- 1Department of Endocrinology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China
- 2Key Laboratory of Environmental, Medicine Engineering, Ministry of Education, school of Public Health, Southeast University, Nanjing, China
- 3Department of Endocrinology, Changji Branch, First Affiliated Hospital of Xinjiang Medical University, Xinjiang, China
- 4Department of Endocrinology, Hunan Provincial People’s Hospital (First Affiliated Hospital of Hunan Normal University), Changsha, China
- 5Department of Gastroenterology, Hebei General Hospital, Shijiazhuang, China
- 6Department of Endocrinology, Yixing Second People’s Hospital, Wuxi, China
- 7Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China
Background: Numerous studies validated frequent glucose dysfunction in patients with acute pancreatitis (AP). However, the prevalence of new-onset diabetes in individuals after a first episode of AP varies widely among previous studies. This study aims to determine the incidence of post-acute pancreatitis diabetes mellitus (PPDM-A) in Chinese people and further identify potential risk factors that influence diabetes development in patients with AP.
Methods: This was a multi-center retrospective cohort study including 6009 inpatients with a first attack of AP. A total of 1804 patients with AP without known endocrine pancreatic disorders or other pancreatic exocrine diseases were eligible for analysis. Data was collected from medical records by hospital information system and telephone follow-ups after discharge. The multiple logistic regression analysis was established to evaluate the potential influencing factors of PPDM-A.
Results: The prevalence of newly diagnosed diabetes after a first episode of AP in China was 6.2%. Data showed that patients who developed PPDM-A were more likely to be younger (X2 = 6.329, P = 0.012), experienced longer hospital stays (X2 = 6.949, P = 0.008) and had a higher frequency of overweight or obesity (X2 = 11.559, P = 0.003) compared to those with normal glycemia. The frequency of stress hyperglycemia on admission (X2 = 53.815, P < 0.001), hyperlipidemia (X2 = 33.594, P < 0.001) and non-alcoholic fatty liver disease (NAFLD) (X2 = 36.335, P < 0.001) were significantly higher among individuals with PPDM-A compared with control group. Also, patients with PPDM-A were more likely to be hyperlipidemic AP (X2 = 16.304, P = 0.001) and show a higher degree of severity (X2 = 7.834, P = 0.020) and recurrence rate (X2 = 26.908, P < 0.001) of AP compared to those without diabetes. In addition, multiple logistic regression analysis indicated that stress hyperglycemia, hyperlipidemia, NAFLD and repeated attacks of AP were the independent influence factors for developing PPDM-A.
Conclusion: Our study first demonstrated the prevalence of secondary diabetes in Chinese patients after AP. The disorder of glucose metabolism in individuals with AP should be regularly evaluated in clinical practice. Further studies are needed to verify the relationship between liver and pancreas in keeping glucose homeostasis under AP condition.
Introduction
Acute pancreatitis (AP), with a significantly increased incidence, is one of the most common gastrointestinal diseases characterized by a local and systemic inflammatory response and has a complex and variable clinical course which is difficult to evaluate the prognosis at an early stage (1). Although mild AP seems to be self-limiting and usually recover within one week, approximately 20% of patients develop moderate or severe acute pancreatitis, accompanied by pancreatic or peripancreatic necrosis or organ failure, or both, and the mortality range varying between 20% and 40% (2, 3).
The pancreas is an accessory organ of digestion known to have dual functions in the exocrine and endocrine systems, which are closely linked anatomically and physiologically (4).One manifestations of this interplay is the development of diabetes of the exocrine pancreas (DEP), also known as type 3c DM (T3cDM) (5). Recently a retrospective cohort study indicated that DEP, after type 2 diabetes, has become the second most common type of adults-onset diabetes, with a higher prevalence than type 1 diabetes (6). As a core feature of DEP, post-acute pancreatitis diabetes mellitus (PPDM-A) has not attracted much attention in clinical practice so far. A considerable proportion of patients often suffer from a “brittle diabetic state” (great variability in glucose homeostasis and additional risk of hypoglycemia), malabsorption of nutrients and micronutrients due to pancreatic exocrine insufficiency, severe gastrointestinal symptoms (including steatorrhea and flatulence), and muscle atrophy (7, 8). Improper diagnosis and treatment strategies further significantly influence their quality of life.
A growing body of evidence concerning the incidence of PPDM-A is still in dispute. A recently published systematic review and meta-analysis by our workgroup reported that new-onset diabetes occurred in 23% of patients with AP (9), in line with previous research conducted by Das et al (10). However, other studies provided inconsistent prevalence rates (11). In particular, few studies provide insight into the frequency of PPDM-A in Chinese patients (12). In addition, the risk factors associated with PPDM-A progression also remain controversial. Some features including the severity of pancreatitis, etiology, the extent of necrosis, presence of infection and other metabolic indicators were considered to be related with the development of diabetes in some of previous research but made no sense in other studies (11). More evidence is needed to verify these contradictory results and help improving optimal management strategies of this special type of diabetes.
This study aims to establish the prevalence of new-onset diabetes in Chinese adults after AP through medical records collection and telephone follow-ups. Further, we try to analyze the potential risk factors accounting for glucose homeostasis of patients who experienced AP in order to identify susceptible populations and provide reasonable basis for development of management guidelines and recommendations on PPDM-A.
Research Design and Methods
Study Design and Ethics Statement
This was a multi-center retrospective cohort study including all inpatients with a first attack of AP from 1 January 2016 to 31 December 2020 at Zhongda Hospital of Southeast University, Nanjing Drum Tower Hospital, Yixing Second People’s Hospital, First Affiliated Hospital of Xinjiang Medical University, Hunan Provincial People’s Hospital and Hebei General Hospital. Written informed consent was obtained from all participants. This study was approved by the ethics committee of all participating institutions. Prior to analysis, all patient information was anonymized and deidentified for privacy.
Study Population
The cohort eligible for this study comprised all adult individuals (>18 years) experienced a first attack of AP during the study period. In accordance with the revised Atlanta classification, the diagnosis of AP is based on two of the following three criteria (13): (1) a sudden onset of persistent and severe upper abdominal pain, often radiating to the back (2) serum amylase or lipase levels (or both) of at least three times the upper limit of normal, or (3) characteristic imaging findings consistent with AP on contrast-enhanced CT, MRI, or transabdominal ultrasound. The exclusion criteria were as follows: I. not admitted to hospital within 48 hours; II. recurrent or chronic pancreatitis; III. previous history of diabetes or glucose-lowering therapy; IV. abnormal glycated hemoglobin (HbA1c) during hospitalization; V. other pancreatic injury including trauma, pancreatectomy, neoplasm, cystic fibrosis, hemochromatosis, fibrocalculous pancreatopathy or rare genetic disorders; VI. severe cardiac, hepatic or renal insufficiency or malignant diseases; VII. immune system disorders or under hormone treatment; VIII. pregnancy or lactation; IX. data deficiency >10% due to mental illness or other causes failing to the follow-up call.
Data Collection
This multi-center retrospective cohort study was conducted in Chinese inpatients with first attack of AP who were follow up from the date of admission to the time they developed diabetes or the end of the observation period (31 December 2020). Information on the demographic parameters (gender, age, ABO blood type), hospital stays, medical and family history, severity and etiology of AP, trypsin (amylase and lipase), fasting and random blood glucose, serum calcium, C-reactive protein (CRP), hepatic and renal functions, lipid profiles, infection condition and treatment options were obtained through hospital information system (HIS). During telephone follow-ups after discharge, data including diagnosis of DM, blood glucose levels, glucose-lowering therapy, recurrence of AP, history of cigarette smoking, alcohol consumption, dietary habits and physical activity were recorded.
Definitions and Classifications
Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). According to the Guidelines for Prevention and Control of Overweight and Obesity in Chinese adults, overweight was defined as BMI greater than or equal to 24 kg/m2, and obesity was defined as BMI greater than or equal to 28 kg/m2. The severity of AP was defined as mild, moderately severe or severe according to the revised Atlanta classification (13). Patients were classified as Mild AP in the absence of local or systemic complications and organ failure. Moderately severe AP was defined as the case of local complications or systemic complications, and in the absence of persistent organ failure (<48h). In the case of persistent single or multiple organ failure (>48h), patients were considered severe AP. 2012 International Association of Pancreatology (IAP)/American Pancreatic Association (APA) guidelines recommend identification of the etiology of AP as early as possible (14). Patients were classified as biliary, hyperlipidemic, alcoholic or idiopathic AP, respectively. On the basis of the 2021 American Diabetes Association (ADA) standards, PPDM-A was defined as new-onset diabetes after AP in the absence of a history of pre-existing diabetes before the AP episode (5). In accordance with the ADA consensus definition, stress hyperglycemia was defined in hospital-related hyperglycemia with an admission HbA1c <47.5 mmol/mol(6.5%) and fasting glucose≥7.0 mmol/L or random glucose≥11.1mmol/L without evidence of previous diabetes (15). Hyperlipidemia was defined as hypertriglyceridemia, hypercholesterolemia, or both, on the basis of the Guidelines for Prevention and Treatment of Hyperlipidemia in Chinese adults. Endoscopic retrograde cholangiopancreatography (ERCP) refers to a therapy to extract the stone from choledochal duct by endoscopic sphincterotomy or duodenal papillary balloon dilatation. Surgical therapies included, but not limited to, cholecystectomy, necrectomy, debridement, drainage.
Statistical Analyses
All analyses were performed in SPSS Statistics (Version 22.0, IBM Corp., Armonk, NY, USA). Continuous variables were presented as means ± standard deviation (SD) or medians [interquartile range (IQR)] in accordance with normality tests, and categorical variables were presented as numbers [percentages (%)]. The independent-samples t-test, Mann-Whitney U test, or chi-square test were used to determine differences between groups. The multiple logistic regression analysis was conducted to analyze the potential risk factors of PPDM-A. All tests were two-sided, and P<0.05 was considered to be statistically significant.
Results
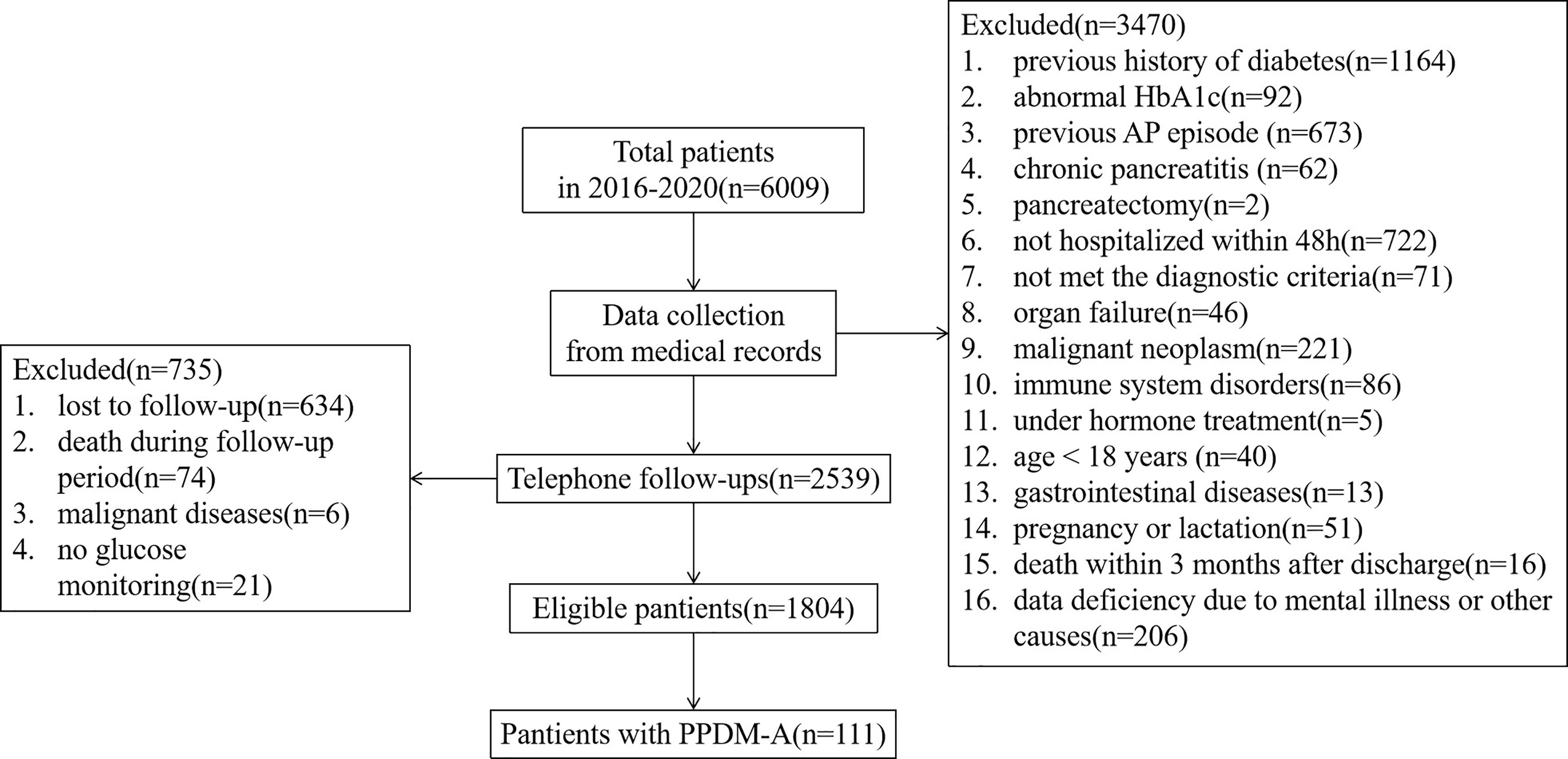
During the period from 2016 to 2020, we identified 6009 individuals with a diagnosis of pancreatitis (Figure 1). Among these individuals, 1256 have been diagnosed diabetes previously or had an abnormal HbA1c(≥6.5%) during hospitalization; 737 had experienced recurrent pancreatitis, chronic pancreatitis or other pancreatic injury; 793 were not hospitalized timely (within 48h) or met the diagnostic criteria; 273 had a previous history of severe organ insufficiency or malignant neoplasm; 91 suffered from immune system disorders or long-term hormone treatment; 40 were under 18 years; 19 had a history of mental illness or gastrointestinal diseases; 51 were during pregnancy or lactation; 90 were died in hospital or follow-up period; 221 had incomplete medical records; and 634 were lost to follow-up. After exclusion of above 4205 cases, the final study cohort comprised 1804 eligible new diagnoses of adult-onset pancreatitis with a median follow-up of 3.04(IQR 1.73, 4.47) years.
Baseline Clinical Characteristics of Patients
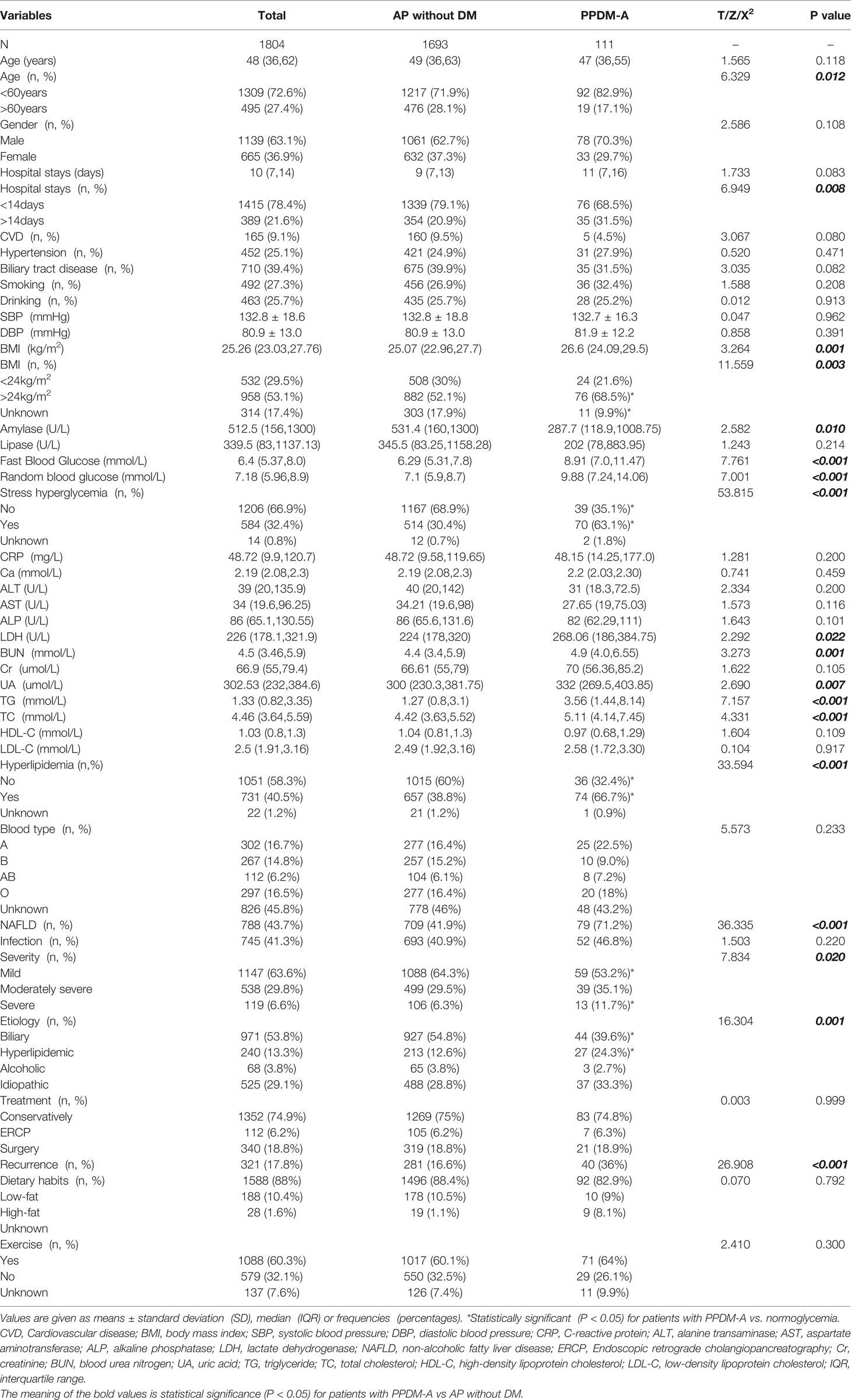
Demographic and clinical characteristics of the patients are reported in Table 1. Of the 1804 patients finally enrolled, a male predominance was observed (63.1%) and the median age was 48 (IQR 36, 62) years on admission. Patients had a median hospital stays of 10(IQR 7, 14) days and a median BMI of 25.26(IQR 23.03, 27.76) kg/m2 at the date of admission. There were 958 patients (53.1%) who were either overweight or obese among all subjects. Cardiovascular disease (CVD) was combined in 165(9.1%) patients and hypertension was diagnosed in 452 (25.1%) patients. 710 (39.4%) patients had a history of biliary tract diseases. There were 492 (27.3%) smokers and 463 (25.7%) drinkers among them. Hyperlipidemia was detected in 731 (40.5%) patients, and 788 (43.7%) patients were accompanied by non-alcoholic fatty liver disease (NAFLD). 1790 patients had fasting or random blood glucose records, and 584 (32.4%) were considered to have stress hyperglycemia. Regarding the etiology of AP, 971 (53.8%) had biliary AP, 240 (13.3%) had hyperlipidemic AP, 68 (3.8%) had alcoholic AP, and 525 (29.1%) had idiopathic AP. As for the severity of AP, 1147(63.6%) were classified as mild AP, 538 (29.8%) as moderately severe AP, and 119 (6.6%) as severe AP. During hospitalization, 1352 (74.9%) patients received conservative medical treatment, 112 (6.2%) underwent ERCP, and 340 (18.8%) underwent surgical operation.
Follow-Up Outcomes and Incidence of PPDM-A
111 patients were defined as developing diabetes after AP, and the overall prevalence of PPDM-A for the entire cohort was 6.2% (111/1804). The patients were divided into two groups according to incidence of diabetes. Of all individuals, 321(17.8%) presented relapse in the follow-up period, and individuals with PPDM-A showed a higher recurrence rate of AP (36.0%) compared with individuals free from diabetes (16.6%) (X2 = 26.908, P < 0.001). Patients with PPDM-A were more likely to be younger (X2 = 6.329, P= 0.012) and had a higher frequency of overweight or obesity (X2 = 11.559, P = 0.003) compared with control group. The frequency of stress hyperglycemia (X2 = 53.815, P < 0.001), hyperlipidemia (X2 = 33.594, P < 0.001) and NAFLD (X2 = 36.335, P < 0.001) were significantly higher among patients with PPDM-A compared to control group. Also, individuals with PPDM-A were more likely to experience longer hospital stays (X2 = 6.949, P= 0.008), higher lactate dehydrogenase (LDH), blood urea nitrogen (BUN), uric acid (UA), triglyceride (TG) and total cholesterol (TC) levels (all P < 0.05), and have a higher degree of severity of AP (X2 = 7.834, P= 0.020) compared to those without diabetes. In addition, individuals with PPDM-A were more likely to have a higher occurrence of hyperlipidemic AP (X2 = 16.304, P= 0.001) compared with those with normal glucose levels. However, two groups showed similar distributions of infectious complications, treatments and lifestyle (all P > 0.05). The patients’ characteristics and detailed results are listed in Table 1.
Influencing Factors of PPDM-A
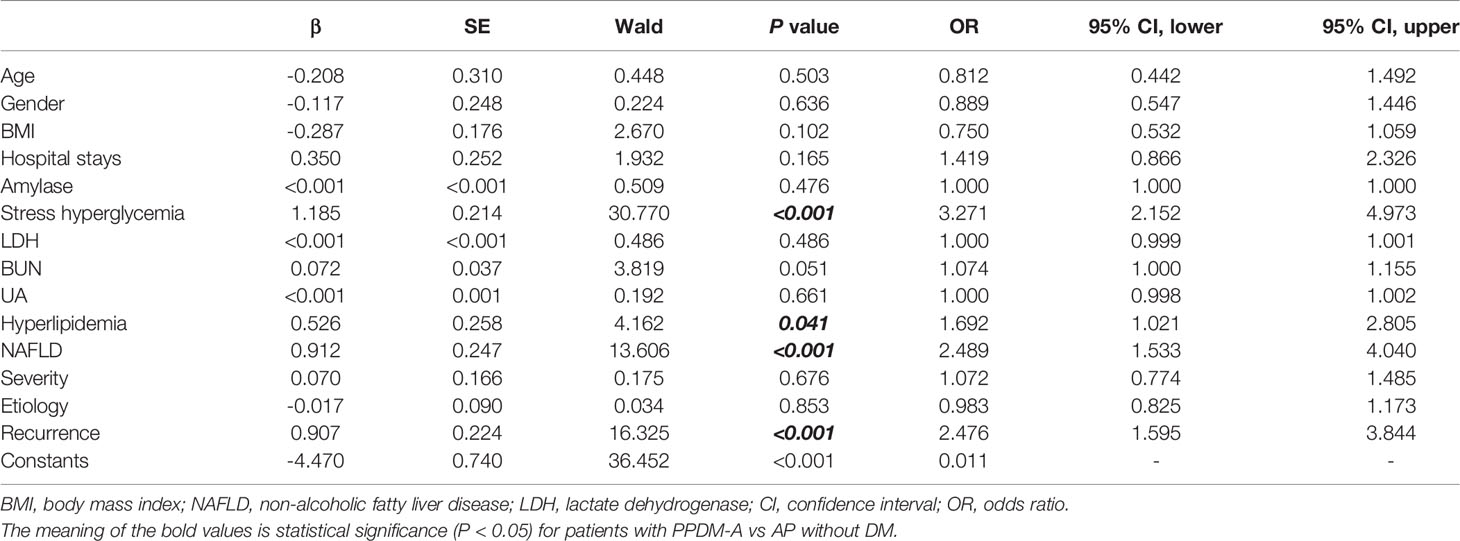
The clinical characteristics mentioned above were included into the multiple logistic regression analysis to investigate the potential contributing factors of PPDM-A. Results suggested that stress hyperglycemia, hyperlipidemia, NAFLD, and recurrent pancreatitis were proven to be the independent risk factors for PPDM-A development (see in Table 2).
Discussion
Recent evidence has gradually focused on the endocrine insufficiency in patients with pancreatic exocrine diseases, but the pathological mechanism of DEP, especially PPDM-A (one of the biggest contributors to DEP), has not been clarified to date (16). Previous studies revealed that exocrine illnesses could damage pancreatic parenchyma and further impair endocrine function of the pancreas (17). Abnormal blood glucose is a sign of endocrine dysfunction or diabetes occurrence in patients experiencing AP onset. Once progression to permanent hyperglycemia, various diabetes complications will gradually emerge and lower their quality of life. Thus, determining the risk factors regarding glucose metabolism after AP is considered to be helpful to correct hyperglycemia and optimize therapeutic strategy for DEP. In this retrospective cohort study, we investigated the outcomes on recurrence, diabetes and other complications among patients with AP. Data showed that patients who developed PPDM-A were featured by younger age, longer hospital stays, a higher frequency of overweight or obesity, stress hyperglycemia on admission, higher LDH, BUN and UA levels, accompanied by hyperlipidemia and NAFLD, more likely to be hyperlipidemic AP, and had a higher degree of severity and recurrence rate of AP compared to those with normal glycemia. Regression analysis further indicated that stress hyperglycemia, hyperlipidemia, NAFLD and recurrent pancreatitis were the independent influence factors for developing PPDM-A. Stress hyperglycemia in the early phase was crucial to permanent hyperglycemia progression after AP, which might be explained by the insulin resistance induced by endothelial dysfunction and decreased insulin biosynthesis and secretion caused by oxidative stress (18, 19). However, elevated blood glucose after AP attack is difficult to recognize because that the changes are insidious and variable, which may result in the long-term neglect of the glucose monitoring and management in patients with AP and even misclassification as type 2 diabetes.
A meta-analysis reported that approximately 23% (95%CI:16%-31%) of patients with AP developed diabetes (10). In our study, the prevalence of new-onset diabetes in patients with AP was 6.2%, which was lower than the results of the meta-analysis. On the one hand, the difference might be related to the strict exclusion criteria of our study, in which the subjects with a history of recurrent or chronic pancreatitis, pancreatic trauma, surgery, neoplasm, hypoplasia, cystic fibrosis, fibrocalculous pancreatopathy or other pancreatic impairments were excluded. Also, we eliminated those with cardiovascular, cerebrovascular, hepatic, renal or malignant diseases, not promptly hospitalized within 48 hours, previously diagnosed diabetes or received glucose-lowering therapy, abnormal HbA1c during or within 90 days of pancreatitis, immune system disorders or under hormone treatment. According to the diagnostic algorithm to identify PPDM proposed by Petrov MS and Basina M recently, new-onset diabetes after pancreatitis (NODAP) should meet impaired glucose metabolism more than 90 days to rule out stress hyperglycemia which may appear during the course of pancreatitis or within 90 days after hospitalization. Such temporary hyperglycemia may reflect the acute stress reaction or be a response of treatment of pancreatitis such as parenteral nutrition or intravenous infusion of dextrose (20). 24 previous prospective clinical studies included in the above meta-analysis didn’t meet all eligibility criteria in our study. We set such strict exclusion criteria to avoid confounding factors as far as possible and try to approach the real incidence of PPDM-A. In addition, the meta-analysis published by Das et al. only included prospective clinical studies. Since our retrospective study design had inevitable recall bias, we cannot ensure that the prevalence will be similar to what reported by Das et al. even if using similar exclusion criteria. However, we will answer this question in our subsequent prospective studies. In spite of some limitations, it is still the first study about the prevalence of new-onset diabetes in Chinese adults after AP, to the best of our knowledge. On the other hand, regional and racial differences might contribute to inconsistent prevalence rates among studies. Of 145 Finnish patients with severe AP, 43% prevalence of newly diagnosed DM is observed (21). Angelini et al. found that in Italy, the incidence of PPDM-A is 5% among 19 patients with severe AP (22) and 8% among 118 patients with AP (including 83 severe AP and 35 mild AP) (23). A study of 112 patients with severe or mild AP in Turkey showed that the morbidity of DM is 12% (24), and the impaired glucose tolerance was not associated with necrosis or disease severity. Yasuda et al. (25) conducted a prospective cohort study comprising of 41 Japanese patients with severe AP and the development of DM was noted in 39% of individuals. Moreover, multivariate analysis revealed that blood glucose was an independent prognostic factor for the development of DM after severe AP (P < 0.05). Another study from India by Gupta et al (26) pointed out that postoperative DM was present in 20% patients with severe AP, and there was no significant correlation of endocrine insufficiency with alcohol intake or gall stone disease (P= 0.6), infected pancreatitis (P= 0.15) and percent of necrosis (P= 0.28). Bharmal et al (27) determined the cumulative incidence of NODAP in New Zealand was 11.2% through 24 months follow-up of 152 patients, and the authors showed that Anthropometrics, pancreatitis-related characteristics, lipid profile, liver enzymes, and markers of inflammation were not significantly associated with the development of high-increasing glycaemia. The exclusion criteria between our study and these studies were similar, although not identical, considering different types of pancreatic injuries, diabetes status and types, follow-up periods, data integrity, survival conditions during hospitalisation and after discharge, and cognitive states. These discoveries were roughly consistent with our results. However, endocrine function after AP was not associated with fatty liver in these studies, probably due to the lack of data on liver assessments and racial differences. Another study of 310 Chinese patients with AP indicated that hyperglycemia on admission, hyperlipidemia, fatty liver, hypertension, ERCP during hospitalization, high levels of LDH and CK, decreased serum calcium, and AB blood type were correlated with elevated blood glucose, and further proved that hyperlipidemia and hyperglycemia on admission were the independent risk factors for diabetes development (12). There may be some potential mechanisms underlying these differences and correlations which needs to be verified. At present, the prevalence of PPDM-A in Chinese population is still uncertain due to insufficient statistical data involving large samples. Our study may offer useful reference to future research.
Glucose homeostasis is important to allow biological processes to proceed normally. In addition to endocrine and exocrine insufficiency of the pancreas itself, several tissues and organs such as liver, gut, brain, muscle and adipose tissue are involved together in the regulation mechanism of glucose homeostasis for patients with DEP. Thereinto, pancreas and liver play key roles in glucose metabolism and thus are regarded as core glucose-controlling target organs (28). As the first messenger of glucose regulation, blood glucose is not only a regulated variable but also a controlled variable, whose level influences the basic homeostatic loop system composed of controllers (pancreas, liver, gut) and effectors (liver, muscle, adipose tissue). In response to an change in blood glucose, the controllers release hormone signals (including insulin, glucagon and incretins), which act on the effectors to produce glucose uptake, transport and utilization, thereby maintaining a stable plasma glucose level.
The effect of exocrine pancreas on endocrine function under physiological conditions can be reflected in the inhibitory effect of amylase and lipase on insulin secretion. One possible explanation is a regulatory mechanism that acts against insulin overproduction and keeps it at a normal level and further avoids exhaustion of β cells (11). In addition to the regulation of insulin secretion, trypsin may also be involved in islet formation and differentiation (29). Inflammation of the pancreas destroys the parenchyma in pathophysiological conditions, and persistent inflammatory response leads to the exocrine pancreatic insufficiency, in which the production of pancreatic enzymes is greatly reduced and thus insulin secretion is released from the inhibitory effect of exocrine enzymes that leads to hyperinsulinemia which has been considered to be responsible for the development of insulin resistance (30). As the extent of pancreatic necrosis increases, the extensive destruction and functional exhaustion of islet β cells led to insufficient insulin secretion. However, the effect of AP severity and extent of necrosis on the risk of developing endocrine insufficiency or secondary hyperglycemia has remained controversial. Historically, PPDM-A develops mostly in those with severe AP and the extent of necrosis was a decisive factor. While this perspective has been challenged by an increasing number of studies. The meta-analysis published by COSMOS group demonstrated that patients with mild AP were at a high risk of developing diabetes and the severity of AP did not materially affect the risk of developing PPDM (10). Several population-based cohort studies also illustrated that there was no correlation between the adjusted risk of PPDM and severity of pancreatitis (31–33). There may be other mechanisms involved in the incidence of PPDM-A besides pancreatic damage per se which remain to be verified by future evidence.
As one of the major metabolic organs, liver plays a critical role in regulating the internal glucose homeostasis. Through glycogenolysis and glycogenesis, liver functions as both a controller and an effector in the homeostatic circuits. Our results showed that patients with AP accompanied by NAFLD were more likely to develop diabetes, highlighting the potential importance of liver in the pathogenesis of DEP. In line with obesity and diabetes, NAFLD is becoming increasingly popular and shares the common pathological mechanisms, i.e. insulin resistance (IR) (34). It has been indicated that immune cells such as macrophages and neutrophils would reside in or infiltrate liver parenchyma under obese conditions and cause chronic inflammation or metabolic disorders that decrease insulin sensitivity thus leading to the development of IR (35). Accumulated adipose tissue releases a large number of inflammatory mediators which can inhibit both the insulin receptor and the action of insulin through various signaling pathways. The adipocytokine interleukin (IL)-6 has been shown to be associated with chronic hyperglycemia and IR after AP (36). Another study showed that obesity-associated adipokine leucine-rich alpha-2-glycoprotein 1 (LRG1) bound with high selectivity to the liver and exacerbated high fat diet-induced hepatosteatosis and IR by increasing de novo lipogenesis and suppressing fatty acid β-oxidation (37). LRG1 also inhibited hepatic insulin signaling by downregulating insulin receptor expression. Other pro-inflammatory cytokines such as monocyte chemoattractant protein (MCP)-1 and tumor necrosis factor (TNF)-α were also found to be elevated in patients after AP (38). As it was shown in our study, hyperlipidemia, especially hypertriglyceridemia, significantly increased the risk of PPDM, which could be attributed to inflammation-induced lipolysis. Lipolysis is the hydrolysis of triglycerides to free fatty acids (FFA) and glycerol (39), and FFA, to some extent, was recognized as energy source or for ectopic fat storage in organs such as the liver and pancreas, potentially contributing to IR (40). Hyperlipidaemia-induced pancreatitis has been found to cause diabetes in more patients compared to the other etiologies (41), which was consistent with our results. In the setting of severe hypertriglyceridemia, restricted blood flow and accumulations of FFA could lead to impairment of circulation in capillary beds, ischemic disturbance to the acinar structures, and a resultant increasingly acidic environment, in which the pancreatic lipase could more easily seep out of acinar cells and be activated by FFA to cause additional pancreatic injury (12).
Accordingly, chronic low-grade inflammation and secondary lipolysis contribute to the critical mechanism of IR. When NAFLD continuously progress to chronic inflammation, fibrosis, and cirrhosis, damaged hepatocytes result in the decline in hepatic glycogen synthesis, storage, decomposition, and the ability to regulate blood glucose homeostasis, manifested as impaired islet function and decreased insulin secretion (42). In addition, giving that insulin is mainly metabolized by the liver, the scavenging effect of liver on insulin decreases under pathological conditions, and clinically presenting with hyperinsulinemia, characterized by frequent fasting hypoglycemia and postprandial hyperglycemia. The results of this study reported that patients with PPDM-A were often accompanied by overweight/obesity, hyperlipidemia and NAFLD, suggesting that the hepatopancreatic dialogue may have a potential effect in the development and progression of DEP. Therefore, the intricate information communication between the liver and pancreas seems to be an important autoregulatory mechanism of glucose homeostasis. It is necessary to explore the internal connections to further illustrate the pathogenesis of DEP and provide a new perspective for improving the clinical therapeutic strategy.
The concept of the gut-islet axis, introduced by Unger and Eisentraut in 1969, described the close connection between the gastrointestinal tract and the islets, containing nutrient, neural and hormonal signals from the gut to islet cells(i.e. the gastrointestinal tract could regulate the activity of islet cells by releasing bioactive substances (43). There are dozens of endocrine cells in the gastrointestinal mucosa which secrete a variety of gastrointestinal hormones released into the blood circulation or play a role in regulating insulin secretion and blood glucose homeostasis in the form of local paracrine (44). For example, once the corresponding glucose set point after dietary stimulation was reached, intestinal L cells would initiate glucose regulation at a threshold of about 5.5mmol/L and secrete glucagon-like peptide-1(GLP-1) in response to blood glucose fluctuations, which is an important controller of blood glucose homeostasis (45). Therefore, the crosstalk between the intestine and the pancreas is also an important regulation mechanism of blood glucose homeostasis.
GLP-1 receptor, a seven-transmembrane G protein-coupled receptor, widely distributed in islet cells, cardiomyocytes, liver, kidney, vascular endothelial cells, skin, hypothalamus and other tissues and organs, suggesting that it plays an important extrapancreatic role in addition to the effect of glucose-dependent insulin secretion and glucagon inhibition (46). GLP-1 receptor agonists (GLP-1RA), a new hypoglycemic drug, has been increasingly proved its efficacy and safety in individuals with NAFLD, which can not only reduce hepatic steatosis and inflammation, but improve non-alcoholic steatohepatitis (NASH) (47). A clinical trial involving patients with NASH showed that treatment with semaglutide resulted in a significantly higher percentage of patients with NASH resolution than placebo (48). D-LIFT trial revealed that dulaglutide significantly reduced liver fat content (LFC) and improved γ-glutamyl transpeptidase (GGT) levels in participants with NAFLD (49). These results provide evidence of evidence-based medicine for the close relationship between liver and intestine, indicating that incretin plays an important role in the homeostasis of glucose and lipid metabolism.
Based on our current analysis, people with recurrent AP are at a significantly increased risk for developing PPDM in comparison with those free from repeated attacks of AP, consistent with previous findings from two population-based studies (33) (50). The difference might be ascribed to the pancreas volume variation. A MRI study by the COSMOS group on pancreas volume in individuals after AP demonstrated a significant 22% reduction in total pancreas volume in patients after two or more recurrences of AP whereas no obvious change in those with one or no recurrence (51). It was worth noting that pancreas tail was known to have the highest proportion of the islet of Langerhans, instead of head or body. After two or more recurrences of AP, the pancreas tail was significantly reduced which was directly proportional to the reduction in β-cell mass (52).
Some limitations are present in this study. First, given the discharge glucose values got from telephone follow-ups not blood tests, the retrospective study design consisting of inpatients may suffer from recall bias and thus not be enough to represent the true prevalence of PPDM-A among overall patients in China. Hence, large-scale prospective population-based studies need to be conducted in the future. Second, the pathological features of PPDM-A will be better explained if pancreas islet function can be included in follow-up period, despite it is hard to be realized due to multiple blood collections of insulin or C-peptide releasing test. Third, only 112 patients (6.2%) in the cohort experienced ERCP treatment and 340 patients (18.8%) received surgery, from which we were unable to measure the effects of therapy methods. Thus, whether different treatments of AP could contribute to different consequences regarding the development of hyperglycemia in the long term still needs further verification. Lastly, the mechanisms underlying the association between liver and endocrine pancreas under DEP conditions remain unclear and to be answered by more evidence-based medical researches.
Conclusion
Our study first determined the prevalence of secondary diabetes in Chinese patients after AP. Stress hyperglycemia, hyperlipidemia, NAFLD and recurrent attacks of AP appear to be the independent risk factors for developing PPDM-A. The disorder of glucose and lipid metabolism in patients with AP should be paid more attention in clinical practice. Furthermore, the mechanisms responsible for the potential relationship between liver and endocrine pancreas need more in-depth studies to explain.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongda Hospital of Southeast University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YL: Conceptualization, Data curation, and Writing-original draft preparation. JZ: Conceptualization and SPSS Statistics. TYang: Conceptualization and Data curation. JS: Visualization and Investigation. JH, ZC and XHY: Data curation. XLY and XL: Investigation. TX and TYu: SPSS Statistics. XS, GL and CZ: Writing-Reviewing and Editing. LL: Conceptualization, Methodology, and Writing-Reviewing and Editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (81970717 and 82170845).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
First, we thank all the patients in this study. Second, we thank our team members for their excellent work. Third, we would like to thank the National Natural Science Foundation of China (81970717 and 82170845) for providing funding support for this study.
Abbreviations
AP, acute pancreatitis; PPDM-A, post-acute pancreatitis diabetes mellitus; DEP, diabetes of the exocrine pancreas; NAFLD, non-alcoholic fatty liver disease; CVD, Cardiovascular disease; NODAP, new-onset diabetes after pancreatitis; IR, insulin resistance; HIS, hospital information system; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; BMI, body mass index; CRP, C-reactive protein; ERCP, Endoscopic retrograde cholangiopancreatography; HbA1c, glycated hemoglobin; ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; Cr, creatinine; BUN, blood urea nitrogen; UA, uric acid; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FFA, free fatty acids; SD, standard deviation; IQR, interquartile range; CI, confidence interval; OR, odds ratio.
References
1. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute Pancreatitis. Lancet (2020) 396(10252):726–34. doi: 10.1016/S0140-6736(20)31310-6
2. Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Dutch Pancreatitis Study Group. Impact of Characteristics of Organ Failure and Infected Necrosis on Mortality in Necrotising Pancreatitis. Gut (2019) 68(6):1044–51. doi: 10.1136/gutjnl-2017-314657
3. Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med (2016) 375(20):1972–81. doi: 10.1056/NEJMra1505202
4. Lv Y, Wei Q, Yuan X, Sun J, Zhang J, Qi L, et al. Two Sides of the Pancreas: Exocrine Insufficiency Is Correlated With Endocrine Dysfunction in Type 2 Diabetes. Clin Chim Acta (2021) 523:81–6. doi: 10.1016/j.cca.2021.09.008
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S15–33. doi: 10.2337/dc21-S002
6. Woodmansey C, McGovern AP, McCullough KA, Whyte MB, Munro NM, Correa AC, et al. Incidence, Demographics, and Clinical Characteristics of Diabetes of the Exocrine Pancreas (Type 3c): A Retrospective Cohort Study. Diabetes Care (2017) 40(11):1486–93. doi: 10.2337/dc17-0542
7. Viggers R, Jensen MH, Laursen HVB, Drewes AM, Vestergaard P, Olesen SS. Glucose-Lowering Therapy in Patients With Postpancreatitis Diabetes Mellitus: A Nationwide Population-Based Cohort Study. Diabetes Care (2021) 44(9):2045–52. doi: 10.2337/dc21-0333
8. Larger E, Philippe MF, Barbot-Trystram L, Radu A, Rotariu M, Nobécourt E, et al. Pancreatic Exocrine Function in Patients With Diabetes. Diabetes Med (2012) 29(8):1047–54. doi: 10.1111/j.1464-5491.2012.03597.x
9. Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of New Onset Diabetes Mellitus Secondary to Acute Pancreatitis: A Systematic Review and Meta-Analysis. Front Physiol (2019) 10:637. doi: 10.3389/fphys.2019.00637
10. Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly Diagnosed Diabetes Mellitus After Acute Pancreatitis: A Systematic Review and Meta-Analysis. Gut (2014) 63(5):818–31. doi: 10.1136/gutjnl-2013-305062
11. Gál E, Dolenšek J, Stožer A, Czakó L, Ébert A, Venglovecz V. Mechanisms of Post-Pancreatitis Diabetes Mellitus and Cystic Fibrosis-Related Diabetes: A Review of Preclinical Studies. Front Endocrinol (Lausanne) (2021) 12:715043. doi: 10.3389/fendo.2021.715043
12. Yuan L, Tang M, Huang L, Gao Y, Li X. Risk Factors of Hyperglycemia in Patients After a First Episode of Acute Pancreatitis: A Retrospective Cohort. Pancreas (2017) 46(2):209–18. doi: 10.1097/MPA.0000000000000738
13. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Acute Pancreatitis Classification Working Group. Classification of Acute Pancreatitis–2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut (2013) 62(1):102–11. doi: 10.1136/gutjnl-2012-302779
14. Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence-Based Guidelines for the Management of Acute Pancreatitis. Pancreatology (2013) 13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063
15. Dungan KM, Braithwaite SS, Preiser JC. Stress Hyperglycaemia. Lancet (2009) 373(9677):1798–807. doi: 10.1016/S0140-6736(09)60553-5
16. Pendharkar SA, Mathew J, Petrov MS. Age- and Sex-Specific Prevalence of Diabetes Associated With Diseases of the Exocrine Pancreas: A Population-Based Study. Dig Liver Dis (2017) 49(5):540–4. doi: 10.1016/j.dld.2016.12.010
17. Sheikh S, Gudipaty L, De Leon DD, Hadjiliadis D, Kubrak C, Rosenfeld NK, et al. Reduced β-Cell Secretory Capacity in Pancreatic-Insufficient, But Not Pancreatic-Sufficient, Cystic Fibrosis Despite Normal Glucose Tolerance. Diabetes (2017) 66(1):134–44. doi: 10.2337/db16-0394
18. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of Oxidative Stress by Acute Glucose Fluctuations Compared With Sustained Chronic Hyperglycemia in Patients With Type 2 Diabetes. JAMA (2006) 295(14):1681–7. doi: 10.1001/jama.295.14.1681
19. Pinkney JH, Stehouwer CD, Coppack SW, Yudkin JS. Endothelial Dysfunction: Cause of the Insulin Resistance Syndrome. Diabetes (1997) 46 Suppl 2:S9–13. doi: 10.2337/diab.46.2.s9
20. Petrov MS, Basina M. DIAGNOSIS OF ENDOCRINE DISEASE: Diagnosing and Classifying Diabetes in Diseases of the Exocrine Pancreas. Eur J Endocrinol (2021) 184(4):R151–63. doi: 10.1530/EJE-20-0974
21. Halonen KI, Pettilä V, Leppäniemi AK, Kemppainen EA, Puolakkainen PA, Haapiainen RK. Long-Term Health-Related Quality of Life in Survivors of Severe Acute Pancreatitis. Intensive Care Med (2003) 29(5):782–6. doi: 10.1007/s00134-003-1700-8
22. Angelini G, Pederzoli P, Caliari S, Fratton S, Brocco G, Marzoli G, et al. Long-Term Outcome of Acute Necrohemorrhagic Pancreatitis. A 4-Year Follow-Up. Digestion (1984) 30(3):131–7. doi: 10.1159/000199097
23. Angelini G, Cavallini G, Pederzoli P, Bovo P, Bassi C, Di Francesco V, et al. Long-Term Outcome of Acute Pancreatitis: A Prospective Study With 118 Patients. Digestion (1993) 54(3):143–7. doi: 10.1159/000201028
24. Kaya E, Dervisoglu A, Polat C. Evaluation of Diagnostic Findings and Scoring Systems in Outcome Prediction in Acute Pancreatitis. World J Gastroenterol (2007) 13(22):3090–4. doi: 10.3748/wjg.v13.i22.3090
25. Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, et al. Long-Term Outcome of Severe Acute Pancreatitis. J Hepatobiliary Pancreat Surg (2008) 15(4):397–402. doi: 10.1007/s00534-007-1266-x
26. Gupta R, Wig JD, Bhasin DK, Singh P, Suri S, Kang M, et al. Severe Acute Pancreatitis: The Life After. J Gastrointest Surg (2009) 13(7):1328–36. doi: 10.1007/s11605-009-0901-z
27. Bharmal SH, Cho J, Alarcon Ramos GC, Ko J, Stuart CE, Modesto AE, et al. Trajectories of Glycaemia Following Acute Pancreatitis: A Prospective Longitudinal Cohort Study With 24 Months Follow-Up. J Gastroenterol (2020) 55(8):775–88. doi: 10.1007/s00535-020-01682-y
28. Kotas ME, Medzhitov R. Homeostasis, Inflammation, and Disease Susceptibility. Cell (2015) 160(5):816–27. doi: 10.1016/j.cell.2015.02.010
29. Wei C, Geras-Raaka E, Marcus-Samuels B, Oron Y, Gershengorn MC. Trypsin and Thrombin Accelerate Aggregation of Human Endocrine Pancreas Precursor Cells. J Cell Physiol (2006) 206(2):322–8. doi: 10.1002/jcp.20459
30. Gray SL, Donald C, Jetha A, Covey SD, Kieffer TJ. Hyperinsulinemia Precedes Insulin Resistance in Mice Lacking Pancreatic Beta-Cell Leptin Signaling. Endocrinology (2010) 151(9):4178–86. doi: 10.1210/en.2010-0102
31. Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of Diabetes Mellitus After First-Attack Acute Pancreatitis: A National Population-Based Study. Am J Gastroenterol (2015) 110(12):1698–706. doi: 10.1038/ajg.2015.356
32. Lee YK, Huang MY, Hsu CY, Su YC. Bidirectional Relationship Between Diabetes and Acute Pancreatitis: A Population-Based Cohort Study in Taiwan. Med (Baltimore) (2016) 95(2):e2448. doi: 10.1097/MD.0000000000002448
33. Ho TW, Wu JM, Kuo TC, Yang CY, Lai HS, Hsieh SH, et al. Change of Both Endocrine and Exocrine Insufficiencies After Acute Pancreatitis in Non-Diabetic Patients: A Nationwide Population-Based Study. Med (Baltimore) (2015) 94(27):e1123. doi: 10.1097/MD.0000000000001123
34. Wang Y, Chen P, Xiao W. The Role of Hepatic Macrophage in the Development of Hepatic Insulin Resistance. Chin J Diabetes (2021) 29(07):544–8. doi: 10.3969/j.issn.1006-6187.2021.07.011
35. Lauterbach MA, Wunderlich FT. Macrophage Function in Obesity-Induced Inflammation and Insulin Resistance. Pflugers Arch (2017) 469(3-4):385–96. doi: 10.1007/s00424-017-1955-5
36. Gillies N, Pendharkar SA, Asrani VM, Mathew J, Windsor JA, Petrov MS. Interleukin-6 Is Associated With Chronic Hyperglycemia and Insulin Resistance in Patients After Acute Pancreatitis. Pancreatology (2016) 16(5):748–55. doi: 10.1016/j.pan.2016.06.661
37. He S, Ryu J, Liu J, Luo H, Lv Y, Langlais PR, et al. LRG1 is an Adipokine That Mediates Obesity-Induced Hepatosteatosis and Insulin Resistance. J Clin Invest (2021) 131(24):e148545. doi: 10.1172/JCI148545
38. Pendharkar SA, Singh RG, Petrov MS. Pro-Inflammatory Cytokine-Induced Lipolysis After an Episode of Acute Pancreatitis. Arch Physiol Biochem (2018) 124(5):401–9. doi: 10.1080/13813455.2017.1415359
39. Prentki M, Madiraju SR. Glycerolipid/free Fatty Acid Cycle and Islet β-Cell Function in Health, Obesity and Diabetes. Mol Cell Endocrinol (2012) 353(1-2):88–100. doi: 10.1016/j.mce.2011.11.004
40. Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, et al. Dose-Response Effect of Elevated Plasma Free Fatty Acid on Insulin Signaling. Diabetes (2005) 54(6):1640–8. doi: 10.2337/diabetes.54.6.1640
41. Wu D, Xu Y, Zeng Y, Wang X. Endocrine Pancreatic Function Changes After Acute Pancreatitis. Pancreas (2011) 40(7):1006–11. doi: 10.1097/MPA.0b013e31821fde3f
42. Shen X, Yan S. Clinical Features and Treatment of Common Liver Diseases With Abnormal Glucose Metabolism. Chin J Diabetes Mellitus (2021) 13(1):4–10. doi: 10.3760/cma.j.cn115791-20200823-00527
43. Unger RH, Eisentraut AM. Entero-Insular Axis. Arch Intern Med (1969) 123(3):261–6. doi: 10.1001/archinte.123.3.261
44. Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N, et al. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol Rev (2017) 97(1):411–63. doi: 10.1152/physrev.00031.2014
45. Reimann F, Gribble FM. Glucose-Sensing in Glucagon-Like Peptide-1-Secreting Cells. Diabetes (2002) 51(9):2757–63. doi: 10.2337/diabetes.51.9.2757
46. Waser B, Reubi JC. Radiolabelled GLP-1 Receptor Antagonist Binds to GLP-1 Receptor-Expressing Human Tissues. Eur J Nucl Med Mol Imaging (2014) 41(6):1166–71. doi: 10.1007/s00259-013-2684-4
47. Bifari F, Manfrini R, Dei Cas M, Berra C, Siano M, Zuin M, et al. Multiple Target Tissue Effects of GLP-1 Analogues on Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH). Pharmacol Res (2018) 137:219–29. doi: 10.1016/j.phrs.2018.09.025
48. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med (2021) 384(12):1113–24. doi: 10.1056/NEJMoa2028395
49. Kuchay MS, Krishan S, Mishra SK, Choudhary NS, Singh MK, Wasir JS, et al. Effect of Dulaglutide on Liver Fat in Patients With Type 2 Diabetes and NAFLD: Randomised Controlled Trial (D-LIFT Trial). Diabetologia (2020) 63(11):2434–45. doi: 10.1007/s00125-020-05265-7
50. Cho J, Scragg R, Petrov MS. The Influence of Cholecystectomy and Recurrent Biliary Events on the Risk of Post-Pancreatitis Diabetes Mellitus: A Nationwide Cohort Study in Patients With First Attack of Acute Pancreatitis. HPB (Oxford) (2021) 23(6):937–44. doi: 10.1016/j.hpb.2020.10.010
51. DeSouza SV, Priya S, Cho J, Singh RG, Petrov MS. Pancreas Shrinkage Following Recurrent Acute Pancreatitis: An MRI Study. Eur Radiol (2019) 29(7):3746–56. doi: 10.1007/s00330-019-06126-7
Keywords: acute pancreatitis, glucose homeostasis, risk factors, non-alcoholic fatty liver disease, diabetes of the exocrine pancreas
Citation: Lv Y, Zhang J, Yang T, Sun J, Hou J, Chen Z, Yu X, Yuan X, Lu X, Xie T, Yu T, Su X, Liu G, Zhang C and Li L (2022) Non-Alcoholic Fatty Liver Disease (NAFLD) Is an Independent Risk Factor for Developing New-Onset Diabetes After Acute Pancreatitis: A Multicenter Retrospective Cohort Study in Chinese Population. Front. Endocrinol. 13:903731. doi: 10.3389/fendo.2022.903731
Received: 24 March 2022; Accepted: 20 April 2022;
Published: 25 May 2022.
Edited by:
Paulo Matafome, University of Coimbra, PortugalReviewed by:
Ana Mendes, Coimbra Hospital and University Center, PortugalJosé Teixeira, University of Coimbra, Portugal
Copyright © 2022 Lv, Zhang, Yang, Sun, Hou, Chen, Yu, Yuan, Lu, Xie, Yu, Su, Liu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Li, dr_liling@126.com; Chi Zhang, zhangcynthia@163.com; Gaifang Liu, liugaifang65@126.com
†ORCID: Ling Li, orcid.org/0000-0003-0083-6978
‡These authors contributed equally to this work
 Yingqi Lv1‡
Yingqi Lv1‡ Zhiwei Chen
Zhiwei Chen Ling Li
Ling Li