Predictors of permanent pacemaker implantation in aortic valve diseases after TAVI with vitaFlow liberty system
- 1Department of Cardiology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Cardiology, The First Affiliated Hospital of Wannan Medical College, Wuhu, China
Introduction: Permanent pacemaker implantation (PPI) is a known complication in patients with aortic stenosis following transcatheter aortic valve implantation (TAVI). However, there is limited research on TAVI for pure aortic regurgitation (PAR), and more investigation is needed to determine the occurrence of postoperative cardiac conduction block and the need for PPI in this population. Therefore, this retrospective analysis aimed to evaluate the incidence of cardiac conduction block and the necessity of PPI after TAVI in patients with different types of aortic valve disease, including pure aortic stenosis (PAS), aortic stenosis with regurgitation (ASR), and PAR.
Methods: Clinical data of 100 patients who TAVI were analyzed retrospectively. The incidence of conduction block was assessed, and clinical factors were examined to predict the necessity of PPI.
Results: Cardiac conduction block was found to be a common complication following TAVI, particularly in patients with PAR. PAR was identified as an independent risk factor for requiring PPI. Additionally, first-degree atrioventricular block emerged as a sensitive predictor for PPI in patients with PAR.
Discussion: These findings provide valuable insights into the safety and effectiveness of TAVI, which can help enhance patient management and reduce complications.
Introduction
After more than two decades of research and development, transcatheter aortic valve implantation (TAVI) has become increasingly common. Previously, TAVI was primarily reserved for individuals with severe aortic stenosis who were deemed high-risk or unsuitable for surgery. However, it is now increasingly employed to treat patients with lower and moderate risk profiles (1), Although TAVI has been used in high-risk patients with pure aortic regurgitation (PAR) in some case series (2), this off-label use still necessitates additional research to ascertain the procedure's safety and the risk of postoperative complications.
To explore these issues, we conducted a retrospective analysis of 100 patients who underwent TAVI at our hospital over the past two years. Specifically, we observed cardiac conduction block in patients with different types of aortic valve disease and explored clinical factors that may predict the need for new permanent pacemaker implantation (PPI) after TAVI. Through this analysis, our aim is to acquire a more comprehensive understanding of the safety and effectiveness of TAVI in diverse patient groups and to pinpoint strategies for reducing potential complications.
Materials and methods
Research subjects
This single-center retrospective study involved the analysis of 100 consecutively enrolled patients who received TAVI in our hospital from February 2021 to February 2023. The study samples included 49 males and 51 females, with a mean age of 73.75 ± 7.75 years, ranging from 53 to 87 years old. One week after TAVI, patients were classified into two groups: the PM group (patients with new PPI) and the NPM group (patients without new PPI). According to the type of aortic disease, three subgroups were identified: 33 cases of pure aortic stenosis (PAS), 23 cases of aortic stenosis with regurgitation (ASR), and 44 cases of pure aortic regurgitation (PAR). The inclusion criteria for this study were patients suffering from symptomatic aortic valve disease who had been diagnosed with severe AS, with or without PAR, by echocardiography before surgery or only severe PAR and Society of Thoracic Surgeons (STS) risk score ≥4% (high risk in surgery). Meanwhile, patients with left ventricular thrombus, left ventricular outflow tract obstruction, and anatomical morphology were not suitable for TAVI, contraindications to anticoagulation, and a life expectancy of less than 12 months after correcting valve diseases were excluded from the study. The Ethics Committee of our hospital reviewed and approved this study, and all patients signed informed consent forms before participating in this study.
TAVI procedure
The TAVI procedure was performed using the VitaFlow Liberty system via the femoral artery. Close monitoring of EKG changes was conducted post-procedure to identify any significant bradycardia necessitating PPI. Skilled interventional cardiologists performed all procedures according to standard care protocols. The size of the prosthesis was selected based on CT scanning measurements of the aortic ring area. The positioning of the aortic valve was guided by angiography and transesophageal echocardiography (TEE), and the valve was released at the level of the right coronary sinus under very fast pacing (≥160 beats/min). Following the procedure, patients received management in accordance with local standard care practices, with subsequent transthoracic echocardiography (TTE) and EKG evaluations conducted at discharge (3, 4).
Procedural assessment
All patients underwent TTE and EKG within one week before the interventional procedure. The diameter and wall thickness of each chamber, the diameter of the ascending aorta, and the left ventricular ejection fraction (LVEF) were measured using biplane Simpson. The width of the vena contracta (VC) and the effective regurgitant orifice area (EROA) were measured using PISA. All patients underwent the implantation of a temporary pacemaker via the right internal jugular vein before TAVI. The temporary pacemaker provided rapid ventricular pacing during valve deployment to reduce cardiac output, ensuring the position of the balloon and valve. Continuous monitoring was performed postoperatively to prevent the occurrence of severe cardiac conduction block. If there were no new cardiac conduction blocks within three days after the procedure, the temporary pacemaker was removed. For patients who developed high-degree or complete atrioventricular block (AVB) during or after the procedure and did not recover within one-week, permanent pacemaker was implanted. Additionally, 12-lead EKG exams were performed on all patients before and after the procedure. AV interval, QRS width, and the presence of right bundle branch block (RBBB) and left bundle branch block (LBBB) were recorded before and after the procedure. Patients with type 2 second-degree or advanced AVB were implanted with permanent pacemakers if they did not recover after one week (5) (Figure 1).
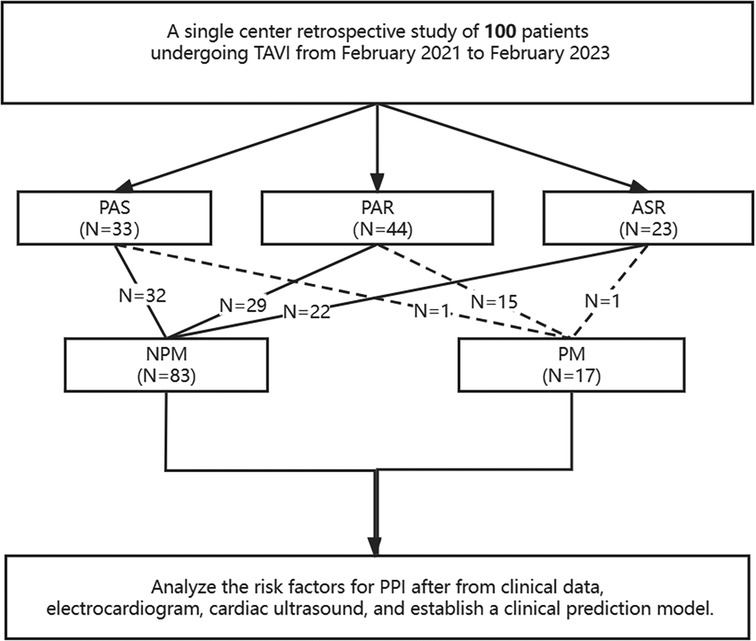
Figure 1. A schematic diagram showing permanent pacemaker implantation after TAVI. PAR, pure aortic regurgitation; PAS, pure aortic stenosis; ASR, aortic stenosis & regurgitation.
Statistical methods
Statistical software SPSS 26.0 was used for statistical analysis of data. The measurement data were assessed for normality and homogeneity of variance and were expressed as , and inter-group comparisons were conducted using the t-test. Categorical variables are presented as counts and percentages and were compared using the χ2 test or Fisher's exact test, depending on the minimum number of observations. All statistical tests were two-sided, and a p-value <0.05 was considered statistically significant.
Results
Comparison of clinical parameters
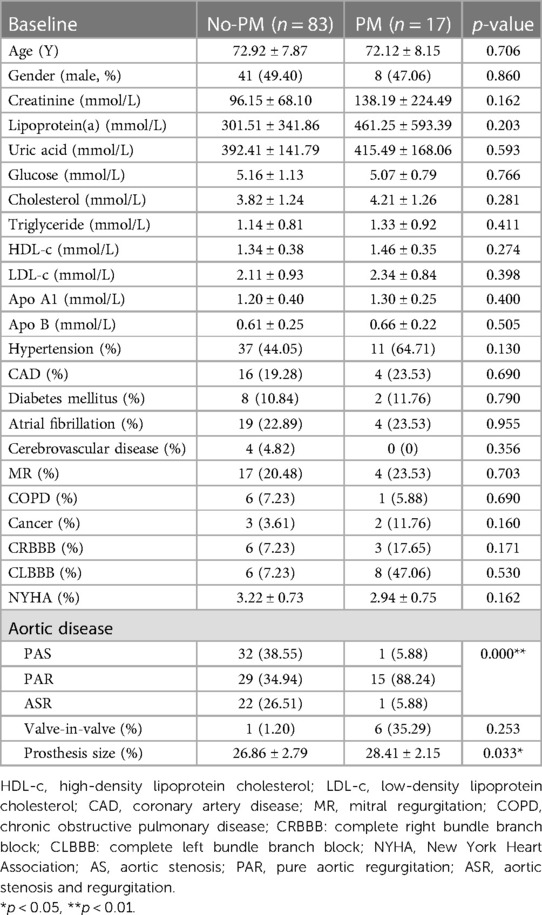
Among the 100 patients who underwent TAVI, 17 patients received PPI after the procedure. There was no statistical difference between the two groups in terms of gender, age, renal function, blood lipid analysis, and the presence of complications such as hypertension, coronary heart disease, type 2 diabetes, atrial fibrillation, obstructive emphysema, mitral insufficiency, and New York heart function classification. However, the valve diameter implanted in PM group was significantly larger compared to the NPM group. Further subgroup analysis revealed a significant difference in pacemaker rate among the AS, ASR, and PAR groups. Specifically, the PAR group had a significantly higher pacemaker rate compared to the other two groups (Table 1).
Comparison of preoperative cardiac ultrasound parameters
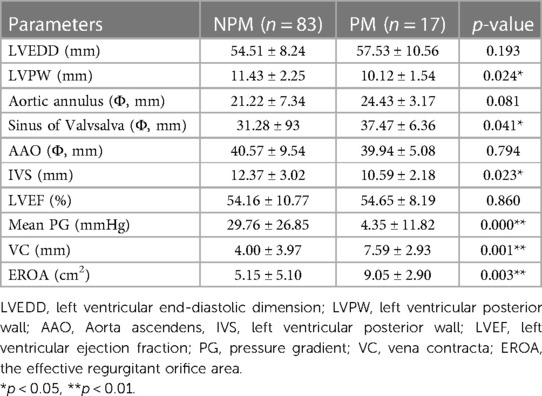
Cardiac ultrasound revealed that there were significant differences (p < 0.05) in LVPW, sinus of valsalva, IVS, mean pressure gradient, VC and EROA between the PM and NPM groups. However, there were no significant differences in LVEDD, LVEF, AAO, and aortic annulus (p > 0.05) between the groups (Table 2).
The impact of TAVI on the conduction system
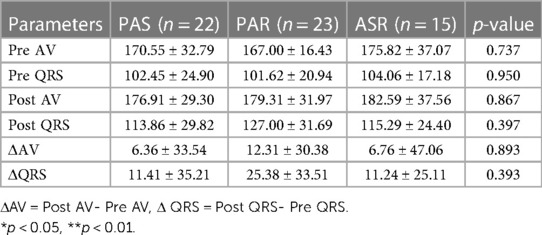
After excluding 17 patients who received new PPI and 23 patients with atrial fibrillation, a total of 60 patients were further analysed. Following TAVI for various types of aortic valve diseases, there were no significant differences in the pre- and post-AV (atrioventricular) interval, QRS width changes, ΔAV interval (post-AV—pre-AV), and ΔQRS (post-QRS—pre-QRS) among the groups (Table 3).
PPI in cardiac conduction block of TAVI
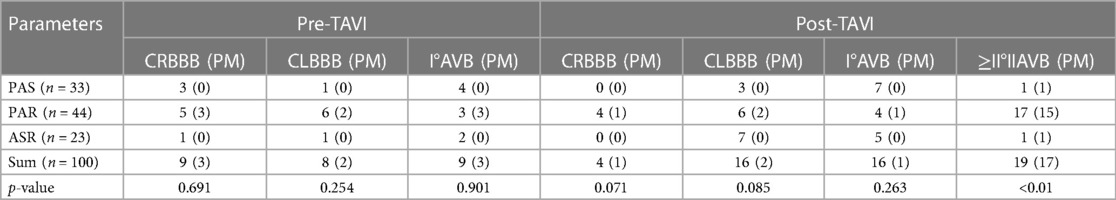
Before TAVI, there were a total of 26 cases with cardiac conduction block, including 9 cases of RBBB, 8 cases of LBBB, and 9 cases of I° AVB. Among these, in the PAR group alone, 3 cases of RBBB, 2 cases of LBBB, and 3 cases of I° AVB underwent PPI after TAVI. However, there were no patients with cardiac conduction block in the PAS and ASR groups who underwent PPI.
After TAVI, there was no significant difference observed between different aortic valve diseases in terms of the occurrence of 16 cases of LBBB and 16 cases of I° AVB. However, four new cases of RBBB were reported, and all of them were in the PAR group, with one case receiving PPI. Additionally, 19 new cases of type II or above AVB were reported, among which 17 cases underwent PPI.
The overall incidence of PPI after TAVI was 17%, but there was a significant difference (p < 0.01) among the PAS, PAR, and ASR groups. The incidence of PPI in the PAR group alone was 34.09% (Table 4).
Independent predictors of pacing outcome
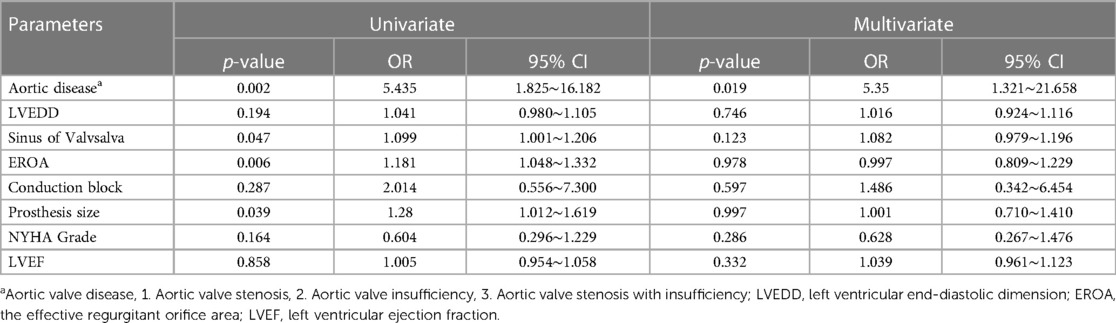
As shown in Table 5, univariate and multivariate regression analyses were performed to analyze the relevant indicators with differences between the PM and NPM groups. The following indicators were examined: type of aortic valve disease, sinus of valsalva, EROA, cardiac conduction block, and implanted prosthesis size.
Among these indicators, the presence of PAR was found to be an independent risk factor for PPI after TAVI. The odds ratio (OR) was calculated to be 5.350, with a 95% confidence interval (CI) ranging from 1.321 to 21.658. This suggests that patients with PAR are more likely to require pacemaker implantation after TAVI compared to those without AR.
Sensitivity and specificity analysis of cardiac conduction block
After TAVI, I° AVB has been found to have a sensitivity of 100% and a specificity of 70.7% in predicting the need for PPI in patients with PAR. Additionally, when compared to RBBB and LBBB, I° AVB demonstrates superior predictive ability for PPI in PAR patients (Table 6).
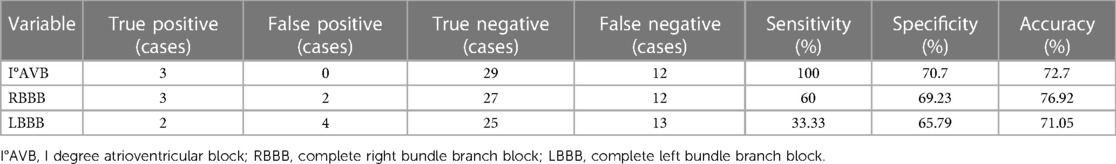
Table 6. Sensitivity and specificity analysis of conduction block prediction in patients with aortic regurgitation for new PPI after TAVI.
Discussion
This study found that cardiac conduction block is commonly observed after TAVI. Moreover, the type of aortic valve diseases, mean pressure gradient, EROA and implanted prosthesis size are related to PPI. The incidence of PPI in PAR patients is 34%, which is an independent factor for PPI after TAVI. Conduction block is indeed a common complication not only in patients with PAS but also in those with PAR who undergo TAVI. Furthermore, the incidence of AVB tends to be higher in these cases. The most common type of cardiac conduction block is newly diagnosed LBBB. Previous research has shown that the incidence rates of LBBB with Edwards SAPIEN and CoreValve ReValving during TAVI are 14.8% and 25%, respectively (6). The meta-analysis has also demonstrated that newly diagnosed LBBB ranges from 13.3% to 37% after TAVI (6). This study found that the incidence of LBBB after TAVI with VitaFlow Liberty™ was lower at 16% compared to 77% with Lotus™ Valve System (7) and 52% with Edwards SAPIEN XT 100 (8). There were no significant differences observed in cardiac conduction block among different aortic valve diseases after TAVI. Previous studies have indicated that nearly 90% of new LBBB cases occur either during or within 24 h after TAVR, potentially due to mechanical damage to the conduction system during balloon dilation and valve implantation. This injury is often temporary, and some newly diagnosed LBBB cases could recover within hours or days (9, 10). RBBB occurs less frequently than LBBB and may be associated with the PAR group. AVB is another common type of cardiac conduction block following TAVI (11). Approximately 22% of patients who undergo TAVI develop new-onset AVB, which is associated with a five-fold higher risk of permanent AVB requiring a PPI (12). This study found that the incidence of advanced II°AVB was 38.4% after TAVI with VitaFlow Liberty™, with a PPI rate of 34.09% in the PAR group. These rates were significantly higher than those observed in the PAS group, which had an AVB incidence of 3.03%, and the PPI rates ranged from 6.1% to 27.3% in PAR patients (2, 13). As for the type of PPI, current reports mainly focus on conventional single- or dual-chamber lead pacemakers. However, patients undergoing TAVI often have poor physical conditions, such as advanced age, frailty, and oral antiplatelet or anticoagulant medications. These factors increase the risk of bleeding and infection associated with traditional pacemakers. In this context, leadless pacemakers may be a preferable choice (14, 15).
Pre-existing cardiac conduction block can be used to predict the risk of PPI after TAVI. Pre-existing RBBB (7, 16–18) and prolonged PR interval (19, 20) were found to be significant predictors for PPI in PAS patients. In this study, 60% of pre-existing RBBB and all pre-existing AV ≥ 200 ms in patients with AR necessitating PPI. Consistent with previous findings, pre-existing LBBB did not predict the need for a pacemaker. The distribution of the His bundle is such that 50% is located on the right side of the membranous septum, 30% on the left side, and 20% within the septum. TAVI can potentially cause injury to the His bundle in the latter two cases, resulting in complete AVB (8, 21). This can reasonably explain that LBBB is not a risk factor for predicting PPI but rather the most common arrhythmia after TAVI. Our findings demonstrate that PR prolongation is the most sensitive predictor for PPI after TAVI.
This study also shows that the anatomy of the left ventricular outflow tract (LVOT) and the size of the valve diameter are related to PPI. Similar to previous studies, patients with PPI have a larger diameter of the aortic sinus (22), thinner IVS and LVPW, and larger diameter replacement valves (23), which are more common in PAR; The larger valve compresses the thinner membranous septal atrioventricular bundle, thereby inducing mechanical damage and increasing the risk of PPI after TAVI (24). On the contrary, in patients without PPI, thicker IVS and LVPW, and smaller diameter replacement valves, which is more common in PAR. The smaller valve compresses the thicker membranous septal atrioventricular tract, resulting in less mechanical damage and reducing the risk of pacemaker implantation after TAVI (25).
Different types of implanted aortic valves can have varying effects on cardiac conduction blocks. For the balloon expandable valves (BEV) and self-expanding valves (SEV), the rate of new LBBB post-TAVR is reported to be approximately 27% for the SEV CoreValve system and 11% for the BEV Edwards valve (26). In terms of PPI, the incidence of PPI is lower for BEV compared to SEV (OR 0.50, 95% CI 0.32 to 0.79) in patients with AS (27). However, for patients with PAR undergoing TAVI, the rates of new PPI can be varied. It is reported to be 16.35% for self-expanding CoreValve (28), 35.1% for balloon expandable Sapien3 (4), 2.3% for J-Valve (29), and 20% for Jena valve prosthesis (30). Interestingly, previous studies indicate a lack of reduction, or even an increase, in the rate of conduction system disturbances associated with these new-generation valves (26, 31, 32). This could potentially be explained by the continuous radial force exerted by the nitinol stent in SEV and the possibility of deeper implantation, leading to mechanical compression and injury to the His bundle (33, 34). In the case of VitaFlow Liberty™, a repositionable BEV, the incidence of PPI is higher in patients with PAR compared to those with PAS. This difference may be attributed to the incorporation of an external fabric cuff in the inferior part of the valve, which is intended to minimize paravalvular leak but may result in greater mechanical damage to the His bundle in patients with PAR.
Conclusion
LBBB and AVB are the most common cardiac conduction blocks of TAVI with VitaFlow Liberty system, PAR is an independent risk factor for PPI in patients with aortic valve diseases undergoing TAVI, and PR > 200 ms is the most sensitive indicator for PPI after TAVI procedure.
Limitations
Although the study offers valuable insights, it is important to acknowledge certain limitations. Firstly, the small sample size may restrict the generalizability of the results. Secondly, the single-center design could introduce bias. Conducting multi-center studies could yield more comprehensive data and validate our findings. Thirdly, as a retrospective study, there may be potential for selection and information bias, and establishing cause-and-effect relationships can be more challenging. Lastly, we lack follow-up data on conduction recovery one month or longer after TAVI with PPI. These limitations underscore the necessity for larger, multi-center, prospective studies with rigorous data collection to confirm our findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Wannan Medical College (Yijishan Hospital). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CJ: Data curation, Methodology, Writing – original draft. XX: Methodology, Supervision, Writing – review & editing. SC: Supervision, Writing – review & editing. ST: Conceptualization, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the key specialty project of Anhui Province Medical and Health.
Acknowledgments
We would like to express their gratitude to EditSprings (https://www.editsprings.cn/) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tagliari AP, Petersen Saadi R, Keller Saadi E. Transcatheter aortic valve implantation for pure native aortic regurgitation: the last frontier. J Clin Med. (2022) 11(17):5181. doi: 10.3390/jcm11175181
2. Kong M, Hong Z, Liu X, Zhu X, Wang J, Dong A. 30-day outcomes after surgical or transapical aortic valve replacement in symptomatic aortic regurgitation. J Cardiovasc Dev Dis. (2022) 9(7):224. doi: 10.3390/jcdd9070224
3. Paraggio L, Burzotta F, Graziani F, Aurigemma C, Romagnoli E, Pedicino D, et al. Transcatheter aortic valve implantation in pure aortic regurgitation: hemodynamic and echocardiographic findings in bioprosthesis vs. Native valve. Catheter Cardiovasc Interv. (2022) 99(5):1599–608. doi: 10.1002/ccd.30082
4. Delhomme C, Urena M, Zouaghi O, Campelo-Parada F, Ohlmann P, Rioufol G, et al. Transcatheter aortic valve implantation using the sapien 3 valve to treat aortic regurgitation: the French multicentre S3ar study. Arch Cardiovasc Dis. (2023) 116(2):98–105. doi: 10.1016/j.acvd.2022.12.003
5. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, et al. 2018 Acc/Aha/Hrs guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. (2019) 140(8):e382–482. doi: 10.1161/CIR.0000000000000628
6. Katsanos S, van Rosendael P, Kamperidis V, van der Kley F, Joyce E, Debonnaire P, et al. Insights into new-onset rhythm conduction disorders detected by multi-detector row computed tomography after transcatheter aortic valve implantation. Am J Cardiol. (2014) 114(10):1556–61. doi: 10.1016/j.amjcard.2014.08.020
7. Zaman S, McCormick L, Gooley R, Rashid H, Ramkumar S, Jackson D, et al. Incidence and predictors of permanent pacemaker implantation following treatment with the repositionable Lotus transcatheter aortic valve. Catheter Cardiovasc Interv. (2017) 90(1):147–54. doi: 10.1002/ccd.26857
8. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current Status and future perspectives. Circulation. (2017) 136(11):1049–69. doi: 10.1161/CIRCULATIONAHA.117.028352
9. Chang S, Liu X, Lu ZN, Yao J, Yin C, Wu W, et al. Feasibility study of temporary permanent pacemaker in patients with conduction block after tavr. Front Cardiovasc Med. (2023) 10:978394. doi: 10.3389/fcvm.2023.978394
10. Pelargonio G, Scacciavillani R, Donisi L, Narducci ML, Aurigemma C, Pinnacchio G, et al. Atrioventricular conduction in pm recipients after transcatheter aortic valve implantation: implications using wenckebach point measurement. Front Cardiovasc Med. (2022) 9:904828. doi: 10.3389/fcvm.2022.904828
11. Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. (2011) 32(2):198–204. doi: 10.1093/eurheartj/ehq339
12. Bleiziffer S, Ruge H, Hörer J, Hutter A, Geisbüsch S, Brockmann G, et al. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. (2010) 3(5):524–30. doi: 10.1016/j.jcin.2010.01.017
13. Takagi H, Hari Y, Kawai N, Ando T, Group A. Meta-analysis and meta-regression of transcatheter aortic valve implantation for pure native aortic regurgitation. Heart Lung Circ. (2020) 29(5):729–41. doi: 10.1016/j.hlc.2019.04.012
14. Mitacchione G, Schiavone M, Gasperetti A, Arabia G, Tundo F, Breitenstein A, et al. Sex differences in leadless pacemaker implantation: a propensity-matched analysis from the I-leaper registry. Heart Rhythm. (2023) 20:S1547-5271(23)02538-9. doi: 10.1016/j.hrthm.2023.07.061
15. Mitacchione G, Schiavone M, Gasperetti A, Arabia G, Breitenstein A, Cerini M, et al. Outcomes of leadless pacemaker implantation following transvenous lead extraction in high-volume referral centers: real-world data from a large international registry. Heart Rhythm. (2023) 20(3):395–404. doi: 10.1016/j.hrthm.2022.12.002
16. Erkapic D, Kim WK, Weber M, Mollmann H, Berkowitsch A, Zaltsberg S, et al. Electrocardiographic and further predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. Europace. (2010) 12(8):1188–90. doi: 10.1093/europace/euq094
17. Koos R, Mahnken AH, Aktug O, Dohmen G, Autschbach R, Marx N, et al. Electrocardiographic and imaging predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. J Heart Valve Dis. (2011) 20(1):83–90.21404902
18. Kaneko H, Hoelschermann F, Seifert M, Tambor G, Okamoto M, Moeller V, et al. Predictors of permanent pacemaker implantation after transcatheter aortic valve implantation for aortic stenosis using medtronic new generation self-expanding corevalve evolut R. Heart Vessels. (2019) 34(2):360–7. doi: 10.1007/s00380-018-1236-z
19. Siontis GCM, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing tavr: a meta-analysis. J Am Coll Cardiol. (2014) 64(2):129–40. doi: 10.1016/j.jacc.2014.04.033
20. Tichelbacker T, Bergau L, Puls M, Friede T, Mutze T, Maier LS, et al. Insights into permanent pacemaker implantation following TAVR in a real-world cohort. PLoS One. (2018) 13(10):e0204503. doi: 10.1371/journal.pone.0204503
21. Hamdan A, Guetta V, Klempfner R, Konen E, Raanani E, Glikson M, et al. Inverse relationship between membranous septal length and the risk of atrioventricular block in patients undergoing transcatheter aortic valve implantation. JACC Cardiovasc Interv. (2015) 8(9):1218–28. doi: 10.1016/j.jcin.2015.05.010
22. Piayda K, Bauer T, Beckmann A, Bekeredjian R, Bleiziffer S, Ensminger S, et al. Procedural results of patients undergoing transcatheter aortic valve implantation with aortic annuli diameter >/=26 mm: insights from the German aortic valve registry. Am J Cardiol. (2022) 164:111–7. doi: 10.1016/j.amjcard.2021.10.021
23. Nijhoff F, Agostoni P, Amrane H, Latib A, Testa L, Oreglia JA, et al. Transcatheter aortic valve implantation in patients with severe aortic valve stenosis and large aortic annulus, using the self-expanding 31-mm medtronic corevalve prosthesis: first clinical experience. J Thorac Cardiovasc Surg. (2014) 148(2):492–9e1. doi: 10.1016/j.jtcvs.2013.09.059
24. Routh JM, Joseph L, Marthaler BR, Bhave PD. Imaging-based predictors of permanent pacemaker implantation after transcatheter aortic valve replacement. Pacing Clin Electrophysiol. (2018) 41(1):81–6. doi: 10.1111/pace.13249
25. Ciardetti N, Ciatti F, Nardi G, Di Muro FM, Demola P, Sottili E, et al. Advancements in transcatheter aortic valve implantation: a focused update. Medicina (Kaunas). (2021) 57(7):711. doi: 10.3390/medicina57070711
26. van der Boon RM, Nuis R-J, Van Mieghem NM, Jordaens L, Rodés-Cabau J, van Domburg RT, et al. New conduction abnormalities after tavi–frequency and causes. Nat Rev Cardiol. (2012) 9(8):454–63. doi: 10.1038/nrcardio.2012.58
27. Elgendy IY, Gad MM, Mahmoud AN, Dvir D, Kapadia SR, Alfonso F, et al. Meta-analysis comparing outcomes of self-expanding versus balloon-expandable valves for transcatheter aortic valve implantation. Am J Cardiol. (2020) 128:202–9. doi: 10.1016/j.amjcard.2020.05.007
28. Roy DA, Schaefer U, Guetta V, Hildick-Smith D, Mollmann H, Dumonteil N, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol. (2013) 61(15):1577–84. doi: 10.1016/j.jacc.2013.01.018
29. Shi J, Wei L, Chen Y, Wang X, Ye J, Qin C, et al. Transcatheter aortic valve implantation with J-valve: 2-year outcomes from a multicenter study. Ann Thorac Surg. (2021) 111(5):1530–6. doi: 10.1016/j.athoracsur.2020.06.139
30. Schlingloff F, Schafer U, Frerker C, Schmoeckel M, Bader R. Transcatheter aortic valve implantation of a second-generation valve for pure aortic regurgitation: procedural outcome, haemodynamic data and follow-up. Interact Cardiovasc Thorac Surg. (2014) 19(3):388–93. doi: 10.1093/icvts/ivu155
31. Urena M, Rodés-Cabau J. Managing heart block after transcatheter aortic valve implantation: from monitoring to device selection and pacemaker indications. EuroIntervention. (2015) 11( Suppl W):W101–5. doi: 10.4244/EIJV11SWA30
32. Yin WH, Lee YT, Tsao TP, Lee KC, Hsiung MC, Wei J. Outcomes of transcatheter aortic valve replacement for pure native aortic regurgitation with the use of newer- vs. early-generation devices. Ann Transl Med. (2022) 10(1):24. doi: 10.21037/atm-21-6936
33. Regueiro A, Abdul-Jawad Altisent O, Del Trigo M, Campelo-Parada F, Puri R, Urena M, et al. Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. Circ Cardiovasc Interv. (2016) 9(5):e003635. doi: 10.1161/CIRCINTERVENTIONS.115.003635
Keywords: transcatheter aortic valve implantation, permanent pacemaker implantation, cardiac conduction block, aortic regurgitation, aortic stenosis
Citation: Ju C, Xie X, Tang S and Cao S (2023) Predictors of permanent pacemaker implantation in aortic valve diseases after TAVI with vitaFlow liberty system. Front. Cardiovasc. Med. 10:1277528. doi: 10.3389/fcvm.2023.1277528
Received: 14 August 2023; Accepted: 12 September 2023;
Published: 29 September 2023.
Edited by:
Federico Migliore, University of Padua, ItalyReviewed by:
Gianfranco Mitacchione, Luigi Sacco Hospital, ItalyFederico Ronco, Azienda ULSS 3 Serenissima, Italy
© 2023 Ju, Xie, Tang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiping Cao csp2012@126.com
 Changlin Ju
Changlin Ju Xiangrong Xie2
Xiangrong Xie2  Shengxin Tang
Shengxin Tang