- 1School of Sports Health, Shenyang Sport University, Shenyang, Liaoning, China
- 2School of Physical Education, Liaoning Normal University, Dalian, Liaoning, China
Apelin, a novel endogenous ligand of the G-protein-coupled receptor APJ, is encoded by the APLN gene and can be hydrolyzed into multiple subtypes, with Apelin-13 being one of the most active subtypes of the Apelin family. Recent studies have revealed that Apelin-13 functions as an adipokine that participates in the regulation of different biological processes, such as oxidative stress, inflammation, apoptosis, and energy metabolism, thereby playing an important role in the prevention and treatment of various metabolic diseases. However, the results of recent studies on the association between Apelin-13 and various metabolic states remain controversial. Furthermore, Apelin-13 is regulated or influenced by various forms of exercise and could therefore be categorized as a new type of exercise-sensitive factor that attenuates metabolic diseases. Thus, in this review, our purpose was to focus on the relationship between Apelin-13 and related metabolic diseases and the regulation of response movements, with particular reference to the establishment of a theoretical basis for improving and treating metabolic diseases.
1 Introduction
The increasing prevalence of metabolic diseases, especially those associated with a sharp increase in the incidence of obesity-related diseases, poses a major threat to human health worldwide (1). These diseases, which are mainly caused by local or systemic metabolic abnormalities, due to an imbalance in energy metabolism (2), include diabetes mellitus (DM), obesity, and metabolic bone disease, among others (3, 4). Such metabolic disorders are accompanied by the abnormal secretion and/or dysfunction of many cytokines (5).
Apelin is mainly synthesized and secreted by white adipocytes (6), and is expressed in metabolic tissues, such as cardiovascular, skeletal, renal, etc (7, 8), while its expression in white adipose tissue (WAT) is higher than that in liver, muscle and brown adipose tissue(BAT), and with the differentiation of adipocytes, the expression of Apelin also increases significantly. Therefore, it is called “adipokine” (6, 9, 10). Apelin-13 is considered the most functional subtype of the Apelin family, and it actively participates in regulating bone marrow mesenchymal stem cell (BMSC) apoptosis, osteoblast differentiation, glucose and lipid metabolism, and other physiological processes (11–13), which means that it has an important role in the prevention and treatment of metabolic diseases such as obesity, diabetes and osteoporosis; however, there is still some dispute regarding its regulatory effect on metabolic diseases (14, 15). Therefore, it is extremely important to explore the physiological mechanisms through which Apelin-13 affects various metabolic diseases.
In addition, Apelin-13 can also serve as a “motion-sensitive factor” and can be used to increase the potential of exercise (16). Long-term exercise can also promote the expression of Apelin-13 in the serum of obese individuals (17), suggesting that exercise might regulate metabolic diseases through Apelin-13. Therefore, this article focuses on the role of Apelin-13 in metabolism and its potential relationship with exercise, to provide new ideas for improving metabolic diseases through exercise.
2 Overview
2.1 Apelin-13 structure
In 1998, Tatemoto et al. extracted a peptide called Apelin from bovine gastric secretions (18), and this was found to be translated from the APLN gene located on chromosomes Xq25-26.1. Subsequent studies showed that APLN in humans, mice, and cattle have high structural homology (19). Apelin has also been confirmed to be an endogenous ligand of the G-protein-coupled receptors APJ and Elabela (ELA) (20, 21); moreover, it activates the receptor signaling pathway by binding to APJ and performs specific biological functions (21). Apelin can be hydrolyzed into subtypes of different lengths (Apelin-12, Apelin-13, Apelin-17, Apelin-36, etc.) (22). The N-terminal residue of pro-Apelin can be translated, modified by endogenous peptidases, and cleaved with high catalytic efficiency to remove phenylalanine at the C-terminus to form Apelin-36, which is then cleaved in a similar manner into smaller Apelin-17 and Apelin-13 fragments (23–25). These subtypes always retain their significantly biologically active C-terminus during this process (26, 27). Moreover, the endogenous peptidases that have a predominant role in this process typically include angiotensin converting enzyme 2 (ACE2) and neutral endopeptidases, among others (28). In addition, these subtypes can all bind APJ receptors, but different conformational states of their receptors might affect protein activity (29). The activity of each subtype is different, and shorter subtypes can more effectively activate the APJ receptor. Accordingly, the activity of Apelin-13 and Apelin-17 is much stronger than that of Apelin-36, and the different binding affinities for their receptor determine the different APJ signaling pathways activated in cells (30, 31). Therefore, Apelin-13 is considered to be the most biologically active affinity fragment among Apelin subtypes (32), and related research on this form is relatively extensive.
2.2 Biological functions of Apelin-13
2.2.1 Apelin-13 affects food intake by regulating the central nervous system
The active substances secreted by adipose tissue can pass through the blood–brain barrier and are released into the central system to exert their effects and regulate energy homeostasis by integrating the internal environment and cellular signals to generate behavioral responses that can initiate or terminate feeding (33–35). One study indicated that the chronic intracerebroventricular (third ventricle) injection of Apelin-13 (1 μg, administered via slow infusion over 24 h for 10 consecutive days) significantly increased the intake of food and water, as well as the body temperature and body weight of wild-type mice (36). However, another study found that the acute intracerebroventricular (2 μg, injection within 15 min) injection of Apelin-13 significantly reduced the intake of food and water, as well as the rate of respiratory exchange in C57BL/6 rats (37); the discrepancy between these two studies was mainly attributed to inconsistencies in the injection speed. Interestingly, the intracerebroventricular injection of Apelin-13 did not exert a significant effect on the food intake and body weight in rats fed a high-fat diet (37). In their attempts to determine possible reasons for this, the authors found that the mRNA expression of APJ in the hypothalamus, which had been significantly increased in the high-fat diet group, was suppressed by the injection of Apelin-13, whereas this was not observed in rats fed a normal diet (37). This indicated that APJ might be involved in the regulation of diet and also that APJ could be affected by different physiological and pathological states. Conversely, the acute intracerebroventricular injection of the receptor APJ-specific antagonist Apelin-13 (F13A) and the corticotropin-releasing factor (CRF) receptor antagonist α-helical CRF9-41 had no significant effect on food intake in Kunming mice (Derived from Swiss mice) but significantly alleviated the reduction in food intake caused by the acute injection of Apelin-13 (38). This indicated that Apelin-13 might be involved in activating central APJ receptors, as well as CRF receptors, thereby directly affecting the activity of the hypothalamus–pituitary–adrenal axis, which reduces food intake, which may be affected by different genetic backgrounds. The above report indicates that Apelin-13 can stimulate the central nervous system, thereby affecting food intake, and may be involved in maintaining energy balance in the body under physiological conditions.
2.2.2 Apelin-13 participates in regulating the inflammatory response
Numerous studies have shown that Apelin-13 not only participates in the central regulation of food intake but also exerts anti-inflammatory effects in various pathological states (39, 40). After subarachnoid hemorrhage, the protein expression of Apelin-13 and APJ in the left basal cortex of the brain is significantly increased. Moreover, the exogenous injection of Apelin-13 was found to significantly reduce thioredoxin interaction protein (TXNIP), nucleotide binding oligomeric domain like receptor protein 3 (NLRP3), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) levels. In addition, the suppression of APJ actually eliminates the protective effects of Apelin-13 on the brain with respect to myeloperoxidase and reactive oxygen species production (41). Another study found that Apelin-13 can also reduce IL-1β and TNF-α levels in the hippocampus of rats with streptozotocin-induced Alzheimer’s disease. Further, the expression of β-lactamase reduces hippocampal cell loss, thereby alleviating cognitive impairment (42). As the degree of human intervertebral disc degeneration worsens, the expression of APJ in the nucleus pulposus tissue (NP) gradually decreases. Recombinant Apelin-13 activates the APJ receptor of nucleus NP and increases the expression of type II collagen, aggrecan, sex determining region Y-box protein 9 (SOX9), matrix metalloproteinase-3 (MMP-3), and MMP-13, in addition to reducing the expression of IL-6 and TNF- α in NP cells. Moreover, the use of Ala13 (an inhibitor of Apelin-13) can reduce the expression of phosphatidylinositol 3-kinase (PI3K) and protein kinase B (AKT), whereas LY294002 can diminish the effect of Apelin-13 on NP cells (43). This indicates that Apelin-13 can inhibit the inflammatory response through the PI3K/Akt signaling pathway, thereby improving intervertebral disc degeneration. These results demonstrate the anti-inflammatory effect of Apelin-13, indicating its role as a “protective factor”, but most studies are limited to its associated phenotype and have not delved into the underlying molecular mechanisms.
2.2.3 Role of Apelin-13 in autophagy and cell apoptosis
Autophagy is a dynamic recycling system for intracellular degradation, through which intracellular substances are transported to lysosomes and degraded, thereby accelerating cell renewal (44). A clinical data showed that mRNA and protein expression of Apelin in NP tissues of patients with intervertebral disc herniation were significantly lower than those of the control group, suggesting that Apelin-13 may play a role in disc herniation. Subsequently, the author found that in the H2O2 induced oxidative stress model of NP cells, Apelin-13 treatment increased mRNA and protein expression of collagen II and polysaccharides in NP cells, and increased autophagy flux (LC3II/I increased and p62 decreased) (45), indicating that Apelin-13 may be a beneficial factor in preventing and treating intervertebral disc herniation. Similarly, the injection of Apelin-13 into the right ventricle significantly increases Bcl-2/Bax to alleviate neuronal apoptosis in mice with cerebral ischemia/reperfusion. This treatment also inhibits the expression of LC3B in the hippocampus and upregulates p62 expression. Acridine orange staining showed that Apelin-13 treatment significantly inhibits the increase of autophagic vacuoles (46), suggesting that it can prevent ischemic stroke injury by inhibiting excessive autophagy and cell apoptosis. Further, Wang et al. found that Apelin-13 treatment significantly reduces the mRNA expression of malondialdehyde (MDA), superoxide, and nitrotyrosine in the myocardium of mice with cardiac ischemia/reperfusion and reduces the contents of redox indicators (cytoplasmic lactate/the pyruvate ratio). However, the use of PI3K inhibitors actually eliminates this effect (47). Therefore, the targeted regulation of Apelin-13 inhibits autophagy and cell apoptosis, providing new insights into the modulation of this protein to improve body homeostasis. It is worth noting that the relevant molecular mechanism of apelin-13 improving different types of diseases through autophagy remains to be studied, which has a strong guiding significance for the prevention and treatment of metabolic diseases.
3 Role of Apelin-13 in metabolic diseases
3.1 Apelin-13 participates in the regulation of obesity
Obesity, a chronic and progressive process that affects the energy balance in the body, not only causes the abnormal or excessive accumulation of body fat (48) but also induces inflammation and fibrosis of the WAT, leading to local or systemic metabolic dysfunction (49). Aggravation linked to obesity is closely related to the release of bioactive adipokines (50). Adipokines released into the circulation act as classic hormones affecting the tissue and organ metabolism via specific receptors on the surfaces of target cells (51), thereby regulating energy metabolism (52), as well as immune system activities (53).
3.1.1 Apelin-13 inhibits obesity
A large amount of data indicates that, obesity usually results in subcutaneous and visceral fat dilation (54). As one of the components of visceral fat, cardiac epicardial fat (EAT) is mainly composed of WAT, and dietary and exercise interventions can partially promote its browning (55, 56). The thickness of the EAT in obese patients is significantly higher than that in normal individuals. Moreover, the expression of Apelin in the EAT is 2.6 times higher than that in subcutaneous adipose tissue, and it is positively correlated with left ventricular diastolic function (57). This suggests that Apelin-13 might play a role in EAT and could serve as a new target for the prevention and treatment (55). Animal studies have found that amidation-modified Apelin-13 might significantly reduce the average diameter of adipocytes, mRNA and protein expression of peroxisome proliferator-activated receptor γ (PPARγ) levels and perilipin 1 (PLIN1), thereby improving the dyslipidemia caused by obesity (14). Cell-based experiments have also shown a similar trend and found that Apelin-13 reduced lipid content and total cholesterol content (14), indicating that amidation-modified Apelin-13 might downregulate the expression of PPARγ, thereby inhibiting the differentiation of adipocytes, resulting in the regulation of PLIN1 expression to promote lipolysis in vivo, which ameliorated obesity symptoms. Reducing lipid storage in the body is another important mechanism that reduces obesity (58). Aquaporin 7 (AQP7), a transporter protein present in the plasma membrane of adipocytes, is positively correlated with the degree of obesity (59). AQP7 deficiency in adipocytes increases glycerol kinase activity and accelerates triglyceride synthesis, ultimately leading to obesity (60). In vitro experiments have confirmed that Apelin-13 significantly upregulates the expression of AQP7 in palmitic acid-treated hypertrophic adipocytes and reduces the accumulation of triglycerides in the cytoplasm, which could be reversed using the PI3K inhibitor LY294002 (61), suggesting that Apelin-13 might suppress lipid storage in hypertrophic adipocytes and thereby reduce obesity by upregulating AQP7 expression via the PI3K signaling pathway. These findings indicate that Apelin-13 can inhibit adipocyte differentiation, promote fat breakdown, and reduce lipid storage, thereby alleviating the pathological changes caused by obesity.
Apoptosis is a highly regulated form of cell death that enables specific cells to be sacrificed for the greater good of the organism (62, 63). An increase in the volume of adipose tissues, which causes obesity, could be due to an increase in the number and/or size of adipocytes (64). Moreover, an increase in adipocyte apoptosis might prevent the excessive accumulation of adipose tissue, thereby ensuring the stability of the physical condition in the body (65). Altered adipokine production could affect the balance between apoptosis and survival, ultimately determining cell fates. According to one study, the activity and quantity of adipocytes in obese rats are significantly higher than those in a normal control group (66). Further, following the tail vein injection of amidation-modified Apelin-13 into obese rats, the serum levels of blood glucose, free fatty acids, triglycerides, total cholesterol, and low-density lipoprotein cholesterol, as well as the expression of B-cell lymphoma-2 (BCL-2) in adipose tissues, decrease, whereas those of caspase-3 increase (67). In addition, intervention using amidation-modified Apelin-13 also diminishes the activity of 3T3-L1 preadipocytes and enhances cell apoptosis (67), indicating that amidation-modified Apelin-13 might alleviate obesity by promoting adipocyte apoptosis.
3.1.2 Apelin-13 accelerates obesity
The conclusion found by Suat et al. (15) is contrary to the above results (61, 67). Apelin-13 can increase serum levels of total cholesterol and low-density lipoprotein cholesterol in a dose-dependent manner, thereby accelerating lipid metabolism disorders and significantly reducing mRNA expression levels of uncoupling protein 1 in white adipose tissue and brown adipose tissue at the scapula, as well as uncoupling protein 3 in the biceps brachii muscle (15), The increase in uncoupling protein 3 content is positively correlated with the mitochondrial content of skeletal muscle (68), indicating that Apelin-13 may reduce adipose tissue browning, mitochondrial content of skeletal muscle, and energy consumption, thereby exacerbating obesity. The report also pointed out that Apelin-13 can increase the food intake and body weight of mice (15), and this difference may be attributed to changes in their eating behavior and pathological state.
Generally, Apelin-13 mainly improves obesity by inhibiting adipocyte differentiation、 promoting fat decomposition and cell apoptosis (Figure 1). However, there are also different sounds, that is, exogenous injection of apelin-13 can also reduce energy consumption in mice, but due to the lack of existing reference basis, it still needs further verification. At present, the effect of the central and circulatory injection of Apelin-13 on different pathological conditions remains an important issue that requires a resolution. Studies focusing on the regulatory effects of Apelin-13 on obesity are relatively limited, and the molecular mechanisms underlying this process require further exploration.
3.2 Apelin-13 participates in regulating diabetes
Epidemiological data indicate that DM, one of the most common and increasingly prevalent metabolic diseases worldwide, could affect 693 million adults by 2045 (69). According to its clinical characteristics, DM can be divided into four types, as follows: type 1 diabetes mellitus (T1DM); type 2 diabetes mellitus (T2DM); gestational diabetes mellitus (GDM); and specific types of DM with other causes (70). Although researchers have made great progress in understanding and treating DM, the morbidity and mortality caused by this disease and related complications continue to increase (71, 72). Therefore, effective and simple biomarkers that enable the early diagnosis, progress monitoring, and targeted treatment of DM are urgently needed.
3.2.1 Apelin-13 and T2DM
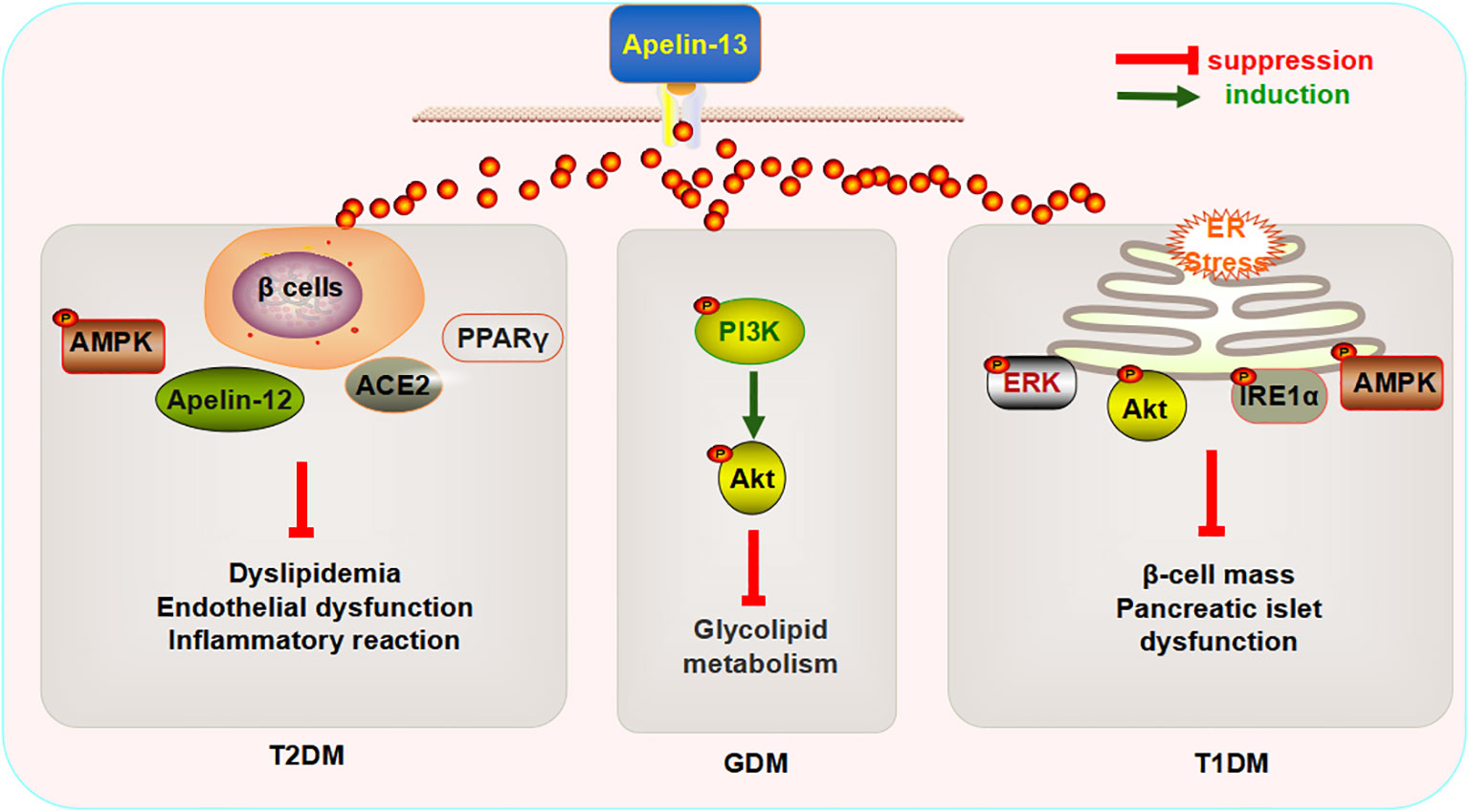
Research has found that the intraperitoneal injection of Apelin-13 (200 μg/kg/day, 4 weeks) can enhance the expression of Apelin-12, glucose transporter 4 (GLUT4), and AMP-activated protein kinase (AMPK) α-2 in the serum, as well as in the myocardial and aortic tissues of rats with DM. I can also improve cardiac functions (decreased heart rate and left ventricular end systolic pressure, and increased maximum rise/fall rate of the left ventricular pressure), insulin resistance, endothelial function (decreased endothelin-1 (ET-1) and increased NO and constitutive nitric oxide synthase (cNOS) activities), and the inflammatory response in rats with DM (73). Sabry et al., reported that the subcutaneous injection of Apelin-13 (0.1 mol/kg/day, 6 weeks) reduces the mRNA expression of angiotensin II (Ang II)-type 1 receptor (ATR1) in the adipose tissue of rats with T2DM and increases the levels of angiotensin-converting enzyme 2 (ACE2) and NO. The application of L-NAME, an inhibitor of cNOS, reverses the significant effects exerted by Apelin-13 (74). ACE2 converts Ang II to Ang1-7 or Ang I to Ang1-9, thereby counteracting the effects of the renin–angiotensin system on the cardiovascular system (75), suggesting that the beneficial effects of Apelin-13 on glucose and lipid metabolism in rats with DM are mainly mediated by the NO activation pathway and/or ACE1/Ang (1-7). The intraperitoneal injection of Apelin-13 (0.1 µmol/kg/day,10 weeks) decreases the glycogen content and increases the mRNA expression of GLUT4 in the myocardium, while increasing the insulin (INS) content in serum and improving the function of pancreatic β cells (76). Apelin-13 affects pathological changes in T2DM by modulating glucose metabolism. In addition, Apelin-13 decreases the fatty acid (FA) content in myocardial tissue (76). The FA content in myocardial tissue mainly depends on the uptake, oxidation, and utilization of glucose (77, 78). This function can be realized by activating PPAR-α in the heart (79, 80). Researchers have further found that Apelin-13 might also reduce the mRNA expression of FA transporters, cluster of differentiation 36 (CD36), carnitine palmitoyl transferase-1 (CPT-1), and PPAR-α in myocardial tissue (76), indicating that the mechanism underlying the regulatory effects of Apelin-13 on FAs might occur via PPAR-α inhibition, which affects lipid metabolism, thus reducing myocardial FA uptake and oxidation. Altered mitochondrial functions comprise another process through which the utilization of cardiac FAs is increased (81). To explore the effect of Apelin-13 on the biogenesis of myocardial mitochondria in rats with T2DM, Feng et al. detected the expression of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1-α) and citrate synthase in the myocardium and found that Apelin-13 did not affect the expression of either (76), suggesting that Apelin-13 might not affect the biogenesis of myocardial mitochondria in rats with T2DM. Notably, they also reported that Apelin-13 might not cause significant changes in serum FAs and blood lipid indicators (76), and this conclusion contradicted the results of previous studies (67, 73). This observation could be attributed to the minimal effect of Apelin-13 on basal fat decomposition in vivo or resistance to Apelin (82), which implies that the association between Apelin-13 and lipid metabolism needs to be further elucidated. These findings confirmed that Apelin-13 might attenuate the pathological changes caused by T2DM by regulating multiple signaling pathways, and thus, this has potential as a new clinical marker.
3.2.2 Apelin-13 and T1DM
Similarly, Apelin-13 exerts a similar protective effect on T1DM and GDM. The intravenous injection of Apelin-13 (400 pmol/kg twice per day for 10 weeks) significantly improves islet mass and INS levels in Akita mice with spontaneous T1DM Akita and inhibits the expression of inositol-requiring protein 1α (IRE1α) and phosphorylation of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), two endoplasmic reticulum (ER) stress receptors in pancreatic tissues. Thus, the inhibition of c-Jun N-terminal kinase (JNK) phosphorylation and expression of the pro-apoptotic transcription factor C/EBP homologous protein (CHOP) (83) indicates that Apelin-13 might inhibit ER stress-dependent cell death in the pancreas of Akita mice to some extent. Next, the authors explored the mechanism through which Apelin-13 regulates of ER stress and found that it increases the phosphorylation levels of extracellular regulated protein kinases (ERK), protein kinase B (Akt), and AMPK (83), suggesting that Apelin-13 might widely regulate multiple pathways, thereby improving ER stress caused by T1DM.
3.2.3 Apelin-13 and GDM
Another study found that the subcutaneous injection of Apelin-13 (2 mg/kg) decreases serum superoxide dismutase (SOD) and glutathione peroxidase (GPx) expression in mice with GDM, which was found to significantly improve glucose and lipid metabolism disorders, oxidative stress, and inflammatory responses. Subsequently, the authors found that the phosphorylation levels of PI3K and Akt in the placenta of mice with GDM had increased significantly following the injection of Apelin-13. Moreover, the injection of LY294002, a PI3K inhibitor, reversed this process (84), suggesting that Apelin-13 might improve glucose and lipid metabolism, reduce oxidative stress and inflammation, and improve GDM by activating the PI3K/Akt signaling pathway. Human studies have shown that the serum levels of Apelin-13, Apelin-36, and NO are higher in patients with GDM than in healthy mothers in the second trimester of pregnancy, who suffer from glucose and lipid metabolism disorders (85). However, the causal relationship between abnormal Apelin-13 expression and health is worthy of further investigation.
The aforementioned data indicate that the exogenous injection of Apelin-13 can improve glucose and lipid metabolism disorders, endothelial dysfunction, inflammatory reactions, and pancreatic islet functions in the context of diabetes by regulating multiple signaling pathways, β-cell quality, ER stress, and oxidative stress, and this protein could therefore become a biomarker for the prevention and treatment of metabolic diseases such as DM (Figure 2).
3.3 Apelin-13 is involved in the regulation of metabolic bone disease
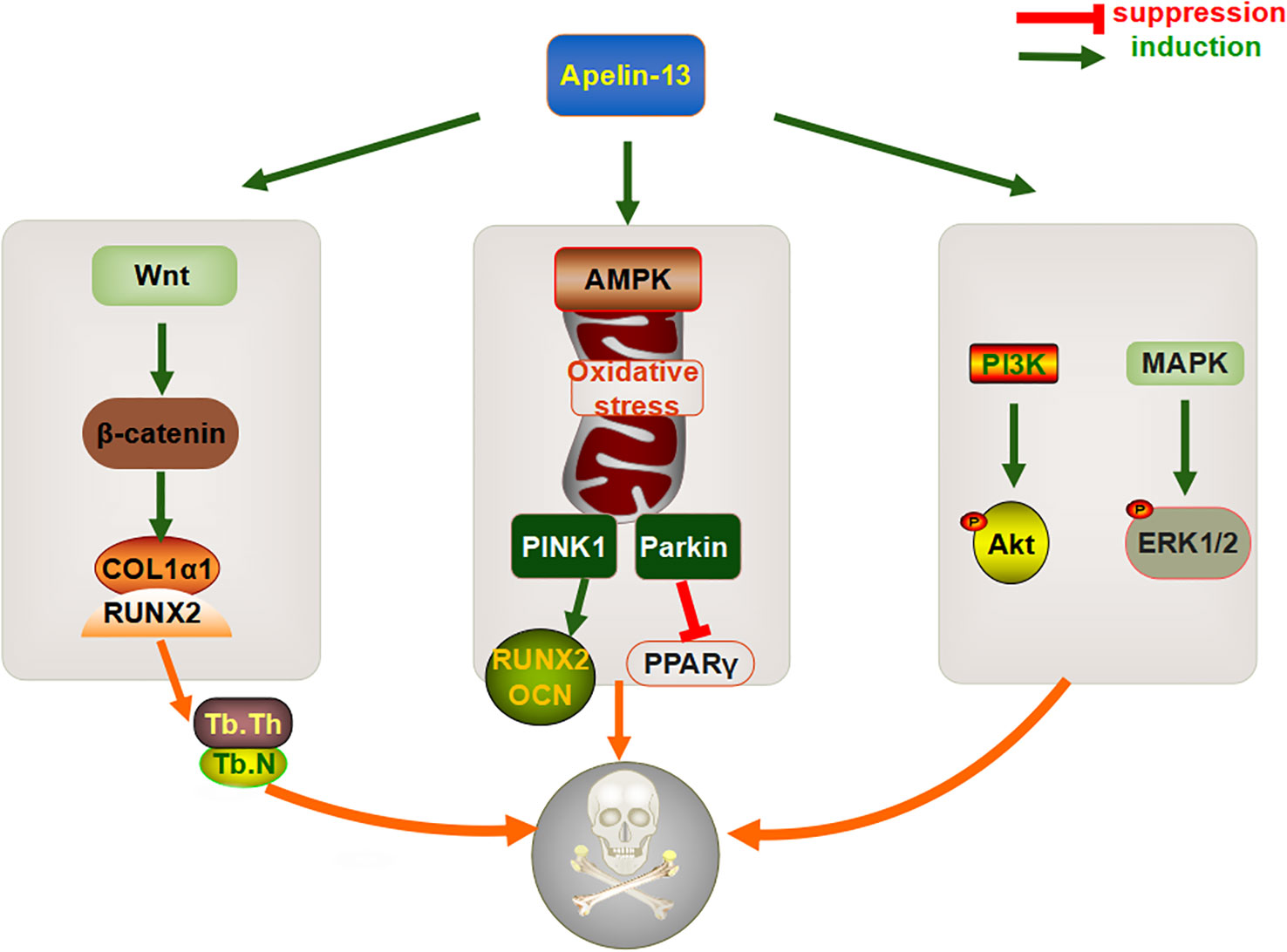
Osteoporosis is characterized by uncoupled bone resorption, resulting in low bone mass, microstructural damage, and structural deterioration, thereby increasing the possibility of a fracture (86). In some individuals, osteoporosis is caused by chronic hyperglycemia, advanced glycation end products, and oxidative stress (87). One study found that the serum levels of Apelin-13 and 25-hydroxyvitamin D3 (25(OH)D3) are significantly lower in patients with osteoporosis than in healthy people (88), suggesting that Apelin-13 might be associated with bone metabolism. Moreover, a clinical trial based on T2DM patients found that the levels of Apelin-13, bone formation sensitive factor procollagen type I N-propeptide (PINP), and bone mineral density (BMD) in the serum of T2DM patients with osteoporosis were significantly lower than those in T2DM patients with normal bone mass, whereas the bone resorption marker type I collagen carboxyterminal telopeptide (ICTP) showed the opposite trend (89). A correlation analysis has also shown that Apelin-13 levels are positively correlated with BMD and PINP levels and negatively correlated with ICTP levels (89). This suggests that Apelin-13 might be useful as a biomarker for monitoring bone metabolism, although the mechanism underlying the interaction between Apelin-13 and bone formation or bone resorption remains unclear.
The increase in bone mass mediated by adipokines is a multifactorial process, involving various pathways that regulate the osteogenic and adipogenic differentiation of BMSCs and bone resorption (90). A large volume of data indicates that multiple signaling pathways, such as Wnt/beta-catenin (β-catenin) and PI3K/Akt, might be directly involved in the regulation of bone metabolic processes (91, 92). Studies have also shown that the total bone marrow cavity area, periosteum, and intracortical bone surfaces are decreased in Apelin-13-deficient mice, whereas the expression levels of collagen type III alpha 1 (Col3α1) and collagen maturation associated genes, namely loxl3 and loxl4, are significantly downregulated (88), suggesting that Apelin-13 might play an important role in bone homeostasis. In vitro experiments revealed that the expression levels of Wnt-target factors, specifically Axin2, CyclinD1, Smad6, and Wisp2, as well as downstream β-catenin, are significantly downregulated in primary bone cells isolated from Apelin-13−/− mice (88), indicating that Apelin-13 could be involved in regulating signaling via the canonical Wnt pathway to prevent osteoporosis. Similarly, the exogenous injection of Apelin-13 was found to reduce the gap distance of cortical bone defects in a rat tibial osteotomy model and increase the number and thickness of trabeculae to accelerate bone healing. Further, in vitro studies found that the administration of recombinant Apelin-13 to human BMSCs upregulates expression levels of the osteogenesis-related gene Col1α1 and the runt-related transcription factor 2 (Runx2) (12). In terms of the underlying mechanism, Apelin-13 might upregulate the levels and activity of total β-catenin in cells, which is then eliminated when an appropriate concentration of DKK1, an effective inhibitor of Wnt/β-catenin signaling, is reached. Similar to that with its exogenous expression, the endogenous overexpression of Apelin-13 via a lentiviral vector also increases the expression levels of osteogenesis-related genes, and alkaline phosphatase (ALP) and alizarin red S staining showed that calcium deposition is accelerated under these conditions (12). This suggested that Apelin-13 might promote the osteogenic differentiation of BMSCs via the Wnt/β-catenin signaling pathway.
Mitochondrial autophagy is the main cellular stress response system that damages organelles and protein immunity, and it plays a key role in the differentiation, apoptosis, and survival of BMSCs (93). Liang et al. found that compared with that in wild-type rats, the expression of Apelin-13 and its receptor APJ is significantly downregulated in the distal femur of ovariectomized rats with osteoporosis. Moreover, after H2O2 treatment, PINK1, Parkin, Bax, and cytochrome C (Cyt-C) are increased, which is accompanied by a decrease in the expression of the anti-apoptotic protein Bcl-2. The exogenous injection of Apelin-13 promotes the activation of mitochondrial autophagy proteins (LC3-II, PINK1, and Parkin), thereby alleviating oxidative stress, mitochondrial dysfunction, and apoptosis in BMSCs, restoring bone mass and microstructure, increasing levels of the osteogenic genes RUNX2, COL1α1, and OCN, and downregulating expression of lipid marker PPARγ. In vitro experiments also showed that AMPK-α gene knockout partially suppresses the role of Apelin-13 in mitotic activation and anti-apoptotic mechanisms (94), suggesting that the AMPK pathway serves as an important pathway through which Apelin-13 promotes BMSC proliferation and osteogenic differentiation. This also suggests that Apelin-13 could have an important role in the adipogenic differentiation of BMSCs.
The maintenance of adult bone mass is regulated by the state of the balance between osteoblasts and osteoclasts and changes in their life cycle caused by changes in apoptosis (95), which result in the destruction of at least 60% of osteoblasts (96). Therefore, the regulation of apoptosis in BMSCs, to attenuate bone metabolism disorders, is now being considered as an important means to alleviate osteoporosis. Although the exogenous injection of Apelin-13 significantly decreases serum deprivation-induced apoptosis, mitochondrial depolarization, cytochrome c release, caspase-3 activation, and reactive oxygen species production in a concentration-dependent manner, thereby increasing ERK1/2 phosphorylation and Akt protein expression, these protective effects can be effectively reversed by the PI3K inhibitor wortmannin or the ERK blocker UO126 (97). It has been suggested that Apelin-13 might mediate apoptosis by regulating a variety of signaling pathways. However, the in vitro injection of recombinant Apelin-13 failed to affect the protein expression of p-Akt, p-ERK, and p-JNK in human BMSCs (12); however, the researchers did not further explore these results, which are probably be attributed to different sources of BMSCs and different types of Apelin-13.
In summary, Apelin-13 might affect osteogenic differentiation, based on effects on bone metabolism, directly via the Wnt/β-catenin pathway or indirectly via mitochondrial autophagy and apoptosis (Figure 3). However, its dual regulatory role in bone metabolism and related molecular mechanisms still require more systematic and in-depth research, especially with respect to osteoclasts, to provide a theoretical basis for the development of Apelin-related drugs.
4 Association between Apelin-13 and exercise
To further promote the utility of exercise for the prevention and control of metabolic diseases, it is necessary to study the molecular mechanisms associated with exercise-sensitive factors (98). Exercise can reduce or increase corresponding tissue-specific exercise factors, such as adiponectin (ADPN), irisin, and fibroblast growth factor 21 (FGF21) (99–101),, thereby attenuating a variety of metabolic diseases (102). One study found that peak oxygen consumption and serum Apelin-13 levels in obese men are increased significantly after undergoing resistance training, which involved 55 min of weight training, exercise at 3 days/week for 12 weeks, followed by a rest period of 4 weeks, and intermittent aerobic training, at 80–90% of the maximum heart rate × 4 min, with 3 min intervals between each session, at intervals of 65% of the maximum heart rate. After 4 weeks of training, the subjects maintained Apelin-13 levels, and neither their body fat rate nor their waist circumference returned to the pre-exercise state (17), suggesting that resistance training and intermittent aerobic training could directly affect Apelin-13 expression and promote its increase following exercise. The absence of an obvious difference in serum Apelin-13 levels between obese men and lean subjects at the beginning of the experiment compelled the authors to conclude that Apelin-13 expression in the body is not sufficiently related to the degree of obesity (17). Another study involved a maximum incremental load treadmill test, with a warm-up at 8 km/h for 2 min, followed by an increase to 10 km/h, with the slope maintained at 0% for the first 3 min, which was then increased by 1.5% per min, until the subject was unable to continue (volitional failure), at which time the test was ended. This experiment showed that although the serum level of Apelin-13 in some male professional soccer players was temporarily increased for 30 min after the exercise, in another group, this level decreased to a certain extent and showed individual differences (16). This result might have been related to the test status of each research subject. In addition, the rate at which Apelin-13 increased was found to be positively correlated with the maximum metabolic equivalent, relative maximum oxygen consumption, and maximum circulating power (16), indicating that Apelin-13 could be associated with sports potential, although this type of sports performance might also be due to the adaptation that occurs in professional athletes after long-term training. In conclusion, exercise can significantly upregulate Apelin-13 levels in the serum of male subjects, and these are closely associated with physical function, whole-body oxygen consumption, and cardiac pumping capacity. However, information regarding Apelin-13 levels in the circulation of women appears to be lacking, and the issue of gender dimorphism needs to be studied in relation to Apelin-13.
Most studies have indicated that Apelin-13 might regulate bodily activities via the central nervous system. The chronic intraventricular injection of Apelin-13 (1 μg/day; injection time of more than 24 h) significantly increases the nocturnal activities of female mice (36), suggesting that Apelin-13 might enhance the excitation of mouse motor neuron centers, thereby promoting increased activity. Although Ying et al. reported that the injection of Apelin-13 can enhance the discharge frequency and motor behavior of pale neurons and that unilateral injection can cause contralateral posture deflection in mice, thereby suggesting that Apelin-13 might directly participate in the regulation of limb movement in these animals (103), they did not elaborate on this. In terms of pathological conditions, Apelin-13 injection into the tail vein of male rats with cerebral ischemia was found to prevent NO depletion, thereby reducing neuronal death and the cerebral infarct volume and significantly improving sensorimotor balance defects (104).
In conclusion, different forms of exercise might upregulate the expression of Apelin-13 in serum. Under various physiological and pathological conditions, Apelin-13 could regulate motor centers, promote muscle contraction, and increase bodily activity. This effect implies that it plays a potential role in the neuromuscular system. Apelin-13 might also mediate the increase in energy expenditure during exercise, thereby improving and regulating metabolic diseases. In addition, cross-talk between Apelin-13 and exercise-associated factors, as well as the mechanisms underlying such crosstalk, remains unclear, indicating that the specific role of Apelin-13 in a variety of pathological states and the manifestation of exercise might need further evaluation.
5 Summary and outlook
In conclusion, Apelin-13, which is an adipokine, might play a heterogeneous role in the regulation of different pathological states, such as obesity, diabetes, osteoporosis, and other metabolic diseases. Although injecting Apelin-13 at a physiological level can cause metabolic disorders, under pathological conditions, it can also protect against metabolic diseases by regulating homeostasis via a variety of signaling pathways. Although the current study reviewed a variety of biological functions that involve Apelin-13, complete characterization of the specific mechanisms underlying the function of Apelin-13 has not been achieved. This also requires an in-depth exploration of the dynamic changes in the Apelin-13 regulatory network under different pathological states and the identification of key nodes. In addition, research on the regulatory effects of exercise on Apelin-13 is still in its formative stages. The regulatory effects of different types, intensities, and amounts of exercise on Apelin-13, which result in a protective effect on the body, remain unknown at present. Thus, an in-depth exploration of the multiple regulatory mechanisms associated with Apelin-13 and the development of related clinical therapeutic drugs will provide more new ideas and individualized targets for the prevention and treatment of metabolic diseases.
Author contributions
RW: Writing – original draft. RH: Writing – review & editing. KX: Conceptualization, Investigation, Writing – review & editing. YC: Project administration, Supervision, Writing – review & editing. XY: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research work was funded by the National Natural Science Foundation of China (No. 12072202).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism: Clin Exp (2022) 133:155217. doi: 10.1016/j.metabol.2022.155217
2. Jia W, Wei M, Rajani C, Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell (2021) 12:411–25. doi: 10.1007/s13238-020-00804-9
3. Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology (2021) 160:573–99. doi: 10.1053/j.gastro.2020.10.057
4. Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Yavropoulou MP, Makras P. Postmenopausal osteoporosis coexisting with other metabolic diseases: Treatment considerations. Maturitas (2021) 147:19–25. doi: 10.1016/j.maturitas.2021.02.007
5. Green CD, Maceyka M, Cowart LA, Spiegel S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab (2021) 33:1293–306. doi: 10.1016/j.cmet.2021.06.006
6. Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology (2005) 146:1764–71. doi: 10.1210/en.2004-1427
7. Lin T, Zhao Y, Guo S, Wu Z, Li W, Wu R, et al. Apelin-13 protects neurons by attenuating early-stage postspinal cord injury apoptosis in vitro. Brain Sci (2022) 12. doi: 10.3390/brainsci12111515
8. Gao Z, Zhong X, Tan YX, Liu D. Apelin−13 alleviates diabetic nephropathy by enhancing nitric oxide production and suppressing kidney tissue fibrosis. Int J Mol Med (2021) 48. doi: 10.3892/ijmm.2021.5008
9. Cirillo P, Ziviello F, Pellegrino G, Conte S, Cimmino G, Giaquinto A, et al. The adipokine apelin-13 induces expression of prothrombotic tissue factor. Thromb Haemostasis (2015) 113:363–72. doi: 10.1160/TH14-05-0451
10. Dai L, Smith PM, Kuksis M, Ferguson AV. Apelin acts in the subfornical organ to influence neuronal excitability and cardiovascular function. J Physiol (2013) 591:3421–32. doi: 10.1113/jphysiol.2013.254144
11. Niknazar S, Abbaszadeh HA, Peyvandi H, Rezaei O, Forooghirad H, Khoshsirat S, et al. Protective effect of [Pyr1]-apelin-13 on oxidative stress-induced apoptosis in hair cell-like cells derived from bone marrow mesenchymal stem cells. Eur J Pharmacol (2019) 853:25–32. doi: 10.1016/j.ejphar.2019.03.012
12. Hang K, Ye C, Xu J, Chen E, Wang C, Zhang W, et al. Apelin enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly through Wnt/β-catenin signaling pathway. Stem Cell Res Ther (2019) 10:189. doi: 10.1186/s13287-019-1286-x
13. Enoki Y, Nagai T, Hamamura Y, Osa S, Nakamura H, Taguchi K, et al. The G protein-coupled receptor ligand apelin-13 ameliorates skeletal muscle atrophy induced by chronic kidney disease. J Cachexia Sarcopenia Muscle (2023) 14:553–64. doi: 10.1002/jcsm.13159
14. Wang S, Gao G, He Y, Li Q, Li Z, Tong G. Amidation-modified apelin-13 regulates PPARγ and perilipin to inhibit adipogenic differentiation and promote lipolysis. Bioinorganic Chem Appl (2021) 2021:3594630. doi: 10.1155/2021/3594630
15. Tekin S, Erden Y, Sandal S, Etem Onalan E, Ozyalin F, Ozen H, et al. Effects of apelin on reproductive functions: relationship with feeding behavior and energy metabolism. Arch Physiol Biochem (2017) 123:9–15. doi: 10.1080/13813455.2016.1211709
16. Ligetvári R, Szokodi I, Far G, Csöndör É., Móra Á., Komka Z, et al. Apelin as a potential regulator of peak athletic performance. Int J Mol Sci (2023) 24. doi: 10.3390/ijms24098195
17. Nikseresht M, Hafezi Ahmadi MR, Hedayati M. Detraining-induced alterations in adipokines and cardiometabolic risk factors after nonlinear periodized resistance and aerobic interval training in obese men. Appl Physiology Nutrition Metab = Physiologie Appliquee Nutr Metabolisme (2016) 41:1018–25. doi: 10.1139/apnm-2015-0693
18. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun (1998) 251:471–6. doi: 10.1006/bbrc.1998.9489
19. Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochemistry (2000) 74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x
20. Yang Y, Zhang K, Huang S, Chen W, Mao H, Ouyang X, et al. Apelin-13/APJ induces cardiomyocyte hypertrophy by activating the Pannexin-1/P2X7 axis and FAM134B-dependent reticulophagy. J Cell Physiol (2022) 237:2230–48. doi: 10.1002/jcp.30685
21. Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Sci (New York N.Y.) (2014) 343:1248636. doi: 10.1126/science.1248636
22. Park J, Park MY, Kim Y, Jun Y, Lee U, Oh CM. Apelin as a new therapeutic target for COVID-19 treatment. QJM Monthly J Assoc Physicians (2022). doi: 10.1093/qjmed/hcac229
23. Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem (2000) 275:21061–7. doi: 10.1074/jbc.M908417199
24. Chapman FA, Nyimanu D, Maguire JJ, Davenport AP, Newby DE, Dhaun N. The therapeutic potential of apelin in kidney disease. Nat Rev Nephrol (2021) 17:840–53. doi: 10.1038/s41581-021-00461-z
25. Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther (2005) 107:198–211. doi: 10.1016/j.pharmthera.2005.04.001
26. Warner FJ, Smith AI, Hooper NM, Turner AJ. Angiotensin-converting enzyme-2: a molecular and cellular perspective. Cell Mol Life Sci CMLS (2004) 61:2704–13. doi: 10.1007/s00018-004-4240-7
27. Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, McLean B, et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates pyr-apelin-13 and apelin-17: physiological effects in the cardiovascular system. Hypertension (Dallas Tex. 1979) (2016) 68:365–77. doi: 10.1161/HYPERTENSIONAHA.115.06892
28. de Oliveira AA, Vergara A, Wang X, Vederas JC, Oudit GY. Apelin pathway in cardiovascular, kidney, and metabolic diseases: Therapeutic role of apelin analogs and apelin receptor agonists. Peptides (2022) 147:170697. doi: 10.1016/j.peptides.2021.170697
29. Respekta N, Pich K, Dawid M, Mlyczyńska E, Kurowska P, Rak A. The apelinergic system: apelin, ELABELA, and APJ action on cell apoptosis: anti-apoptotic or pro-apoptotic effect? Cells (2022) 12. doi: 10.3390/cells12010150
30. Lv X, Kong J, Chen WD, Wang YD. The role of the apelin/APJ system in the regulation of liver disease. Front Pharmacol (2017) 8:221. doi: 10.3389/fphar.2017.00221
31. Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta (2001) 1538:162–71. doi: 10.1016/S0167-4889(00)00143-9
32. Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochemistry (2003) 84:1162–72. doi: 10.1046/j.1471-4159.2003.01587.x
33. Duquenne M, Folgueira C, Bourouh C, Millet M, Silva A, Clasadonte J, et al. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Nat Metab (2021) 3:1071–90. doi: 10.1038/s42255-021-00432-5
34. Imbernon M, Saponaro C, Helms HCC, Duquenne M, Fernandois D, Deligia E, et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab (2022) 34:1054–1063.e7. doi: 10.1016/j.cmet.2022.06.002
35. Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients (2019) 11. doi: 10.3390/nu11112704
36. Valle A, Hoggard N, Adams AC, Roca P, Speakman JR. Chronic central administration of apelin-13 over 10 days increases food intake, body weight, locomotor activity and body temperature in C57BL/6 mice. J Neuroendocrinol (2008) 20:79–84. doi: 10.1111/j.1365-2826.2007.01617.x
37. Clarke KJ, Whitaker KW, Reyes TM. Diminished metabolic responses to centrally-administered apelin-13 in diet-induced obese rats fed a high-fat diet. J Neuroendocrinol (2009) 21:83–9. doi: 10.1111/j.1365-2826.2008.01815.x
38. Lv SY, Yang YJ, Qin YJ, Mo JR, Wang NB, Wang YJ, et al. Central apelin-13 inhibits food intake via the CRF receptor in mice. Peptides (2012) 33:132–8. doi: 10.1016/j.peptides.2011.11.011
39. Yuan Y, Wang W, Zhang Y, Hong Q, Huang W, Li L, et al. Apelin-13 attenuates lipopolysaccharide-induced inflammatory responses and acute lung injury by regulating PFKFB3-driven glycolysis induced by NOX4-dependent ROS. J Inflammation Res (2022) 15:2121–39. doi: 10.2147/JIR.S348850
40. Xia F, Chen H, Jin Z, Fu Z. Apelin-13 protects the lungs from ischemia-reperfusion injury by attenuating inflammatory and oxidative stress. Hum Exp Toxicol (2021) 40:685–94. doi: 10.1177/0960327120961436
41. Xu W, Li T, Gao L, Zheng J, Yan J, Zhang J, et al. Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J Neuroinflamm (2019) 16:247. doi: 10.1186/s12974-019-1620-3
42. Luo H, Xiang Y, Qu X, Liu H, Liu C, Li G, et al. Apelin-13 suppresses neuroinflammation against cognitive deficit in a streptozotocin-induced rat model of alzheimer's disease through activation of BDNF-TrkB signaling pathway. Front Pharmacol (2019) 10:395. doi: 10.3389/fphar.2019.00395
43. Liu W, Niu F, Sha H, Liu LD, Lv ZS, Gong WQ, et al. Apelin-13/APJ system delays intervertebral disc degeneration by activating the PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci (2020) 24:2820–8. doi: 10.26355/eurrev_202003_20643
44. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell (2011) 147:728–41. doi: 10.1016/j.cell.2011.10.026
45. Jiang W, Zhao P, Zhang X. Apelin Promotes ECM Synthesis by Enhancing Autophagy Flux via TFEB in Human Degenerative NP Cells under Oxidative Stress. BioMed Res Int (2020) 2020:4897170. doi: 10.1155/2020/4897170
46. Shao ZQ, Dou SS, Zhu JG, Wang HQ, Wang CM, Cheng BH, et al. Apelin-13 inhibits apoptosis and excessive autophagy in cerebral ischemia/reperfusion injury. Neural Regeneration Res (2021) 16:1044–51. doi: 10.4103/1673-5374.300725
47. Wang C, Liu N, Luan R, Li Y, Wang D, Zou W, et al. Apelin protects sarcoplasmic reticulum function and cardiac performance in ischaemia-reperfusion by attenuating oxidation of sarcoplasmic reticulum Ca2+-ATPase and ryanodine receptor. Cardiovasc Res (2013) 100:114–24. doi: 10.1093/cvr/cvt160
48. Tham KW, Abdul Ghani R, Cua SC, Deerochanawong C, Fojas M, Hocking S, et al. Obesity in South and Southeast Asia-A new consensus on care and management. Obes Rev Off J Int Assoc Study Obes (2023) 24:e13520. doi: 10.1111/obr.13520
49. Marcelin G, Gautier EL, Clément K. Adipose tissue fibrosis in obesity: etiology and challenges. Annu Rev Physiol (2022) 84:135–55. doi: 10.1146/annurev-physiol-060721-092930
50. He S, Ryu J, Liu J, Luo H, Lv Y, Langlais PR, et al. LRG1 is an adipokine that mediates obesity-induced hepatosteatosis and insulin resistance. J Clin Invest (2021) 131. doi: 10.1172/JCI148545
51. Zorena K, Jachimowicz-Duda O, Ślęzak D, Robakowska M, Mrugacz M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21103570
52. Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest (2019) 129:4041–9. doi: 10.1172/JCI129193
53. Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and obesity: role and clinical implication. Front Endocrinol (2021) 12:585887. doi: 10.3389/fendo.2021.585887
54. Ayton SL, Gulsin GS, McCann GP, Moss AJ. Epicardial adipose tissue in obesity-related cardiac dysfunction. Heart (British Cardiac Society) (2022) 108:339–44. doi: 10.1136/heartjnl-2020-318242
55. Wu Y, Zhang A, Hamilton DJ, Deng T. Epicardial fat in the maintenance of cardiovascular health. Methodist DeBakey Cardiovasc J (2017) 13:20–4. doi: 10.14797/mdcj-13-1-20
56. González N, Moreno-Villegas Z, González-Bris A, Egido J, Lorenzo Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol (2017) 16:44. doi: 10.1186/s12933-017-0528-4
57. Toczylowski K, Hirnle T, Harasiuk D, Zabielski P, Lewczuk A, Dmitruk I, et al. Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J Trans Med (2019) 17:310. doi: 10.1186/s12967-019-2060-7
58. Chaurasia B, Summers SA. Ceramides in metabolism: key lipotoxic players. Annu Rev Physiol (2021) 83:303–30. doi: 10.1146/annurev-physiol-031620-093815
59. Lebeck J, Søndergaard E, Nielsen S. Increased AQP7 abundance in skeletal muscle from obese men with type 2 diabetes. Am J Physiol Endocrinol Metab (2018) 315:E367–e373. doi: 10.1152/ajpendo.00468.2017
60. Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci United States America (2005) 102:10993–8. doi: 10.1073/pnas.0503291102
61. Guo M, Chen F, Lin T, Peng Y, Li W, Zhu X, et al. Apelin-13 decreases lipid storage in hypertrophic adipocytes in vitro through the upregulation of AQP7 expression by the PI3K signaling pathway. Med Sci Monitor Int Med J Exp And Clin Res (2014) 20:1345–52. doi: 10.12659/MSM.890124
62. Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Bioscience Rep (2019) 39. doi: 10.1042/BSR20180992
63. Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol (2021) 18:1106–21. doi: 10.1038/s41423-020-00630-3
64. Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clément K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest (2019) 129:4032–40. doi: 10.1172/JCI129192
65. Wang L, Wang S, Shi Y, Li R, Günther S, Ong YT, et al. YAP and TAZ protect against white adipocyte cell death during obesity. Nat Commun (2020) 11:5455. doi: 10.1038/s41467-020-19229-3
66. Li Q, Hagberg CE, Silva Cascales H, Lang S, Hyvönen MT, Salehzadeh F, et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat Med (2021) 27:1941–53. doi: 10.1038/s41591-021-01501-8
67. Li Z, Wang S, He Y, Li Q, Gao G, Tong G. Regulation of apelin-13 on bcl-2 and caspase-3 and its effects on adipocyte apoptosis. Evidence-Based Complementary Altern Med eCAM (2021) 2021:1687919. doi: 10.1155/2021/1687919
68. Ntessalen M, Procter NEK, Schwarz K, Loudon BL, Minnion M, Fernandez BO, et al. Inorganic nitrate and nitrite supplementation fails to improve skeletal muscle mitochondrial efficiency in mice and humans. Am J Clin Nutr (2020) 111:79–89. doi: 10.1093/ajcn/nqz245
69. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol (2020) 16:377–90. doi: 10.1038/s41581-020-0278-5
70. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care (2023) 46:S19–s40. doi: 10.2337/dc23-S002
71. LeRoith D, Biessels GJ, Braithwaite SS, Casanueva FF, Draznin B, Halter JB, et al. Treatment of diabetes in older adults: an endocrine society* Clinical practice guideline. J Clin Endocrinol Metab (2019) 104:1520–74. doi: 10.1210/jc.2019-00198
72. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
73. Li M, Fang H, Hu J. Apelin−13 ameliorates metabolic and cardiovascular disorders in a rat model of type 2 diabetes with a high−fat diet. Mol Med Rep (2018) 18:5784–90. doi: 10.3892/mmr.2018.9607
74. Sabry MM, Mahmoud MM, Shoukry HS, Rashed L, Kamar SS, Ahmed MM. Interactive effects of apelin, renin-angiotensin system and nitric oxide in treatment of obesity-induced type 2 diabetes mellitus in male albino rats. Arch Physiol Biochem (2019) 125:244–54. doi: 10.1080/13813455.2018.1453521
75. Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, et al. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev (2018) 98:505–53. doi: 10.1152/physrev.00023.2016
76. Feng J, Zhao H, Du M, Wu X. The effect of apelin-13 on pancreatic islet beta cell mass and myocardial fatty acid and glucose metabolism of experimental type 2 diabetic rats. Peptides (2019) 114:1–7. doi: 10.1016/j.peptides.2019.03.006
77. Menendez-Montes I, Abdisalaam S, Xiao F, Lam NT, Mukherjee S, Szweda LI, et al. Mitochondrial fatty acid utilization increases chromatin oxidative stress in cardiomyocytes. Proc Natl Acad Sci United States America (2021) 118. doi: 10.1073/pnas.2101674118
78. Cortassa S, Caceres V, Tocchetti CG, Bernier M, de Cabo R, Paolocci N, et al. Metabolic remodelling of glucose, fatty acid and redox pathways in the heart of type 2 diabetic mice. J Physiol (2020) 598:1393–415. doi: 10.1113/JP276824
79. Regueira M, Riera MF, Galardo MN, Pellizzari EH, Cigorraga SB, Meroni SB. Activation of PPAR α and PPAR β/δ regulates Sertoli cell metabolism. Mol Cell Endocrinol (2014) 382:271–81. doi: 10.1016/j.mce.2013.10.006
80. Quarta C, Stemmer K, Novikoff A, Yang B, Klingelhuber F, Harger A, et al. GLP-1-mediated delivery of tesaglitazar improves obesity and glucose metabolism in male mice. Nat Metab (2022) 4:1071–83. doi: 10.1038/s42255-022-00617-6
81. Tao J, Chen H, Wang YJ, Qiu JX, Meng QQ, Zou RJ, et al. Ketogenic diet suppressed T-regulatory cells and promoted cardiac fibrosis via reducing mitochondria-associated membranes and inhibiting mitochondrial function. Oxid Med Cell Longevity (2021) 2021:5512322. doi: 10.1155/2021/5512322
82. Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation (2003) 108:1432–9. doi: 10.1161/01.CIR.0000091235.94914.75
83. Chen H, Zheng C, Zhang X, Li J, Li J, Zheng L, et al. Apelin alleviates diabetes-associated endoplasmic reticulum stress in the pancreas of Akita mice. Peptides (2011) 32:1634–9. doi: 10.1016/j.peptides.2011.06.025
84. Zheng XD, Huang Y, Li H. Regulatory role of Apelin-13-mediated PI3K/AKT signaling pathway in the glucose and lipid metabolism of mouse with gestational diabetes mellitus. Immunobiology (2021) 226:152135. doi: 10.1016/j.imbio.2021.152135
85. Karagoz ZK, Aydin S, Ugur K, Tigli A, Deniz R, Baykus Y, et al. Molecular communication between Apelin-13, Apelin-36, Elabela, and nitric oxide in gestational diabetes mellitus. Eur Rev Med Pharmacol Sci (2022) 26:3289–300. doi: 10.26355/eurrev_202205_28748
86. Wu D, Cline-Smith A, Shashkova E, Perla A, Katyal A, Aurora R. T-cell mediated inflammation in postmenopausal osteoporosis. Front Immunol (2021) 12:687551. doi: 10.3389/fimmu.2021.687551
87. Rabe OC, Winther-Jensen M, Allin KH, Svendsen OL. Fractures and osteoporosis in patients with diabetes with charcot foot. Diabetes Care (2021) 44:2033–8. doi: 10.2337/dc21-0369
88. Han XF, Zhang XX, Liu KM, Zhang Q. Apelin-13 deficiency alters cortical bone geometry, organic bone matrix, and inhibits Wnt/β-catenin signaling. Gen Comp Endocrinol (2018) 267:29–35. doi: 10.1016/j.ygcen.2018.05.024
89. Liu S, Wang W, Yin L, Zhu Y. Influence of Apelin-13 on osteoporosis in Type-2 diabetes mellitus: A clinical study. Pakistan J Med Sci (2018) 34:159–63. doi: 10.12669/pjms.341.14135
90. Wang ZX, Luo ZW, Li FX, Cao J, Rao SS, Liu YW, et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun (2022) 13:1453. doi: 10.1038/s41467-022-29191-x
91. Shen G, Ren H, Shang Q, Zhao W, Zhang Z, Yu X, et al. Foxf1 knockdown promotes BMSC osteogenesis in part by activating the Wnt/β-catenin signalling pathway and prevents ovariectomy-induced bone loss. EBioMedicine (2020) 52:102626. doi: 10.1016/j.ebiom.2020.102626
92. Zhao SJ, Kong FQ, Jie J, Li Q, Liu H, Xu AD, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics (2020) 10:17–35. doi: 10.7150/thno.36930
93. Zhang S, Xie Y, Yan F, Zhang Y, Yang Z, Chen Z, et al. Negative pressure wound therapy improves bone regeneration by promoting osteogenic differentiation via the AMPK-ULK1-autophagy axis. Autophagy (2022) 18:2229–45. doi: 10.1080/15548627.2021.2016231
94. Chen L, Shi X, Xie J, Weng SJ, Xie ZJ, Tang JH, et al. Apelin-13 induces mitophagy in bone marrow mesenchymal stem cells to suppress intracellular oxidative stress and ameliorate osteoporosis by activation of AMPK signaling pathway. Free Radical Biol Med (2021) 163:356–68. doi: 10.1016/j.freeradbiomed.2020.12.235
95. Li Z, Li D, Chen R, Gao S, Xu Z, Li N. Cell death regulation: A new way for natural products to treat osteoporosis. Pharmacol Res (2023) 187:106635. doi: 10.1016/j.phrs.2022.106635
96. Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone And Mineral Res Off J Am Soc Bone Mineral Res (2007) 22:1492–501. doi: 10.1359/jbmr.070518
97. Zeng X, Yu SP, Taylor T, Ogle M, Wei L. Protective effect of apelin on cultured rat bone marrow mesenchymal stem cells against apoptosis. Stem Cell Res (2012) 8:357–67. doi: 10.1016/j.scr.2011.12.004
98. Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol (2022) 18:273–89. doi: 10.1038/s41574-022-00641-2
99. Khalafi M, Hossein Sakhaei M, Kheradmand S, Symonds ME, Rosenkranz SK. The impact of exercise and dietary interventions on circulating leptin and adiponectin in individuals who are overweight and those with obesity: A systematic review and meta-analysis. Adv Nutr (Bethesda Md.) (2023) 14:128–46. doi: 10.1016/j.advnut.2022.10.001
100. Bao JF, She QY, Hu PP, Jia N, Li A. Irisin, a fascinating field in our times. Trends Endocrinol Metabolism: TEM (2022) 33:601–13. doi: 10.1016/j.tem.2022.06.003
101. Jin L, Geng L, Ying L, Shu L, Ye K, Yang R, et al. FGF21-sirtuin 3 axis confers the protective effects of exercise against diabetic cardiomyopathy by governing mitochondrial integrity. Circulation (2022) 146:1537–57. doi: 10.1161/CIRCULATIONAHA.122.059631
102. Porflitt-Rodríguez M, Guzmán-Arriagada V, Sandoval-Valderrama R, Tam CS, Pavicic F, Ehrenfeld P, et al. Effects of aerobic exercise on fibroblast growth factor 21 in overweight and obesity. A Systematic Review. Metabolism: Clin Exp (2022) 129:155137. doi: 10.1016/j.metabol.2022.155137
103. Wang Y, Xue Y, Liu C, Chen L. Apelin-13 regulates electrical activity in the globus pallidus and induces postural changes in rats. Neural Regeneration Res (2021) 16:2264–8. doi: 10.4103/1673-5374.310694
Keywords: adipokine, Apelin-13, adipose tissue, metabolic disease, exercise
Citation: Wen R, Huang R, Xu K, Cheng Y and Yi X (2023) Beneficial effects of Apelin-13 on metabolic diseases and exercise. Front. Endocrinol. 14:1285788. doi: 10.3389/fendo.2023.1285788
Received: 30 August 2023; Accepted: 13 November 2023;
Published: 28 November 2023.
Edited by:
Junhao Huang, Guangzhou Sport University, ChinaReviewed by:
Oscar Lorenzo, Health Research Institute Foundation Jimenez Diaz (IIS-FJD), SpainSøren Madsen, The University of Sydney, Australia
Copyright © 2023 Wen, Huang, Xu, Cheng and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuejie Yi, yixuejie8387@163.com
†These authors have contributed equally to this work and share first authorship
 Ruiming Wen
Ruiming Wen Ruiqi Huang2†
Ruiqi Huang2† Ke Xu
Ke Xu Xuejie Yi
Xuejie Yi