Pneumococci in biofilms are non-invasive: implications on nasopharyngeal colonization
- Department of Microbiology and Immunology, Center for Airway Inflammation Research, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
Streptococcus pneumoniae (the pneumococcus) is an opportunistic pathogen that colonizes the human nasopharynx asymptomatically. Invasive pneumococcal disease develops following bacterial aspiration into the lungs. Pneumococci within the nasopharynx exist as biofilms, a growth phenotype characterized by surface attachment, encasement within an extracellular matrix, and antimicrobial resistance. Experimental evidence indicates that biofilm pneumococci are attenuated vs. their planktonic counterpart. Biofilm pneumococci failed to cause invasive disease in experimentally challenged mice and in vitro were shown to be non-invasive despite being hyper-adhesive. This attenuated phenotype corresponds with observations that biofilm pneumococci elicit significantly less cytokine and chemokine production from host cells than their planktonic counterparts. Microarray and proteomic studies show that pneumococci within biofilms have decreased metabolism, less capsular polysaccharide, and reduced production of the pore-forming toxin pneumolysin. Biofilm pneumococci are predominately in the transparent phenotype, which has elevated cell wall phosphorylcholine, an adhesin subject to C-reactive protein mediated opsonization. Herein, we review these changes in virulence, interpret their impact on colonization and transmission, and discuss the notion that non-invasive biofilms are principal lifestyle of S. pneumoniae.
Introduction
Streptococcus pneumoniae (the pneumococcus) is a leading cause of community-acquired pneumonia (CAP), sepsis, and meningitis throughout the world despite the existence of multiple effective vaccines (Bennett et al., 2014). This Gram-positive, encapsulated bacterium asymptomatically colonizes the human nasopharynx where carriage can last for months (Gray et al., 1980). In susceptible individuals, usually the very young and elderly, aspiration of pneumococci can lead to pneumonia and subsequently invasive pneumococcal disease (IPD). At any given time approximately 40% of children and 15% of adults are colonized (Crook et al., 2004; Huang et al., 2009). Annual global IPD burden is roughly 14.5 million cases resulting in 800,000 deaths in children under the age of 5 and a case fatality rate surpassing 20% in the elderly (O'Brien et al., 2009; Heron, 2012; Naucler et al., 2013).
S. pneumoniae in sputum and blood samples from individuals with IPD are primarily in the form of lancet-shaped diplococci; the same morphology observed when grown planktonically in media. Growth as diplococci or short chains is now recognized to help S. pneumoniae evade stochastic alternative pathway mediated complement deposition and opsonophagocytosis (Dalia and Weiser, 2011). Within the past 15 years it has become evident that the pneumococcus also forms biofilms in vivo during nasopharyngeal colonization (Figure 1) and otitis media (Hoa et al., 2009; Reid et al., 2009). Biofilms are aggregates of surfaced attached bacteria encased within an extracellular matrix (ECM). The ECM, which in vivo is composed of host factors, polysaccharides, and extracellular DNA, is now understood to protect bacteria from the host immune system and desiccation (Moscoso et al., 2006); the latter being important during pneumococcal fomite transmission (Walsh and Camilli, 2011). Importantly, biofilm pneumococci have been shown to be decisively less virulent than their planktonic counterparts. This review focuses on how S. pneumoniae modulates its virulence during biofilm formation and why this may promote long-term, asymptomatic colonization. We also discuss the increasingly evident role biofilms play during pneumococcal transmission on fomites.
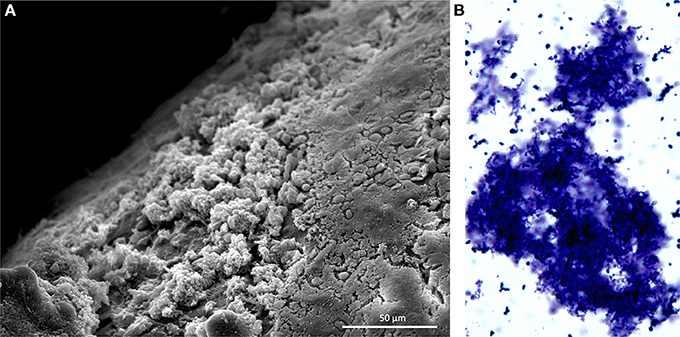
Figure 1. Pneumococcal biofilms form in the nasopharynx. (A) Scanning electron microscopy image of S. pneumoniae biofilms formed on the nasal septum of a mouse. Mice were experimentally colonized 7 days prior. Biofilms are the non-contiguous aggregates on the left. (B) S. pneumoniae biofilm aggregate in nasopharyngeal lavage fluid. Sample was collected from mouse 14 days after experimental colonization. Pneumococci were stained with crystal violet and visualized with a light microscope at 400X. Image credit: Krystle Blanchette.
Biofilm Pneumococci are Avirulent
Given the importance of biofilms in recalcitrant infections and for S. pneumoniae in the middle ear during otitis media (Reid et al., 2009; Chauhan et al., 2014), initial studies examining pneumococcal biofilms sought to associate the ability to form biofilms with enhanced virulence (Munoz-Elias et al., 2008; Lizcano et al., 2010). However, the ability to form biofilms in vitro could not be linked to the anatomical site from which a clinical isolate was obtained (i.e., nasopharynx of an asymptomatic carrier or blood from individual with IPD), nor the ability of the isolate to cause bacteremia in an infectious mouse model (Hall-Stoodley et al., 2008; Lizcano et al., 2010). Importantly, these and other studies have shown that in vitro biofilm formation was most enhanced for mutants that lacked capsular polysaccharide (CPS) (Moscoso et al., 2006). CPS mutants are avirulent due to their inability to prevent opsonophagocytosis (Hyams et al., 2010). Thus, the fact that unencapsulated mutants form more robust biofilms suggested a direct disconnect between pneumococcal biofilm formation and its propensity for invasive disease.
To directly test if pneumococci within biofilms were virulent, Sanchez et al. intratracheally challenged mice with equal colony forming units (CFU) of a virulent serotype 4 isolate grown to exponential (mid-logarithmic) phase in media or as a 3-day biofilm in a continuous flow-through reactor. They observed that only mice infected with planktonic pneumococci progressed to bacteremia while most of those challenged with biofilm pneumococci had negative blood cultures (Figure 2A) (Sanchez et al., 2011b). Studies by Blanchette-Cain et al. showed that pneumococci grown as a biofilm were hyper-adhesive yet uninvasive when tested in vitro on Detroit-562 pharyngeal epithelial cells (Figure 2B) (Blanchette-Cain et al., 2013). Marks et al. had similar results and showed that pneumococci grown as biofilms on fixed and live NCI-H292 bronchial epithelial cells neither invaded nor were internalized. Of note, Marks et al. showed that pneumococci recently dispersed from a biofilm due to an inflammatory signal, such as viral infection, were hyper-virulent with substantially greater capacity to cause invasive disease in mice than either biofilm pneumococci or pneumococci grown for a sustained period planktonically (Marks et al., 2013). Why recently dispersed pneumococci are more virulent than their sustained planktonic counterparts is not immediately clear, albeit two possibilities are that these bacteria carry biofilm ECM components that enhance their adhesive capacity, and major changes in gene expression profiles (Pettigrew et al., 2014). This observation helps to explain why viral infection is an established risk factor for the development of pneumococcal pneumonia (Brundage, 2006; McCullers, 2006).
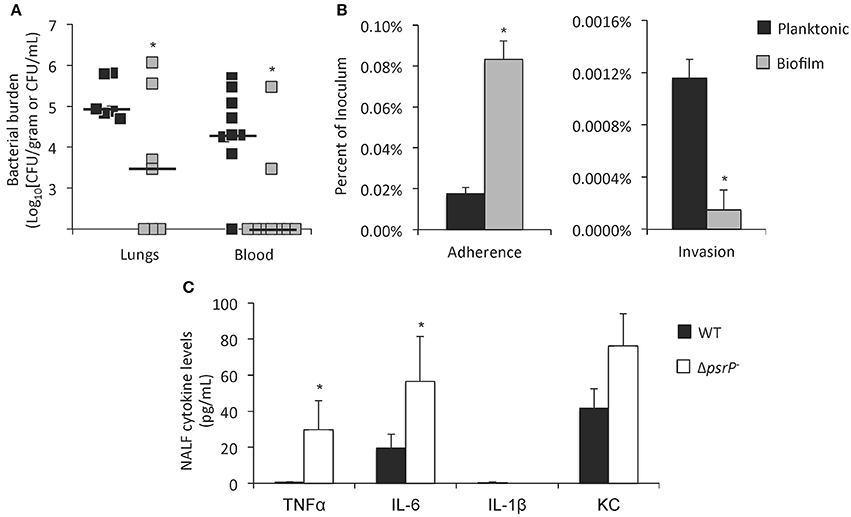
Figure 2. Biofilm pneumococci are less virulent and elicit a weaker immune response than their planktonic counterparts. (A) Bacterial titers in the lungs and blood of BALB/c mice challenged intratracheally with 105 CFU of planktonic or biofilm derived S. pneumoniae (each square = individual mouse; n = 6–8). From: Sanchez et al. (2011b). (B) Percentage of planktonic and biofilm derived S. pneumoniae that attached and invaded Detroit-562 pharyngeal cells in vitro. Percentages were calculated from the total inoculum. (C) Cytokine levels in nasopharyngeal lavage fluid (NALF) of colonized mice. Mice were challenged with wild type (WT) and PsrP-deficient (ΔpsrP−) S. pneumoniae and NALF collected 7 days later. Note that ΔpsrP− does not form biofilms during colonization (n = 5). Panels (B,C) from: Blanchette-Cain et al. (2013). Asterisks denote a statistically significant difference (P < 0.05).
Reduced Capsular Polysaccharide During Biofilm Growth
CPS is the principal virulence determinant for S. pneumoniae and exists in >90 identified serotypes (Bennett et al., 2014). In addition to resisting opsonophagocytosis (Melin et al., 2010), the negative or neutral charge of CPS plays an important role in helping the pneumococcus evade entrapment in mucus (Nelson et al., 2007). The necessity of CPS for IPD is exhibited by the fact that all invasive strains of S. pneumoniae are encapsulated whereas unencapsulated pneumococci are infrequent and usually only associated with topical eye infection (Barker et al., 1999).
Multiple investigators have reported an inhibitory role for CPS during in vitro biofilm formation with capsule deficient mutants forming substantially more robust biofilms than their encapsulated parent strain (Moscoso et al., 2006; Qin et al., 2013). Allergucci and Sauer showed that biofilms formed by a serotype 3 isolate were in large part composed of spontaneous mutants deficient in CPS related genes (Allegrucci and Sauer, 2007). Marks et al. have added evidence that this may occur in vivo by showing that unencapsulated pneumococci form more robust biofilms on the surface of epithelial cell monolayers (Marks et al., 2012). In fact, the presence of a capsule was shown to inhibit unencapsulated pneumococci from forming robust biofilms in mixed in vitro cultures (Domenech et al., 2009). Yet, CPS production is required for efficient in vivo colonization (Shainheit et al., 2014), indicating that during colonization the pneumococcus must strike a balance between CPS hindrance of biofilm formation and resistance to host defense.
Gene expression analyses using qRT-PCR and microarrays have shown that genes within the CPS operon were downregulated during in vitro biofilm formation vs. planktonic growth (Oggioni et al., 2006; Sanchez et al., 2011b). Moreover, the amount of capsule detected and the enzymes responsible for CPS production were substantially lower for biofilm vs. planktonic grown pneumococci as detected by ELISA and MALDI-TOF (Sanchez et al., 2011a,b). In agreement with dynamic changes in CPS production, pneumococci reduce capsule thickness once in contact with epithelial cells (Hammerschmidt et al., 2005). This is supported by microarray gene analysis of cells in contact with respiratory epithelial cells in vitro (Orihuela et al., 2004b). Thus, biofilm pneumococci reduce levels of CPS making them more susceptible to phagocytosis following aspiration.
Phase Variation
S. pneumoniae oscillates between an opaque phase variant that produces high levels of CPS and low levels of cell wall teichoic acid, and a transparent phase variant with low CPS and high cell wall teichoic acid (Weiser et al., 1994). The basis for phase variation is now understood to be epigenetic, with alternate methylation patterns on genes (Manso et al., 2014). Due to negative selection for the transparent phase by phagocytes, opaque variants predominate in the blood (Kim and Weiser, 1998). In contrast, the transparent phenotype is better able to adhere to cells and thus predominates in the nasopharynx (Weiser et al., 1994). Of note, Sanchez et al. have shown that in vitro biofilms are primarily composed of the transparent variant, despite the seed cultures used to initiate the biofilm being mostly opaque (Sanchez et al., 2011b).
In its transition from opaque to transparent, the pneumococcus loses virulence potential while enhancing its ability to adhere to host cells. As discussed, loss or a reduction in CPS enhances susceptibility to opsonophagocytosis yet is required for the exposure of surface adhesins that mediate bacterial attachment to host cells (Ring et al., 1998). Critically, cell invasion occurs for planktonic but not biofilm pneumococci (Blanchette-Cain et al., 2013). The increased amount of teichoic acid carried by the transparent variant also makes it subject to recognition by C-reactive protein (CRP), resulting in activation of complement (Kim et al., 1999). However, phosphorylcholine residues present on teichoic acid allow the pneumococcus to bind to the host ligand platelet-activating factor (PAFr) receptor on host cells (Cundell et al., 1995).
Despite loss of capsule and increased exposure of teichoic acid, pneumococci in biofilms are resistant to opsonophagocytosis (Yuste et al., 2007). One reason for this includes that CRP binding to phosphorylcholine is competed with by members of the choline-binding protein family (Mukerji et al., 2012), such as the adhesin Choline-binding protein A (CbpA) which is upregulated during transparent phase growth as well as in biofilms (Sanchez et al., 2011b). CbpA is also a key inhibitor of complement deposition through its binding to Factor H and complement component C3 (Cheng et al., 2000; Dave et al., 2001). Another choline-binding protein that plays a key role in complement inhibition includes Pneumococcal surface protein A (PspA), which prevents classical complement activation in a C1q dependent manner (Tu et al., 1999; Yuste et al., 2007; Mukerji et al., 2012). Importantly, the opaque variant has been suggested to play a critical role in the formation of the ECM (Trappetti et al., 2011). Of note, gene expression studies for biofilms and transparent pneumococci do not entirely overlap. Thus, phase variation is an important aspect of pneumococcal biofilm formation but is not entirely responsible for its phenotype.
Downregulation of Metabolic Processes and Modulated Virulence Gene Expression
Antimicrobial resistance is one of the defining properties of biofilms and has been extensively documented for biofilm pneumococci, particularly in the context of recurring otitis media (Stewart and Costerton, 2001; Hall-Stoodley et al., 2008). Why, the ability of an isolate to form well-structured biofilms in vivo was correlated with resistance to high concentrations of gentamycin (Marks et al., 2012). Enhanced resistance to antimicrobials in biofilm pneumococci may be due to a decrease in metabolic rate, which also confers resistance to antimicrobials targeting cell wall, protein synthesis, and DNA replication. The ECM also serves as an inhibitor or off-target for antimicrobials. This topic is extensively reviewed elsewhere (Domenech et al., 2012). Once aspirated, a reduced metabolic rate would presumably impair the ability of biofilm pneumococci to respond in a timely fashion to hostile host factors present in the lower respiratory tract.
Along such lines, planktonic and biofilm S. pneumoniae are now recognized to have distinct protein and gene expression profiles. Using qRT-PCR, Oggioni et al. showed that the gene expression profile of virulence-associated genes of different strains isolated from the blood were more similar to that of planktonic growth in broth, whereas the same strain isolated from the lungs, brain, or nasopharynx of infected mice was more similar to that of in vitro biofilms (Oggioni et al., 2006). Microarray analysis of in vitro grown planktonic vs. biofilm pneumococci showed that biofilm pneumococci downregulated genes involved in protein synthesis, energy production, metabolism, CPS production; along with the virulence genes that encode the pneumococcal pilus, which has been shown to be an invasin (Barocchi et al., 2006), and the pore-forming toxin pneumolysin (Sanchez et al., 2011b). Pneumolysin has been demonstrated to be required for systemic bacteremia and host cell damage and inflammation (Orihuela et al., 2004a; Mitchell and Dalziel, 2014), thus its down regulation would most likely compromise virulence. Yet pneumolysin has also been shown to contribute toward in vitro biofilm formation (Shak et al., 2013). Thus, and like that for CPS, pneumolysin production is most likely fine-tuned to strike a balance with the host during colonization. In contrast, the genes encoding the adhesins PsrP, PavA as well as the previously discussed CbpA, were detected as being upregulated during biofilm growth (Sanchez et al., 2011b; Qin et al., 2013). These proteins may play a role in intra-species aggregation such as observed during in vivo biofilms, either by binding to other pneumococci directly or through bridging molecules such as fibronectin (Blanchette and Orihuela, 2012). The, why biofilm pneumococci do not invade cells remains unclear.
Mass spectroscopy (MS) based identification of proteins isolated from biofilm and planktonic cell lysates confirm profound differences between these two physiological growth states (Allegrucci et al., 2006; Sanchez et al., 2011a). One important caveat to this approach is that pneumococcal biofilms are in part composed of dead pneumococci and proteomic studies don't distinguish between proteins from live bacteria or those dead bacteria that have accumulated within the biofilm. When alive, these dead bacteria may have had a substantially different proteome. Nonetheless, and in agreement with microarray studies, MS of biofilm and planktonic cell lysates by Sanchez et al. found that the frequency of peptides corresponding to enzymes involved in protein synthesis and processing, energy metabolism, CPS production, and proteins involved in transcription, regulation and DNA binding, as well as the virulence determinants enolase, pyruvate oxidase (produces hydrogen peroxide), and pneumolysin were less frequent in biofilm lysates than planktonic lysates (Sanchez et al., 2011a). The extent to which major differences occur in the proteome is further highlighted by the finding that antiserum from humans who recovered from IPD robustly recognized proteins in planktonic cell lysates but not biofilm cell lysates when tested by Western blot (Sanchez et al., 2011a). This provides evidence that the in vivo antigen protein profiles for colonization vs. invasive disease are considerably different, and that the overall productions of factors that mediate a response to a host or subvert the host response are altered.
Host Response to Biofilm Pneumococci
Only recently have investigators begun to examine how the host responds to biofilm pneumococci. Studies by Blanchette-Cain et al. have shown that biofilm pneumococci elicit significantly less Interleukin (IL)-6 and IL-8 from Detroit-562 pharyngeal epithelial cells than planktonic cultures. Similarly, biofilm pneumococci elicited less IL-6, IL-1β, and TNFα, from J774A.1 macrophages. In vivo, mutant pneumococci lacking the biofilm determinant PsrP, and thus unable to form in vivo biofilms, elicited greater TNFα, IL-6, IL-1β, and KC production in the nasopharynx of 7-day colonized mice vs. its parent strain (Figure 2C) (Blanchette-Cain et al., 2013). This was credited to the reduced tissue invasiveness of biofilm pneumococci, but as indicated may also involve reduced production of the toxin pneumolysin. Importantly, pneumococci may also actively suppress the host response in a way that has not yet been determined. For example studies have shown that Group B Streptococcus interacts with Siglec-5 and this dampens the host response (Carlin et al., 2009). Future studies examining this possibility are warranted.
Given the fact that the majority of individuals are colonized asymptomatically, we speculate that non-invasive pneumococci within biofilms promote long-term colonization and transmission through less vigorous activation of the innate immune response and therefore a delay in the onset of the adaptive response and their clearance. Yet, direct evidence for this is lacking with intranasal challenge of mice with PsrP-deficient or other mutants that are biofilm deficient not resulting in reduced bacterial titers in the nasopharynx when measured by CFU or qRT-PCR (Blanchette-Cain et al., 2013). Thus, studies are warranted to determine what the true physiological advantage of this immunoquiescent phenotype actually is and if it impacts the number of bacteria in the nasopharynx or long-term carriage.
Biofilms as Source of Fomite Transmission and Conclusions
Multiple studies have shown that biofilm-derived pneumococci are more resistant to desiccation than their planktonic counterparts (Walsh and Camilli, 2011), with viable cells isolated from fomites over a period ten times longer than planktonic (Marks et al., 2014). Additionally, viable pneumococci have been recovered from a variety of desiccated surfaces in a day care setting: hands, books, and both hard and soft toys. Importantly, desiccated pneumococci recovered from fomites still retain colonization capabilities in a murine model, even with a normal inoculum (Walsh and Camilli, 2011; Marks et al., 2014). As is shown in Figure 1B, the pneumococcal aggregates can be sloughed from the nasopharynx and these aggregates most likely are a vehicle for transmission, possibly providing the bacteria with moisture and nutrients for an extended period outside the body. We point out that dispersed bacteria, as shown by Marks et al. (2013), are planktonic and may be a second vehicle for transmission following an inflammatory episode such as virus infection.
Most individuals carrying S. pneumoniae are colonized asymptomatically, thus the biofilm state is the major form by which the pneumococcus interacts with its host. Herein we have discussed how biofilm pneumococci are distinct from their planktonic counterparts. Specifically, pneumococci downregulate CPS, enhance expression of adhesins, shift toward the transparent phenotype, and lower the expression of metabolic processes and key virulence determinants that elicit a robust host response. Therefore, biofilm pneumococci seem to be exquisitely honed to the colonization phenotype at the expense of the invasive phenotype. There are many questions that remain to be answered; for example, direct evidence that the immunoquiescent phenotype confers a colonization advantage is lacking. This may be due to limitations in the current model systems and/or our ability to quantify bacteria in vivo. Additionally, does the pneumococcus rely on dispersal of biofilm aggregates or the spread of highly invasive biofilm dispersed planktonic pneumococci as the principle method for transmission, or are both effective? Perhaps different strains rely differently on these transmission methods. There are also infections that seem to be a mix of biofilms and planktonic bacteria, for example during otitis media. How these two physiological states impact the course of disease is unclear and warrants attention. In summary, a myriad of functional reasons can and do exist for why biofilm pneumococci are less virulent. A better understanding of the short-term survival and long-term evolutionary advantages would substantially enhance our understanding of pneumococcal biology, and may permit us to develop novel targets for bacterial clearance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allegrucci, M., Hu, F. Z., Shen, K., Hayes, J., Ehrlich, G. D., Post, J. C., et al. (2006). Phenotypic characterization of Streptococcus pneumoniae biofilm development. J. Bacteriol. 188, 2325–2335. doi: 10.1128/JB.188.7.2325-2335.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Allegrucci, M., and Sauer, K. (2007). Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 189, 2030–2038. doi: 10.1128/JB.01369-06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barker, J. H., Musher, D. M., Silberman, R., Phan, H. M., and Watson, D. A. (1999). Genetic relatedness among nontypeable pneumococci implicated in sporadic cases of conjunctivitis. J. Clin. Microbiol. 37, 4039–4041.
Barocchi, M. A., Ries, J., Zogaj, X., Hemsley, C., Albiger, B., Kanth, A., et al. (2006). A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. U.S.A. 103, 2857–2862. doi: 10.1073/pnas.0511017103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bennett, J. E., Dolin, R., and Blaser, M. J. (2014). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Cambridge, UK: Elsevier.
Blanchette, K. A., and Orihuela, C. J. (2012). Future perspective on host-pathogen interactions during bacterial biofilm formation within the nasopharynx. Future Microbiol. 7, 227–239. doi: 10.2217/fmb.11.160
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Blanchette-Cain, K., Hinojosa, C. A., Akula Suresh Babu, R., Lizcano, A., Gonzalez-Juarbe, N., Munoz-Almagro, C., et al. (2013). Streptococcus pneumoniae biofilm formation is strain dependent, multifactorial, and associated with reduced invasiveness and immunoreactivity during colonization. MBio 4, e00745–e00713. doi: 10.1128/mBio.00745-13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brundage, J. F. (2006). Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect. Dis. 6, 303–312. doi: 10.1016/S1473-3099(06)70466-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Carlin, A. F., Chang, Y. C., Areschoug, T., Lindahl, G., Hurtado-Ziola, N., King, C. C., et al. (2009). Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J. Exp. Med. 206, 1691–1699. doi: 10.1084/jem.20090691
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chauhan, A., Bernardin, A., Mussard, W., Kriegel, I., Esteve, M., Ghigo, J. M., et al. (2014). Preventing biofilm formation and associated occlusion by biomimetic glycocalyxlike polymer in central venous catheters. J. Infect. Dis. 210, 1347–1356. doi: 10.1093/infdis/jiu249
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cheng, Q., Finkel, D., and Hostetter, M. K. (2000). Novel purification scheme and functions for a C3-binding protein from Streptococcus pneumoniae. Biochemistry 39, 5450–5457. doi: 10.1021/bi992157d
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Crook, D. W., Brueggmann, A. B., Sleeman, K. L., and Peto, T. E. A. (2004). Pneumococcal Carriage. The Pneumococcus. Washington, DC: ASM Press.
Cundell, D. R., Gerard, N. P., Gerard, C., Idanpaan-Heikkila, I., and Tuomanen, E. I. (1995). Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377, 435–438. doi: 10.1038/377435a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dalia, A. B., and Weiser, J. N. (2011). Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe 10, 486–496. doi: 10.1016/j.chom.2011.09.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dave, S., Brooks-Walter, A., Pangburn, M. K., and McDaniel, L. S. (2001). PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69, 3435–3437. doi: 10.1128/IAI.69.5.3435-3437.2001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Domenech, M., Garcia, E., and Moscoso, M. (2009). Versatility of the capsular genes during biofilm formation by Streptococcus pneumoniae. Environ. Microbiol. 11, 2542–2555. doi: 10.1111/j.1462-2920.2009.01979.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Domenech, M., Garcia, E., and Moscoso, M. (2012). Biofilm formation in Streptococcus pneumoniae. Microb. Biotechnol. 5, 455–465. doi: 10.1111/j.1751-7915.2011.00294.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gray, B. M., Converse, G. M. 3rd., and Dillon, H. C. Jr. (1980). Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142, 923–933. doi: 10.1093/infdis/142.6.923
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hall-Stoodley, L., Nistico, L., Sambanthamoorthy, K., Dice, B., Nguyen, D., Mershon, W. J., et al. (2008). Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 8:173. doi: 10.1186/1471-2180-8-173
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hammerschmidt, S., Wolff, S., Hocke, A., Rosseau, S., Muller, E., and Rohde, M. (2005). Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 73, 4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hoa, M., Syamal, M., Sachdeva, L., Berk, R., and Coticchia, J. (2009). Demonstration of nasopharyngeal and middle ear mucosal biofilms in an animal model of acute otitis media. Ann. Otol. Rhinol. Laryngol. 118, 292–298. doi: 10.1177/000348940911800410
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Huang, S. S., Hinrichsen, V. L., Stevenson, A. E., Rifas-Shiman, S. L., Kleinman, K., Pelton, S. I., et al. (2009). Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124, e1–e11. doi: 10.1542/peds.2008-3099
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hyams, C., Camberlein, E., Cohen, J. M., Bax, K., and Brown, J. S. (2010). The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 78, 704–715. doi: 10.1128/IAI.00881-09
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kim, J. O., Romero-Steiner, S., Sorensen, U. B., Blom, J., Carvalho, M., Barnard, S., et al. (1999). Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67, 2327–2333.
Kim, J. O., and Weiser, J. N. (1998). Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177, 368–377. doi: 10.1086/514205
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lizcano, A., Chin, T., Sauer, K., Tuomanen, E. I., and Orihuela, C. J. (2010). Early biofilm formation on microtiter plates is not correlated with the invasive disease potential of Streptococcus pneumoniae. Microb. Pathog. 48, 124–130. doi: 10.1016/j.micpath.2010.01.002
Manso, A. S., Chai, M. H., Atack, J. M., Furi, L., De Ste Croix, M., Haigh, R., et al. (2014). A random six-phase switch regulates pneumococcal virulence via global epigenetic changes. Nat. Commun. 5, 5055. doi: 10.1038/ncomms6055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marks, L. R., Davidson, B. A., Knight, P. R., and Hakansson, A. P. (2013). Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio 4, 4. doi: 10.1128/mBio.00438-13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marks, L. R., Parameswaran, G. I., and Hakansson, A. P. (2012). Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect. Immun. 80, 2744–2760. doi: 10.1128/IAI.00488-12
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marks, L. R., Reddinger, R. M., and Hakansson, A. P. (2014). Biofilm formation enhances fomite survival of Streptococcus pneumoniae and Streptococcus pyogenes. Infect. Immun. 82, 1141–1146. doi: 10.1128/IAI.01310-13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McCullers, J. A. (2006). Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19, 571–582. doi: 10.1128/CMR.00058-05
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Melin, M., Trzcinski, K., Meri, S., Kayhty, H., and Vakevainen, M. (2010). The capsular serotype of Streptococcus pneumoniae is more important than the genetic background for resistance to complement. Infect. Immun. 78, 5262–5270. doi: 10.1128/IAI.00740-10
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mitchell, T. J., and Dalziel, C. E. (2014). The biology of pneumolysin. Subcell. Biochem. 80, 145–160. doi: 10.1007/978-94-017-8881-6_8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moscoso, M., Garcia, E., and Lopez, R. (2006). Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J. Bacteriol. 188, 7785–7795. doi: 10.1128/JB.00673-06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mukerji, R., Mirza, S., Roche, A. M., Widener, R. W., Croney, C. M., Rhee, D. K., et al. (2012). Pneumococcal surface protein A inhibits complement deposition on the pneumococcal surface by competing with the binding of C-reactive protein to cell-surface phosphocholine. J. Immunol. 189, 5327–5335. doi: 10.4049/jimmunol.1201967
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Munoz-Elias, E. J., Marcano, J., and Camilli, A. (2008). Isolation of Streptococcus pneumoniae biofilm mutants and their characterization during nasopharyngeal colonization. Infect. Immun. 76, 5049–5061. doi: 10.1128/IAI.00425-08
Naucler, P., Darenberg, J., Morfeldt, E., Ortqvist, A., and Henriques Normark, B. (2013). Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax 68, 571–579. doi: 10.1136/thoraxjnl-2012-203106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nelson, A. L., Roche, A. M., Gould, J. M., Chim, K., Ratner, A. J., and Weiser, J. N. (2007). Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 75, 83–90. doi: 10.1128/IAI.01475-06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
O'Brien, K. L., Wolfson, L. J., Watt, J. P., Henkle, E., Deloria-Knoll, M., McCall, N., et al. (2009). Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902. doi: 10.1016/S0140-6736(09)61204-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Oggioni, M. R., Trappetti, C., Kadioglu, A., Cassone, M., Iannelli, F., Ricci, S., et al. (2006). Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol. Microbiol. 61, 1196–1210. doi: 10.1111/j.1365-2958.2006.05310.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Orihuela, C. J., Gao, G., Francis, K. P., Yu, J., and Tuomanen, E. I. (2004a). Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190, 1661–1669. doi: 10.1086/424596
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Orihuela, C. J., Radin, J. N., Sublett, J. E., Gao, G., Kaushal, D., and Tuomanen, E. I. (2004b). Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72, 5582–5596. doi: 10.1128/IAI.72.10.5582-5596.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pettigrew, M. M., Marks, L. R., Kong, Y., Gent, J. F., Roche-Hakansson, H., and Hakansson, A. P. (2014). Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza a virus infection. Infect. Immun. 82, 4607–4619. doi: 10.1128/IAI.02225-14
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Qin, L., Kida, Y., Imamura, Y., Kuwano, K., and Watanabe, H. (2013). Impaired capsular polysaccharide is relevant to enhanced biofilm formation and lower virulence in Streptococcus pneumoniae. J. Infect. Chemother. 19, 261–271. doi: 10.1007/s10156-012-0495-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Reid, S. D., Hong, W., Dew, K. E., Winn, D. R., Pang, B., Watt, J., et al. (2009). Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J. Infect. Dis. 199, 786–794. doi: 10.1086/597042
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ring, A., Weiser, J. N., and Tuomanen, E. I. (1998). Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest. 102, 347–360. doi: 10.1172/JCI2406
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sanchez, C. J., Hurtgen, B. J., Lizcano, A., Shivshankar, P., Cole, G. T., and Orihuela, C. J. (2011a). Biofilm and planktonic pneumococci demonstrate disparate immunoreactivity to human convalescent sera. BMC Microbiol. 11:245. doi: 10.1186/1471-2180-11-245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sanchez, C. J., Kumar, N., Lizcano, A., Shivshankar, P., Dunning Hotopp, J. C., Jorgensen, J. H., et al. (2011b). Streptococcus pneumoniae in biofilms are unable to cause invasive disease due to altered virulence determinant production. PLoS ONE 6:e28738. doi: 10.1371/journal.pone.0028738
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shainheit, M. G., Mule, M., and Camilli, A. (2014). The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect. Immun. 82, 694–705. doi: 10.1128/IAI.01289-13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shak, J. R., Ludewick, H. P., Howery, K. E., Sakai, F., Yi, H., Harvey, R. M., et al. (2013). Novel role for the Streptococcus pneumoniae toxin pneumolysin in the assembly of biofilms. MBio 4, e00655–e00613. doi: 10.1128/mBio.00655-13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stewart, P. S., and Costerton, J. W. (2001). Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138. doi: 10.1016/S0140-6736(01)05321-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Trappetti, C., Ogunniyi, A. D., Oggioni, M. R., and Paton, J. C. (2011). Extracellular matrix formation enhances the ability of Streptococcus pneumoniae to cause invasive disease. PLoS ONE 6:e19844. doi: 10.1371/journal.pone.0019844
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tu, A. H., Fulgham, R. L., McCrory, M. A., Briles, D. E., and Szalai, A. J. (1999). Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67, 4720–4724.
Walsh, R. L., and Camilli, A. (2011). Streptococcus pneumoniae is desiccation tolerant and infectious upon rehydration. MBio 2, e00092–e00011. doi: 10.1128/mBio.00092-11
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weiser, J. N., Austrian, R., Sreenivasan, P. K., and Masure, H. R. (1994). Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62, 2582–2589.
Yuste, J., Botto, M., Bottoms, S. E., and Brown, J. S. (2007). Serum amyloid P aids complement-mediated immunity to Streptococcus pneumoniae. PLoS Pathog. 3:1208–1219. doi: 10.1371/journal.ppat.0030120
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: Streptococcus pneumoniae, biofilms, colonization, virulence, transmission
Citation: Gilley RP and Orihuela CJ (2014) Pneumococci in biofilms are non-invasive: implications on nasopharyngeal colonization. Front. Cell. Infect. Microbiol. 4:163. doi: 10.3389/fcimb.2014.00163
Received: 26 September 2014; Accepted: 21 October 2014;
Published online: 06 November 2014.
Edited by:
Pietro Speziale, University degli Studi di Pavia, ItalyReviewed by:
Nick Stephen Jakubovics, Newcastle University, UKAnders P. Hakansson, University at Buffalo, State University of New York, USA
Copyright © 2014 Gilley and Orihuela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos J. Orihuela, Department of Microbiology and Immunology, The University of Texas Health Science Center at San Antonio, Mail Code 8259, 8403 Floyd Curl Drive, San Antonio, TX 78229-3900, USA e-mail: orihuela@uthscsa.edu
 Ryan P. Gilley
Ryan P. Gilley Carlos J. Orihuela
Carlos J. Orihuela