The use of robots in stroke rehabilitation: A narrative review
Abstract
BACKGROUND:
Stroke is among the leading causes of acquired disability in the United States, affecting nearly 800,000 Americans annually. The identification of more effective treatments for hemiparesis has been recognized as a top research priority. Intelligent, motor-driven devices for rehabilitation, or rehabilitation robotics, represent an exciting frontier with considerable potential to address these concerns.
PURPOSE:
This article presents a state of the science review regarding selected robotic technologies that are representative of current robot-aided rehabilitation strategies, the evidence surrounding their efficacy, barriers to widespread dissemination, and technologies in development.
METHODS:
Narrative Review.
CONCLUSIONS:
Based on this synthesis, we suggest that robotic rehabilitation tools are neither the standard of care, nor entirely experimental, but rather a clinically innovative therapy of some utility.
1Introduction
Stroke is among the leading causes of acquired disability in the United States, affecting nearly 800,000 Americans annually (Winstein, Stein, et al., 2016). As survival rates have improved and cost containment pressures have grown, optimization of recovery following stroke has become even more critical. The identification of more effective treatments for hemiparesis has been recognized as a top research priority by stroke survivors, caregivers and health professionals (Pollock, St George, Fenton, & Firkins, 2012). Intelligent, motor-driven devices for rehabilitation, or rehabilitation robotics, represent an exciting frontier with considerable potential to address theseconcerns.
Traditional stroke rehabilitation integrates a variety of treatment strategies, with varying degrees of evidence-based support. Often, treatment incorporates repetitive exercise, used to facilitate motor learning and build muscle strength. In the acute phase, treatment may integrate passive range of motion, in order to maintain the integrity of physical structures in anticipation of subsequent neurological recovery. As recovery occurs, therapeutic exercise typically advances to active-assistive movements, in which a clinician uses physical cues and graded support to aid completion of simple movements. In an effort to improve efficiency due to limited one-on-one treatment time with patients, clinicians may delegate many of these exercises to support staff, provide them in a group setting, or ask clients to complete them independently outside of formal therapy hours.
Robotic devices are well-suited to assist in this area, based on their ability to carry out simple, repetitive tasks with consistency. Robots can be programmed to guide a patient through a series of specific motions, while maintaining a prescribed level of support and restricting undesired (or contraindicated) movements. In this capacity, they represent a reliable option to “stand-in” for the skilled clinician. Robots also present an additive value, in that they are capable of performing repetitive movements without fatiguing, while simultaneously collecting objective quantitative data.
Robotic devices can also provide a level of patient engagement during repetitive physical tasks that may be difficult to achieve during conventional exercise therapy. Many devices now incorporate software that transforms potentially tedious physical movements into compelling games and physical challenges that keep users motivated and engaged.
This review is not intended as an exhaustive review of the scientific literature on robot-aided rehabilitation, but rather focuses on specific representative technologies and the rationale and evidence supporting their clinical use for stroke rehabilitation, in order to provide the reader with an overall understanding of the field, its current state, and future directions.
2Robots as exercise devices
In the current state of rehabilitation robotics, exercise-based treatments are most often delivered through relatively large workstation devices. Workstation devices are typically comprised of a mechanical component and a computer display for patient engagement and to provide visual feedback to the user. These workstation devices fall into two main categories: end-effector devices and exoskeletal workstation devices.
End-effector devices were the first robotic technologies developed specifically for stroke rehabilitation and their relatively robust level of formal study reflects this history. Examples of end-effector devices include the MIT-Manus now commercialized as the InMotion Shoulder-Elbow robot, (Bionik, Inc, Toronto), the Reo Go (Motorika, Israel), and the G-Eo (Reha Technologies, Switzerland). End-effector devices rely on a single distal point of contact to guide the entire limb. For example, an end-effector device for the upper limb may make contact at the hand and forearm, facilitating elbow and shoulder movements in-turn. Theoretically, this model enables the robot to support natural motion without undue constraint of the limb and allows the device to accommodate a range of users with minimal mechanical adjustments.
In practice, end-effector systems may be limited by the movement patterns and structural restrictions of the neurologically-impaired limb. For example, robot-assisted movement of the forearm forward may be used to generate elbow extension. However, for a patient with severe spasticity or an elbow contracture, this same movement may inadvertently result in compensatory flexion of the trunk instead of elbow extension. The freedom of movement provided by the end-effector design can therefore act to a patient’s benefit, enabling supported, unencumbered movement, or to their detriment, by permitting undesirable compensatory movement patterns.
In contrast, exoskeletal workstations devices provide direct control of each segment of the limb, with separate motors controlling each plane of motion. Examples of exoskeletal workstations include the Armeo Power (Hocoma, Switzerland), and the Lokomat (Hocoma). This design enables precise control of the limb and restriction of unwanted movement patterns. This degree of control comes at a certain cost, however. Exoskeletal workstations are typically large and bulky devices designed to achieve control of multiple limb segments. The resulting mass and inertia of the device can only be partially offset by the device itself, impacting the fluidity of motion. While advances have been made in this area, these devices have not yet achieved a level of speed and fluidity that accurately mimics natural motion.
In the near-term, exoskeletal workstation devices remain costly machines that are essentially restricted to rehabilitation clinics and centers, and are unrealistic to deploy in a home setting. Despite improvements in efficiency, switching from patient to patient often requires adjustments of various parameters due to differences in limb length and dimensions. This, coupled with the complexity of these devices requiring direct supervision by a clinician during their use, limits their impact on improving productivity, and therefore their widespread deployment in clinical settings.
3Robots for upper limb exercise
3.1MIT-MANUS
One of the best-studied end-effector robots for the upper limb is the MIT-MANUS robotic system, commercially available as the InMotion series of devices (InMotion/Bionik). This modular system consists of proximal and distal components, which can be used individually or in concert to train the upper limb. These configurations include a module for elbow and shoulder movement in the horizontal plane, shoulder and hand grasp in the vertical plane, and wrist movement in all planes. Typically, the device utilizes an assist-as-needed paradigm, continually sensing motion of the limb and initiating or completing movements to complete a programmed simulated task. The device’s most studied mode or “therapeutic exercise game” achieves approximately 1000 movements in a single session, using a simple targeted reaching task similar to reaching around the face of a clock.
In the sub-acute phase of recovery, the MIT-MANUS has demonstrated efficacy for reducing motor impairment, improving function, and eliciting enduring change (Fasoli et al., 2004; Lo et al., 2010; Volpe et al., 1999). In one of the largest randomized controlled trials of rehabilitation robotics to date, the Department of Veterans Affairs investigated the value of MIT-MANUS for chronic stroke (Lo et al., 2010). The study assigned 127 individuals with moderate to severe upper limb impairment to robot-assisted therapy, intensive human-delivered therapy mimicking robotic movements, or usual care for a period of twelve weeks. Researchers found no statistically significant difference between robotic and human-delivered therapy groups at the conclusion of treatment, suggesting that robotic therapy provides a similar, but not superior, benefit for motor performance as compared to human-delivered treatment.
3.2Armeo Power
Perhaps the most advanced robotic exoskeletal workstation device for the upper limb currently on the market is the Armeo Power, the commercial version of the ARMin device, marketed by Hocoma, Inc. The device is a large workstation with an exoskeleton enveloping the user’s arm, which can be adjusted for shoulder height and limb length. The device provides arm weight support, which offsets the weight of the device and a designated proportion of a patient’s limb weight. The Armeo Power employs custom software, which enables the device to be used in various ways. Currently, it offers a mobilization mode, 2D gaming, 3D gaming, and functional training in the form of simulated activities of daily living. Its sister product, the ArmeoSpring, functions similarly with the use of springs to offset the device’s and the user’s upper limb weight, instead of using motors to assist movement.
The Armeo Power excels in the area of patient-engagement, employing robust graphics and simple, yet engaging, games to promote repetitive movement. The software enables the clinician to select the appropriate challenge by controlling the complexity of the visual field, defining the range of motion required, and designating the pace of gameplay.
Similar to the MIT-MANUS, the Power employs an assist-as-needed model, allowing the clinician to provide the optimal challenge at all levels of recovery. Additionally, this technology enables stabilization of specific joints during gameplay, enabling the clinician to select a modular or composite approach to treatment, as desired.
In 2014, a study was published assessing the efficacy of the Armeo Power in a multi-center randomized trial (Klamroth-Marganska et al., 2014). Seventy-seven chronic stroke patients with moderate to severe paresis were randomized to robotic or conventional therapy for a period of eight weeks. The researchers found that all participants showed improved motor function, but that patients in the robotic therapy group had greater improvements in motor function compared with dose-matched conventional upper limb therapy, with a mean difference of 0.78 points on the Fugl-Meyer. While this change was statistically significant, the difference between robotic and dose-matched conventional therapy is insufficient to be clinically meaningful. Moreover, similar to other studies of upper limb robotic therapy for individuals with chronic hemiparesis, the magnitude of the overall motor improvement is of marginal clinical significance (3.25 points on the upper extremity Fugl-Meyer scale), as compared with the minimum clinically important difference for patients with mild to moderate impairment, approximately 5 points (Page, Fulk, & Boyne, 2012).
The Armeo Power is being used in an ongoing, multicenter trial, SMARTS 2, which couples the exoskeleton’s suspension mode with a novel software platform designed to elicit motor exploration through a game controlling an animated dolphin. The study explores the impact of dose-matched, intensive training (robotic and conventional) in the acute phase of recovery, with patients beginning treatment no more than six weeks following stroke (Krakauer, 2014).
3.3Bilateral devices – Bi-Manu-Track robotic arm trainer
An alternative approach to upper limb robotic treatment is a bilateral treatment strategy. An example of this approach is seen in the Bi-Manu-Track (Reha-Stim, Germany), which consists of dual forearm troughs mounted on a tabletop workstation. The Bi-Manu-Track provides mirrored movements to the upper limbs including forearm pronation/supination, wrist flexion/extension, and metacarpophalangeal extension. The device can provide passive bilateral movement, mirror movements produced by the unaffected/affected arm, or provide resistance to movement.
A small randomized controlled trial (n = 20) compared Bi-Manu-Track therapy coupled with functional training to dose-matched conventional therapy in chronic stroke patients (Liao, Wu, Hsieh, Lin, & Chang, 2012). After four weeks of intensive therapy (90–105 minutes, 5 days/week), the robot-assisted therapy group demonstrated significantly increased motor function, hemiplegic arm activity (as measured by self-report of functional capabilities) and bilateral arm coordination as compared with the control group.
3.4DIEGO
A third, less widely used design concept in workstation robotics, utilizes cables to support and mobilize the upper limbs. The commercially-available DIEGO (Tyromotion, Graz, Austria) utilizes an overhead boom with four suspended cables, which connect to slings at the wrist and elbow. The device can be applied unilaterally or bilaterally and employs “intelligent gravity compensation” to unweight the limb and facilitate motion in three dimensions, much like a mobile arm support. Support can be withdrawn over time as patients progress from passive to active therapymodes.
The DIEGO expands upon the idea of gaming as a means of patient engagement by integrating specially designed cognitive games, in an effort to pair upper limb training with cognitive remediation. Most notably, the device can also be utilized to enable supported performance of actual tabletop functional tasks.
The design of the DIEGO enables it to offer the versatility and rapid setup of an end-effector device, with the added benefit of direct application to functional activities. This unique capability underscores a new approach in robotic therapy – integrating robotic support with actual task practice in context. The Armeo Boom (Hocoma) is similar in some regards, although it relies purely on mechanical support of the upper limb, rather than robotic controls.
3.5Amadeo
Rehabilitation of the hand represents a substantial challenge for robotic devices, based on the hand’s size and mechanical complexity.
The Amadeo by Tyromotion is an end-effector device designed for the hand and one of very few options presently available in the commercial market. The Amadeo consists of a forearm trough and individual digit actuators, which attach to the fingers via magnets affixed using adhesive bandages. The individual digit supports move along a track to flex and extend the digits.
The Amadeo has demonstrated feasibility and preliminary efficacy for stroke in the subacute phase (Sale, Lombardi, & Franceschini, 2012). A recent randomized controlled study of seventeen patients compared traditional occupational therapy with Amadeo robotic therapy (Orihuela-Espina et al., 2016). After forty sessions, both groups showed significant improvement, however, the robotic intervention resulted in a larger effect size on the Fugl-Meyer and Motricity Index with respect to hand function.
3.6Hand of Hope
The Hand of Hope (Rehab-Robotics, Hong Kong) is a semi-wearable hand exoskeletal device. The Hand of Hope utilizes a biofeedback approach, detecting a user’s intent through surface electromyography and responding with exoskeleton-driven grasp or release. In a study of ten subjects with chronic stroke, a training program using the Hand of Hope resulted in statistically significant improvements in functional performance as well as enhanced muscle coordination, as measured by EMG (Hu et al., 2013).
3.7Other devices
There are a number of other commercially available upper limb workstation devices, such as the ReoGo (Motorika), Hand Mentor Pro (Motus Nova), The Kinarm (BKIN), and the Proficio (Barrett). While a comprehensive review of these devices is beyond the scope of this paper, each has specific design features that may have advantages over competing devices in certain circumstances.
4Robots for lower limb exercise
4.1Lokomat
The Lokomat (Hocoma) is the most widely studied robotic gait training device on the market. This workstation device consists of a treadmill, a bodyweight support system, and bilateral exoskeletal components, which provide actuation at the hips and knees (Van Kammen, Boonstra, Van Der Woude, Reinders-Messelink, & Den Otter, 2016). Optional elastic foot lifters provide additional support at the ankle if needed.
The Lokomat software supports a range of “guidance” parameters. At its maximal level, the device guides the limbs through a predefined movement pattern, established by studies of normal gait. As guidance is reduced, the device permits increased deviation from this trajectory before providing assistance.
Studies in the stroke population have supported the use of Lokomat training to supplement the effects of conventional treatment. A small study (n = 30) conducted in 2007 comparing a four-week course of Lokomat training plus physical therapy with dose (time)-matched physical therapy found comparable gains in functional ambulation among acute stroke patients. Additionally, the Lokomat group demonstrated improved gait characteristics, specifically a significantly longer single stance phase on the paretic leg in over-ground ambulation (Husemann, Muller, Krewer, Heller, & Koenig, 2007). A larger study (n = 67) comparing these training paradigms in the subacute phase found superior outcomes in the Lokomat group after a six-week training period with respect to functional and motor outcomes (Schwartz et al., 2009).
Studies of the chronic stroke population echo these findings. A 2014 study (n = 107) assessing both subacute and chronic patients in an intensive training protocol made up of conventional physical therapy or robotic therapy and physical therapy found enhanced outcomes in the Lokomat group (Dundar, Toktas, Solak, Ulasli, & Eroglu, 2014). Most recently, a small study of chronic stroke patients comparing combined treatment with traditional treadmill training in an intensive four-week protocol reported an advantage of robotic treatment, with significant improvements in gait speed, cadence, step length and balance, as compared to the standard treatment group. The double limb support period was also found to be significantly lower among subjects who completed Lokomat therapy (Bang &Shin, 2016).
Other studies have suggested that Lokomat training is less effective than dose-matched gait training. Hornby et al. found that single therapist-assisted locomotor training was more effective than dose-matched robotic training with respect to gait speed and symmetry after a 12 session program (Hornby et al., 2008).
The Lokomat device is essentially a robotic implementation of body-weight supported treadmill training, conventionally performed manually by a physical therapist. Emerging evidence suggests that this training paradigm may not be optimal for the stroke population. Despite a seemingly robust conceptual foundation (Dobkin & Duncan, 2014), body-weight supported treadmill training has proved disappointing in a major clinical trial.The LEAPS trial evaluated the efficacy of therapist-assisted, body-weight supported locomotor training for 408 patients with acute stroke. The study found that therapist-assisted body-weight supported locomotor training was no more effective than a therapist-directed home exercise program emphasizing balance and mobility at one year post-stroke (Duncan et al., 2011). While this study did not directly assess the efficacy of a comparable robotic intervention, such as the Lokomat, it does call into question the validity of body-weight supported locomotor training in any capacity as an evidence-based intervention post-stroke. As a result, this therapy is not widely used at present. Dobkin and Duncan (2014) argue that early-stage conceptual research, rather than costly mass-production of complex devices may expedite the development of novel interventions with greater effiacy. They further contend that workstation robotic devices may ultimately be insufficient to simulate the environment and task-specific advantages of overground training in a natural context.
4.2G-EO system
The G-EO System Robot (Reha Technologies) is a commercially available, end-effector system developed specifically for stroke rehabilitation. The device is conceptually similar to an elliptical machine, with two footplates that move along a designated path, in addition to a bodyweight support system. The device senses a patient’s effort to move and responds by producing the prescribed gait pattern. It also offers a Partial Movement mode, which enables isolation and repetition of gait components. The G-EO enables customization of ambulation parameters such as step length, step height, and foot angles during toe off and initial contact.
The G-EO expands on the work of the research device the HapticWalker, delivering the first commercial robotic treadmill device capable of simulating stair-climbing in a format feasible in the clinical environment. Initial research exploring this stair climbing mode suggests that the device facilitates muscle activation patterns in ambulatory stroke patients that are comparable with patterns recorded during genuine stair climbing tasks (Hesse, Waldner, & Tomelleri, 2010).
While no randomized controlled trials have been conducted with this technology to date, preliminary findings suggest that the G-EO may be feasible and beneficial for gait and stair climbing ability for the subacute population (Hesse, Tomelleri, Bardeleben, Werner, & Waldner, 2012). Additionally, a recently published uncontrolled, multicenter study suggests the feasibility of the G-EO for chronic stroke patients with respect to gait recovery (Mazzoleni et al., 2017).
4.3TPAD
The Tethered Pelvic Assist Device (TPAD) is a research device that applies forces via cables attached to a pelvic belt worn by a patient during treadmill training. Using force plate and motion capture data, the device ascertains the appropriate level of force for a patient and delivers forces along an adjustable vector during prescribed phase(s) of gait. In preliminary studies, this technology has been utilized to facilitate weight shifting, or loading of the affected limb, for individuals with hemiparesis (Bishop et al., 2017).
The TPAD demonstrates the potential of robotic devices to function as a training signal, providing haptic cues to the user without providing substantial concurrent physical assistance. This can be programmed in an “assist as needed” mode similar to other robotic devices, or an error augmentation mode in order to elicit an adaptive response from the user. This versatility is advantageous as a means of studying different forms of force feedback during post-stroke gait training, which may help identify more effective strategies for optimizing motor learning following stroke.
4.4Other devices
Other lower limb workstation devices are commercially available, such as the KineAssist-MX (HDT Global) and the Walkbot (P&S Mechanics), but are beyond the scope ofthis review.
5Robots as assistive devices
There are several strategies employed by robotic systems to retrain movement after stroke. Workstation devices can either use simple games to encourage and guide movements, or integrate simulations of real-life tasks such as cooking or cleaning to create the perception of performing a functional task. An alternative strategy is to use wearable robotic devices to facilitate the performance of actual functional tasks. This can be conceived either as a training system to encourage restoration of motor abilities after removal of the device, or as an assistive device to assist the user on an ongoing basis. This latter approach is sometimes termed a powered orthosis, or else a neuroprosthesis.
6Upper limb wearable devices
6.1Myomo
The MyoPro Motion-G is a lightweight, wearable orthosis for the upper limb. The device detects electomyographic signals from the limb to sense a user’s intention and responds by providing assisted elbow flexion/extension or grasp/release. In a recent study of individuals with chronic stroke, the device was found to have an neuroprosthetic effect on motor performance while in use, with a mean increase of 8.72 points on the Fugl-Meyer Scale (Peters, Page, & Persch, 2016). Performance also improved during functional tasks selected by the research team.
6.2MyHand
The MyHand device, currently in development, targets individuals with gross grasp, but insufficient functional release of the hand (Meeker, Park, Bishop, Stein, & Ciocarlie, 2017). The device seeks to capitalize on the residual capacities of the upper limb post-stroke in order to provide both a training mechanism and neuroprosthetic utility for home-use. The MyHand device is designed to be low-cost and to accommodate patients with a range of residual capacities, including those with insufficient EMG to operate other devices. A number of control mechanisms, including contralateral and ipsilateral manifestations, are in development.
A logical extension of this work may be the after-effect of utilizing wearable robotics. Since these devices enable practice in context, they may elicit improvements in motor function distinct from workstation robotic devices.
6.3Compensatory devices
The majority of robotic devices in the stroke space are engineered to facilitate motor recovery. However, another type of device uses robots as a compensatory instrument for individuals with severe motor impairments. Kinova Robotics markets a commercially available, lightweight robotic arm (the “JACO”) designed to be mounted to a wheelchair base or other steady surface, and a similar device is sold by Exact Dynamics (the “iARM”). These devices may hold potential for individuals with large brainstem strokes resulting in profound disability by linking the physical capabilities of a robotic arm with user-friendly controls. More sophisticated interfaces that simplify the demands on the user to control the device may ultimately grant new freedom to individuals with severe physical limitations.
7Lower limb wearable devices
Most of the wearable exoskeleton technologies for the lower limbs have their genesis in the spinal cord injury population, where the devices were intended to restore ambulation to individuals with paraplegia. The ReWalk is a wearable exoskeleton that integrates a lightweight frame actuated at the hip and knee. The device offers variable assistance and initiates ambulation by a tilt sensor, which senses forward motion at the trunk. Another commercial device intended for individuals with spinal cord injury is the Indego (Parker Hannifin), which uses a modular design for easy setup and breakdown.
The Ekso GT is the first wearable exoskeletal device cleared by the FDA for use in stroke treatment. The device consists of a wearable exoskeleton powered at the hips and knees, with a backpack component containing batteries and controllers. The Ekso GT software allows clinician to adjust the amount of support provided to each limb. An upgrade to this software, SmartAssist, has been released in Europe, and includes “pre-gait” activities such as weight shifting and squats.
Evidence supporting the therapeutic value of these devices post-stroke remain limited. In an open-label case series of 23 stroke patients in the subacute and chronic phases, a four-week (12 session) training period with the use of the device resulted in significant improvement on the Motricity Index, Functional Ambulation Scale, 10-meter walk, and 6-minute walk, with additional improvements on the Trunk Control Test and Walking Handicap Scale for the subacute population (Molteni et al., 2017).
7.1H2
The Exo-H2 (Technaid S.L., Spain) is an experimental overground exoskeleton originally developed for incomplete spinal cord injury. The H2 is differentiated by six points of actuation, including actuation of the ankle, which is designed to limit foot drop during ambulation. The device uses an assist-as-needed paradigm, which enables a specified amount of deviation from an idealized gait pattern before applying a corrective force. The technology can be used in a modular fashion to provide unilateral or joint-specific support, based on a user’s needs. The device also features an open architecture, which can be paired with neural interfaces or other technologies to expand research capabilities related to stroke recovery. In a small pre-clinical study examining feasibility of the device with three chronic stroke patients, the H2 was found to be well-tolerated and capable of producing a more symmetrical gait pattern (Bortole et al., 2015).
7.2AlterG Bionic Leg
The AlterG Bionic Leg is a commercially available, unilateral, powered knee orthosis designed for the treatment of neurological and orthopedic conditions. The device can be used for a variety of tasks including transfers, ambulation on even surfaces and stair climbing/descent. The device utilizes multiple sensors, including force sensors in the shoe, to interpret a user’s movement and respond with appropriate assistance. In a randomized controlled pilot study of individuals with chronic stroke, an 18 hour, 6 week intervention with the Bionic Leg was found to be no more effective in improving gait velocity than sham physical therapy (Stein, Bishop, Stein, & Wong, 2014).
7.3HAL
The Hybrid Assistive Limb (HAL) suit by Cyberdyne is a Japanese device originally developed for older adults with muscle weakness (Ueba et al., 2013). The device has a modular design, which allows it to provide support unilaterally or bilaterally at the hip and/or knee. The hybrid system supports autonomous control, driven by weight shift, or voluntary control, driven by activation of specific muscles, as determined by surface EMG. Initial studies of the HAL suit have been mixed in the stroke population, with some patients experiencing marked difficulty adjusting to the technology (Maeshima et al., 2011) and others suggesting initial feasibility for patients with severe gait impairment when paired with a bodyweight support system (Nilsson et al., 2014).
7.4Other devices
The REX (REX Bionics, New Zealand) is the first commercially available wearable exoskeleton to achieve self-balancing, freeing the user from the need for upper limb support during ambulation. This new feature may hold unique promise in early stroke rehabilitation by providing a stable base for trunk strengthening and balance training, without the need for additional staff, although clinical studies have not yet been reported in this population.
8Considerations on efficacy
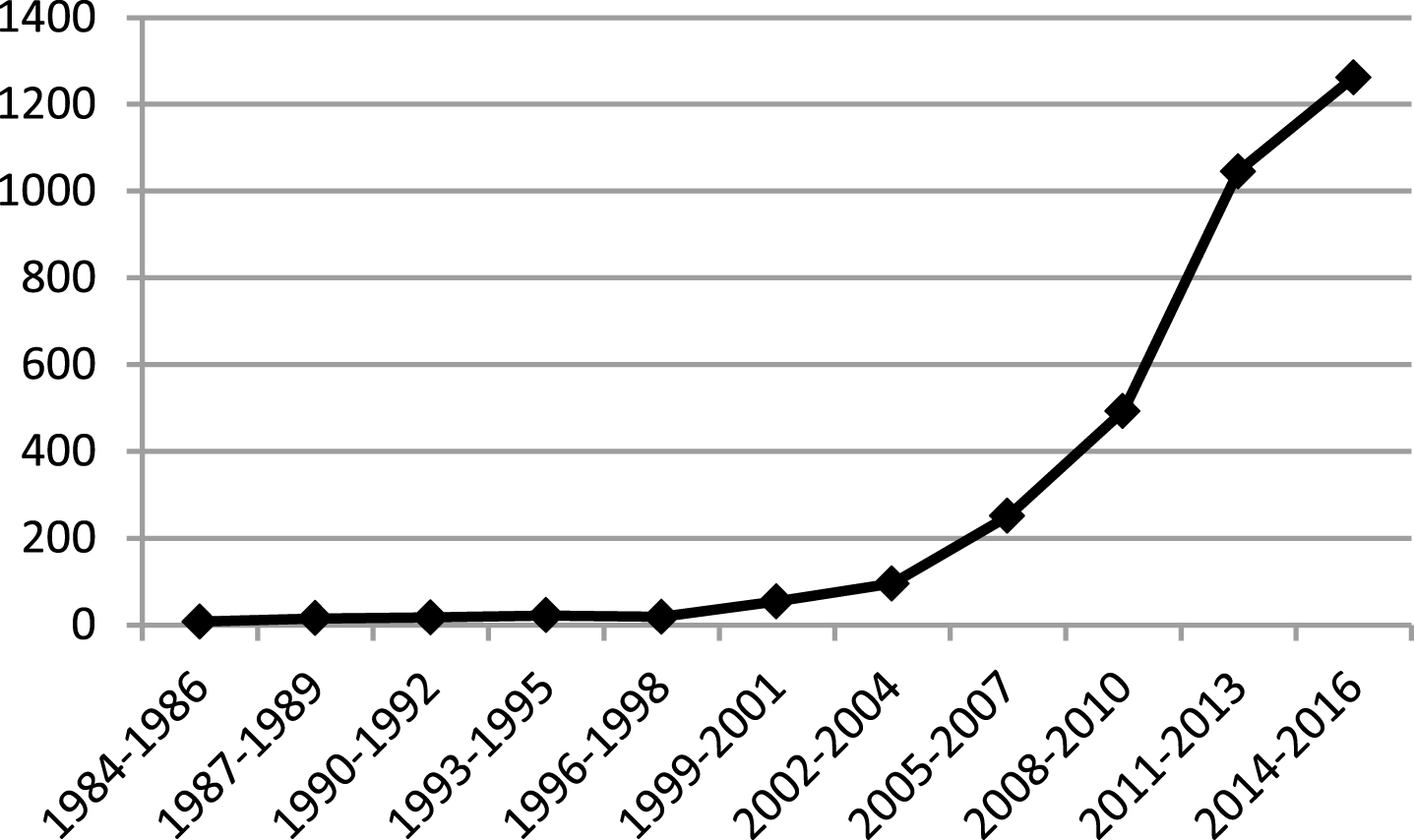
Research surrounding robotic technologies in rehabilitation has ballooned over the past twenty years, with the number of ongoing trials listed on clinicaltrials.gov jumping from 18 between 1996 and 1998 to 1,262 between 2014 and 2016. Despite this explosion in the research arena, definitive evidence regarding the efficacy of these devices following stroke remains limited.
Two recent meta-analyses looking at upper limb training found that robotic interventions have a small, but positive effect on motor control and muscle strength post-stroke (Mehrholz, Pohl, Platz, Kugler, & Elsner, 2015; Veerbeek, Langbroek-Amersfoort, van Wegen, Meskers, & Kwakkel, 2017). The impact of these interventions on activities of daily living remains unclear, with authors reporting conflicting conclusions. In contrast, a 2016 systematic review contended that robot-assisted therapy not be recommended for clinical use, based on insufficient evidence of efficacy. They instead advocate the use of well-supported interventions including conventional muscle strengthening exercises, constraint-induced movement therapy, mirror therapy and botulinum toxin to aid upper limb rehabilitation (Hatem et al., 2016).
The body of evidence for lower limb training is similarly weak. A 2017 Cochrane review found no significant effect of robotic therapy on gait velocity or capacity, but suggested a small effect on recovery of independent ambulation when coupled with traditional physical therapy (Mehrholz et al., 2017).
If robotic therapies are poised to deliver the highly repetitive movement ostensibly required for motor recovery, why isn’t the data more compelling?
Emerging research hints at several possible answers, including the idea that “more” may not equate with “better” for the stroke population. ICARE, a large, multi-site study examined the impact of dose on upper limb motor performance in sub-acute stroke (Winstein, Wolf, et al., 2016). The study compared two control groups - usual care occupational therapy and dose-equivalent occupational therapy - with a novel, evidence-based method stressing repetition and task-oriented training in intensive doses (30 hours/10 weeks). Researchers found that the investigational intervention was no more effective than standard, dose-matched therapy with respect to motor outcomes at 12 months post-randomization and that neither intensive treatment regimen was more effective than standard care, despite a more than two-fold disparity in dose (mean of 27 vs. 11 hours per week). These findings suggest that developing more effective therapy may require more than increasing the dose delivered.
Alternatively, the treatment provided by current robotic technologies may be somehow suboptimal. Many devices employ a modular approach to motor learning in that they break down complex actions (e.g. reaching) into joint-specific, repetitive movement patterns (e.g. elbow flexion/extension). This approach attempts to focus a patient’s efforts in order to heighten learning. While this strategy may be intuitive, it is incongruent with our understanding of human development, in which movement is learned holistically through trial and error. The modular approach is also at odds with clinical thinking, which has shifted away from repetitive exercise towards meaningful occupation and functional activities in context. Robotic designers have adapted by linking workstation robotics with increasingly life-like simulations, promoting user engagement through gaming, and moving towards wearable technologies, however, it remains to be seen if these strategies will suffice to provide therapy that is motivating for patients and readily generalized to daily life.
While robotic technologies incorporate a range of designs, a majority of robotic devices employ a single mode of motor facilitation – assist-as-needed support. The assist-as-needed model affords the user the opportunity to practice ideal movements in a repetitive context without reliance on compensatory strategies. In theory, this model allows a user to reduce error and internalize a new barometer of “normal” for reference during daily life. However, research demonstrates that users can become overly reliant on this feedback, limiting their performance when it is removed (Bishop et al., 2017).
Competing therapeutic approaches, such as error augmentation, are based on the strategy that the alteration or exaggeration of errors during training may be best suited to optimize learning. This model has been integrated into newer technologies such as the TPAD for the purpose of better understanding how individuals learn after stroke. Ultimately, robotic devices employing versatile software that can be programmed to test a range of theories, may be best positioned to discern which training paradigm is most effective and to reproduce these principles therapeutically as our understanding evolves.
It bears consideration that the failure of robotic interventions to achieve more dramatic results following stroke may rest not in the limitation of robotic technologies, but perhaps in the biological limits of human recovery. Regenerative therapies or biological interventions (e.g. stem cells) may represent an additional or alternative option to catalyze recovery in the future.
9Factors affecting adoption of technologies
Medical devices in the United States are subject to oversight by the Federal Food and Drug Administration (FDA), while devices in the European Union are managed by the European Commission (EC). In both the United States and Europe, devices are stratified into categories based upon their intended use and risk profile, with the degree of regulation and oversight rising with each class (Van Norman, 2016).
The majority of rehabilitation robotics are considered moderate-risk devices(Römer & Stuyt, 2007), which carry limited requirements to obtain FDA Approval or Conformite Europeenne (CE) Marking. In both the United States and Europe, devices that are considered to be “substantially similar” to already-approved “predicate devices” are absolved from clinical testing requirements, or required to perform limited, less rigorous testing (Van Norman,2016).
As a result of this low barrier to entry, small players in the robotics market are able to innovate without the financial burden of clinical testing. However, questions have been raised as to the sufficiency of these standards regulating medical devices to ensure patient safety by bodies such as the Institute of Medicine and U.S. Congress. Critics also suggest that limited testing requirements may discourage manufacturers from sufficiently investigating their products before bringing them to market (Van Norman, 2016) or leave consumers with limited information regarding the efficacy of new products.
From a business perspective, the allure of new technologies may be an important marketing tool and a point of differentiation in the increasingly competitive healthcare market. However, widespread adoption of robotic technologies has been hindered by several factors, not the least of which is economics. Rehabilitation devices have capitalized on the decreasing costs of advanced components, but they remain costly, with significant overhead and carrying costs for maintenance. Many devices are also quite large, which means that they occupy therapy space that may otherwise be used more efficiently.
One proposed economic advantage of robotic devices is their capacity to mitigate the physical load and intensive staffing requirements of rehabilitation. Like the Continuous Passive Motion (CPM) machine has altered the landscape of orthopedic rehabilitation, these devices may offer similar capacities for provision of basic, physically intensive components of neurological rehabilitation. However, robotics may not ultimately increase productivity or free clinicians for more advanced therapy, as most of the current technologies require ongoing clinical oversight for patient safety.
From a financial perspective, robotic technologies remain inaccessible for many patients. Robotic treatment is costly and considered experimental, based on the limited evidence base, which means insurers do not typically cover it. An initial analysis of the VA Robotics study suggests that the short-term cost of robotic intervention may be offset by lower medical costs over time (Wagner et al., 2011), however, more robust study is warranted to better understand their economic impact.
Fig. 1
Armeo Power upper limb workstation exoskeleton device.
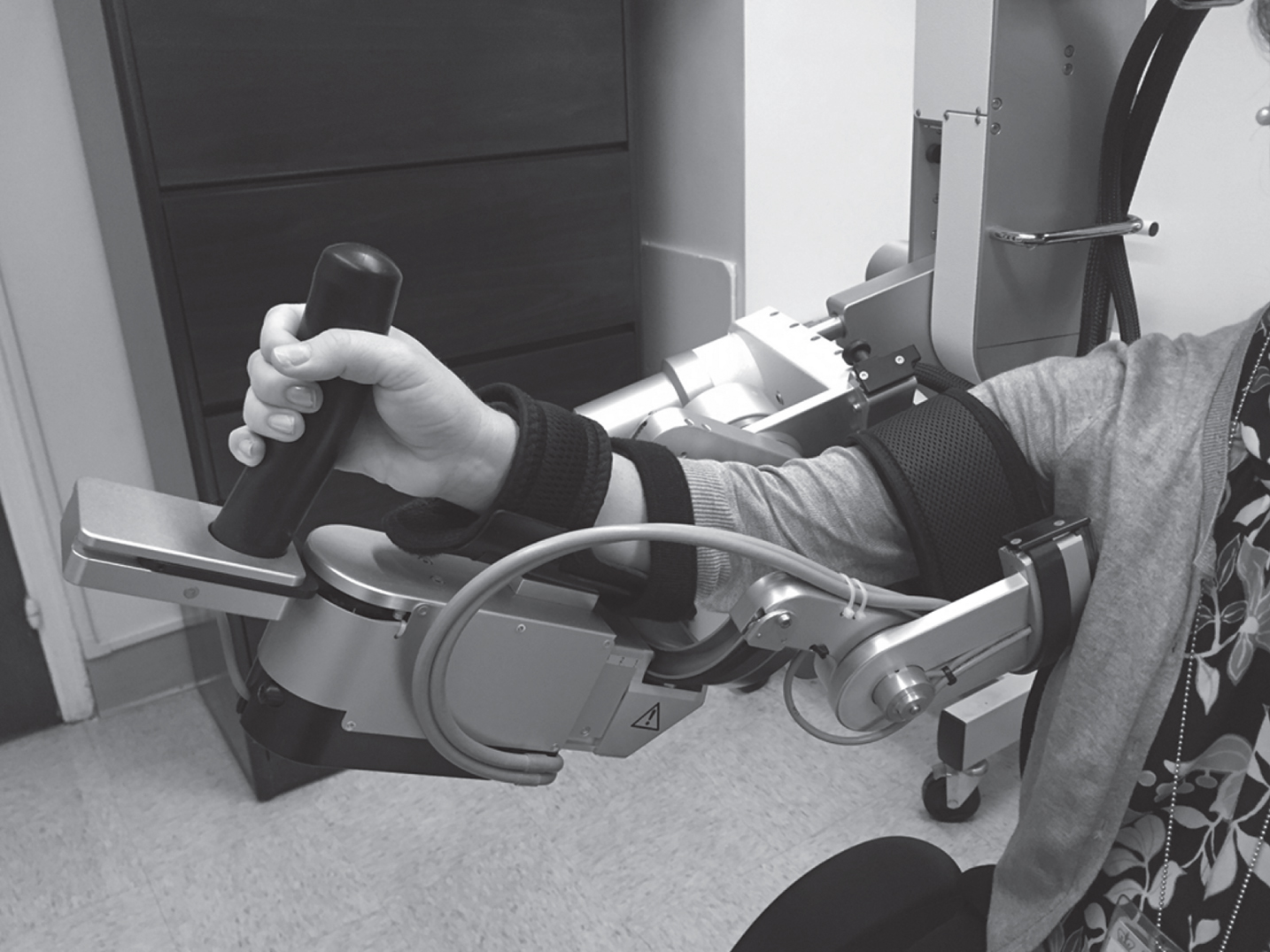
Fig. 2
Tethered Pelvic Assist Device (TPAD). Tethers enhanced digitally to aid visibility.

Fig. 3
Number of Publications with Keywords “Robotics” and “Rehabilitation” on ClinicalTrials.gov by Year.
10New frontiers
Historically, robotic rehabilitation devices have relied on rigid materials for their physical strength and predictable performance. The budding field of soft robotics seeks to upend this model through the use of compliant materials, informed by the biological structures of agile organisms like the octopus (Rus & Tolley, 2015). Soft robotic materials more closely match the physical structures and characteristics of the human body, offering theoretical advantages in patient safety, fit and mobility. In the rehabilitation space, soft robotics may enable more human-like joint function and enhanced adaptability for precise functional tasks, such as grasp.
Still, various challenges lay ahead in this fledgling field. Soft materials, such as silicone rubber, present unique mechanical challenges in that the degrees of freedom they afford make them both incredibly appealing for rehabilitation modalities and tremendously difficult to control. Additionally, soft robotics often employ pneumatic or hydraulic control mechanisms, which are prone to slow actuation rates and require pumps or reservoirs for actuation. At this juncture, soft technologies remain largely beholden to “hard” electronic platforms for their control, which limit their utility. However, so-called soft electronics have gained much attention in the research space as of late, opening up the possibility of a range of technologies, such as proprioceptive stretch sensors, for future integration into stroke rehabilitation. Alternative technologies such as brain-computer interfaces and pattern recognition software may also offer more intuitive control mechanisms for patients in the not-so-distant future.
Among the most exciting aspects of rehabilitation robotics lie in their ability to collect large amounts of data on the kinematics and other aspects of movement. Robotic devices therefore may help us answer the many questions surrounding stroke recovery. Many conventional measures of stroke recovery address specific movement patterns or arbitrary functional tasks, with limited ability to parse out true motor recovery from learned compensation. Kinematic data, on the other hand, provides a direct measurement, which may be distilled into new insight on stroke recovery.
11Conclusions
The American Heart Association’s “2016 Guidelines for Adult Stroke Rehabilitation and Recovery” contains a recommendation that “Robot-assisted movement training to improve motor function and mobility after stroke in combination with conventional therapy may be considered,” based upon the current evidence (Winstein, Stein, et al., 2016). This concisely summarizes the current role of robotics in post-stroke rehabilitation, as neither the standard of care, nor entirely experimental.
Significant barriers to broader dissemination include limited incremental clinical benefit, high cost, and complexity to implement. The ability of scholars and designers to overcome these barriers is likely to dictate the evolution of this important field.
Conflict of interest
Joel Stein, MD: Consultant, Tyromotion, Consultant, Rex Bionics.
Lynne Weber, MA, OTR/L: No disclosures.
References
1 | Bang, D.-H. , & Shin, W.-S. ((2016) ). Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: A randomized controlled pilot trial. NeuroRehabilitation, 38: (4), 343–349, 10.3233/NRE-161325 |
2 | Bishop, L. , Khan, M. , Martelli, D. , Quinn, L. , Stein, J. , & Agrawal, S. ((2017) ). Exploration of two training paradigms using forced induced weight shifting with the tethered pelvic assist device to reduce asymmetry in individuals after stroke case reports. American Journal of Physical Medicine and Rehabilitation, 96: (10),S135–S140. 10.1097/PHM.0000000000000779 |
3 | Bortole, M. , Venkatakrishnan, A. , Zhu, F. , Moreno, J. C. , Francisco, G. E. , Pons, J. L. , & Contreras-Vidal, J. L. ((2015) ). The H2 robotic exoskeleton for gait rehabilitation after stroke: Early findings from a clinical study. Journal of NeuroEngineering and Rehabilitation, 12: (12). 10.1186/s12984-015-0048-y |
4 | Dobkin, B. H. , Duncan, P. W. ((2012) ). Should body-weight supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate?. Neurorehabilitation Neural Repair, 26: (4), 308–317, doi: 10.1177/1545968312439687 |
5 | Duncan, P. W. , Sullivan, K. J. , Behrman, A. L. , Azen, S. P. , Wu, S. S. , Nadeau, S. E. , & Hayden, S. K. ((2011) ). Body-weight– supported treadmill rehabilitation after stroke. New England Journal of Medicine, 364: (21), 2026–2036, 10.1056/NEJMoa1010790 |
6 | Dundar, U. , Toktas, H. , Solak, O. , Ulasli, A. M. , & Eroglu, S. ((2014) ). A comparative study of conventional physiotherapy versus robotic training combined with physiotherapy in patients with stroke. Topics in Stroke Rehabilitation, 21: (6), 453–461, 10.1310/tsr2106-453 |
7 | Fasoli, S. E. , Krebs, H. I. , Stein, J. , Frontera, W. R. , Hughes, R. , & Hogan, N. ((2004) ). Robotic therapy for chronic motor impairments after stroke: Follow-up results. Physical Medicine and Rehabilitation, 85: (7), 1106–1111, 10.1016/j.apmr.2003.11.028 |
8 | Hatem, S. M. , Saussez, G. , della Faille, M. Prist, V. , Zhang, X. , Dispa, D. , & Bleyenheuft, Y. ((2016) ). Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Frontiers in Human Neuroscience, 10: (442), 10.3389/fnhum.2016.00442 |
9 | Hesse, S. , Tomelleri, C. , Bardeleben, A. , Werner, C. , & Waldner, A. ((2012) ). Robot-assisted practice of gait and stair climbing in nonambulatory stroke patients. The Journal of Rehabilitation Research and Development, 49: (4), 613. 10.1682/JRRD.2011.08.0142 |
10 | Hesse, S. , Waldner, A. , & Tomelleri, C. ((2010) ). Innovative gait robot for the repetitive practice of floor walking and stair climbing up and down in stroke patients. Jounal of NeuroEngineering and Rehabilitation, 7: (1), 30. 10.1186/1743-0003-7-30 |
11 | Hornby, T. G. , Campbell, D. D. , Kahn, J. H. , Demott, T. , Moore, J. L. , & Roth, H. R. ((2008) ). Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: A randomized controlled study. Stroke, 39: (6), 1786–1792, 10.1161/STROKEAHA.107.504779 |
12 | Hu, X. L. , Tong, K. Y. , Wei, X. J. , Rong, W. , Susanto, E. A. , & Ho, S. K. ((2013) ). The effects of post-stroke upper-limb training with an electromyography (EMG)-driven hand robot. Journal of Electromyography and Kinesiology. 10.1016/j.jelekin.2013.07.007 |
13 | Husemann, B. , Muller, F. , Krewer, C. , Heller, S. , & Koenig, E. ((2007) ). Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: A randomized controlled pilot study. Stroke, 38: (2), 349–354, 10.1161/01.STR.0000254607.48765.cb |
14 | Klamroth-Marganska, V. , Blanco, J. , Campen, K. , Curt, A. , Dietz, V. , Ettlin, T. , & Riener, R. ((2014) ). Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial. The Lancet Neurology. 10.1016/S1474-4422(13)70305-3 |
15 | Krakauer, J. W. ((2014) ). Study to Enhance Motor Acute Recovery With Intensive Training After Stroke (SMARTS2), Retrieved January 1, (2017) , https://clinicaltrials.gov/ct2/show/NCT02292251 |
16 | Liao, W. W. , Wu, C. Y. , Hsieh, Y. W. , Lin, K. C. , & Chang, W. Y. ((2012) ). Effects of robot-assisted upper limb rehabilitation on daily function and real-world arm activity in patients with chronic stroke: A randomized controlled trial. Clinical Rehabilitation, 26: (2), 111–120, 10.1177/0269215511416383 |
17 | Lo, A. C. , Guarino, P. D. , Richards, L. G. , Haselkorn, J. K. , Wittenberg, G. F. , Federman, D. G. , & Peduzzi, P. ((2010) ). Robot-assisted therapy for long-term upper-limb impairment after stroke. New England Journal of Medicine, 10.1056/NEJMoa0911341 |
18 | Maeshima, S. , Osawa, A. , Nishio, D. , Hirano, Y. , Takeda, K. , Kigawa, H. , & Sankai, Y. ((2011) ). Efficacy of a hybrid assistive limb in post-stroke hemiplegic patients: A preliminary report. BMC Neurology, 11. 10.1186/1471-2377-11-116 |
19 | Mazzoleni, S. , Focacci, A. , Franceschini, M. , Waldner, A. , Spagnuolo, C. , Battini, E. , & Bonaiuti, D. ((2017) ). Robot-assisted end-effector-based gait training in chronic stroke patients: A multicentric uncontrolled observational retrospective clinical study. NeuroRehabilitation, 40: (4), 483–492, 10.3233/NRE-161435 |
20 | Meeker, C. , Park, S. , Bishop, L. , Stein, J. , & Ciocarlie, M. ((2017) ). EMG pattern classification to control a hand orthosis for functional grasp assistance after stroke, In 2017 International Conference on Rehabilitation Robotics (ICORR), (pp. 1203–1210), 10.1109/ICORR.2017.8009413 |
21 | Mehrholz, J. , Pohl, M. , Platz, T. , Kugler, J. , & Elsner, B. ((2015) ). Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. In Cochrane Database of Systematic Reviews, 10.1002/14651858.CD006876.pub4 |
22 | Mehrholz, J. , Thomas, S. , Werner, C. , Kugler, J. , Pohl, M. , & Elsner, B. ((2017) ). Electromechanical-assisted training for walking after stroke. Cochrane Database of Systematic Reviews, 10.1002/14651858.CD006185.pub4 |
23 | Molteni, F. , Gasperini, G. , Gaffuri, M. , Colombo, M. , Giovanzana, C. , Lorenzon, C. , & Guanziroli, E. ((2017) ). Wearable robotic exoskeleton for over-ground gait training in sub-acute and chronic hemiparetic stroke patients: Preliminary results. European Journal of Physical and Rehabilitation Medicine, 10.23736/S1973-9087.17.04591-9 |
24 | Nilsson, A. , Vreede, K. S. , Haglund, V. , Kawamoto, H. , Sankai, Y. , & Borg, J. ((2014) ). Gait training early after stroke with a new exoskeleton - The hybrid assistive limb: A study of safety and feasibility. Journal of NeuroEngineering and Rehabilitation, 11: (1), 10.1186/1743-0003-11-92 |
25 | Orihuela-Espina, F. , Roldán, G. F. , Sánchez-Villavicencio, I. , Palafox, L. , Leder, R. , Sucar, L. E. , & Hernández-Franco, J. ((2016) ). Robot training for hand motor recovery in subacute stroke patients: A randomized controlled trial. Journal of Hand Therapy, 29: (1), 51–57, 10.1016/j.jht.2015.11.006 |
26 | Page, S. J. , Fulk, G. D. , & Boyne, P. ((2012) ). Clinically important differences for the upper-extremity fugl-meyer scale in people with minimal to moderate impairment due to chronic stroke. Physical Therapy, 92: (6), 791–798, 10.2522/ptj.20110009 |
27 | Peters, H. T. , Page, S. J. , & Persch, A. ((2016) ). Giving them a hand: Wearing a myoelectric elbow-wrist-hand orthosis reduces upper extremity impairment in chronic stroke. Archives of Physical Medicine and Rehabilitation, 10.1016/j.apmr.2016.12.016 |
28 | Pollock, A. , St George, B. , Fenton, M. , & Firkins, L. ((2012) ). Top ten research priorities relating to life after stroke. Lancet Neurology, 11: (3), 209. 10.1016/S1474-4422(12)70029-7 |
29 | Römer, G. R. B. E. , & Stuyt, H. J. A. ((2007) ). Compiling a medical device file and a proposal for an international standard for rehabilitation robots, In 2007 IEEE 10th International Conference on Rehabilitation Robotics, ICORR’07, 10.1109/ICORR.2007.4428471 |
30 | Rus, D. , & Tolley, M. T. ((2015) ). Design, fabrication and control of soft robots. Nature, 10.1038/nature14543 |
31 | Sale, P. , Lombardi, V. , & Franceschini, M. ((2012) ). Hand robotics rehabilitation: Feasibility and preliminary results of a robotic treatment in patients with hemiparesis. Stroke Research and Treatment, 2012: , 820931. 10.1155/2012/820931 |
32 | Schwartz, I. , Sajin, A. , Fisher, I. , Neeb, M. , Shochina, M. , Katz-Leurer, M. , & Meiner, Z. ((2009) ). The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: A randomized controlled trial. PM and R, 1: (6), 516–523, 10.1016/j.pmrj.2009.03.009 |
33 | Stein, J. , Bishop, L. , Stein, D. J. , & Wong, C. K. ((2014) ). Gait training with a robotic leg brace after stroke. American Journal of Physical Medicine & Rehabilitation, 93: (11), 987–994, 10.1097/PHM.0000000000000119 |
34 | Ueba, T. , Hamada, O. , Ogata, T. , Inoue, T. , Shiota, E. , & Sankai, Y. ((2013) ). Feasibility and safety of acute phase rehabilitation after stroke using the hybrid assistive limb robot suit. Neurol Med Chir (Tokyo), 53: (287), 287–Tokyo, https://www.jstage.jst.go.jp/article/nmc/53/5/53_2012-0314/_pdf |
35 | Van Kammen, K. , Boonstra, A. M. , Van Der Woude, L. H. V. , Reinders-Messelink, H. A. , & Den Otter, R. ((2016) ) The combined effects of guidance force, bodyweight support and gait speed on muscle activity during able-bodied walking in the Lokomat, 10.1016/j.clinbiomech.2016.04.013 |
36 | Van Norman, G. A. ((2016) ). Drugs and devices: Comon of European and U.S. approval processes. JACC: Basic to Translational Science, 1: (5), 399–412, 10.1016/J.JACBTS.2016.06.003 |
37 | Veerbeek, J. M. , Langbroek-Amersfoort, A. C. , van Wegen, E. E. H. , Meskers, C. G. M. , & Kwakkel, G. ((2017) ). Effects of robot-assisted therapy for the upper limb after stroke: A systematic review and meta-analysis. Neurorehabilitation and Neural Repair, 31: (2), 107–121, 10.1177/1545968316666957 |
38 | Volpe, B. T. , Krebs, H. I. , Hogan, N. , Edelsteinn, L. , Diels, C. M. , & Aisen, M. L. ((1999) ). Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology, 53: (8), 1874–1876, 10.1212/WNL.53.8.1874 |
39 | Wagner, T. H. , Lo, A. C. , Peduzzi, P. , Bravata, D. M. , Huang, G. D. , Krebs, H. I. , & Guarino, P. D. ((2011) ). An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke, 10.1161/STROKEAHA.110.606442 |
40 | Winstein, C. J. , Stein, J. , Arena, R. , Bates, B. , Cherney, L. R. , Cramer, S. C. , & Zorowitz, R. D. ((2016) ). Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 10.1161/STR.0000000000000098 |
41 | Winstein, C. J. , Wolf, S. L. , Dromerick, A. W. , Lane, C. J. , Nelsen, M. A. , Lewthwaite, R. , & Azen, S. P. ((2016) ). Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: The ICARE randomized clinical trial. Jama, 315: (6), 10.1001/jama.2016.0276 |



