Analyses of Brucella pathogenesis, host immunity, and vaccine targets using systems biology and bioinformatics
- 1 Unit for Laboratory Animal Medicine, Department of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, MI, USA
- 2 Center for Computational Medicine and Bioinformatics, University of Michigan Medical School, Ann Arbor, MI, USA
Brucella is a Gram-negative, facultative intracellular bacterium that causes zoonotic brucellosis in humans and various animals. Out of 10 classified Brucella species, B. melitensis, B. abortus, B. suis, and B. canis are pathogenic to humans. In the past decade, the mechanisms of Brucella pathogenesis and host immunity have been extensively investigated using the cutting edge systems biology and bioinformatics approaches. This article provides a comprehensive review of the applications of Omics (including genomics, transcriptomics, and proteomics) and bioinformatics technologies for the analysis of Brucella pathogenesis, host immune responses, and vaccine targets. Based on more than 30 sequenced Brucella genomes, comparative genomics is able to identify gene variations among Brucella strains that help to explain host specificity and virulence differences among Brucella species. Diverse transcriptomics and proteomics gene expression studies have been conducted to analyze gene expression profiles of wild type Brucella strains and mutants under different laboratory conditions. High throughput Omics analyses of host responses to infections with virulent or attenuated Brucella strains have been focused on responses by mouse and cattle macrophages, bovine trophoblastic cells, mouse and boar splenocytes, and ram buffy coat. Differential serum responses in humans and rams to Brucella infections have been analyzed using high throughput serum antibody screening technology. The Vaxign reverse vaccinology has been used to predict many Brucella vaccine targets. More than 180 Brucella virulence factors and their gene interaction networks have been identified using advanced literature mining methods. The recent development of community-based Vaccine Ontology and Brucellosis Ontology provides an efficient way for Brucella data integration, exchange, and computer-assisted automated reasoning.
Introduction
Brucella abortus is a Gram-negative, facultative intracellular bacterium that causes brucellosis in humans and many animals (Corbel, 1997). The brucellae are taxonomically placed in the alpha-2 subdivision of the class Proteobacteria. There are 10 species of Brucella based on preferential host specificity: B. melitensis (goats), B. abortus (cattle), B. suis (swine), B. canis (dogs), B. ovis (sheep), B. neotomae (desert mice), B. cetacea (cetacean), B. pinnipedia (seal), B. microti (voles), and B. inopinata (unknown) (O’Callaghan and Whatmore, 2011). Of 10 recognized species of Brucella, B. abortus, B. melitensis, B. suis, and B. canis are pathogenic to humans. Human infections with B. canis are rare. B. abortus, B. melitensis, and B. suis are the most pathogenic to humans, have been identified as agents amenable for use in bio-terrorism, and are listed as category B priority pathogens by the US Center for Disease Control (CDC). Brucellosis is one of the most common zoonotic diseases. It infects annually approximately 500,000 humans worldwide. Upon entry into human or animals, the bacteria invade the blood stream and lymphatics where they multiply inside phagocytic cells and eventually cause septicemia. Symptoms include undulant fever, abortion, asthenia, endocarditis, and encephalitis.
Brucella lacks well-known bacterial virulence factors such as cytolysins, capsules, exotoxins, secreted proteases, fimbriae, phage-encoded toxins, and virulence plasmids (DelVecchio et al., 2002; Paulsen et al., 2002). The brucellae infect phagocytic macrophages and non-phagocytic epithelial cells (e.g., HeLa cells) in vivo and in vitro (Ko and Splitter, 2003; Kohler et al., 2003; Roop et al., 2004). Brucella virulence relies on the ability to survive and replicate in the vacuolar phagocytic compartments of macrophages. Many Brucella virulent factors, such as lipopolysaccharide (LPS; Lapaque et al., 2005), type IV secretion system (T4SS; O’Callaghan et al., 1999; de Jong et al., 2008), and the BvrR/BvrS two-component system (Guzman-Verri et al., 2002), have been identified to be critical in the intracellular process of Brucella inside macrophages (Xiang et al., 2006). While these virulence factors may not directly mediate clinical manifestations of brucellosis, they are critical for Brucella to survive and replicate inside host cells. While prolonged persistence of the brucellae in macrophages leads to the chronic infection, extensive replication of the bacteria in placental trophoblasts results in acute reproductive tract pathology and abortion in natural hosts (Roop et al., 2009). Specifically, the Brucella lifecycle contains two phases: (i) chronic infection of phagocytic macrophage leading to Brucella survival and replication, and (ii) acute infection of non-phagocytic epithelial cells leading to reproductive tract pathology and abortion. Spleen and liver are the organs that contain many bacterial cells after Brucella invasion. After a majority of Brucella cells are killed in vivo, the remaining Brucella cells will persist and live for a long time in vivo (Hort et al., 2003).
Although antibodies specific for the O-antigen (i.e., O polysaccharide or O-side chain) of the lipopolysaccharide can confer partial protection in some host species, cell-mediated immunity (CMI) plays a critical role in protection against virulent Brucella infection. The maturation and proinflammatory production of cytokines of dendritic cells is critical for controlling Brucella infections (Macedo et al., 2008). Recently we found that B. abortus vaccine strain RB51 and B. suis vaccine candidate VTRS1 induce caspase-2-mediated apoptotic and necrotic macrophage cell death (Chen and He, 2009; Chen et al., 2011). The programmed cell death is inhibited by virulent Brucella strains. Caspase-2-mediated cell death induced by vaccine strain RB51 may promote an effective Brucella antigen presentation by a cross-priming mechanism (Bevan, 2006; Chen and He, 2009). Passive transfer assays with mice suggest that both CD4+ and CD8+ T cells are important in protective immunity against brucellosis (Araya et al., 1989; Araya and Winter, 1990). To confer protection against B. abortus infection, immune CD4+ T cells secrete many cytokines, including gamma interferon (IFN-γ) that stimulates the antimicrobial activity of macrophages (Jiang and Baldwin, 1993; Zhan and Cheers, 1993; He et al., 2001). A crucial role of IFN-γ in the resistance to Brucella infection was demonstrated in mice by in vivo antibody neutralization experiments (Zhan and Cheers, 1993) and an IFN-γ knockout mouse study (Murphy et al., 2001). CD8+ cytotoxic T lymphocytes (CTL) are critical in killing Brucella-infected target cells (Oliveira and Splitter, 1995; He et al., 2001).
Brucella abortus strain RB51 and strain 19 and B. melitensis strain Rev. 1 have been used as commercial animal brucellosis vaccines (Schurig et al., 2002). Strain 19 is the first effective live attenuated Brucella vaccine widely used in the world. This smooth strain induces anti-O-antigen antibody in the host. Since this serological response is used for brucellosis diagnosis in the field, Strain 19-induced antibody response is often misdiagnosed as the sign of virulent Brucella infection. Cattle brucellosis vaccine strain RB51 is a rough live attenuated B. abortus strain derived from smooth virulent strain 2308 (Schurig et al., 1991). RB51 does not induce an anti-O-antigen serological antibody response, thus does not interfere with serological diagnosis. Rev. 1 protects sheep and goats from infection with B. melitensis. However, these vaccine strains cannot be used in humans due to their pathogenicity. There is no safe, effective human brucellosis vaccine. However, such a human vaccine is desired for improving public health and biosafety. To rationally design a safe and effective brucellosis vaccine, it is important to further understand the mechanisms of Brucella pathogenesis and protective Brucella immunity.
Systems biology aims to understand biological systems on a system level using interdisciplinary technologies. In contrast to the traditional reductionist molecular approach, which focuses on understanding the roles of single genes or proteins, systems biology applies a more holistic approach by studying networks and the interactions between individual components of networks (Kuster et al., 2011). The goal of systems biology is to understand the structure, dynamics, and interactions of whole cells rather than portions thereof. Systems biology treats an organism (e.g., Brucella and human) as an integrated cellular system consisting of an interacting network of genes, proteins, and molecular cellular components including their biochemical/biophysical reactions. Biological data and software tools for data analysis are two basic ingredients in systems biology. The high throughput experimental “omics” (Omics) technologies, including genomics, transcriptomics, proteomics, and metabolomics, are major driving forces of systems biology (Kay and Wren, 2009; Zhang et al., 2010). The development of genome-scale computational and bioinformatic tools allows analysis and modeling of metabolic, regulatory and signaling networks of the cell at the systems-level.
Bioinformatics is the application of a combination of computer science, statistics, mathematics, and information technology to the field of biology and medicine. Bioinformatics enables the discovery of new biological insights and creates a global perspective of unifying principles in biology. Bioinformatics emerged as a scientific field in 1990s when large amounts of nucleotide and amino acid sequences were generated. At the time, bioinformatics took a role of generating and maintaining databases to store biological information and to support sequence data analyses. Subsequently, bioinformatics has evolved leading to the development of new computational algorithms, statistics methods, and tools to integrate, manage, and analyze various biological data including high throughput Omics data and literature data.
Brucella research has benefited from the application of cutting edge systems biology and bioinformatics technologies. The availability of Brucella and host (e.g., human and mouse) genomes allow comparative genomic analyses of host specificity, virulence analysis, and rational vaccine target design. High throughput array technologies have been developed for analyses of transcriptomics and proteomics gene expression profiles of host and Brucella in vitro and in vivo. These analyses have resulted in a better understanding of host–Brucella interactions and Brucella pathogenesis. Advanced literature mining approaches are also used to identify Brucella virulence factors and genetic interaction pathways. Various Brucella databases are publicly accessible for query and analysis of structured data. Recently, ongoing ontology studies have facilitated Brucella data integration and computer-assisted automated reasoning. This article provides a comprehensive review of the applications of advanced systems biology and bioinformatics to the study of Brucella pathogenesis, host–Brucella interactions, and for the development of Brucella vaccines.
Comparative Brucella Genomics for Understanding Brucella Genetic Conservation, Variability, and Host Specificity
While the mechanism of Brucella host specificity is still unclear, comparative Brucella genomics has permitted identification of gene variability among different Brucella species and strains, resulting in a better understanding of Brucella virulence and adaptation in different hosts.
The genome of B. melitensis strain 16 M was first sequenced and published in 2002 (DelVecchio et al., 2002). Since then, B. suis strain 1330 (Paulsen et al., 2002), and B. abortus strains 2308 (Chain et al., 2005) and 9-941 (Halling et al., 2005), and vaccine B. abortus strain 19 (Crasta et al., 2008) have been sequenced and published in peer-reviewed journals. As of October 11, 2011, the NCBI genome sequence site has been found to contain 14 sequenced Brucella genomes1. Furthermore, 25 additional Brucella genomes have been sequenced by the Broad Institute. These are available for public query, download, and further analysis at URL: http://www.broadinstitute.org/annotation/genome/brucella_group/GenomeStats.html. Therefore, at least 39 Brucella strains have been sequenced. With the increasing number of sequenced bacterial genomes, it becomes possible to conduct a systematic comparative analysis of whole genomes of different Brucella strains and to dissect their genetic conservation and variability.
Each Brucella genome contains two circular chromosomes. The size of Chromosome I and II approximates 2.2 and 1.1 Mb, respectively. There are about 3200–3400 genes in each genome. Based on DNA–DNA hybridization studies, the genus Brucella is a highly homogeneous group (>90% DNA identity among all nomenspecies; Verger et al., 2000). Ratushna et al. (2006) compared the genome sequences of B. abortus strain 9-941, B. melitensis strain 16 M, and B. suis strain 1330. A majority (>90%) of annotated genes in these three genomes share 98–100% sequence identity at a nucleotide level. A majority of differentiating genes among these three species are located in large (∼20 kb) regions (Ratushna et al., 2006). Whatmore et al. (2007) examined nine discrete genomic loci that correspond to 4396 bp of sequence from 160 Brucella isolates. A phylogeny analysis using the multilocus sequences showed that four classical Brucella species, B. abortus, B. melitensis, B. ovis, and B. neotomae are well-separated clusters in the phylogenic tree structure. With the exception of biovar 5, B. suis isolates cluster together. B. canis isolates are located on a phylogenic branch closely related to B. suis biovar 3 and 4 isolates. Marine mammal isolates represent a distinct cluster (Whatmore et al., 2007). The major conclusion of the phylogenic tree analysis was verified by another maximum likelihood phylogenetic analysis of the 10 Brucella strains (Wattam et al., 2009). That B. suis is a single species has been questioned since it has a broader host specificity but does not have any identified species-specific markers (Moreno et al., 2002).
Since limited genome diversity exists among different Brucella species, a comparison of Brucella species whole genomes is a powerful tool to identify Brucella gene variability that is responsible for differences in host preference and virulence restriction. The sequence insertion/deletion events may contribute to host specificity between different Brucella species. Rajashekara et al. (2004) used the complete genome sequence of B. melitensis 16 M, a strain highly pathogenic to humans, to construct a genomic microarray. Hybridization of labeled genomic DNA from different Brucella strains to this microarray identified 217 open reading frames (ORFs) that were altered in five Brucella species, including B. abortus, B. suis, B. canis, B. ovis, and B. neotomae. Many of the ORFs are located in the 16 M genome in nine regions (genomics islands) ranging in size from 5 to 44 kb. Genomic islands lost in a given species are often restricted to that particular species (Rajashekara et al., 2004). The genomic islands missing in B. ovis are present in Brucella species pathogenic to humans. However, B. neotomae, a non-pathogenic species in humans and domestic animals, also possesses these islands. Interestingly, the genetic islands identified do not encode adhesins or secreted virulence factors that contribute to host specificity in other bacterial species (Moon et al., 1977; Tsolis et al., 1999; Inatsuka et al., 2005). It is likely that adhesins and secreted virulence factors are encoded in conserved loci where they are differentially expressed or inactivated by point mutations. As seen in Bordetella species (Parkhill et al., 2003), gene inactivation or altered gene regulation may contribute to differences in host range and virulence of Brucella species in humans.
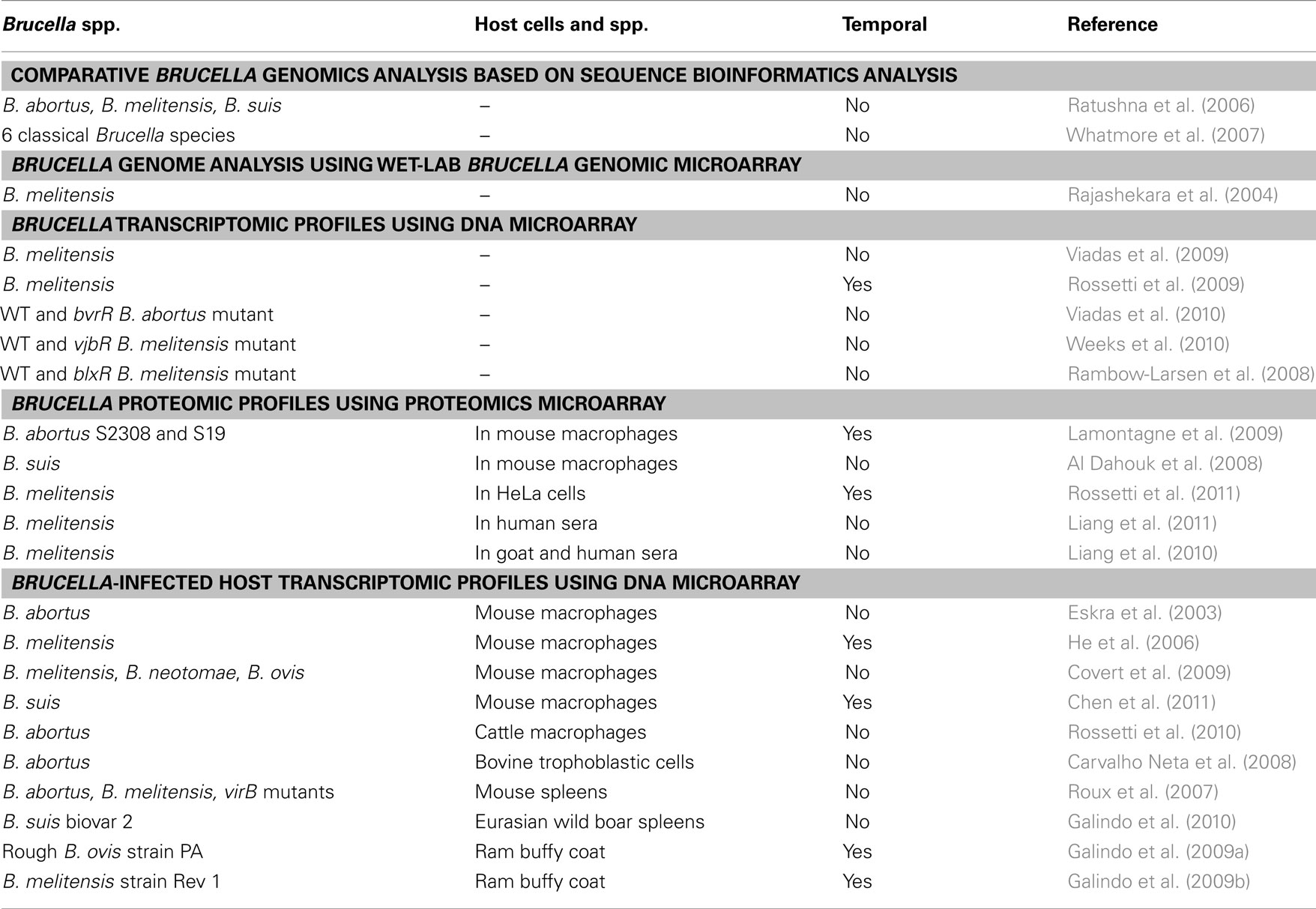
Table 1 lists studies published on Brucella pathogenesis and host immunity using high throughput transcriptomics and proteomics methods. These diverse studies are described in detail in the following sections.
Analysis of Brucella Gene Expression Profiles and Regulatory Responses from in vitro Cultures Using DNA Microarrays
DNA microarrays have been used to delineate Brucella pathogenesis mechanisms. Viadas et al. (2009) generated a Brucella whole-genome DNA microarray based on a comprehensive collection of B. melitensis ORF clones or ORFeomes. The Brucella DNA microarray was used to determine the global transcriptional profile of B. abortus grown under laboratory conditions. Ribosomal proteins, Krebs cycle, and oxidative phosphorylation enzymes were found to have overexpressed transcripts. T4SS virB operon, flagellar components, and other genes related to virulence and intracellular growth were poorly transcribed. This report demonstrated the usefulness of the ORFeome for the construction of a PCR product microarray for analysis of global gene expression in Brucella and may be applied to other microorganisms as well.
Rossetti et al. (2009) found that B. melitensis cells grown in the late-log growth phase are more invasive in HeLa (a representative epithelial cell line) cells compared to the brucellae grown to the mid-log or stationary growth phase. To identify candidate pathogen genes involved in invasion of epithelial cells, cDNA microarrays were used to characterize genome-wide transcript changes of B. melitensis genes in late-log growth phase (the most invasive culture) compared to the stationary growth phase (the least invasive culture). At the late logarithmic growth phase, virulent B. melitensis is more invasive in HeLa epithelial cells than the mid-logarithmic or stationary growth phases. Compared to the stationary growth phase, 414 up- and 40 down-regulated genes were identified in late logarithmic growth phase. The majority of up-regulated genes in the late-log phase cultures were associated with growth, including DNA replication, transcription, translation, intermediate metabolism, energy production and conversion, membrane transport, and biogenesis of the cell envelope and outer membrane. Down-regulated genes were distributed among several functional categories (Rossetti et al., 2009).
The two-component BvrR/BvrS system is essential for B. abortus virulence. To determine the genes regulated by BvrR/BvrS, Viadas et al. (2010) performed a whole-genome microarray analysis using B. abortus RNAs obtained from wild type and bvrR mutant cells grown in vitro under the same conditions. Among 127 differentially expressed genes, 83 were up-regulated and 44 were down-regulated in the bvrR mutant. Many genes involved in cell envelope or outer membrane biogenesis, including the outer membrane proteins (OMPs; Omp25a and Omp25d), lipoproteins, stress response proteins, chaperones, flagellar genes, ABC transport protein, and genes for lipopolysaccharide (LPS) and fatty acid biosynthesis, were differentially expressed. Ten genes related to carbon metabolism (e.g., pckA and fumB) were up-regulated in the bvrR mutant. Three denitrification genes (nirK, norC, and nosZ) were also regulated. The two-component system also affects seven transcriptional regulators including VjbR, ExoR, and OmpR. Therefore, the Brucella BvrR/BvrS system modulates cell envelope biogenesis, controls the carbon and nitrogen metabolism, and interact with other regulators to ensure the survival of Brucella in an extracellular environment as well as an intracellular niche (Viadas et al., 2010).
The quorum sensing (QS) communication system regulates gene expression in response to population density and often regulates virulence determinants as well. QS typically follows production of an auto inducer such as acyl-homoserine lactone (AHL). Among the proteobacteria, the AHL signal is synthesized by luxI and interacts with the transcriptional regulator LuxR. Deletion of Brucella vjbR, a LuxR-like transcriptional regulator, greatly attenuates intracellular survival of B. melitensis. To better define the role of VjbR and QS in Brucella virulence and survival, Weeks et al. (2010) used microarrays to analyze gene expression profiles of Brucella under the control of VjbR and an AHL signal (N-dodecanoyl homoserine lactone, C12-HSL). Specifically, wild type B. melitensis and isogenic ΔvjbR transcriptomes were grown in the presence and absence of exogenous C12-HSL. A comparison of VjbR and C12-HSL transcriptomes identified shared regulation of 127 genes. Of these genes, all but three genes were inversely regulated. These results suggest that C12-HSL functions via VjbR to reverse gene expression. In the absence of VjbR and in the presence of C12-HSL, 48 genes were up-regulated at the stationary growth phase. The differentially regulated genes included adhesins, proteases, antibiotic and toxin resistance genes, stress survival aids, transporters, membrane biogenesis genes, amino acid metabolism and transport, transcriptional regulators, energy production genes, and fliF and virB operons. Many of the differentially regulated genes have been identified as virulence factors in other bacterial pathogens. Therefore, it can be concluded that VjbR and C12-HSL contribute to virulence and survival by regulating expression of virulence mechanisms (Weeks et al., 2010).
In addition to VjbR, the first LuxR-type regulatory protein identified in Brucella, Rambow-Larsen et al. (2008) identified a second LuxR-type regulatory protein (BlxR) in Brucella. Microarray analysis of a blxR mutant suggests that BlxR regulates the expression of genes encoding the T4SS and flagella. These results were confirmed by experimental evidence by deletion of blxR in B. melitensis. Both BlxR and VjbR are positively auto-regulated and cross-regulate the expression of each other (Rambow-Larsen et al., 2008).
Analysis of Brucella Gene Expression Profiles Inside Host Cells
The virulence of Brucella relies heavily on their ability to survive and replicate within the vacuolar phagocytic compartments of macrophages (Baldwin and Winter, 1994; Roop et al., 2009). After phagocytosis by macrophages, the brucellae reside within a vacuole that interacts with early endosomes. These Brucella-containing vacuoles (BCVs) avoid further interactions with the endocytic pathway and interact with endoplasmic reticulum (ER). After sustained interaction and fusion with the ER, mature BCVs become replicative compartments (i.e., replicative phagosomes) with ER-like properties. This late maturation event (for the biogenesis of an ER-derived replicative organelle) requires a functional T4SS (Celli et al., 2003). Virulent brucellae successfully fuse with ER cysternae and survive and multiply. However, attenuated brucellae fail to fuse with ER and are destroyed inside of the host phagolysosomes.
To investigate physiological adaptations of virulent Brucella in its intracellular lifecycle, Lamontagne et al. (2009) infected murine macrophages with virulent B. abortus 2308 or attenuated B. abortus vaccine strain 19 and then compared the proteomes of intracellular Brucella recovered at 3, 20, and 44 h after macrophage infections. In total, 190 Brucella proteins were differentially expressed in the time course of infections. Ninety Brucella proteins were uniquely differentially expressed by strain 2308. Thirty proteins were only differentially expressed by strain 19. The remaining 70 proteins were differentially expressed by both strains. In virulent strain 2308, carbohydrate based carbon utilization and protein synthesis processes were initially reduced when the cells switched to alternative energy sources and low oxygen tension respiration. In the later stages of strain 2308 infection, the expressions of proteins related to key metabolic processes, protein synthesis, iron acquisition, and transport were significantly up-regulated, and its cell envelope actively modified. In contrast, strain 19 adjusted its metabolic profile to a lower degree in the early stage of infection. In the later stage of infection, strain 19 was unable to revert to pre-infection protein expression levels in key processes (Lamontagne et al., 2009).
Al Dahouk et al. (2008) used a 2-D DIGE approach to characterize the intramacrophagic proteome of B. suis at alate stage of in vitro infection. Compared to extracellularly grown, stationary-phase bacteria, the concentrations of 168 proteins were altered. The majority of the 44 proteins differentially regulated at the late stage of infection participated in bacterial metabolism. Of these, 40% were down-regulated. These results indicate that intramacrophagic B. suis has an adaptive response in terms of quantitative reduction of processes involving energy, protein, and nucleic acid metabolism.
Brucella infects hosts primarily by adhering and penetrating mucosal epithelium surfaces. Similar to the kinetics profile of Brucella inside macrophages, virulent Brucella have an initial adaption period followed by a replicative phase inside epithelial cells. Using cDNA microarray analysis, Rossetti et al. (2011) characterized the transcriptional profile of the intracellular pathogen B. melitensis at 4 h (adaptation period) and at 12 h (replicative phase) following infection of HeLa cells. The study found that 161 and 115 Brucella genes were differentially expressed at 4 and 12 h, respectively, post infection. Most of the genes expressed were involved in pathogen growth and metabolism. At the adaptation period, 126 (78% of 161) genes were down-regulated. At the replicative phase, 86 (75% of 115) genes were up-regulated.
Macrophage Immune Responses to Brucella Infections Based on Omics Gene Expression Data Analysis
The above section reviewed two proteomics and one transcriptomics studies concerning profiling gene expression patterns of different Brucella strain inside infected macrophages (Al Dahouk et al., 2008; Lamontagne et al., 2009). DNA microarray analysis has been used frequently to analyze transcriptomic gene expression profiles in murine macrophages infected with virulent Brucella strains (Eskra et al., 2003; He et al., 2006). Five studies on this topic have been reported and summarized below.
Using Affymetrix murine U74A gene microarrays, Eskra et al. (2003) found that over 140 genes, of the >6000 genes, were reproducibly differentially transcribed in RAW264.7 macrophages infected with B. abortus for 4 h. Initially, an increase in the transcription of a number of proinflammatory cytokines and chemokines, such as TNF-α, IL-1α, and IL-1β, was observed. However, transcription of receptors and cytokines associated with antigen presentation, e.g., MHC class II and IL-12p40, were not found at 4 h post infection. Virulent B. melitensis also inhibited transcription of various host genes involved in apoptosis and intracellular vesicular trafficking. It appears that Brucella utilizes specific mechanisms to inhibit many cell pathways (Eskra et al., 2003).
Covert et al. (2009) subsequently demonstrated that the infections with B. melitensis, B. neotomae, and B. ovis bacteria for 4 h elicit common and distinctive defense transcriptional responses of RAW 264.7 macrophages. Although few B. melitensis and B. neotomae cells enter macrophages, B. ovis cells are readily ingested by macrophages. Macrophages infected with these different Brucella species demonstrated common changes in gene expression compared to uninfected macrophages. Compared to uninfected macrophages, macrophage infections with all three Brucella species induced increased transcript levels of 72 genes including chemokines and defense response genes (e.g., IL-1β, MIP-1α, Fas, and TNF). Meanwhile, decreased transcript levels of 68 genes, such as genes associated with vesicular trafficking (e.g., Rab3d) and response to external stimulus (e.g., IL-17a), were identified in macrophages infected with all three Brucella species. Genes with altered transcript levels of Brucella-infected macrophages may correlate with Brucella species-specific host defenses and intracellular survival strategies. B. melitensis, but not B. neotomae or B. ovis, is pathogenic to human. Correspondingly, the infection with B. melitensis, but the other two Brucella spp., induced decreased gene expression in growth arrest (Gas2), immunoglobulin receptor (Fc gamma RI), and chemokine receptor (Cxcr4) genes (Covert et al., 2009).
He et al. (2006) analyzed the time course response of J774.A1 macrophages during infection with virulent B. melitensis strain 16 M using Affymetrix mouse 430 2.0 array containing more than 39,000 genes. Transcriptions of 243 up-regulated and 1053 down-regulated genes were identified at 4 h post infection compared to uninfected macrophages. However, compared to uninfected macrophages, only 12 genes were found up- or down-regulated after 24 h, and no genes were found differentially regulated at 48 h post infection. Although many pro-apoptosis genes were up-regulated, it is noteworthy that the caspase cascade pathways were not activated. These results suggest that some upstream component(s) that induces caspase activation is suppressed. Interestingly, caspase-2, a caspase that regulates the release of cytochrome c from the mitochondria, was down-regulated. Furthermore, 106 mitochondria-associated genes were down-regulated while only 4 mitochondria-associated genes were up-regulated at 4 h post infection (He et al., 2006). It seems that B. melitensis 16 M may prevent apoptosis in macrophages by suppressing mitochondrial gene expression involved in cytochrome c release, reactive oxygen species (ROS) production, and mitochondrial membrane permeability, thereby preventing activation of caspase cascades. Prevention of apoptosis in macrophages by B. melitensis strain 16 M ensures extensive replication after the initial killing stage. Such inhibition may contribute to the ability of Brucella spp. to persist chronically in the reticuloendothelial system of infected humans and animals. Many of the hypotheses generated from the microarray analyses were later confirmed by other studies (Chen and He, 2009; Chen et al., 2011). For example, wet-lab experiments from the same group found that smooth B. abortus strain 2308 prevents mitochondrial permeability and the release of cytochrome c from mitochondria. Smooth virulent Brucella strains that contain intact LPS are capable of inhibiting programmed cell death in infected human and mouse macrophages (Gross et al., 2000; Tolomeo et al., 2003; He et al., 2006). Rough attenuated Brucella strains, which lack O-antigen or produce extremely low levels of the antigen, cannot survive inside macrophages and indeed induce programmed cell death (Fernandez-Prada et al., 2003; Rittig et al., 2003; Pei and Ficht, 2004). The author’s laboratory found that rough and live attenuated B. abortus strains RB51 (the current cattle vaccine) and RA1 induced a caspase-2-mediated apoptotic and necrotic macrophage cell death (Chen and He, 2009). An inhibition of caspase-2 prevents cytochrome c release and almost completely inhibited cell death induced by these rough strains.
Brucella suis primarily infects pigs and is pathogenic to humans. Our studies reveal that smooth virulent B. suis strain 1330 (S1330) prevents programmed cell death of infected macrophages. However, rough attenuated B. suis strain VTRS1 (a vaccine candidate) induces a high level of macrophage cell death. Like B. abortus vaccine strain RB51, VTRS1 has a Brucella wboA gene mutation, which results in the deficiency of LPS O-antigen as well as the rough phenotype (Winter et al., 1996). An Affymetrix microarray study was conducted to analyze temporal transcriptional responses of murine macrophage-like J774.A1 cells infected with S1330 or VTRS1, 17,685 probe sets were significantly up- or down-regulated depending on Brucella strain, time, and the interaction between the strain and time (Chen et al., 2011). A miniTUBA dynamic Bayesian network analysis predicted that VTRS1-induced macrophage cell death was mediated by a proinflammatory gene TNF-α, an NF-κB pathway gene IκB-α, and caspase-2. Compared to S1330, VTRS1-induced a dramatically higher level of proinflammatory response as indicated by increased transcriptions of 40 proinflammatory genes. Increased protein level production of TNF-α and IL-1β were detected in the supernatants in VTRS1-infected macrophage cell culture. Inhibition and knockout mouse studies further confirmed that VTRS1 induces a proinflammatory, caspase-2- and NF-κB-mediated macrophage cell death. Interestingly, caspase-1 does not play any obvious role in the VTRS1-induced macrophage cell death in studies utilizing a caspase-1 inhibitor (Chen et al., 2011). This novel caspase-2-mediated proinflammatory cell death differs from apoptosis (which is not proinflammatory), and differs from classical caspase-1-mediated pyroptosis. The details of the mechanism for the cell death pathway and the biological relevance of this pathway in Brucella pathogenesis and protective Brucella immunity are currently under active investigations.
Using a cDNA microarray technology, Rossetti et al. (2010) compared the early transcriptome of B. abortus-infected monocyte-derived macrophages (MDMs) from cattle naturally resistant (R) or susceptible (S) to brucellosis. The MDMs isolated from peripheral blood were infected with virulent B. abortus strain 2308 for 24 h. Their study identified slightly increased genome activation in R MDMs and a down-regulated transcriptome in S MDMs. Specifically, compared to uninfected cells, Brucella infection induced 46 up- and 195 down-regulated genes in S MDMs at 12 h post infection. In R MDMs, 31 genes were up- and 25 genes were down-regulated at 12 h postinfection. R MDMs had the ability to induce a type 1 immune response against B. abortus infection, including up-regulation of CCL4 and reduced expression of EBF1. This ability was impaired in S cells, as demonstrated by decreased expression of HSPA14, TCIRG1, and C1QBP genes. Several inflammation-associated host genes, such as IL-1A, CCL2, and CCL5, were up-regulated in infected S MDMs. These differences may explain the different resistances of MDMs to virulent Brucella infection.
Host Epithelial Cell Responses to Brucella Infections Based on Omics Gene Expression Data Analysis
Brucella abortus induces acute placentitis and abortion in infected animals, key events for transmission of the disease. To better understand the intricate interaction between B. abortus and trophoblastic cells, Carvalho Neta et al. (2008) evaluated the profile of gene expression by bovine trophoblastic cells during infection with B. abortus. Microarray analysis was performed after explants of chorioallantoic membranes were infected with B. abortus strain 2308 for 4 h. Expression of proinflammatory genes by trophoblastic cells was suppressed at 4 h after inoculation. A significant up-regulation of CXC chemokines [CXCL6 (GCP-2) and CXCL8 (interleukin 8)] was observed at 12 (but not at 6 h) after inoculation. Therefore, in trophoblastic cells infected with virulent B. abortus, the expression of proinflammatory mediators was suppressed during the early stages of infection. This was followed by a delayed and mild expression of proinflammatory chemokines. A similar profile of chemokine expression, including up-regulation of CXCL6 and CXCL8, was found in the placentomes of experimentally infected cows. The kinetic trophoblastic response is likely to contribute to the pathogenesis of B. abortus-induced placentitis (Carvalho Neta et al., 2008).
Innate Host Splenocyte Responses to Brucella Infections Based on DNA Microarray Analyses
Soon after Brucella cells invade a host, infectious brucellae migrate to the spleen and liver. In spleen and liver, the course of Brucella infection encompasses four phases. The early preimmune infection phase is characterized by logarithmic Brucella growth and an accumulation of bacteria in the liver and spleen. The second bacteriostatic phase is typically accompanied with the onset of a delayed type hypersensitivity to Brucella antigens and granuloma formation. In the third immune effector phase, up to 90% of the bacteria may be destroyed. This phase is typically followed by a phase of obviously impaired eradication of bacteria (phase IV; Hort et al., 2003).
Spleen is most frequently used for analysis of innate and adaptive immune responses to Brucella infections. To identify host responses specifically regulated by the Brucella T4SS, Roux et al. (2007) used Affymetrix mouse 430 2.0 arrays to compare early transcriptional responses of mouse splenocytes to infection with B. abortus, B. melitensis, and B. abortus virB mutants defective in the T4SS. The largest number of differentially expressed genes occurred in the categories of inflammation and immunity. Galindo et al. (2010) studied gene expression changes in spleens of the wildlife reservoir species Eurasian wild boar (Sus scrofa), which is naturally infected with B. suis biovar 2. B. suis biovars (bv.) 2 is frequently isolated from wild boar and hares and largely restricted to Europe. This study identified 633 up-regulated genes and 1373 down-regulated genes in infected wild boar. B. suis bv. 2 infection induced up-regulation of genes in cell maturation, migration, and/or proliferation in infected animals. The down-regulated genes are associated with impaired activity of several important cellular metabolic pathways including metabolism, cytoskeleton organization and biogenesis, stress, apoptosis, immune response and lysosomal function, and vesicle-mediated transport. These gene expression profiles facilitate intracellular multiplication and the development of chronic infections.
Analysis of Host Blood Cell Immune Responses to Brucella Vaccination and Challenge Using Microarray Technology
Brucella ovis causes ovine brucellosis, characterized by infertility in rams, abortion in ewes, and increased perinatal mortality in lambs. Galindo et al. (2009a) characterized differential transcriptomics gene expression in buffy coat samples of rams experimentally infected with B. ovis strain PA by microarray hybridization and real-time RT-PCR. The buffy coat, the fraction of an anticoagulated blood sample after density gradient centrifugation, contains most of the white blood cells and platelets. Of the 600 ruminant inflammatory and immune response genes, 20 and 14 genes in the buffy coat samples were significantly regulated, with an expression fold change >1.75 with a P-value < 0.05, at 15 and 60 days post-challenge (dpc), respectively. Specifically, in infected rams at 15 dpc, 16 were up-regulated, and 4 were down-regulated. At 60 dpc, 11 and 3 genes, respectively, were up- and down-regulated in infected rams. Four genes, desmoglein, ENaC-alpha, IL18BP, and MIF, were up-regulated at both 15 and 60 dpc. The inflammatory and innate immune pathways were activated in infected animals. The infection of B. ovis up-regulated phagocytosis-associated genes and down-regulated genes related to protective host defense. These responses facilitate the chronicity of B. ovis infection.
Omics can also be used to characterize possible correlates of protective response against Brucella infection. Vaccination with live attenuated B. melitensis Rev 1 vaccine is used to control ovine brucellosis caused by B. ovis in sheep. To identify possible correlates of protective response to B. ovis infection, Galindo et al. (2009b) used microarrays to characterize inflammatory and immune response genes differentially expressed in rams previously immunized with Rev 1 and experimentally challenged with B. ovis. Total RNA was isolated from buffy coat samples before vaccination (T0), 150 days after vaccination and before challenge (T1), and 60 dpc (T2). Protected and susceptible rams did not show significant differences in gene expression prior to vaccination with Rev 1 (timeT0). After vaccination, but prior to challenge (T1), the toll-like receptor 10 (TLR10) was the only gene significantly expressed at higher levels in protected rams as compared to vaccinated rams that were susceptible to B. ovis infection. Concomitantly, 12 proinflammatory and innate immune effectors were up-regulated in vaccinated rams that were susceptible to B. ovis infection. After challenge with B. ovis at time T2, the vaccinated and protected rams showed higher expression levels of Bcl-2-homologous antagonist/killer (Bak), annexin I (ANXI), and interleukin 6 (IL6) genes. These genes provide possible correlates of protective response to B. ovis infection in rams immunized with Rev 1 vaccine.
Analysis of Brucella-Specific Serological Antibody Responses Using Proteomics
Protein expression in bacteria is an important determinant in the induction of Brucella-specific antibodies. A systems biology approach can be used to identify antibody signatures associated with Brucella infections in humans and to predict serodiagnostic antigens. Using a full proteome microarray expressing 3046 cloned B. melitensis genes, Liang et al. (2011) identified 122 immunodominant antigens and 33 serodiagnostic antigens. The reactive antigens had enriched features in terms of membrane association and secretion as indicated by the presence of a signal peptide, a single transmembrane domain, and an outer membrane or periplasmic location. This systems biology approach facilitates the understanding of the breadth and specificity of the immune response to B. melitensis.
In clinical settings, the detection of agglutinating anti-LPS antibodies is the basis for current serological diagnosis of human brucellosis. To better understand the multiplicity of antibody responses that develop after B. melitensis infection, Liang et al. (2010) used a protein microarray containing 1406 predicted B. melitensis proteins to analyze sera from experimentally infected goats and naturally infected humans from an endemic region in Peru. Eighteen antigens were differentially recognized by infected and non-infected goats. Thirteen serodiagnostic antigens were identified that differentiated human patients with acute brucellosis from syndromically similar patients. Only two of the serodiagnostic antigens overlapped between humans and goats. A number of cross-reactive antigens were found in healthy goats and healthy humans (Liang et al., 2010). This study demonstrates that an experimentally infected natural reservoir host and a naturally infected human host produce different immune responses.
Brucella Vaccine Target Prediction Based on Genome Sequence Analysis Using Vaxign Reverse Vaccinology
Reverse vaccinology is an emerging and revolutionary vaccine development strategy that starts with the prediction of vaccine targets by bioinformatics analysis of genome sequences (Rappuoli, 2000; He et al., 2010a). Reverse vaccinology was first applied in the development of a vaccine against serogroup B Neisseria meningitidis (MenB; Pizza et al., 2000). The complete MenB genome was screened for genes coding for putative surface-exposed and secreted proteins. Out of ∼600 novel vaccine candidates, 350 were expressed in Escherichia coli; 28 were found to elicit protective immunity (Pizza et al., 2000). Reverse vaccinology has also been applied successfully to other pathogens such as Streptococcus pneumoniae, Porphyromonas gingivalis, and Chlamydia pneumoniae (Rappuoli, 2000).
To promote vaccine development, the author’s laboratory has developed Vaxign2, the first web-based vaccine design program based on reverse vaccinology (Xiang and He, 2009; He and Xiang, 2010; He et al., 2010b). Predicted features in the Vaxign pipeline include protein subcellular location, transmembrane helices, adhesin probability, sequence conservation among genomes of pathogenic strains, exclusion of sequences in non-pathogenic strains, exclusion of proteins shared in host spp. (e.g., human, mouse, and pigs), and epitope binding to MHC class I and class II. Currently more than 200 genomes have been pre-computed using the Vaxign pipeline. The results are available for query in the Vaxign website. Vaxign also allows dynamic vaccine target prediction based on protein sequences provided by users. A user can register for a private account in Vaxign and save predicted results for further analyses.
Based on the Vaxign reverse vaccinology approach, sequenced Brucella genomes have been used for predicting vaccine targets for Brucella spp. (Xiang and He, 2009; He and Xiang, 2010). An O-sialoglycoprotein endopeptidase was predicted to be a secreted Brucella protein. Among 3034 proteins in B. abortus strain 2308, 32 were identified as OMPs. Two of the 32 OMPs contain more than one transmembrane alpha-helixes. Twenty out of the remaining 30 proteins are predicted as adhesins or adhesin-like proteins. Fifteen of these 20 OMPs are conserved in pathogenic B. abortus, B. suis, and B. melitensis strains. One of the 15 proteins is homologous to a human protein. Among the final 14 proteins are two known Brucella protective antigens (Omp25 and Omp31-1), two flagellar hook proteins FlgE and FlgK, one porin protein Omp2b, two TonB-dependent receptor proteins. Omp2b and Omp31-1 are absent from the genome of B. ovis, a Brucella species non-pathogenic to human (He and Xiang, 2010). The feasibility of using these Brucella proteins for development of a safe and effective human vaccine deserves further investigation.
Literature Mining of Brucella Virulence Factors and Pathogenesis Network
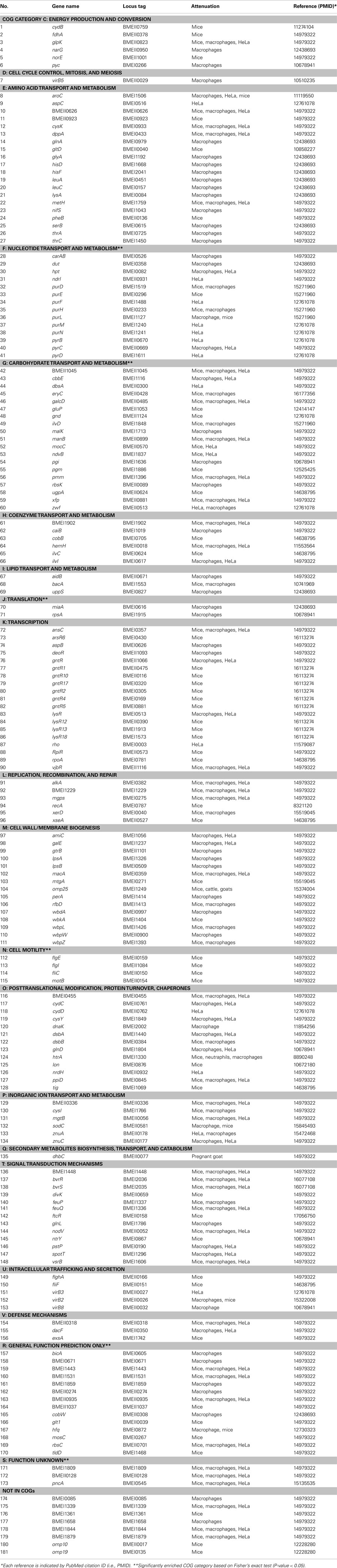
Many virulence factors have been retrieved by literature mining of all Brucella publications found in PubMed. Seventy-five mutated Brucella genes were identified to be attenuated inside macrophages or HeLa cells, or in an in vivo mouse model, using a literature mining and curation system (Limix) as part of the Brucella Bioinformatics Portal (BBP; Xiang et al., 2006). These 75 mutated Brucella genes are essential for Brucella virulence and pathogenesis and are thus treated as Brucella virulence factors (Xiang et al., 2006). Based on the NCBI Clusters of Orthologous Groups (COGs; Tatusov et al., 2000), the 75 Brucella genes have been classified into different categories. This study confirms the well-known pathogenesis mechanisms of Brucella T4SS encoded by the virB operon (O’Callaghan et al., 1999), the BvrR–BvrS two-component regulatory system encoded by bvrR and bvrS (Sola-Landa et al., 1998), and the intact Brucella lipopolysaccharide (Allen et al., 1998). The curation demonstrated an important role of the transport and metabolism of various metabolites including amino acid, carbohydrate, lipid, and inorganic ions. Those Brucella genes participating in these events are essential for intracellular Brucella growth and their survival inside phagosomes of eukaryotic cells.
The updated BBP database contains 181 Brucella virulence factors. These are classified by the mutants’ attenuated characteristics in host cells or in animals in vivo (Table 2). A new statistic COG analysis of these virulence factors confirms many of previous data mining results. Six COG categories are significantly enriched (P-value < 0.05), including: (i) Nucleotide transport and metabolism (COG category F), (ii) Cell motility (COG category N), (iii) Translation (COG category J), (iv) Carbohydrate transport and metabolism (COG category G), (v) General function prediction (COG category R), and (vi) Function unknown (COG category S; Table 2). Beside these groups, many other categories, such as Signal transduction mechanisms (COG category T) and Intracellular trafficking and secretion (COG category U), are also critical for Brucella pathogenesis. These factors may not be crucial for Brucella survival in vitro. However, their presence is critical for Brucella replication in vivo. Many Brucella virulent factors have no defined functions and are classified in the categories of General function prediction, Function unknown, or Not in COGs. How these factors become virulence factors deserves further investigations.
Literature mining approaches can also be used to identify genetic networks crucial for Brucella pathogenesis. Out of 1358 potential interactions available from more than 7000 abstracts and/or full text papers extracted from PubMed, the Limix system found 69 true positive interactions (Xiang et al., 2006). These interactions were automatically displayed using our graphic visualization program. These results allow a more comprehensive investigation of Brucella pathogenesis and the generation of novel hypotheses (Xiang et al., 2006). For example, this study identified a possible interaction between T4SS and the BvrR–BvrS two-component regulatory system. Specifically, the secretion of the N-terminal fragment of BvrR fused to a ribosome binding site and start codon deficient chloramphenicol acetyl transferase (CAT) report gene is diminished in virB1 and virB10 mutants (Marchesini et al., 2004). How the T4SS regulates the BvrR/BvrS system remains unclear. However, Martinez-Nunez et al. (2010) recently found that BvrR/BvrS regulates the expression of the T4SS VirB in B. abortus.
As described below, biomedical ontologies can be used to dramatically improve Brucella literature mining.
Ontology-Based Analysis of Brucella Pathogenesis, Host Immunity, and Vaccine Targets
A biomedical ontology is a consensus-based, controlled vocabulary of terms and relations, with associated definitions that are logically formulated in such a way as to promote automated reasoning (Xiang et al., 2010). Biomedical ontologies structure and interlink knowledge and data from complex biomedical domains in such a fashion as to permit shared understanding of a specific domain among different resources.
Extensive brucellosis research has resulted in a large number of publications encompassing various medical topics ranging from basic Brucella genetic study to vaccine clinical trials. To support data exchange and reasoning, a Brucellosis Ontology (IDOBRU)3 has been developed (Lin et al., 2011). IDOBRU is a biomedical ontology in the brucellosis domain and is an extension ontology of the core infectious disease ontology (IDO-core; Cowell and Smith, 2010). Currently IDOBRU contains more than 1000 ontology terms covering areas such as etiology, transmission, symptoms, virulence factors, pathogenesis, prevention, and treatment. IDOBRU has been used to model different aspects of brucellosis, including host infection and zoonotic disease transmission, symptoms, virulence factors and pathogenesis, diagnosis, intentional release, vaccine prevention, and treatment. IDOBRU is the first reported bacterial IDO that has been developed to model different disease aspects in a formal logical format (Lin et al., 2011). The ontology can serve as a knowledgebase for Brucella and brucellosis. IDOBRU captures the knowledge extracted from published peer-reviewed sources that cover brucellosis bench research, clinical practice, and public health. In addition, IDOBRU has stored all Brucella virulence factors discussed in BBP (Table 2).
The vaccine ontology (VO)4 is an open-access community-supported ontology in the domain of vaccine and vaccination (He et al., 2009). VO represents various vaccines and their relations. VO has collected more than 40 curated Brucella vaccines or vaccine candidates that have been officially licensed or proven to provide protection in animal models. The ontology provides detailed machine-readable information for each Brucella vaccine, such as the vaccine type, manufacturers of licensed vaccines, and host immune responses. Fourteen protective Brucella antigens have been included in VO. In addition, VO has been used to integrate many other vaccine data in the VIOLIN vaccine database and analysis system5 (Xiang et al., 2008).
IDOBRU and VO can be used to support Brucella and brucellosis data exchange, data integration, and automated reasoning. These two ontologies use a machine-readable Web ontology language (OWL) format and thus support OWL-based ontological reasoning. Software programs can be developed to query IDOBRU and VO and to perform statistical and reasoning analyses. One particular research area of note is the application of these ontologies to advanced literature mining. In PubMed vaccine literature indexing is poorly performed due to limited hierarchy of Medical Subject Headings (MeSH) annotation in the vaccine field. SciMiner is a literature mining system that supports literature indexing and gene name tagging (Hur et al., 2009). Our study indicates that application of VO in SciMiner will aid vaccine literature indexing and mining of vaccine–gene interaction networks. Using the abstracts of 14,947 Brucella-related papers, VO-SciMiner identified 140 Brucella genes associated with Brucella vaccines. These genes included known protective antigens, virulence factors, and genes closely related to Brucella vaccines. When a total of 67 Brucella vaccine terms were incorporated into the VO-based SciMiner (VO-SciMiner), the program exhibited a superior performance in retrieving Brucella vaccine-related papers over that obtained with a MeSH-based PubMed literature search. For example, a VO-SciMiner search of “live attenuated Brucella vaccine” returned 922 hits as of April 20, 2011, while a PubMed search of the same query yielded only 74 (Hur et al., 2011). VO has identified 17 live attenuated Brucella vaccines (Hur et al., 2011). Licensed live attenuated vaccines RB51, strain 19, and Rev. 1 have been tested in mouse and large animals. Many live attenuated Brucella vaccines at the research stage have recently been tested in relevant animal models. For example, microencapsulated RB51 (Arenas-Gamboa et al., 2009a) and strain 19 (Arenas-Gamboa et al., 2009b) have recently been tested in red deer. RB51 and RB51 overexpressing superoxide dismutase (sodC) and glycosyltransferase (wboA) genes has been tested in bison (Olsen et al., 2009). These studies provide support toward the development of a safe and effective vaccine for practical animal uses.
Concluding Remarks
During the past decade, systems biology, and bioinformatics approaches have widely been used for study of the mechanisms of Brucella pathogenesis and host protective immunity against Brucella infections and for support of vaccine design. This review article demonstrates that integrative experimental Omics and computational bioinformatics analyses have dramatically advanced our understanding of how different Brucella species infect different host species, how Brucella gene expressions are regulated in cell culture or inside host cells (i.e., macrophages or epithelial cells), and how host cells (macrophages, epithelial cells, splenocytes, and blood cells) respond to Brucella infections. Advanced literature mining provides tools to retrieve and analyze virulence factors, protective antigens, and host–Brucella gene interactions from thousands of Brucella research publications. Machine and human-readable Brucella Ontology and VO have provided more ways to integrate Brucella data with other infectious diseases and vaccine data.
One main message of the review is that systems biology and bioinformatics approaches are able to help to facilitate vaccine development and predict fundamental molecular mechanisms of host–Brucella interactions. With the initial high throughput experimental studies and advanced data analyses, many predictions can be made and used as novel hypotheses for further confirmation by “traditional” experimental approaches. The findings from the Omics studies have opened new avenues of research. Many of these studies confirmed and expanded the results of classical approaches in the areas of Brucella pathogenesis and host immunity against Brucella infection or vaccination. From our literature data mining analysis, known Brucella virulence factors can be retrieved. Compared to any isolated study of Brucella virulence factor(s), a systematical analysis of all possible virulence factors provides a more comprehensive view of how Brucella survives and replicates in a hostile intracellular environment and in vivo. In contrast to the traditional vaccine development strategy of continuous trials after isolated hypotheses, the new strategy that starts with systems biology and bioinformatics analyses make it possible to more rationally design safe, effective, and optimized Brucella vaccines.
Although much progress has been made, many challenges still exist. For example, while different gene expression profiles have been discovered at different experimental conditions, how to integrate these data and make sense of the interconnected host–Brucella interaction mechanism remains a challenge. IDOBRU and VO may provide ontology-based platforms for obtaining a higher level of data and knowledge integration. However, currently IDOBRU and VO only provide proof-of-concept demonstrations for representing Brucella virulence factors and host immune responses (Lin et al., 2011). Additional efforts are required to systematically apply IDOBRU/VO and related semantic web tools to represent and analyze different levels of host–Brucella interaction data and knowledge. Another challenge is how to improve the translation of the knowledge learned from the systems and bioinformatics studies into the generation of new vaccines and drugs against infectious Brucella infections? The Brucella gene expression data obtained under different experimental conditions may be used to better design vaccine protein targets. The host response profiles may facilitate a deeper understanding of the protective immune response in the host. This will require diligent research and development to design new ways to make all these translational outcomes a reality.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript was supported by the NIH–NIAID grant R01AI081062. Critical review of this manuscript by Dr. George W. Jourdian at the University of Michigan is gratefully acknowledged.
Footnotes
References
Al Dahouk, S., Jubier-Maurin, V., Scholz, H. C., Tomaso, H., Karges, W., Neubauer, H., and Köhler, S. (2008). Quantitative analysis of the intramacrophagic Brucella suis proteome reveals metabolic adaptation to late stage of cellular infection. Proteomics 8, 3862–3870.
Allen, C. A., Adams, L. G., and Ficht, T. A. (1998). Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66, 1008–1016.
Araya, L. N., Elzer, P. H., Rowe, G. E., Enright, F. M., and Winter, A. J. (1989). Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143, 3330–3337.
Araya, L. N., and Winter, A. J. (1990). Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect. Immun. 58, 254–256.
Arenas-Gamboa, A. M., Ficht, T. A., Davis, D. S., Elzer, P. H., Wong-Gonzalez, A., and Rice-Ficht, A. C. (2009a). Enhanced immune response of red deer (Cervus elaphus) to live rb51 vaccine strain using composite microspheres. J. Wildl. Dis. 45, 165–173.
Arenas-Gamboa, A. M., Ficht, T. A., Davis, D. S., Elzer, P. H., Kahl-McDonagh, M., Wong-Gonzalez, A., and Rice-Ficht, A. C. (2009b). Oral vaccination with microencapsulated strain 19 vaccine confers enhanced protection against Brucella abortus strain 2308 challenge in red deer (Cervus elaphus elaphus). J. Wildl. Dis. 45, 1021–1029.
Carvalho Neta, A. V., Stynen, A. P., Paixao, T. A., Miranda, K. L., Silva, F. L., Roux, C. M., Tsolis, R. M., Everts, R. E., Lewin, H. A., Adams, L. G., Carvalho, A. F., Lage, A. P., and Santos, R. L. (2008). Modulation of the bovine trophoblastic innate immune response by Brucella abortus. Infect. Immun. 76, 1897–1907.
Celli, J., de Chastellier, C., Franchini, D. M., Pizarro-Cerda, J., Moreno, E., and Gorvel, J. P. (2003). Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198, 545–556.
Chain, P. S., Comerci, D. J., Tolmasky, M. E., Larimer, F. W., Malfatti, S. A., Vergez, L. M., Aguero, F., Land, M. L., Ugalde, R. A., and Garcia, E. (2005). Whole-genome analyses of speciation events in pathogenic brucellae. Infect. Immun. 73, 8353–8361.
Chen, F., Ding, X., Ding, Y., Xiang, Z., Li, X., Ghosh, D., Schurig, G. G., Sriranganathan, N., Boyle, S. M., and He, Y. (2011). Proinflammatory caspase-2-mediated macrophage cell death induced by a rough attenuated Brucella suis strain. Infect. Immun. 79, 2460–2469.
Chen, F., and He, Y. (2009). Caspase-2 mediated apoptotic and necrotic murine macrophage cell death induced by rough Brucella abortus. PLoS ONE 4, e6830. doi: 10.1371/journal.pone.0006830
Covert, J., Mathison, A. J., Eskra, L., Banai, M., and Splitter, G. (2009). Brucella melitensis, B. neotomae and B. ovis elicit common and distinctive macrophage defense transcriptional responses. Exp. Biol. Med. (Maywood) 234, 1450–1467.
Cowell, L. G., and Smith, B. (2010). “Infectious disease ontology,” in Infectious Disease Informatics, ed. V. Sintchenko (New York: Springer), 373–395.
Crasta, O. R., Folkerts, O., Fei, Z., Mane, S. P., Evans, C., Martino-Catt, S., Bricker, B., Yu, G., Du, L., and Sobral, B. W. (2008). Genome sequence of Brucella abortus vaccine strain S19 compared to virulent strains yields candidate virulence genes. PLoS ONE 3, e2193. doi: 10.1371/journal.pone.0002193
de Jong, M. F., Sun, Y. H., den Hartigh, A. B., van Dijl, J. M., and Tsolis, R. M. (2008). Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol. Microbiol. 70, 1378–1396.
DelVecchio, V. G., Kapatral, V., Redkar, R. J., Patra, G., Mujer, C., Los, T., Ivanova, N., Anderson, I., Bhattacharyya, A., Lykidis, A., Reznik, G., Jablonski, L., Larsen, N., D’Souza, M., Bernal, A., Mazur, M., Goltsman, E., Selkov, E., Elzer, P. H., Hagius, S., O’Callaghan, D., Letesson, J. J., Haselkorn, R., Kyrpides, N., and Overbeek, R. (2002). The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. U.S.A. 99, 443–448.
Eskra, L., Mathison, A., and Splitter, G. (2003). Microarray analysis of mRNA levels from RAW264.7 macrophages infected with Brucella abortus. Infect. Immun. 71, 1125–1133.
Fernandez-Prada, C. M., Zelazowska, E. B., Nikolich, M., Hadfield, T. L., Roop, R. M. II, Robertson, G. L., and Hoover, D. L. (2003). Interactions between Brucella melitensis and human phagocytes: bacterial surface O-polysaccharide inhibits phagocytosis, bacterial killing, and subsequent host cell apoptosis. Infect. Immun. 71, 2110–2119.
Galindo, R. C., Munoz, P. M., de Miguel, M. J., Marin, C. M., Blasco, J. M., Gortazar, C., Kocan, K. M., and de la Fuente, J. (2009a). Differential expression of inflammatory and immune response genes in rams experimentally infected with a rough virulent strain of Brucella ovis. Vet. Immunol. Immunopathol. 127, 295–303.
Galindo, R. C., Munoz, P. M., de Miguel, M. J., Marin, C. M., Blasco, J. M., Gortazar, C., Kocan, K. M., and de la Fuente, J. (2009b). Characterization of possible correlates of protective response against Brucella ovis infection in rams immunized with the B. melitensis Rev 1 vaccine. Vaccine 27, 3039–3044.
Galindo, R. C., Munoz, P. M., de Miguel, M. J., Marin, C. M., Labairu, J., Revilla, M., Blasco, J. M., Gortazar, C., and de la Fuente, J. (2010). Gene expression changes in spleens of the wildlife reservoir species, Eurasian wild boar (Sus scrofa), naturally infected with Brucella suis biovar 2. J. Genet. Genomics 37, 725–736.
Gross, A., Terraza, A., Ouahrani-Bettache, S., Liautard, J. P., and Dornand, J. (2000). In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68, 342–351.
Guzman-Verri, C., Manterola, L., Sola-Landa, A., Parra, A., Cloeckaert, A., Garin, J., Gorvel, J. P., Moriyon, I., Moreno, E., and Lopez-Goni, I. (2002). The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc. Natl. Acad. Sci. U.S.A. 99, 12375–12380.
Halling, S. M., Peterson-Burch, B. D., Bricker, B. J., Zuerner, R. L., Qing, Z., Li, L. L., Kapur, V., Alt, D. P., and Olsen, S. C. (2005). Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J. Bacteriol. 187, 2715–2726.
He, Y., Cowell, L., Diehl, A. D., Mobley, H. L., Peters, B., Ruttenberg, A., Scheuermann, R. H., Brinkman, R. R., Courtot, M., Mungall, C., Xiang, Z., Chen, F., Todd, T., Colby, L., Rush, H., Whetzel, T., Musen, M. A., Athey, B. D., Omenn, G. S., and Smith, B. (2009). VO: Vaccine Ontology. The 1st International Conference on Biomedical Ontology (ICBO 2009), Buffalo, NY: Nature Precedings.
He, Y., Rappuoli, R., De Groot, A. S., and Chen, R. T. (2010a). Emerging vaccine informatics. J. Biomed. Biotechnol. 2010, 218590.
He, Y., Xiang, Z., and Mobley, H. L. (2010b). Vaxign: the first web-based vaccine design program for reverse vaccinology and applications for vaccine development. J. Biomed. Biotechnol. 2010, 15.
He, Y., Reichow, S., Ramamoorthy, S., Ding, X., Lathigra, R., Craig, J. C., Sobral, B. W., Schurig, G. G., Sriranganathan, N., and Boyle, S. M. (2006). Brucella melitensis triggers time-dependent modulation of apoptosis and down-regulation of mitochondrion-associated gene expression in mouse macrophages. Infect. Immun. 74, 5035–5046.
He, Y., Vemulapalli, R., Zeytun, A., and Schurig, G. G. (2001). Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect. Immun. 69, 5502–5508.
He, Y., and Xiang, Z. (2010). Bioinformatics analysis of Brucella vaccines and vaccine targets using VIOLIN. Immunome Res. 6(Suppl. 1), S5.
Hort, G. M., Weisenburger, J., Borsdorf, B., Peters, C., Banai, M., Hahn, H., Jacob, J., and Mielke, M. E. (2003). Delayed type hypersensitivity-associated disruption of splenic periarteriolar lymphatic sheaths coincides with temporary loss of IFN-gamma production and impaired eradication of bacteria in Brucella abortus-infected mice. Microbes Infect. 5, 95–106.
Hur, J., Schuyler, A. D., States, D. J., and Feldman, E. L. (2009). SciMiner: web-based literature mining tool for target identification and functional enrichment analysis. Bioinformatics 25, 838–840.
Hur, J., Xiang, Z., Feldman, E. L., and He, Y. (2011). Ontology-based Brucella vaccine literature indexing and systematic analysis of gene-vaccine association network. BMC Immunol. 12, 49. doi: 10.1186/1471-2172-12-49
Inatsuka, C. S., Julio, S. M., and Cotter, P. A. (2005). Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc. Natl. Acad. Sci. U.S.A. 102, 18578–18583.
Jiang, X., and Baldwin, C. L. (1993). Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61, 124–134.
Kay, E., and Wren, B. W. (2009). Recent advances in systems microbiology. Curr. Opin. Microbiol. 12, 577–581.
Ko, J., and Splitter, G. A. (2003). Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16, 65–78.
Kohler, S., Michaux-Charachon, S., Porte, F., Ramuz, M., and Liautard, J. P. (2003). What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 11, 215–219.
Kuster, D. W., Merkus, D., van der Velden, J., Verhoeven, A. J., and Duncker, D. J. (2011). Integrative Physiology 2.0: integration of systems biology into physiology and its application to cardiovascular homeostasis. J. Physiol. 589, 1037–1045.
Lamontagne, J., Forest, A., Marazzo, E., Denis, F., Butler, H., Michaud, J. F., Boucher, L., Pedro, I., Villeneuve, A., Sitnikov, D., Trudel, K., Nassif, N., Boudjelti, D., Tomaki, F., Chaves-Olarte, E., Guzmán-Verri, C., Brunet, S., Côté-Martin, A., Hunter, J., Moreno, E., and Paramithiotis, E. (2009). Intracellular adaptation of Brucella abortus. J. Proteome Res. 8, 1594–1609.
Lapaque, N., Moriyon, I., Moreno, E., and Gorvel, J. P. (2005). Brucella lipopolysaccharide acts as a virulence factor. Curr. Opin. Microbiol. 8, 60–66.
Liang, L., Leng, D., Burk, C., Nakajima-Sasaki, R., Kayala, M. A., Atluri, V. L., Pablo, J., Unal, B., Ficht, T. A., Gotuzzo, E., Saito, M., Morrow, W. J., Liang, X., Baldi, P., Gilman, R. H., Vinetz, J. M., Tsolis, R. M., and Felgner, P. L. (2010). Large scale immune profiling of infected humans and goats reveals differential recognition of Brucella melitensis antigens. PLoS Negl. Trop. Dis. 4, e673. doi: 10.1371/journal.pntd.0000673
Liang, L., Tan, X., Juarez, S., Villaverde, H., Pablo, J., Nakajima-Sasaki, R., Gotuzzo, E., Saito, M., Hermanson, G., Molina, D., Felgner, S., Morrow, W. J., Liang, X., Gilman, R. H., Davies, D. H., Tsolis, R. M., Vinetz, J. M., and Felgner, P. L. (2011). Systems biology approach predicts antibody signature associated with Brucella melitensis infection in humans. J. Proteome Res. 10, 4813–4824.
Lin, Y., Xiang, X., and He, Y. (2011). Brucellosis ontology (IDOBRU) as an extension of the infectious disease ontology. J. Biomed. Semantics 2, 9.
Macedo, G. C., Magnani, D. M., Carvalho, N. B., Bruna-Romero, O., Gazzinelli, R. T., and Oliveira, S. C. (2008). Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J. Immunol. 180, 1080–1087.
Marchesini, M. I., Ugalde, J. E., Czibener, C., Comerci, D. J., and Ugalde, R. A. (2004). N-terminal-capturing screening system for the isolation of Brucella abortus genes encoding surface exposed and secreted proteins. Microb. Pathog. 37, 95–105.
Martinez-Nunez, C., Altamirano-Silva, P., Alvarado-Guillen, F., Moreno, E., Guzman-Verri, C., and Chaves-Olarte, E. (2010). The two-component system BvrR/BvrS regulates the expression of the type IV secretion system VirB in Brucella abortus. J. Bacteriol. 192, 5603–5608.
Moon, H. W., Nagy, B., Isaacson, R. E., and Orskov, I. (1977). Occurrence of K99 antigen on Escherichia coli isolated from pigs and colonization of pig ileum by K99+ enterotoxigenic E. coli from calves and pigs. Infect. Immun. 15, 614–620.
Moreno, E., Cloeckaert, A., and Moriyon, I. (2002). Brucella evolution and taxonomy. Vet. Microbiol. 90, 209–227.
Murphy, E. A., Sathiyaseelan, J., Parent, M. A., Zou, B., and Baldwin, C. L. (2001). Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103, 511–518.
O’Callaghan, D., Cazevieille, C., Allardet-Servent, A., Boschiroli, M. L., Bourg, G., Foulongne, V., Frutos, P., Kulakov, Y., and Ramuz, M. (1999). A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33, 1210–1220.
O’Callaghan, D., and Whatmore, A. M. (2011). Brucella genomics as we enter the multi-genome era. Brief. Funct. Genomics 10, 334–341.
Oliveira, S. C., and Splitter, G. A. (1995). CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 25, 2551–2557.
Olsen, S. C., Boyle, S. M., Schurig, G. G., and Sriranganathan, N. N. (2009). Immune responses and protection against experimental challenge after vaccination of bison with Brucella abortus strain RB51 or RB51 overexpressing superoxide dismutase and glycosyltransferase genes. Clin. Vaccine Immunol. 16, 535–540.
Parkhill, J., Sebaihia, M., Preston, A., Murphy, L. D., Thomson, N., Harris, D. E., Holden, M. T., Churcher, C. M., Bentley, S. D., Mungall, K. L., Cerdeño-Tárraga, A. M., Temple, L., James, K., Harris, B., Quail, M. A., Achtman, M., Atkin, R., Baker, S., Basham, D., Bason, N., Cherevach, I., Chillingworth, T., Collins, M., Cronin, A., Davis, P., Doggett, J., Feltwell, T., Goble, A., Hamlin, N., Hauser, H., Holroyd, S., Jagels, K., Leather, S., Moule, S., Norberczak, H., O’Neil, S., Ormond, D., Price, C., Rabbinowitsch, E., Rutter, S., Sanders, M., Saunders, D., Seeger, K., Sharp, S., Simmonds, M., Skelton, J., Squares, R., Squares, S., Stevens, K., Unwin, L., Whitehead, S., Barrell, B. G., and Maskell, D. J. (2003). Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35, 32–40.
Paulsen, I. T., Seshadri, R., Nelson, K. E., Eisen, J. A., Heidelberg, J. F., Read, T. D., Dodson, R. J., Umayam, L., Brinkac, L. M., Beanan, M. J., Daugherty, S. C., Deboy, R. T., Durkin, A. S., Kolonay, J. F., Madupu, R., Nelson, W. C., Ayodeji, B., Kraul, M., Shetty, J., Malek, J., Van Aken, S. E., Riedmuller, S., Tettelin, H., Gill, S. R., White, O., Salzberg, S. L., Hoover, D. L., Lindler, L. E., Halling, S. M., Boyle, S. M., and Fraser, C. M. (2002). The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. U.S.A. 99, 13148–13153.
Pei, J., and Ficht, T. A. (2004). Brucella abortus rough mutants are cytopathic for macrophages in culture. Infect. Immun. 72, 440–450.
Pizza, M., Scarlato, V., Masignani, V., Giuliani, M. M., Arico, B., Comanducci, M., Jennings, G. T., Baldi, L., Bartolini, E., Capecchi, B., Galeotti, C. L., Luzzi, E., Manetti, R., Marchetti, E., Mora, M., Nuti, S., Ratti, G., Santini, L., Savino, S., Scarselli, M., Storni, E., Zuo, P., Broeker, M., Hundt, E., Knapp, B., Blair, E., Mason, T., Tettelin, H., Hood, D. W., Jeffries, A. C., Saunders, N. J., Granoff, D. M., Venter, J. C., Moxon, E. R., Grandi, G., and Rappuoli, R. (2000). Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287, 1816–1820.
Rajashekara, G., Glasner, J. D., Glover, D. A., and Splitter, G. A. (2004). Comparative whole-genome hybridization reveals genomic islands in Brucella species. J. Bacteriol. 186, 5040–5051.
Rambow-Larsen, A. A., Rajashekara, G., Petersen, E., and Splitter, G. (2008). Putative quorum-sensing regulator BlxR of Brucella melitensis regulates virulence factors including the type IV secretion system and flagella. J. Bacteriol. 190, 3274–3282.
Ratushna, V. G., Sturgill, D. M., Ramamoorthy, S., Reichow, S. A., He, Y., Lathigra, R., Sriranganathan, N., Halling, S. M., Boyle, S. M., and Gibas, C. J. (2006). Molecular targets for rapid identification of Brucella spp. BMC Microbiol. 6, 13. doi: 10.1186/1471-2180-6-13
Rittig, M. G., Kaufmann, A., Robins, A., Shaw, B., Sprenger, H., Gemsa, D., Foulongne, V., Rouot, B., and Dornand, J. (2003). Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J. Leukoc. Biol. 21, 21.
Roop, R. M. II, Bellaire, B. H., Valderas, M. W., and Cardelli, J. A. (2004). Adaptation of the brucellae to their intracellular niche. Mol. Microbiol. 52, 621–630.
Roop, R. M. II, Gaines, J. M., Anderson, E. S., Caswell, C. C., and Martin, D. W. (2009). Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Med. Microbiol. Immunol. 198, 221–238.
Rossetti, C. A., Galindo, C. L., Everts, R. E., Lewin, H. A., Garner, H. R., and Adams, L. G. (2010). Comparative analysis of the early transcriptome of Brucella abortus-infected monocyte-derived macrophages from cattle naturally resistant or susceptible to brucellosis. Res. Vet. Sci. 91, 40–51.
Rossetti, C. A., Galindo, C. L., Garner, H. R., and Adams, L. G. (2011). Transcriptional profile of the intracellular pathogen Brucella melitensis following HeLa cells infection. Microb. Pathog. 51, 338–344.
Rossetti, C. A., Galindo, C. L., Lawhon, S. D., Garner, H. R., and Adams, L. G. (2009). Brucella melitensis global gene expression study provides novel information on growth phase-specific gene regulation with potential insights for understanding Brucella: host initial interactions. BMC Microbiol. 9, 81. doi: 10.1186/1471-2180-9-81
Roux, C. M., Rolan, H. G., Santos, R. L., Beremand, P. D., Thomas, T. L., Adams, L. G., and Tsolis, R. M. (2007). Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell. Microbiol. 9, 1851–1869.
Schurig, G. G., Roop, R. M. D., Bagchi, T., Boyle, S., Buhrman, D., and Sriranganathan, N. (1991). Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28, 171–188.
Schurig, G. G., Sriranganathan, N., and Corbel, M. J. (2002). Brucellosis vaccines: past, present and future. Vet. Microbiol. 90, 479–496.
Sola-Landa, A., Pizarro-Cerda, J., Grillo, M. J., Moreno, E., Moriyon, I., Blasco, J. M., Gorvel, J. P., and López-Goñi, I. (1998). A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29, 125–138.
Tatusov, R. L., Galperin, M. Y., Natale, D. A., and Koonin, E. V. (2000). The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 28, 33–36.
Tolomeo, M., Di Carlo, P., Abbadessa, V., Titone, L., Miceli, S., Barbusca, E., Cannizzo, G., Mancuso, S., Arista, S., and Scarlata, F. (2003). Monocyte and lymphocyte apoptosis resistance in acute and chronic brucellosis and its possible implications in clinical management. Clin. Infect. Dis. 36, 1533–1538.
Tsolis, R. M., Townsend, S. M., Miao, E. A., Miller, S. I., Ficht, T. A., Adams, L. G., and Bäumler, A. J. (1999). Identification of a putative Salmonella enterica serotype typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect. Immun. 67, 6385–6393.
Verger, J. M., Grayon, M., Cloeckaert, A., Lefevre, M., Ageron, E., and Grimont, F. (2000). Classification of Brucella strains isolated from marine mammals using DNA-DNA hybridization and ribotyping. Res. Microbiol. 151, 797–799.
Viadas, C., Rodriguez, M. C., Garcia-Lobo, J. M., Sangari, F. J., and Lopez-Goni, I. (2009). Construction and evaluation of an ORFeome-based Brucella whole-genome DNA microarray. Microb. Pathog. 47, 189–195.
Viadas, C., Rodriguez, M. C., Sangari, F. J., Gorvel, J. P., Garcia-Lobo, J. M., and López-Goñi, I. (2010). Transcriptome analysis of the Brucella abortus BvrR/BvrS two-component regulatory system. PLoS ONE 5, e10216. doi: 10.1371/journal.pone.0010216
Wattam, A. R., Williams, K. P., Snyder, E. E., Almeida, N. F. Jr., Shukla, M., Dickerman, A. W., Crasta, O. R., Kenyon, R., Lu, J., Shallom, J. M., Yoo, H., Ficht, T. A., Tsolis, R. M., Munk, C., Tapia, R., Han, C. S., Detter, J. C., Bruce, D., Brettin, T. S., Sobral, B. W., Boyle, S. M., and Setubal, J. C. (2009). Analysis of ten Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifestyle. J. Bacteriol. 191, 3569–3579.
Weeks, J. N., Galindo, C. L., Drake, K. L., Adams, G. L., Garner, H. R., and Ficht, T. A. (2010). Brucella melitensis VjbR and C12-HSL regulons: contributions of the N-dodecanoyl homoserine lactone signaling molecule and LuxR homologue VjbR to gene expression. BMC Microbiol. 10, 167. doi: 10.1186/1471-2180-10-167
Whatmore, A. M., Perrett, L. L., and MacMillan, A. P. (2007). Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 7, 34. doi: 10.1186/1471-2180-7-34
Winter, A. J., Schurig, G. G., Boyle, S. M., Sriranganathan, N., Bevins, J. S., Enright, F. M., Elzer, P. H., and Kopec, J. D. (1996). Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am. J. Vet. Res. 57, 677–683.
Xiang, Z., Courtot, M., Brinkman, R. R., Ruttenberg, A., and He, Y. (2010). OntoFox: web-based support for ontology reuse. BMC Res. Notes 3, 175. doi: 10.1186/1756-0500-3-175
Xiang, Z., and He, Y. (2009). Vaxign: a web-based vaccine target design program for reverse vaccinology. Procedia Vaccinol. 1, 23–29.
Xiang, Z., Todd, T., Ku, K. P., Kovacic, B. L., Larson, C. B., Chen, F., Hodges, A. P., Tian, Y., Olenzek, E. A., Zhao, B., Colby, L. A., Rush, H. G., Gilsdorf, J. R., Jourdian, G. W., and He, Y. (2008). VIOLIN: vaccine investigation and online information network. Nucleic Acids Res. 36, D923–D928.
Xiang, Z., Zheng, W., and He, Y. (2006). BBP: Brucella genome annotation with literature mining and curation. BMC Bioinformatics 7, 347. doi: 10.1186/1471-2105-7-347
Zhan, Y., and Cheers, C. (1993). Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61, 4899–4901.
Keywords: Brucella, pathogenesis, host immunity, vaccine, systems biology, bioinformatics
Citation: He Y (2012) Analyses of Brucella pathogenesis, host immunity, and vaccine targets using systems biology and bioinformatics. Front. Cell. Inf. Microbio. 2:2. doi: 10.3389/fcimb.2012.00002
Received: 30 October 2011; Paper pending published: 03 December 2011;
Accepted: 12 January 2012; Published online: 01 February 2012.
Edited by:
Thomas A. Ficht, Texas A&M University, USAReviewed by:
Daniel E. Voth, University of Arkansas for Medical Sciences, USAJose A. Bengoechea, Fundacion Caubet–CIMERA Illes Balears, Spain
Copyright: © 2012 He. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Yongqun He, Unit for Laboratory Animal Medicine, Department of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, MI 48109, USA. e-mail: yongqunh@med.umich.edu