Right heart failure after left ventricular assist device: From mechanisms to treatments
- 1Division of Cardiology, Department of Medicine, University of Washington, Seattle, WA, United States
- 2School of Medicine, University of Washington, Seattle, WA, United States
- 3Division of Cardiothoracic Surgery, Department of Surgery, University of Washington, Seattle, WA, United States
Left ventricular assist device (LVAD) therapy is a lifesaving option for patients with medical therapy-refractory advanced heart failure. Depending on the definition, 5–44% of people supported with an LVAD develop right heart failure (RHF), which is associated with worse outcomes. The mechanisms related to RHF include patient, surgical, and hemodynamic factors. Despite significant progress in understanding the roles of these factors and improvements in surgical techniques and LVAD technology, this complication is still a substantial cause of morbidity and mortality among LVAD patients. Additionally, specific medical therapies for this complication still are lacking, leaving cardiac transplantation or supportive management as the only options for LVAD patients who develop RHF. While significant effort has been made to create algorithms aimed at stratifying risk for RHF in patients undergoing LVAD implantation, the predictive value of these algorithms has been limited, especially when attempts at external validation have been undertaken. Perhaps one of the reasons for poor performance in external validation is related to differing definitions of RHF in external cohorts. Additionally, most research in this field has focused on RHF occurring in the early phase (i.e., ≤1 month) post LVAD implantation. However, there is emerging recognition of late-onset RHF (i.e., > 1 month post-surgery) as a significant cause of morbidity and mortality. Late-onset RHF, which likely has a unique physiology and pathogenic mechanisms, remains poorly characterized. In this review of the literature, we will describe the unique right ventricular physiology and changes elicited by LVADs that might cause both early- and late-onset RHF. Finally, we will analyze the currently available treatments for RHF, including mechanical circulatory support options and medical therapies.
Introduction
Heart transplantation still is considered the gold-standard treatment for advanced heart failure (1–3). Yet, limited donor organ supply limits this therapy to a small proportion of those who might benefit from it. Left ventricular assist device (LVAD) therapy has emerged as a viable option for those who cannot be transplanted before an irreversible complication or death occurs. LVAD placement immediately produces lifesaving hemodynamic changes such as normalization of cardiac output and reducing left ventricular (LV) pressures (4). These changes improve end-organ function, functional capacity, and survival (4–6). The evolution of LVAD technology has led to a 2-year event-free survival of around 78% with the latest commercial LVAD iteration, the HeartMate 3 (Abbott) (7, 8).
However, due to mechanisms that remain incompletely characterized, a significant number of patients develop right heart failure (RHF) following LVAD implantation. Depending on the definition, up to 40% of people supported with LVAD develop RHF, which is associated with poor outcomes (9–12). Based on the Interagency Registry of Mechanical Circulatory Support (INTERMACS) 2020 annual report, heart failure and multisystem organ failure were among the most significant causes of death among LVAD patients, with RHF likely playing a pivotal role in many (13). Thus, the development of RHF is one of the complications observed in patients chronically supported with LVADs that limits the full potential benefit from device therapy.
The treatments and understanding of most LVAD complications have evolved over the years. However, RHF remains poorly characterized and, most importantly, lacks medical treatment options. Despite the numerous compounds developed to successfully treat the failing LV, a paucity of research on RHF, including research into the essential physiological, phenotypic, histologic, and molecular differences between the right and left ventricles, has contributed to the lack of specific pharmacological agents for RHF. This manuscript aims to review the latest literature on right ventricular (RV) physiology, RHF pathophysiology in the presence of LVAD, and options to treat this complication.
Right ventricular physiology
The right and left ventricles work together as an interdependent system and share many similarities (14–16). Nonetheless, there are key dissimilarities, including the development of cardiomyocytes of each ventricle from different embryological progenitors, as well as differences in geometry, wall thickness, and loading conditions, that underscore critical differences between the ventricles (17, 18).
The RV is a thin-walled crescentic-shaped chamber that wraps around the LV (Figure 1) (19–21). Since both ventricles are connected as a series of “pumps” in a normal heart, the RV must deliver the same stroke volume as the LV to maintain normal circulation. Like the LV, the RV systolic function also follows the Frank-Starling law, which governs the interaction between venous return (preload), pulmonary vascular resistance (afterload), and myocardial contractility. Compared to the LV, the RV functions at higher ventricular volumes and lower pressure and impedance, i.e., the pulmonary circulation (22, 23). This different hemodynamic environment allows the RV to provide the appropriate stroke volume, even though its myocardial mass is only one-sixth that of the LV.

Figure 1. Reconstruction of a normal RV illustrating the three-dimensional relationships with the LV. The mesh surface represents the LV, and the continuous blue surface illustrates the RV. LV, left ventricle; P, pulmonary valve; RV, right ventricle; T, tricuspid valve. Reproduced from Sheehan and Redington (19).
Additional unique characteristics of the RV are that it is more afterload sensitive and compliant than the LV. Increasing afterload leads to a disproportionate increase in energy expenditure and decreased efficiency of the RV as compared to the LV, resulting in a reduction of the RV stroke volume at a faster rate than the LV (Figure 2A). The higher compliance of the RV allows it to handle a large amount of blood return fluctuations without significant changes in stroke work compared to the LV (Figure 2B) (23–25).
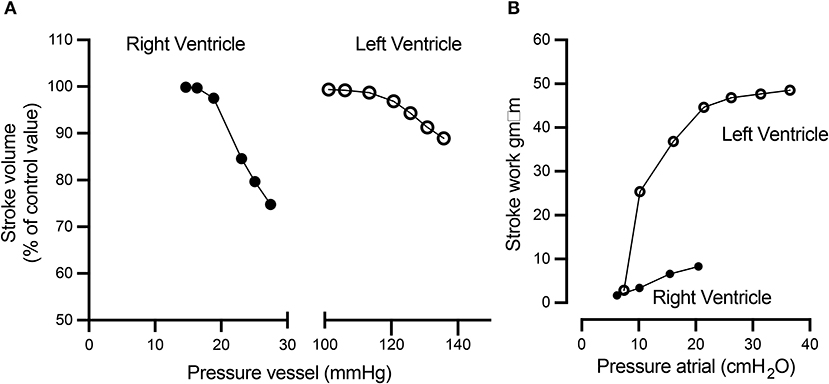
Figure 2. The RV and LV respond differently to increased afterload and preload. (A) Compared to the LV, with a 10 mmHg afterload difference (between 20 and 30 mmHg), the RV displays a rapid decline in the stroke volume, while the LV remains relatively stable. Conversely, (B) the LV stroke work rapidly increases as the preload increases, while the RV stroke work response is more modest to similar preload changes. This figure was published in: Braunwald (24). Reproduced by permission from Braunwald (24).
Ventricular interdependence is crucial for the systolic and diastolic function of the RV. Ventricular interdependence refers to the size, shape, and compliance of one ventricle affecting the other through direct interaction (26, 27). Through ventricular interdependence, the LV contributes ~20–40% of the RV systolic pressure, with the RV contributing about 4–10% of the LV systolic pressure (26, 28). The interventricular septum appears to be the main contributor to ventricular interdependence. However, other factors such as muscle fibers that connect both ventricles, shared coronary blood flow, and the pericardium also play essential roles in this cooperative process (26, 29).
Post LVAD RHF pathophysiology
Placement of a fully functional LVAD into an advanced heart failure patient rapidly elicits favorable hemodynamic changes, including restoration of cardiac output and reductions in left ventricular and pulmonary arterial pressures (4). This new hemodynamic profile renders better end-organ perfusion, functional capacity, survival, and quality of life (4–6). The decreased pulmonary arterial pressure with LVAD, via immediate reduction of left-sided filling pressures and more long-term remodeling of fixed pulmonary hypertension, lowers the pulmonary vascular resistance, thereby improving right-sided afterload and RV function (4, 30–32). This LV unloading also improves RV function by decreasing functional mitral valve regurgitation and reversing an excessive shift of the septum into the RV as a result of the higher LV volume (33–35). Despite these corrective modifications of cardiogenic shock obtained with LVAD, other coexisting forces can lead to RHF (Figure 3). The pathophysiology of post-LVAD RHF is multifactorial, including post-surgical hemodynamic and geometric changes of the heart, along with perioperative and patient-related factors. Factors such as changes in preload and alterations in ventricular interdependence through pericardiotomy and interventricular septal function have been considered to play a central role in RHF pathophysiology.
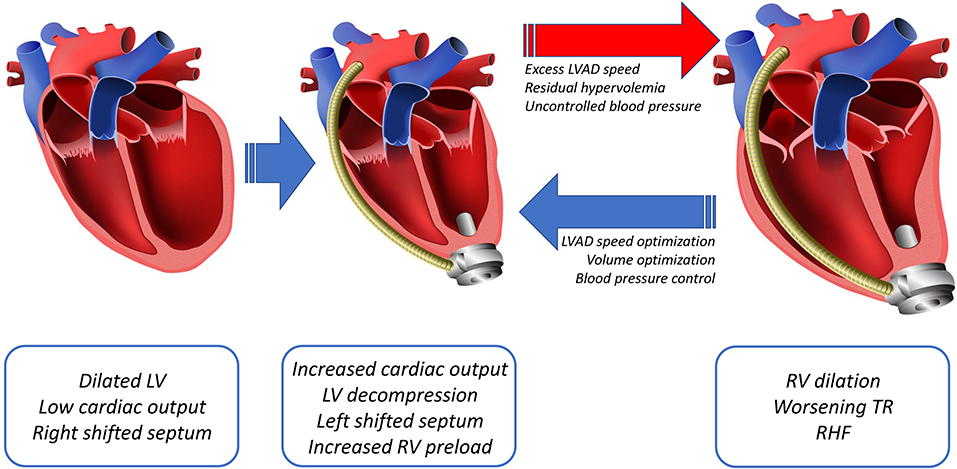
Figure 3. Diagram showing the main changes post LVAD, including an increased cardiac output that leads to decompression of the LV and a leftward shift of the septum with subsequently increased preload to the RV. The increased right-sided preload and a leftward shift of the septum, dilates the RV, worsening the tricuspid regurgitation (TR), resulting in RHF.
The role of preload in RHF
As LVAD support decreases right-sided afterload, it also raises preload. This can ultimately lead to a volume overloaded and more afterload-sensitive RV (36–38). The increase in RV preload is due to an increase in LV output due to LVAD support (4, 38, 39). While the RV appropriately increases the stroke volume to match the new left-sided supported cardiac output via a Frank-Starling mechanism, the capacity of the RV to handle a higher preload is limited by pre-existing RV functional reserve. RV distension due to a greater preload also can induce tricuspid annular dilatation, thereby worsening pre-existing tricuspid regurgitation (39–41). In essence, whenever the LVAD delivers more volume than the RV can accommodate, the result is chronic RV pressure/volume overload, eventually resulting in RHF. This occurs with excess LVAD speed, residual hypervolemia, systemic hypertension, or a combination of these factors. Tricuspid regurgitation also can be exacerbated by tricuspid valve tethering due to leftward septal shift upon LV decompression with the LVAD support, further aggravating RV pressure/volume overload (Figure 3) (42).
The role of pericardium in RHF
One of the roles of the pericardium is to maintain biventricular morphology and interdependence, both essential features for normal RV function and geometry (26, 43). It has been widely reported that disruption of the pericardium during cardiac surgery is associated with RV dysfunction (44–46). Thus, pericardiotomy during LVAD implantation likely contributes to RHF development. Along these lines, there has been interest in using a less invasive surgical approach, such as left lateral thoracotomy, for LVAD implantation. Observational studies and the single-arm, prospective LATERAL clinical trial have suggested that with this less invasive surgical approach, LVAD implantation appears safe; and is associated with fewer blood transfusions, shorter hospital stays, and lower rates of RHF (47–52). These data suggest that preserving pericardial integrity during LVAD implantation might be protective for the RV.
The role of the interventricular septum in RHF
The interventricular septum is a significant contributor to the RV function through its contraction and ventricular interdependence (53). Longitudinal contraction of the interventricular septum accounts for nearly 80% of normal RV function, and the oblique contraction that is responsible for the twisting motion of this chamber allows it to overcome higher afterload (54, 55). Following a cardiac surgery with pericardiotomy, the contraction pattern changes, switching to mainly transverse shortening of the interventricular septum (56). This change in the contraction pattern leads to an adaptive enhancement of transverse contractile function, maintaining a relatively normal RV function. However, these alterations make the RV more afterload sensitive. In addition to changes in interventricular contractile dynamics after pericardiotomy, the leftward septal shift due to LV decompression further limits the contribution of the interventricular septum to RV function, putting this chamber at higher risk of failure (57).
Definitions and epidemiology of RHF
The definition of RHF has evolved, starting with the INTERMACS 2008, which required a central venous pressure of >18 mmHg, cardiac index < 2.0 L/min/m2, and a treatment for RHF such as RV mechanical circulatory support (MCS), inotrope or inhaled nitric oxide for more than a week. A version that included more variables, INTERMACS 2014, required central venous pressure of >16 mmHg or evidence of elevated central venous pressure on echocardiogram or physical exam, as well as laboratory manifestations of high central venous pressure. Finally, the 2020 consensus statement of the Mechanical Circulatory Support Academic Research Consortium (MCS-ARC) proposed a more complex and comprehensive definition of RHF. This latest definition requires signs of elevated right-sided pressures or the presence of manifestations suggestive of RHF, as well as either inotropic or mechanical intervention for this complication. Additionally, MCS-ARC RHF events are categorized as early acute, early post-implant, and late-onset, depending on the timing of RHF presentation (Figure 4) (58, 59).
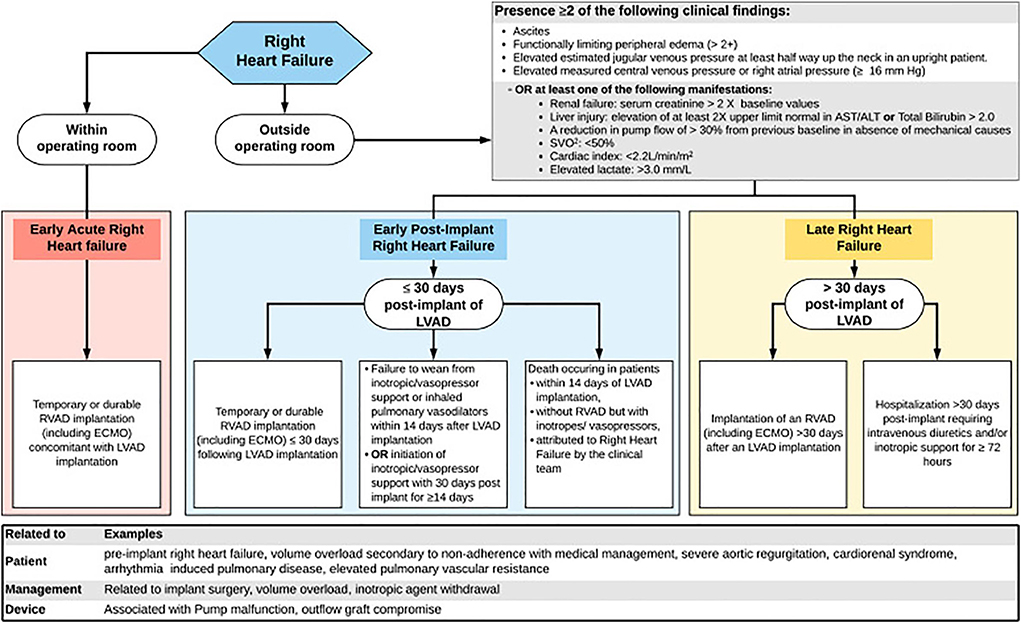
Figure 4. Algorithm illustrating the MCS-ARC diagnostic criteria for RHF (58). The RHF events are classified as early acute if it occurs immediately after LVAD implantation, early if it is within the first 30 days post-surgery, and late if it happened > 30 days post-surgery. Additionally, for early and late RHF, the new criteria require the presence of clinical findings or manifestations suggestive of RHF, in addition to an intervention to treat this complication. Reproduced from Kormos (58).
Given the differing definitions and modified versions of contemporary definitions of RHF used across studies, it is challenging to understand the true burden of post-LVAD RHF and to compare its incidence across different cohorts (7, 12, 60–64). Thus, observational studies using different definitions have reported the prevalence of RHF with LVAD support ranging from 5 to 44%. However, all have reported that RHF is associated with increased morbidity, mortality, and longer hospital stay (7, 9–12, 63–67). Furthermore, most studies have focused on characterizing early onset RHF, i.e., which occurs soon after the LVAD implantation. However, there is growing evidence that RHF may manifest following patient discharge, called late-onset RHF. Late-onset RHF is less well-characterized, and it remains unknown if late-onset RHF represents part of a continuum from early RHF or a completely different entity with different etiologic factors (64, 66, 68–70).
Prediction of RHF
There has been a significant effort to develop predictive tools for RHF in patients undergoing LVAD implantation. Pre-preoperative characteristics, echocardiographic measurements, hemodynamic parameters, and biomarkers have been associated with post-LVAD RHF incidence (71–75). Moreover, various complex scoring systems include several of those identified independent risk factors. Some of the most studied models are the Michigan RHF score system, Penn RHF risk score, Heartmate II RHF model, Utah RHF risk score, Pittsburg decision tree, CRITT score, and EuroMACS score (12, 60, 63, 76–78). Unfortunately, none of those risk scoring systems have performed as expected in external validation studies, limiting their applicability in clinical practice. One of the limitations of these studies is the heterogeneous definitions of RHF, which could partly explain the inconsistency in external validation (79).
Some hemodynamic parameters or calculations, such as high CVP, low RV stroke work index, CVP to pulmonary capillary wedge pressure ratio, pulmonary artery pulsatility index (PAPi), elevated pulmonary vascular resistance, and diastolic pulmonary gradient, have been associated with RHF (12, 61, 77, 80–84). Although, some of these parameters are widely used and cited in the clinical practice, these need further external validation using a standardized and contemporary definition of RHF.
Treatments of RHF
Early recognition and treatment of RHF are crucial to preserve end-organ function and improve outcomes (85). Since randomized controlled clinical trials are largely lacking in this area, most evidence for RHF management is based on observational studies and personal experience, with significant variation among centers. RHF management generally comprises pulmonary artery catheter-guided therapy, volume optimization with diuretics or renal replacement treatment, pulmonary vasodilators, inotropes, heart rate or rhythm management, or mechanical RV support.
Medical management of RHF
Volume optimization is crucial to restoring normal RV preload and afterload (86). In the setting of RHF, both low cardiac index and renal venous congestion as a consequence of an elevated central venous pressure can limit diuretic response (87). Patients refractory to diuretic treatment can benefit from ultrafiltration with renal replacement therapies (86). Additionally, in the acute setting, a reduction in pulmonary pressure by correcting hypoxia and acidosis and using pharmacologic pulmonary vasodilators can help decrease the pulmonary vascular resistance (88–91). In order to maximize RV health, it is imperative to use invasive hemodynamic optimization of LVAD speed, filling pressures, and systemic mean arterial pressure. Excess LVAD speed, residual hypervolemia, and suboptimal control of essential hypertension all contribute to chronic RV pressure/volume overload, ultimately resulting in chronic RV failure (Figure 3).
Inotropes are another palliative treatment option to provide temporary RV support by augmenting RV contractility and decreasing RV end-diastolic volume and pressure. Dobutamine and milrinone are the two most commonly utilized inotropes and have similar safety profiles regarding arrhythmogenicity (92, 93). However, milrinone has the advantage of leading to a more significant reduction in RV pressures because of its potent pulmonary and systemic vasodilatory effect compared with dobutamine (94, 95). Another relevant aspect in managing RHF is avoidance of systemic hypotension to maintain coronary artery perfusion and prevent or reduce RV ischemia (96, 97).
Atrial and ventricular tachyarrhythmias are common complications in patients with advanced heart failure. The LVAD, by unloading the LV, decreasing the adrenergic drive, and inducing reverse remodeling of the heart, has a positive impact on arrhythmogenicity. Additionally, LVAD support allows patients to remain stable and even asymptomatic while having significant atrial or ventricular arrhythmias. However, the persistence of tachyarrhythmias can be harmful to the unsupported RV contributing to the development of RHF (41, 98, 99). Therefore, although no randomized clinical trial has demonstrated that arrhythmia treatment decreases RHF incidence; it is common practice to address arrhythmia promptly and to make efforts to maintain sinus rhythm to preserve the RV function.
Mechanical circulatory support for RHF
The timing for RV MCS placement varies among centers; some institutions offer this early on, even preventively in high-risk patients. In contrast, other hospitals offer RV MCS once medical therapy has failed. The specific support device selected also varies based on the level of support needed and the center's practice and experience.
Impella RP
The Impella RP (Abiomed Inc, Danvers, MA) (100) is a microaxial flow device inserted percutaneously through the femoral vein, with the distal tip positioned in the pulmonary artery. This device drains blood from the inferior vena cava and propels it into the pulmonary artery, and can provide as much as 4 L/min of flow (100, 101). Insertion of this device can be challenging due to the need to navigate the tricuspid valve and outflow tract infundibulum, and the device can be prone to migration and hemolysis.
Protek duo
The Protek Duo (LivaNova, London, UK) (102) is a dual lumen cannula with an inflow and an outflow limb. It is typically inserted via the internal jugular or subclavian veins under fluoroscopic guidance. This catheter works in conjunction with an extracorporeal centrifugal pump, the TandemHeart (LivaNova, London, UK). Advantages of this device are the relative ease of insertion and the fact that it can be inserted in an upper venous system, thereby allowing for ambulation. Disadvantages are that it also is prone to migration and, due to its diameter, can induce pulmonary valve regurgitation, thereby leading to re-circulation and ineffective RV support (103, 104).
Peripheral VA-ECMO
Peripheral VA-ECMO is another option for temporary RV support. However, since this strategy reduces LVAD preload by unloading the RV and increases LVAD afterload, it will negatively impact LVAD function (105). Therefore, careful LVAD/VA-ECMO adjustments must be made to avoid competition between the two devices.
Paracorporeal CentriMag RV assist device
The paracorporeal CentriMag RV Assist Device is a surgically implanted system that involves the placement of a right atrial or RV venous inflow cannula and a pulmonary artery arterial outflow cannula. The cannulae are connected to a paracorporeal CentriMag pump (Abbott, Chicago, IL) (106). This approach has the advantage of allowing for very effective RV support. The cannulae can be tunneled under the costal margin, allowing chest closure and ambulation. However, the most significant disadvantage of this configuration is that a sternotomy is required for insertion, and a second surgery is typically required to remove the system (107, 108).
Durable biventricular assist devices
There is no FDA-approved, long-term durable RV support system for LVAD patients with refractory RHF. However, small case series have reported using an LVAD system for RV support, leaving the patient with long-term dischargeable biventricular support. For right-sided device placement, the inflow cannula is placed in the right atrium or RV while the outflow graft is connected to the pulmonary artery (109, 110). Despite the feasibility of this surgical intervention, there are several concerns, including thrombus generation, especially in the venous system, and a higher risk for inflow cannula malposition and obstruction.
Conclusion
Advances in LVAD technology have improved outcomes for patients with advanced heart failure receiving these devices. Despite these innovations, surgery-related events and the hemodynamic implications inherent to left-sided univentricular support cause cardiac morphology and dynamics changes that may eventually result in early- or late-onset RHF. There has been significant progress in managing and understanding several LVAD complications, including gastrointestinal bleeding, driveline infection, and stroke. However, RHF remains the least characterized and understood of all LVAD complications. In addition, the utilization of differing RHF definitions across studies has contributed to difficulties in thoroughly characterizing the risk factors for and pathologic mechanisms underlying this important LVAD complication. Fortunately, there is growing interest in the scientific community to fill this knowledge gap, which includes the proposal of a more inclusive and comprehensive RHF definition by the MCS-ARC. Patients with RHF after an LVAD currently receive pharmacologic treatment or MCS that maintains end-organ perfusion while the right-sided hemodynamics are optimized, allowing the RV to recover. However, we currently lack a long-term and sustainable therapy for refractory RHF, leaving only a few options for those unfortunate patients. As heart transplantation remains a very limited resource, leaving LVAD as the only option for many patients with refractory heart failure, there is an urgent need to advance research in this area.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was funded by CB: Bristol Myers Squibb Foundation, Robert A. Winn Diversity in Clinical Trials Career Development Award, Raisbeck Gift, and R25HL145817. KO'B: NIH 5R01HL144937-03.
Conflict of interest
Author CM is an investigator and consultant for Abbott, Abiomed, and Carmat.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al. A fully magnetically levitated left ventricular assist device - final report. N Engl J Med. (2019) 380:1618–27. doi: 10.1056/NEJMoa1900486
2. Khush KK, Hsich E, Potena L, Cherikh WS, Chambers DC, Harhay MO, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-eighth adult heart transplantation report - 2021; focus on recipient characteristics. J Heart Lung Transplant. (2021) 40:1035–49. doi: 10.1016/j.healun.2021.07.015
3. Colvin M, Smith JM, Ahn Y, Skeans MA, Messick E, Bradbrook K, et al. Optn/Srtr 2020 annual data report: heart. Am J Transplant. (2022) 22(Suppl. 2):350–437. doi: 10.1111/ajt.16977
4. McCarthy PM, Savage RM, Fraser CD, Vargo R, James KB, Goormastic M, et al. Hemodynamic and physiologic changes during support with an implantable left ventricular assist device. J Thorac Cardiovasc Surg. (1995) 109:409–17; discussion 17–8. doi: 10.1016/S0022-5223(95)70271-7
5. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. (2001) 345:1435–43. doi: 10.1056/NEJMoa012175
6. Cowger JA, Naka Y, Aaronson KD, Horstmanshof D, Gulati S, Rinde-Hoffman D, et al. Quality of life and functional capacity outcomes in the momentum 3 trial at 6 months: a call for new metrics for left ventricular assist device patients. J Heart Lung Transplant. (2018) 37:15–24. doi: 10.1016/j.healun.2017.10.019
7. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. (2018) 378:1386–95. doi: 10.1056/NEJMoa1800866
8. Berardi C, Bravo CA, Li S, Khorsandi M, Keenan JE, Auld J, et al. The history of durable left ventricular assist devices and comparison of outcomes: heartware, heartmate II, heartmate 3, and the future of mechanical circulatory support. J Clin Med. (2022) 11:2022. doi: 10.3390/jcm11072022
9. Grant AD, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol. (2012) 60:521–8. doi: 10.1016/j.jacc.2012.02.073
10. Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. (2015) 34:1123–30. doi: 10.1016/j.healun.2015.06.015
11. Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. (2006) 25:1–6. doi: 10.1016/j.healun.2005.07.008
12. Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, et al. Right ventricular failure in patients with the heartmate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. (2010) 139:1316–24. doi: 10.1016/j.jtcvs.2009.11.020
13. Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, et al. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg. (2021) 111:778–92. doi: 10.1016/j.athoracsur.2020.12.038
14. Appleyard RF, Glantz SA. Pulmonary model to predict the effects of series ventricular interaction. Circ Res. (1990) 67:1225–37. doi: 10.1161/01.RES.67.5.1225
15. Slinker BK, Glantz SA. End-systolic and end-diastolic ventricular interaction. Am J Physiol. (1986) 251:H1062–75. doi: 10.1152/ajpheart.1986.251.5.H1062
16. Smerup M, Nielsen E, Agger P, Frandsen J, Vestergaard-Poulsen P, Andersen J, et al. The three-dimensional arrangement of the myocytes aggregated together within the mammalian ventricular myocardium. Anat Rec (Hoboken). (2009) 292:1–11. doi: 10.1002/ar.20798
17. Wang GY, McCloskey DT, Turcato S, Swigart PM, Simpson PC, Baker AJ. Contrasting inotropic responses to alpha1-adrenergic receptor stimulation in left versus right ventricular myocardium. Am J Physiol Heart Circ Physiol. (2006) 291:H2013–7. doi: 10.1152/ajpheart.00167.2006
18. Irlbeck M, Muhling O, Iwai T, Zimmer HG. Different response of the rat left and right heart to norepinephrine. Cardiovasc Res. (1996) 31:157–62. doi: 10.1016/S0008-6363(95)00188-3
19. Sheehan F, Redington A. The right ventricle: anatomy, physiology and clinical imaging. Heart. (2008) 94:1510–5. doi: 10.1136/hrt.2007.132779
20. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
21. Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart. (2006) 92(Suppl. 1):i2–13. doi: 10.1136/hrt.2005.077875
22. Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. (1999) 1:7–21. doi: 10.3109/10976649909080829
23. Sarnoff SJ, Berglund E. Ventricular function. I starling's law of the heart studied by means of simultaneous right and left ventricular function curves in the dog. Circulation. (1954) 9:706–18. doi: 10.1161/01.CIR.9.5.706
24. Braunwald E. Cor pulmonale and pulmonary thromboembolism. In: Heart Disease: A Textbook of Cardiovascular Medicine: Saunders Philadelphia, PA: Saunders/Elsevier (1980).
25. Abel FL. Effects of alterations in peripheral resistance on left ventricular function. Proc Soc Exp Biol Med. (1965) 120:52–6. doi: 10.3181/00379727-120-30441
26. Santamore WP, Dell'Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. (1998) 40:289–308. doi: 10.1016/S0033-0620(98)80049-2
27. Maughan WL, Sunagawa K, Sagawa K. Ventricular systolic interdependence: volume elastance model in isolated canine hearts. Am J Physiol. (1987) 253:H1381–90. doi: 10.1152/ajpheart.1987.253.6.H1381
28. Feneley MP, Gavaghan TP, Baron DW, Branson JA, Roy PR, Morgan JJ. Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation. (1985) 71:473–80. doi: 10.1161/01.CIR.71.3.473
29. Dell'Italia LJ. The right ventricle: anatomy, physiology, and clinical importance. Curr Probl Cardiol. (1991) 16:653–720. doi: 10.1016/0146-2806(91)90009-Y
30. Nair PK, Kormos RL, Teuteberg JJ, Mathier MA, Bermudez CA, Toyoda Y, et al. Pulsatile left ventricular assist device support as a bridge to decision in patients with end-stage heart failure complicated by pulmonary hypertension. J Heart Lung Transplant. (2010) 29:201–8. doi: 10.1016/j.healun.2009.09.013
31. Zimpfer D, Zrunek P, Roethy W, Czerny M, Schima H, Huber L, et al. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J Thorac Cardiovasc Surg. (2007) 133:689–95. doi: 10.1016/j.jtcvs.2006.08.104
32. Salzberg SP, Lachat ML, von Harbou K, Zund G, Turina MI. Normalization of high pulmonary vascular resistance with lvad support in heart transplantation candidates. Eur J Cardiothorac Surg. (2005) 27:222–5. doi: 10.1016/j.ejcts.2004.11.001
33. Mandarino WA, Morita S, Kormos RL, Kawai A, Deneault LG, Gasior TA, et al. Quantitation of right ventricular shape changes after left ventricular assist device implantation. ASAIO J. (1992) 38:M228–31. doi: 10.1097/00002480-199207000-00026
34. Kanwar MK, Rajagopal K, Itoh A, Silvestry SC, Uriel N, Cleveland JC Jr, et al. Impact of left ventricular assist device implantation on mitral regurgitation: an analysis from the momentum 3 trial. J Heart Lung Transplant. (2020) 39:529–37. doi: 10.1016/j.healun.2020.03.003
35. Dobrovie M, Spampinato RA, Efimova E, da Rocha E, Fischer J, Kuehl M, et al. Reversibility of severe mitral valve regurgitation after left ventricular assist device implantation: single-centre observations from a real-life population of patients. Eur J Cardiothorac Surg. (2018) 53:1144–50. doi: 10.1093/ejcts/ezx476
36. Houston BA, Kalathiya RJ, Hsu S, Loungani R, Davis ME, Coffin ST, et al. Right ventricular afterload sensitivity dramatically increases after left ventricular assist device implantation: a multi-center hemodynamic analysis. J Heart Lung Transplant. (2016) 35:868–76. doi: 10.1016/j.healun.2016.01.1225
37. Morita S, Kormos RL, Mandarino WA, Eishi K, Kawai A, Gasior TA, et al. Right ventricular/arterial coupling in the patient with left ventricular assistance. Circulation. (1992) 86:II316–25.
38. Moon MR, Castro LJ, DeAnda A, Tomizawa Y, Daughters GT 2nd, Ingels NB Jr, et al. Right ventricular dynamics during left ventricular assistance in closed-chest dogs. Ann Thorac Surg. (1993) 56:54–66; discussion−7. doi: 10.1016/0003-4975(93)90402-4
39. Farrar DJ, Compton PG, Hershon JJ, Fonger JD, Hill JD. Right heart interaction with the mechanically assisted left heart. World J Surg. (1985) 9:89–102. doi: 10.1007/BF01656260
40. Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. (2010) 29:S1–39. doi: 10.1016/j.healun.2010.01.011
41. Brisco MA, Sundareswaran KS, Milano CA, Feldman D, Testani JM, Ewald GA, et al. Incidence, risk, and consequences of atrial arrhythmias in patients with continuous-flow left ventricular assist devices. J Card Surg. (2014) 29:572–80. doi: 10.1111/jocs.12336
42. Badano LP, Hahn R, Rodriguez-Zanella H, Araiza Garaygordobil D, Ochoa-Jimenez RC, Muraru D. Morphological assessment of the tricuspid apparatus and grading regurgitation severity in patients with functional tricuspid regurgitation: thinking outside the box. JACC Cardiovasc Imaging. (2019) 12:652–64. doi: 10.1016/j.jcmg.2018.09.029
43. Burger W, Straube M, Behne M, Sarai K, Beyersdorf F, Eckel L, et al. Role of pericardial constraint for right ventricular function in humans. Chest. (1995) 107:46–9. doi: 10.1378/chest.107.1.46
44. Zanobini M, Loardi C, Poggio P, Tamborini G, Veglia F, Di Minno A, et al. The impact of pericardial approach and myocardial protection onto postoperative right ventricle function reduction. J Cardiothorac Surg. (2018) 13:55. doi: 10.1186/s13019-018-0726-5
45. Unsworth B, Casula RP, Kyriacou AA, Yadav H, Chukwuemeka A, Cherian A, et al. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J. (2010) 159:314–22. doi: 10.1016/j.ahj.2009.11.013
46. Wranne B, Pinto FJ, Hammarstrom E, St Goar FG, Puryear J, Popp RL. Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J. (1991) 66:435–42. doi: 10.1136/hrt.66.6.435
47. Pasrija C, Sawan MA, Sorensen E, Voorhees H, Shah A, Strauss E, et al. Less invasive left ventricular assist device implantation may reduce right ventricular failure. Interact Cardiovasc Thorac Surg. (2019) 29:592–8. doi: 10.1093/icvts/ivz143
48. McGee E Jr, Danter M, Strueber M, Mahr C, Mokadam NA, Wieselthaler G, et al. Evaluation of a lateral thoracotomy implant approach for a centrifugal-flow left ventricular assist device: the lateral clinical trial. J Heart Lung Transplant. (2019) 38:344–51. doi: 10.1016/j.healun.2019.02.002
49. Strueber M, Meyer AL, Feussner M, Ender J, Correia JC, Mohr FW, et al. Minimally invasive off-pump implantation technique for continuous-flow left ventricular assist devices: early experience. J Heart Lung Transplant. (2014) 33:851–6. doi: 10.1016/j.healun.2014.05.016
50. Zhang B, Guo S, Fu Z, Liu Z. Minimally invasive versus conventional continuous-flow left ventricular assist device implantation for heart failure: a meta-analysis. Heart Fail Rev. (2022) 27:1053–61. doi: 10.1007/s10741-021-10102-z
51. Wood KL, Ayers BC, Sagebin F, Vidula H, Thomas S, Alexis JD, et al. Complete sternal-sparing heartmate 3 implantation: a case series of 10 consecutive patients. Ann Thorac Surg. (2019) 107:1160–5. doi: 10.1016/j.athoracsur.2018.10.005
52. Mohite PN, Sabashnikov A, Raj B, Hards R, Edwards G, Garcia-Saez D, et al. Minimally invasive left ventricular assist device implantation: a comparative study. Artif Organs. (2018) 42:1125–31. doi: 10.1111/aor.13269
53. Banka VS, Agarwal JB, Bodenheimer MM, Helfant RH. Interventricular septal motion: biventricular angiographic assessment of its relative contribution to left and right ventricular contraction. Circulation. (1981) 64:992–6. doi: 10.1161/01.CIR.64.5.992
54. Buckberg GD, Group R. The ventricular septum: the lion of right ventricular function, and its impact on right ventricular restoration. Eur J Cardiothorac Surg. (2006) 29 Suppl 1:S272–8. doi: 10.1016/j.ejcts.2006.02.011
55. Brown SB, Raina A, Katz D, Szerlip M, Wiegers SE, Forfia PR. Longitudinal shortening accounts for the majority of right ventricular contraction and improves after pulmonary vasodilator therapy in normal subjects and patients with pulmonary arterial hypertension. Chest. (2011) 140:27–33. doi: 10.1378/chest.10-1136
56. Raina A, Vaidya A, Gertz ZM, Susan C, Forfia PR. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post-surgical assessment of right ventricular function. J Heart Lung Transplant. (2013) 32:777–83. doi: 10.1016/j.healun.2013.05.004
57. Moon MR, Bolger AF, DeAnda A, Komeda M, Daughters GT 2nd, Nikolic SD, et al. Septal function during left ventricular unloading. Circulation. (1997) 95:1320–7. doi: 10.1161/01.CIR.95.5.1320
58. Kormos RL, Antonides CFJ, Goldstein DJ, Cowger JA, Starling RC, Kirklin JK, et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: a consensus statement of the mechanical circulatory support academic research consortium. J Heart Lung Transplant. (2020) 39:735–50. doi: 10.1016/j.healun.2020.03.010
59. Lenderman, JC,. Intermacs Appendices—School of Medicine—Interagency Registry for Mechanically Assisted Circulatory Support – Uab. Available online at: https://www.uab.edu/medicine/intermacs/intermacs-documents (accessed August 3, 2022).
60. Fitzpatrick JR 3rd, Frederick JR, Hsu VM, Kozin ED, O'Hara ML, Howell E, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. (2008) 27:1286–92. doi: 10.1016/j.healun.2008.09.006
61. Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. (2010) 105:1030–5. doi: 10.1016/j.amjcard.2009.11.026
62. Wang Y, Simon MA, Bonde P, Harris BU, Teuteberg JJ, Kormos RL, et al. Decision tree for adjuvant right ventricular support in patients receiving a left ventricular assist device. J Heart Lung Transplant. (2012) 31:140–9. doi: 10.1016/j.healun.2011.11.003
63. Soliman OII, Akin S, Muslem R, Boersma E, Manintveld OC, Krabatsch T, et al. Derivation and validation of a novel right-sided heart failure model after implantation of continuous flow left ventricular assist devices: the euromacs (european registry for patients with mechanical circulatory support) right-sided heart failure risk score. Circulation. (2018) 137:891–906. doi: 10.1161/CIRCULATIONAHA.117.030543
64. Rich JD, Gosev I, Patel CB, Joseph S, Katz JN, Eckman PM, et al. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J Heart Lung Transplant. (2017) 36:50–8. doi: 10.1016/j.healun.2016.08.010
65. Aissaoui N, Morshuis M, Schoenbrodt M, Hakim Meibodi K, Kizner L, Borgermann J, et al. temporary right ventricular mechanical circulatory support for the management of right ventricular failure in critically ill patients. J Thorac Cardiovasc Surg. (2013) 146:186–91. doi: 10.1016/j.jtcvs.2013.01.044
66. Rame JE, Pagani FD, Kiernan MS, Oliveira GH, Birati EY, Atluri P, et al. Evolution of late right heart failure with left ventricular assist devices and association with outcomes. J Am Coll Cardiol. (2021) 78:2294–308. doi: 10.1016/j.jacc.2021.09.1362
67. Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, et al. Incidence and clinical significance of late right heart failure during continuous-flow left ventricular assist device support. J Heart Lung Transplant. (2015) 34:1024–32. doi: 10.1016/j.healun.2015.03.011
68. Ruiz-Cano MJ, Ramazyan L, Schramm R, Lauenroth V, Paluszkiewicz L, Rojas S, et al. Clinical implications of late-onset right ventricular failure after implantation of a continuous-flow left ventricular assist device as bridge to transplantation. Eur J Cardiothorac Surg. (2021) 60:177–85. doi: 10.1093/ejcts/ezab114
69. Baran DA, Mehra MR. Late-onset right heart failure after left ventricular assist device implant: quo vadis? J Heart Lung Transplant. (2017) 36:26–7. doi: 10.1016/j.healun.2016.12.001
70. Hatano M, Jimba T, Fujiwara T, Tsuji M, Bujo C, Ishida J, et al. Late-onset right ventricular failure after continuous-flow left ventricular assist device implantation: case presentation and review of the literature. J Cardiol. (2022) 80:110–5. doi: 10.1016/j.jjcc.2021.12.009
71. Liu H, Jones TE, Jeng E, Peng KL, Peng YG. Risk stratification and optimization to prevent right heart failure during left ventricular assist device implantation. J Cardiothorac Vasc Anesth. (2021) 35:3385–93. doi: 10.1053/j.jvca.2020.09.130
72. Lo Coco V, De Piero ME, Massimi G, Chiarini G, Raffa GM, Kowalewski M, et al. Right ventricular failure after left ventricular assist device implantation: a review of the literature. J Thorac Dis. (2021) 13:1256–69. doi: 10.21037/jtd-20-2228
73. Raina A, Seetha Rammohan HR, Gertz ZM, Rame JE, Woo YJ, Kirkpatrick JN. Postoperative right ventricular failure after left ventricular assist device placement is predicted by preoperative echocardiographic structural, hemodynamic, and functional parameters. J Card Fail. (2013) 19:16–24. doi: 10.1016/j.cardfail.2012.11.001
74. Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. (2002) 106(12 Suppl. 1):I198–202. doi: 10.1161/01.cir.0000032906.33237.1c
75. Potapov EV, Stepanenko A, Dandel M, Kukucka M, Lehmkuhl HB, Weng Y, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant. (2008) 27:1275–81. doi: 10.1016/j.healun.2008.08.012
76. Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. (2008) 51:2163–72. doi: 10.1016/j.jacc.2008.03.009
77. Atluri P, Goldstone AB, Fairman AS, MacArthur JW, Shudo Y, Cohen JE, et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg. (2013) 96:857–63; discussion 63–4. doi: 10.1016/j.athoracsur.2013.03.099
78. Loforte A, Montalto A, Musumeci F, Amarelli C, Mariani C, Polizzi V, et al. Calculation of the alma risk of right ventricular failure after left ventricular assist device implantation. ASAIO J. (2018) 64:e140–e7. doi: 10.1097/MAT.0000000000000800
79. Frankfurter C, Molinero M, Vishram-Nielsen JKK, Foroutan F, Mak S, Rao V, et al. Predicting the risk of right ventricular failure in patients undergoing left ventricular assist device implantation: a systematic review. Circ Heart Fail. (2020) 13:e006994. doi: 10.1161/CIRCHEARTFAILURE.120.006994
80. Kiernan MS, Grandin EW, Brinkley M Jr, Kapur NK, Pham DT, Ruthazer R, et al. Early right ventricular assist device use in patients undergoing continuous-flow left ventricular assist device implantation: incidence and risk factors from the interagency registry for mechanically assisted circulatory support. Circ Heart Fail. (2017) 10:e003863. doi: 10.1161/CIRCHEARTFAILURE.117.003863
81. Imamura T, Kinugawa K, Kinoshita O, Nawata K, Ono M. High pulmonary vascular resistance in addition to low right ventricular stroke work index effectively predicts biventricular assist device requirement. J Artif Organs. (2016) 19:44–53. doi: 10.1007/s10047-015-0867-4
82. Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. (2016) 35:67–73. doi: 10.1016/j.healun.2015.06.009
83. Fukamachi K, McCarthy PM, Smedira NG, Vargo RL, Starling RC, Young JB. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. (1999) 68:2181–4. doi: 10.1016/S0003-4975(99)00753-5
84. Alnsasra H, Asleh R, Schettle SD, Pereira NL, Frantz RP, Edwards BS, et al. Diastolic pulmonary gradient as a predictor of right ventricular failure after left ventricular assist device implantation. J Am Heart Assoc. (2019) 8:e012073. doi: 10.1161/JAHA.119.012073
85. Fitzpatrick JR 3rd, Frederick JR, Hiesinger W, Hsu VM, McCormick RC, Kozin ED, et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg. (2009) 137:971–7. doi: 10.1016/j.jtcvs.2008.09.021
86. Marenzi G, Lauri G, Grazi M, Assanelli E, Campodonico J, Agostoni P. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol. (2001) 38:963–8. doi: 10.1016/S0735-1097(01)01479-6
87. Wencker D. Acute cardio-renal syndrome: progression from congestive heart failure to congestive kidney failure. Curr Heart Fail Rep. (2007) 4:134–8. doi: 10.1007/s11897-007-0031-4
88. Bendjelid K. Does inhaled nitric oxide support the hemodynamic of spontaneous breathing patients with cardiogenic shock related to right ventricular myocardial infarction? J Am Coll Cardiol. (2005) 45:965. doi: 10.1016/j.jacc.2004.12.031
89. Rudolph AM, Yuan S. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J Clin Invest. (1966) 45:399–411. doi: 10.1172/JCI105355
90. Argenziano M, Choudhri AF, Moazami N, Rose EA, Smith CR, Levin HR, et al. Randomized, double-blind trial of inhaled nitric oxide in lvad recipients with pulmonary hypertension. Ann Thorac Surg. (1998) 65:340–5. doi: 10.1016/S0003-4975(97)01307-6
91. Lovich MA, Pezone MJ, Wakim MG, Denton RJ, Maslov MY, Murray MR, et al. Inhaled nitric oxide augments left ventricular assist device capacity by ameliorating secondary right ventricular failure. ASAIO J. (2015) 61:379–85. doi: 10.1097/MAT.0000000000000211
92. Aranda JM Jr, Schofield RS, Pauly DF, Cleeton TS, Walker TC, Monroe VS Jr, et al. Comparison of dobutamine versus milrinone therapy in hospitalized patients awaiting cardiac transplantation: a prospective, randomized trial. Am Heart J. (2003) 145:324–9. doi: 10.1067/mhj.2003.50
93. Mathew R, Di Santo P, Jung RG, Marbach JA, Hutson J, Simard T, et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med. (2021) 385:516–25. doi: 10.1056/NEJMoa2026845
94. Colucci WS, Wright RF, Jaski BE, Fifer MA, Braunwald E. Milrinone and dobutamine in severe heart failure: differing hemodynamic effects and individual patient responsiveness. Circulation. (1986) 73:III175–83.
95. Monrad ES, Baim DS, Smith HS, Lanoue AS. Milrinone, dobutamine, and nitroprusside: comparative effects on hemodynamics and myocardial energetics in patients with severe congestive heart failure. Circulation. (1986) 73:III168–74.
96. Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, et al. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. (2001) 38:1137–42. doi: 10.1016/S0735-1097(01)01496-6
97. Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology. (2011) 258:119–27. doi: 10.1148/radiol.10100725
98. Raasch H, Jensen BC, Chang PP, Mounsey JP, Gehi AK, Chung EH, et al. Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation. Am Heart J. (2012) 164:373–8. doi: 10.1016/j.ahj.2012.06.018
99. Garan AR, Levin AP, Topkara V, Thomas SS, Yuzefpolskaya M, Colombo PC, et al. Early post-operative ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant. (2015) 34:1611–6. doi: 10.1016/j.healun.2015.05.018
100. Anderson MB, Goldstein J, Milano C, Morris LD, Kormos RL, Bhama J, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: the prospective recover right study of the impella Rp device. J Heart Lung Transplant. (2015) 34:1549–60. doi: 10.1016/j.healun.2015.08.018
101. Morgan JA, O'Neill WW. Percutaneous right ventricular assist device support in a patient supported by an lvad. ASAIO J. (2016) 62:e41–2. doi: 10.1097/MAT.0000000000000344
102. Aggarwal V, Einhorn BN, Cohen HA. Current status of percutaneous right ventricular assist devices: first-in-man use of a novel dual lumen cannula. Catheter Cardiovasc Interv. (2016) 88:390–6. doi: 10.1002/ccd.26348
103. Agrawal N, Hameed F, Battel L, Arora A, Kumar S, Akkanti B, et al. Clinical outcomes in acute right ventricular failure with percutaneous right ventricular assist devices: impella Rp and protek duo. J Am Coll Cardiol. (2021) 77(18_Suppl. 1):634. doi: 10.1016/S0735-1097(21)01993-8
104. Badu B, Cain MT, Durham LA 3rd, Joyce LD, Sundararajan S, Gaglianello N, et al. A Dual-lumen percutaneous cannula for managing refractory right ventricular failure. ASAIO J. (2020) 66:915–21. doi: 10.1097/MAT.0000000000001099
105. Riebandt J, Haberl T, Wiedemann D, Moayedifar R, Schloeglhofer T, Mahr S, et al. Extracorporeal membrane oxygenation support for right ventricular failure after left ventricular assist device implantation. Eur J Cardiothorac Surg. (2018) 53:590–5. doi: 10.1093/ejcts/ezx349
106. John R, Long JW, Massey HT, Griffith BP, Sun BC, Tector AJ, et al. Outcomes of a multicenter trial of the levitronix centrimag ventricular assist system for short-term circulatory support. J Thorac Cardiovasc Surg. (2011) 141:932–9. doi: 10.1016/j.jtcvs.2010.03.046
107. Borisenko O, Wylie G, Payne J, Bjessmo S, Smith J, Yonan N, et al. Thoratec centrimag for temporary treatment of refractory cardiogenic shock or severe cardiopulmonary insufficiency: a systematic literature review and meta-analysis of observational studies. ASAIO J. (2014) 60:487–97. doi: 10.1097/MAT.0000000000000117
108. Sultan I, Kilic A, Kilic A. Short-term circulatory and right ventricle support in cardiogenic shock: extracorporeal membrane oxygenation, tandem heart, centrimag, and impella. Heart Fail Clin. (2018) 14:579–83. doi: 10.1016/j.hfc.2018.06.014
109. Krabatsch T, Potapov E, Stepanenko A, Schweiger M, Kukucka M, Huebler M, et al. Biventricular circulatory support with two miniaturized implantable assist devices. Circulation. (2011) 124:S179–86. doi: 10.1161/CIRCULATIONAHA.110.011502
Keywords: right ventricular failure, right heart failure, left ventricular assist device, right ventricle, advanced heart failure
Citation: Bravo CA, Navarro AG, Dhaliwal KK, Khorsandi M, Keenan JE, Mudigonda P, O'Brien KD and Mahr C (2022) Right heart failure after left ventricular assist device: From mechanisms to treatments. Front. Cardiovasc. Med. 9:1023549. doi: 10.3389/fcvm.2022.1023549
Received: 19 August 2022; Accepted: 22 September 2022;
Published: 19 October 2022.
Edited by:
Sofie Gevaert, Ghent University Hospital, BelgiumReviewed by:
Minoru Ono, The University of Tokyo Hospital, JapanAndrea Montisci, Ospedale Civile di Brescia, Italy
Copyright © 2022 Bravo, Navarro, Dhaliwal, Khorsandi, Keenan, Mudigonda, O'Brien and Mahr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio A. Bravo, cbravo11@uw.edu
†These authors share senior authorship
 Claudio A. Bravo
Claudio A. Bravo Andrew G. Navarro2
Andrew G. Navarro2  Jeffrey E. Keenan
Jeffrey E. Keenan Kevin D. O'Brien
Kevin D. O'Brien Claudius Mahr
Claudius Mahr