1) patients with hypertension and/or OH inducing drugs and 2) patients without hypertension and not taking OH-inducing drugs. We adjusted these analyses according to model 1 (age, sex).
RESULTS
Table 1 presents the baseline characteristics in the total cohort (n = 1,882), and in subjects without (n = 1,329; 71%) and with (n = 553; 29%) OH at baseline. Mean age of the population was 64 (SD±8) years old, 43% was female. Subjects with OH at baseline were older, more often female, had a higher BMI, and had higher systolic BP levels. Further, they used lipid lowering drugs more often, and used more drugs in total. In terms of OH-inducing drugs, subjects were more likely to take antidepressant drugs and alpha-blockers, but did not use antipsychotics or long-acting nitrates more often than subjects without OH.
Table 1
Baseline characteristics in subjects with (n = 553) and without (n = 1,329) orthostatic hypotension (OH) at baseline
| Total sample | Without OH | With OH | p | |
| (n = 1,882) | (n = 1,329) | (n = 553) | ||
| Demographics | ||||
| Age, y (mean, SD) | 63.9 (8.2) | 63.1 (8.3) | 65.9 (7.7) | <0.001 |
| Gender (% female) | 42.8 | 42.5 | 43.6 | 0.67 |
| CV risk factors | ||||
| BMI (mean, SD) | 25.9 (4.3) | 25.8 (4.1) | 26.2 (4.5) | 0.10 |
| Current smoker (%) | 17.2 | 16.5 | 18.9 | 0.02 |
| Hypertension (%) | 27.6 | 26.5 | 30.3 | 0.10 |
| Diabetes (%) | 11.1 | 10.5 | 12.3 | 0.24 |
| Hypercholesterolemia (%) | 9.9 | 9.2 | 11.6 | 0.11 |
| SBP (mean, SD) | 149 (20) | 144 (19) | 160 (20) | <0.001 |
| DBP (mean, SD) | 85 (10) | 83 (10) | 89 (11) | <0.001 |
| Cardiovascular disease | ||||
| Stroke, TIA | 7.5 | 6.9 | 8.9 | 0.33 |
| Coronary artery disease | 6.4 | 6.0 | 7.2 | 0.14 |
| Medication | ||||
| Total number of drugs (mean, SD) | 2.4 (2.5) | 2.3 (2.4) | 2.8 (2.6) | <0.001 |
| CVRM drugs | ||||
| Antihypertensive drugs (%) | 33.6 | 33.0 | 35.3 | 0.37 |
| Lipid lowering drugs (%) | 25.5 | 23.3 | 30.7 | <0.001 |
| Antidiabetic drugs (%) | 9.8 | 9.0 | 11.6 | 0.09 |
| OH-inducing drugs | ||||
| Antipsychotics (%) | 5.2 | 5.3 | 4.9 | 0.73 |
| Antidepressant drugs (%) | 11.8 | 10.4 | 15.4 | <0.001 |
| Alpha-blockers (%) | 3.9 | 3.3 | 5.4 | 0.03 |
| Long-acting nitrates (%) | 0.5 | 0.6 | 0.4 | 0.51 |
OH, orthostatic hypotension; CV, cardiovascular; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TIA, transient ischemic accident; CVRM, cardiovascular risk management.
Prevalence of OH according to diagnosis categories
Prevalence of OH increased across the spectrum of cognitive decline; OH was present in 19% of the subjects with SCD, in 28% of patients with MCI, and in 41% of patients with dementia (Fig. 1). In patients with dementia, prevalence of OH ranged from 30% in patients with FTD to 60% in patients with DLB. Median (IQR) SBP drop 1 and 3 minutes after standing ranged from 5 (–3 –14) mmHg in subjects with SCD to approximately 8 and 9 (–1 –19) mmHg in patients with AD and VaD. The largest median (IQR) drop in SBP at 1 and 3 minutes after standing was observed in patients with DLB, namely 19 (7–35) mmHg and 22 (9–37) mmHg. Median DBP tended to rise 2–4 mmHg after standing in all diagnosis groups, except for patients with DLB. In this group, median (IQR) DBP drop 1 and 3 minute after standing was 5 or 7 (0–11) mmHg.
Fig.1
Prevalence of orthostatic hypotension (OH, %), early OH, and delayed and/or prolonged OH in subjects with SCD, and patients with MCI or dementia. *OH (≥20 mmHg drop in systolic BP and/or≥10 mmHg drop in diastolic BP) at 1 and/or 3 minutes after standing, **OH at 1 minute after standing, ***OH at (1 and) 3 minutes after standing. OH, orthostatic hypotension; SCD, subjective cognitive decline; MCI, mild cognitive impairment; AD, Alzheimer’s disease; VaD, vascular dementia; FTD, frontotemporal dementia; DLB, Lewy body dementia.
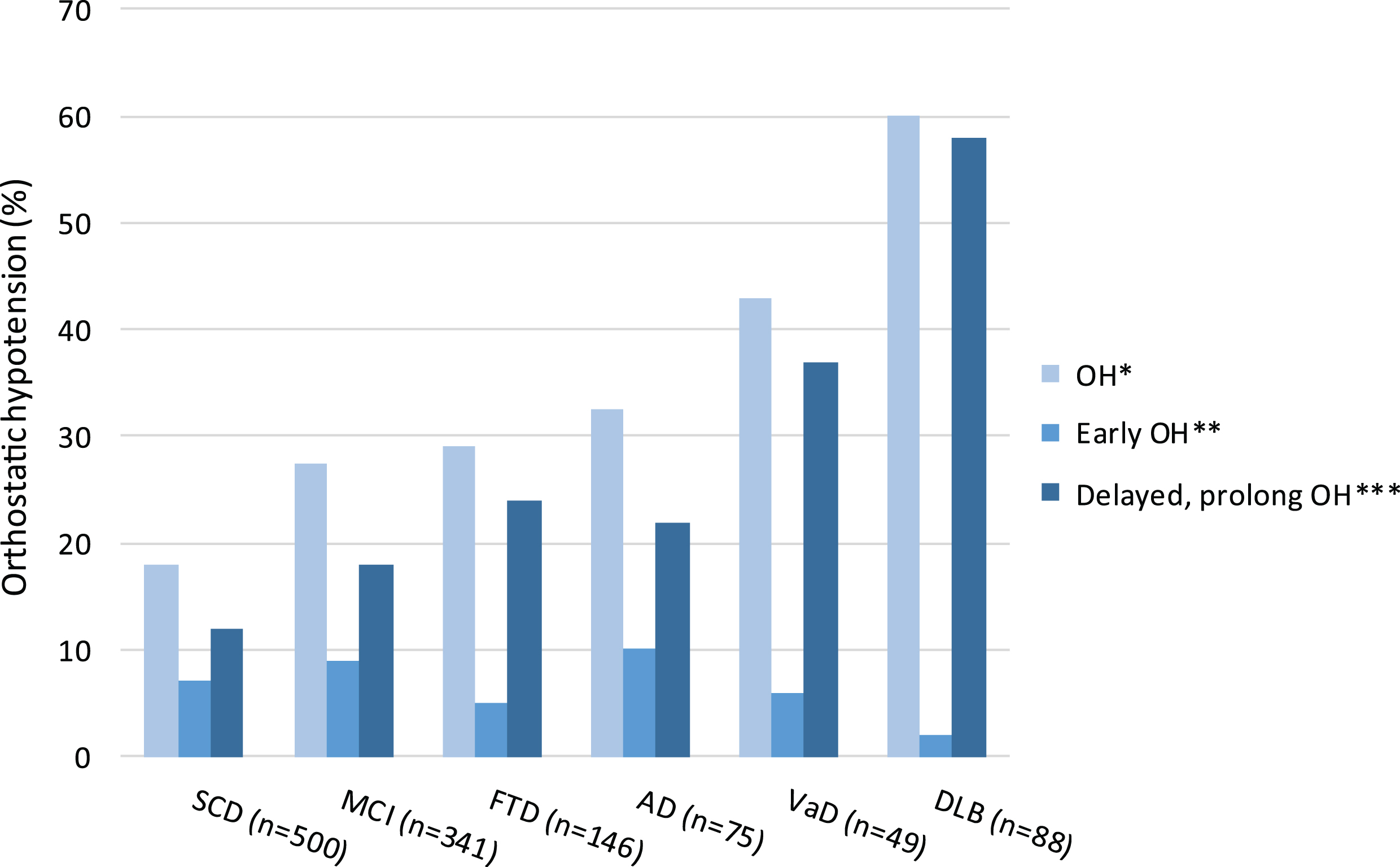
In subjects with SCD, as well as in patients with MCI or dementia, prevalence of delayed and/or prolonged OH was higher than prevalence of early OH. Delayed and/or prolonged OH was most prevalent in patients with VaD or DLB (Fig. 1).
Table 2a presents the odds (95% CI) of having OH in patients with MCI or different types of dementia compared to subjects with SCD. Patients with MCI were 1.4 (1.0–2.0) times more likely to have OH compared to subjects with SCD. In patients with dementia, the odds of having OH ranged from 1.7 (1.3–2.2) to 5.1 (3.1–8.4), with the highest odds in patients with VaD and DLB. When differentiating between early versus delayed and/or prolonged OH, patients with MCI of dementia were more likely to have delayed and/or prolonged OH, but not early OH (Table 2b). These odds were particularly high for patients with VaD and DLB; ORs (95% CI) were 3.5 (1.8–6.8) and 7.9 (4.7–13.4).
Table 2a
Odds (95% CI) of having orthostatic hypotension (OH) in subjects with MCI, and patients with dementia compared to subjects with SCD
| Model 1a | Model 2b | Model 3c | |
| Subjects with SCD | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| MCI | 1.4 (1.0–2.0) | 1.4 (1.0–1.9) | 1.4 (1.0–1.9) |
| AD | 1.7 (1.3–2.2) | 1.6 (1.2–2.2) | 1.6 (1.3–2.2) |
| FTD | 1.7 (1.1–2.6) | 1.6 (1.1–2.5) | 1.6 (1.1–2.5) |
| VaD | 2.6 (1.4–4.7) | 2.4 (1.3–4.4) | 2.3 (1.2–4.3) |
| DLB | 5.1 (3.1–8.4) | 5.0 (3.0–8.2) | 4.9 (3.0–8.1) |
OH, orthostatic hypotension; SCD, subjective cognitive decline; MCI, mild cognitive impairment; AD, Alzheimer’s disease; VaD, vascular dementia; FTD, frontotemporal dementia; DLB, Lewy body dementia. aAdjusted for age, sex; badjusted as in model 1 with additional adjustment for OH-inducing drugs (antihypertensive, antidepressant, antipsychotic drugs, alpha-blockers, long-acting nitrates); cadjusted as in model 2 with additional adjustment for presence of diabetes and cardiovascular disease (stroke/TIA, coronary artery disease).
Table 2b
Odds (95% CI) of having early or delayed orthostatic hypotension (OH) compared to subjects without OH in subjects with MCI and patients with dementia
| Model 1a | Model 2b | Model 3c | ||||
| Early OH | Delayed, prolonged OH | Early OH | Delayed, prolonged OH | Early OH | Delayed, prolonged OH | |
| Subjects with SCD | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| MCI | 1.3 (0.8–2.1) | 1.5 (1.0–2.3) | 1.2 (0.7–2.1) | 1.5 (1.0–2.2) | 1.2 (0.7–2.1) | 1.5 (1.0–2.2) |
| AD | 1.4 (0.9–2.2) | 1.8 (1.3–2.6) | 1.3 (0.8–2.1) | 1.8 (1.3–2.6) | 1.3 (0.9–2.1) | 1.8 (1.3–2.6) |
| FTD | 0.8 (0.3–1.8) | 2.2 (1.4–3.5) | 0.7 (0.3–1.7) | 2.2 (1.4–3.5) | 0.7 (0.3–1.7) | 2.2 (1.4–3.5) |
| VaD | 1.0 (0.3–3.4) | 3.5 (1.8–6.8) | 0.9 (0.3–3.3) | 3.2 (1.6–6.2) | 0.9 (0.2–3.1) | 3.1 (1.6–6.2) |
| DLB | 0.5 (0.1–2.2) | 7.9 (4.7–13.4) | 0.5 (0.1–2.0) | 7.9 (4.6–13.4) | 0.5 (0.1–2.0) | 7.7 (4.6–13.1) |
OH, orthostatic hypotension; SCD, subjective cognitive decline; MCI, mild cognitive impairment; AD, Alzheimer’s disease; VaD, vascular dementia; FTD, frontotemporal dementia; DLB, Lewy body dementia. aAdjusted for age, sex; badjusted as in model 1 with additional adjustment for OH-inducing drugs (antihypertensive, antidepressant, antipsychotic drugs, alpha-blockers, long-acting nitrates); cadjusted as in model 2 with additional adjustment for presence of diabetes and cardiovascular disease (stroke/TIA, coronary artery disease).
Adjustment for OH-inducing drugs (antihypertensive, antidepressant, antipsychotic drugs, alpha-blocking, long-acting nitrates) (model 2), and additionally for presence of diabetes and cardiovascular disease (model 3) did not change the results of the analyses. The results of the cross-sectional analyses stratified by diagnosis of hypertension and/or taking OH-inducing drugs did not differ from the original analyses (Supplementary Table 3a and 3b).
Risk of clinical progression
Compared to the patients with SCD or MCI without follow-up data (n = 365), those who were included in the analyses were older and less often female. Furthermore, patients included in the analyses had a lower BMI, were more likely to use lipid lowering drugs, and more likely to use antipsychotic drugs (Supplementary Table 1). During a mean (SD) follow-up of 2.7 (1.4) years), 23 (13%) subjects with SCD at baseline converted to MCI or dementia and 51 (25%) patients with MCI at baseline converted to dementia (Supplementary Table 2). Table 3a presents the risk (95% CI) of clinical progression, according to OH status. Although borderline statistically significant, subjects with OH at baseline had a 1.4 (0.9–2.2) times increased risk of clinical progression compared with subjects without OH at baseline. This was independent of age, sex, OH-inducing medication, diabetes, or cardiovascular disease. When differentiating between early and delayed and/or prolonged OH, mainly patients with delayed and/or prolonged OH had an increased risk of clinical progression to MCI or dementia; OR (95% CI) was 1.7 (1.1–2.7), but early OH not (Table 3b). When we stratified our analyses for 1) patients with hypertension and/or OH inducing drugs and 2) patients without hypertension and not taking OH-inducing drugs, we noted similar directional associations between (delayed and/or prolonged) orthostatic hypotension and clinical progression to MCI or dementia for both groups. However, the association between OH and clinical progression to MCI or dementia appeared slightly stronger for group 1 (point estimate of 2 versus 1.5) (Supplementary Table 4a and 4b).
Table 3a
Risk (hazard ratio, 95% confidence interval) of clinical progression to mild cognitive impairment or dementia in patients with orthostatic hypotension (OH) at baseline compared to patients without OH at baseline
| Model 1a | Model 2b | Model 3c | |
| Subjects without OH | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| OH* | 1.4 (0.9–2.2) | 1.4 (0.9–2.2) | 1.4 (0.9–2.2) |
*OH (≥20 mmHg drop in systolic BP and/or≥10 mmHg drop in diastolic BP) at 1 and/or 3 minutes after standing. aAdjusted for age, sex; badjusted as in model 1 with additional adjustment for OH-inducing drugs (antihypertensive, antidepressant, antipsychotic drugs, alpha-blockers, long-acting nitrates); cadjusted as in model 2 with additional adjustment for presence of diabetes and cardiovascular disease (stroke/TIA, coronary artery disease).
Table 3b
Risk (hazard ratio, 95% confidence interval) of clinical progression to mild cognitive impairment or dementia in patients with orthostatic hypotension (OH) at baseline compared to subjects without OH at baseline
| Model 1a | Model 2b | Model 3c | |
| Subjects without OH | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Early OH* | 0.8 (0.3–1.9) | 0.8 (0.3–1.9) | 0.8 (0.3–1.9) |
| Delayed, prolonged OH** | 1.7 (1.1–2.7) | 1.7 (1.1–2.7) | 1.7 (1.1–2.7) |
*OH (≥20 mmHg drop in systolic BP and/or≥10 mmHg drop in diastolic BP) at 1 minute after standing, **OH at (1 and) 3 minutes after standing. aAdjusted for age, sex; badjusted as in model 1 with additional adjustment for OH-inducing drugs (antihypertensive, antidepressant, antipsychotic drugs, alpha-blockers, long-acting nitrates); cadjusted as in model 2 with additional adjustment for presence of diabetes and cardiovascular disease (stroke/TIA, coronary artery disease).
DISCUSSION
Within a memory clinic population, we found that 1) compared to SCD, the prevalence of OH, particularly of delayed and/or prolonged OH, was higher in MCI and even higher in dementia, with highest prevalences observed in VaD and DLB, 2) delayed and/or prolonged OH at baseline increased the risk of clinical progression to MCI or dementia in initially non-demented individuals.
In line with previous studies performed in the general population, prevalence of OH was high and increased across the spectrum of SCD, to MCI, and dementia [7, 8]. In most community-based cohort studies [9, 11], however, the prevalence of different types of dementia was too low to assess whether patients with OH are more likely to have any particular type of dementia. We observed that the prevalence of OH was highest in patients with DLB and VaD, which has been previously reported [7, 25], and is to be expected as autonomic dysfunction is a well-known feature in α-synucleinopathies like DLB [26], while OH and VaD are both features of systemic vascular pathology [4]. No earlier studies investigated the association between early and delayed and/or prolonged OH with SCD, MCI, or dementia. We observed that having delayed and/or prolonged OH, which is more likely to be related to autonomic dysfunction than early OH, was associated with incident MCI or dementia. Earlier studies already showed that other autonomic dysfunction related postural BP changes (greater systolic BP variability during postural changes, a greater systolic BP deficit (>30%) and severe OH with a systolic BP≥30 mmHg or diastolic BP≥15 mmHg after standing) were associated with incident dementia [9, 10, 27]. The relation between OH and dementia is not straightforward and could be explained in various ways. Commonly proposed is the causal pathway in which OH is defined as a risk factor for dementia; OH leads to cerebral hypoperfusion, which, if regularly occurring, causes ischemic damage and thereafter cognitive decline or dementia [28, 29]. This causal pathway explains why in our study particularly patients with delayed and/or prolonged OH were at increased risk of converting to MCI or dementia. After all, patients with delayed and/or prolonged OH are more likely to experience longer periods of cerebral hypoperfusion compared to patients with early OH. When we stratified for 1) patients with hypertension and/or OH inducing drugs and 2) patients without hypertension and not taking OH-inducing drugs, we noted similar directional associations between (delayed and/or prolonged) OH and clinical progression to either MCI or dementia for both groups. However, the association appeared slightly stronger for those with a history of hypertension. This is in line with previous findings [5, 26], suggesting the increased risk of OH for developing MCI or dementia is likely to be of particular importance in older adults with hypertension or many (cardiovascular) comorbidities, as they are at increased risk of impaired cerebral autoregulatory mechanisms due to arterial stiffening to start with. A second theory behind the association between OH and dementia defines OH as a marker rather than as a precipitating process for developing either MCI of dementia. Lastly, the relation between OH and dementia can be one of reverse causality, with neurodegeneration in patients with dementia leading to systemic autonomic failure and subsequently OH. Although our observation that delayed and/or prolonged OH preceded development of dementia makes this less likely, we did not have data on OH during follow-up and thus we were not able to rule out this possibility of reverse causality. Also, the follow-up period was too short to introduce a time lag in the longitudinal analyses in order to minimize the possibility of reverse causality. Separating out the different causes is of potential importance because of the possible therapeutic implications. If delayed and/or prolonged OH is implicated in causing or progressing neurodegeneration, then limiting OH might represent an important therapeutic target.
This study has the strengths of being performed in a large sample of memory clinic visiting patients in which dementia subtypes were thoroughly assessed, instead of in a more frequently studied community-based cohort. Particularly in such a high-risk population, it is essential to identify markers to discriminate patients who are likely to progress to MCI or dementia from patients not likely to cognitively deteriorate as it provides opportunities to timely initiate treatment and/or arrange more targeted care planning. Secondly, BP was measured during standardized BP measurements, providing valid data for the assessment of OH. However, a number of limitations must be addressed. First, while population-based studies followed participants ranging from 15–24 years [10, 30, 31], the mean follow-up time of our study was 2.4 years. This is a relatively short period of time to assess the incidence of slowly developing neurodegenerative diseases. However, even after 2.4 years, having delayed and/or prolonged OH at baseline was associated with an increased risk of clinical progression to MCI or dementia, suggesting this association would only be strengthened after a longer follow-up period. For the longitudinal analyses, we included subjects with SCD or MCI in whom data on follow-up diagnosis was available. No data on follow-up (e.g., occurrence of death, reason for loss to follow-up) were available for the other subjects with SCD or MCI at baseline making it unable to censor these subjects. Because sample characteristics between subjects included and excluded from the analyses were not significantly different from each other, the results of our longitudinal analyses are likely to be generalizable to our entire sample. The results of our analyses did not change after adjustment for diabetes and cardiovascular disease. However, because data on pre-existing comorbidities were based on self-report, we potentially underestimated (and therefore under-adjusted our analyses) the actual presence of diabetes and cardiovascular disease. Lastly, the number of patients with SCD or MCI at baseline experiencing clinical progression was not large enough to assess whether these patients with OH were at risk of a certain type of dementia.
The results of our study have important clinical implications, as they point out that early OH can be seen as a benign and brief drop in blood pressure, but delayed and/or prolonged OH should not. Future research is required to assess whether delayed and/or prolonged OH is a risk factor for or a risk indicator of incident dementia. And, whether treatment of delayed and/or prolonged OH can prevent clinical progression to MCI or dementia. Also, assessing the effect of specific antihypertensive drugs and their duration of use may answer lingering questions regarding the influence of different types of antihypertensive drugs (particularly beta-blockers and ACE-inhibiting drugs [32, 33]) in the association between OH and progression to either MCI or dementia.
In conclusion, compared to SCD, the prevalence of OH, particularly of delayed and/or prolonged OH, was higher in MCI and even higher in dementia, with highest prevalences observed in VaD and DLB. Although we were not able to rule out the possibility of reverse causality, in contrast to early OH, delayed and/or prolonged OH increased the risk of clinical progression to MCI or dementia during follow-up. Additional work is required to assess whether repeated and prolonged drops in systemic BP leading to cerebral hypoperfusion in patients with delayed and/or prolonged OH may (partially) explain the pathophysiological link in this association. Also, it is essential to evaluate whether interventions aiming at minimizing the occurrence of OH have an impact on cognitive function.
ACKNOWLEDGMENTS
Research of the VUmc Alzheimer Center is part of the neurodegeneration research programme of the Amsterdam Neuroscience. The VUmc Alzheimer Center is supported by Alzheimer Nederland and Stichting VUmc fonds. The clinical database structure was developed with funding from Stichting Dioraphte. WMvdF holds the Pasman chair.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0402r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-190402.
REFERENCES
[1] | Viramo P , Luukinen H , Koski K , Laippala P , Sulkava R , Kivela SL ((1999) ) Orthostatic hypotension and cognitive decline in older people, J Am Geriatr Soc 47: , 600–604. |
[2] | Mader SL , Josephson KR , Rubenstein LZ ((1987) ) Low prevalence of postural hypotension among community-dwelling elderly, JAMA 258: , 1511–1514. |
[3] | Angelousi A , Girerd N , Benetos A , Frimat L , Gautier S , Weryha G , Boivin JM ((2014) ) Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: A systematic review and meta-analysis, J Hypertens 32: , 1562–1571. |
[4] | Mehrabian S , Duron E , Labouree F , Rollot F , Bune A , Traykov L , Hanon O ((2010) ) Relationship between orthostatic hypotension and cognitive impairment in the elderly, J Neurol Sci 299: , 45–48. |
[5] | Shibao C , Lipsitz LA , Biaggioni I , American Society of Hypertension Writing Group ((2013) ) Evaluation and treatment of orthostatic hypotension, J Am Soc Hypertens 7: , 317–324. |
[6] | Frith J , Parry SW ((2017) ) New Horizons in orthostatic hypotension, Age Ageing 46: , 168–174. |
[7] | Sonnesyn H , Nilsen DW , Rongve A , Nore S , Ballard C , Tysnes OB , Aarsland D ((2009) ) High prevalence of orthostatic hypotension in mild dementia, Dement Geriatr Cogn Disord 28: , 307–313. |
[8] | Labouree F , Mehrabian S , Duron E , Rollot F , Bune A , Traykov L , Hanon O ((2011) ) Relationship between orthostatic hypotension and cognitive impairment in the elderly, Alzheimers Dement 7: , S597. |
[9] | Cremer A , Soumare A , Berr C , Dartigues JF , Gabelle A , Gosse P , Tzourio C ((2017) ) Orthostatic hypotension and risk of incident dementia: Results from a 12-year follow-up of the Three-City Study Cohort, Hypertension 70: , 44–49. |
[10] | Wolters FJ , Mattace-Raso FU , Koudstaal PJ , Hofman A , Ikram MA ((2016) ) Orthostatic hypotension and the long-term risk of dementia: A population-based study, PLoS Med 13: , e1002143. |
[11] | Elmstahl S , Widerstrom E ((2014) ) Orthostatic intolerance predicts mild cognitive impairment: Incidence of mild cognitive impairment and dementia from the Swedish general population cohort Good Aging in Skane, Clin Interv Aging 9: , 1993–2002. |
[12] | Min M , Shi T , Sun C , Liang M , Zhang Y , Wu Y , Sun Y ((2018) ) The association between orthostatic hypotension and dementia: A meta-analysis of prospective cohort studies, Int J Geriatr Psychiatry 33: , 1541–1547. |
[13] | van der Flier WM , Pijnenburg YA , Prins N , Lemstra AW , Bouwman FH , Teunissen CE , van Berckel BN , Stam CJ , Barkhof F , Visser PJ , van Egmond E , Scheltens P ((2014) ) Optimizing patient care and research: The Amsterdam Dementia Cohort, J Alzheimers Dis 41: , 313–327. |
[14] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity, J Intern Med 256: , 183–194. |
[15] | Petersen RC , Stevens JC , Ganguli M , Tangalos EG , Cummings JL , DeKosky ST ((2001) ) Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology, Neurology 56: , 1133–1142. |
[16] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease, Alzheimers Dement 7: , 263–269. |
[17] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease, Neurology 34: , 939–944. |
[18] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EG , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini ML , Rosen H , Prioleau-Latham CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia, Brain 134: , 2456–2477. |
[19] | McKeith IG , Dickson DW , Lowe J , Emre M , O’Brien JT , Feldman H , Cummings J , Duda JE , Lippa C , Perry EK , Aarsland D , Arai H , Ballard CG , Boeve B , Burn DJ , Costa D , Del Ser T , Dubois B , Galasko D , Gauthier S , Goetz CG , Gomez-Tortosa E , Halliday G , Hansen LA , Hardy J , Iwatsubo T , Kalaria RN , Kaufer D , Kenny RA , Korczyn A , Kosaka K , Lee VM , Lees A , Litvan I , Londos E , Lopez OL , Minoshima S , Mizuno Y , Molina JA , Mukaetova-Ladinska EB , Pasquier F , Perry RH , Schulz JB , Trojanowski JQ , Yamada M , Consortium on DLB ((2005) ) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium, Neurology 65: , 1863–1872. |
[20] | McKeith IG , Boeve BF , Dickson DW , Halliday G , Taylor JP , Weintraub D , Aarsland D , Galvin J , Attems J , Ballard CG , Bayston A , Beach TG , Blanc F , Bohnen N , Bonanni L , Bras J , Brundin P , Burn D , Chen-Plotkin A , Duda JE , El-Agnaf O , Feldman H , Ferman TJ , Ffytche D , Fujishiro H , Galasko D , Goldman JG , Gomperts SN , Graff-Radford NR , Honig LS , Iranzo A , Kantarci K , Kaufer D , Kukull W , Lee VMY , Leverenz JB , Lewis S , Lippa C , Lunde A , Masellis M , Masliah E , McLean P , Mollenhauer B , Montine TJ , Moreno E , Mori E , Murray M , O’Brien JT , Orimo S , Postuma RB , Ramaswamy S , Ross OA , Salmon DP , Singleton A , Taylor A , Thomas A , Tiraboschi P , Toledo JB , Trojanowski JQ , Tsuang D , Walker Z , Yamada M , Kosaka K ((2017) ) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium, Neurology 89: , 88–100. |
[21] | Roman GC , Tatemichi TK , Erkinjuntti T , Cummings JL , Masdeu JC , Garcia JH , Amaducci L , Orgogozo JM , Brun A , Hofman A , et al. ((1993) ) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop, Neurology 43: , 250–260. |
[22] | Frith J ((2015) ) Diagnosing orthostatic hypotension: A narrative review of the evidence, Br Med Bull 115: , 123–134. |
[23] | Kamaruzzaman S , Watt H , Carson C , Ebrahim S ((2010) ) The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study, Age Ageing 39: , 51–56. |
[24] | Poon IO , Braun U ((2005) ) High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans, J Clin Pharm Ther 30: , 173–178. |
[25] | Punchick B , Freud T , Press Y ((2016) ) The association between orthostatic hypotension and cognitive state among adults 65 years and older who underwent a comprehensive geriatric assessment, Medicine (Baltimore) 95: , e4264. |
[26] | Stubendorff K , Aarsland D , Minthon L , Londos E ((2012) ) The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson’s disease with dementia, PLoS One 7: , e45451. |
[27] | Hayakawa T , McGarrigle CA , Coen RF , Soraghan CJ , Foran T , Lawlor BA , Kenny RA ((2015) ) Orthostatic blood pressure behavior in people with mild cognitive impairment predicts conversion to dementia, J Am Geriatr Soc 63: , 1868–1873. |
[28] | Wolters FJ , Zonneveld HI , Hofman A , van der Lugt A , Koudstaal PJ , Vernooij MW , Ikram MA , Heart-Brain Connection Collaborative Research Group ((2017) ) Cerebral perfusion and the risk of dementia: A population-based study, Circulation 136: , 719–728. |
[29] | de la Torre JC ((1999) ) Critical threshold cerebral hypoperfusion causes Alzheimer’s disease? Acta Neuropathol 98: , 1–8. |
[30] | Holm H , Nagga K , Nilsson ED , Melander O , Minthon L , Bachus E , Fedorowski A , Magnusson M ((2017) ) Longitudinal and postural changes of blood pressure predict dementia: The Malmo Preventive Project, Eur J Epidemiol 32: , 327–336. |
[31] | Rawlings A , Juraschek S , Heiss G , Hughes T , Meyer M , Selvin E , Sharrett AR , Windham BG , Gottesman R ((2017) ) Orthostatic hypotension is associated with 20-year cognitive decline and incident dementia: The atherosclerosis risk in communities (ARIC) study, Circulation 135: . |
[32] | Canney M , O’Connell MD , Murphy CM , O’Leary N , Little MA , O’Seaghdha CM , Kenny RA ((2016) ) Single agent antihypertensive therapy and orthostatic blood pressure behaviour in older adults using beat-to-beat measurements: The Irish Longitudinal Study on Ageing, PLoS One 11: , e0146156. |
[33] | Jochemsen HM , Teunissen CE , Ashby EL , van der Flier WM , Jones RE , Geerlings MI , Scheltens P , Kehoe PG , Muller M ((2014) ) The association of angiotensin-converting enzyme with biomarkers for Alzheimer’s disease, Alzheimers Res Ther 6: , 27. |



