The Green Roof Microbiome: Improving Plant Survival for Ecosystem Service Delivery
- 1Department of Biological Sciences, University of Toronto Scarborough, Toronto, ON, Canada
- 2Department of Physical and Environmental Sciences, University of Toronto Scarborough, Toronto, ON, Canada
- 3Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, ON, Canada
- 4State Key Laboratory of Biocontrol, College of Ecology and Evolution, Sun Yat-sen University, Guangzhou, China
- 5School of Earth, Environmental and Biological Sciences, Queensland University of Technology, Brisbane, QLD, Australia
Plants are key contributors to ecosystem services delivered by green roofs in cities including stormwater capture, temperature regulation, and wildlife habitat. As a result, current research has primarily focused on their growth in relationship to extensive green roof (e.g., substrates <15 cm depth) ecosystem services. Green roofs are exposed to a variety of harsh abiotic factors such as intense solar radiation, wind, and isolation from ground-level habitats, making survival exceedingly difficult. Plants in natural habitats benefit from a variety of interactions with fungi and bacteria. These plant-microbial interactions improve mechanisms of survival and productivity; however, many green roof substrates are sterilized prior to installation and lack microbial communities with unstudied consequences for green roof plant health and subsequent survival and performance. In this paper, we present six hypotheses on the positive role of microbes in green roof applications. In natural and experimental systems, microbial interactions have been linked to plant (1) drought tolerance, (2) pathogen protection, (3) nutrient availability, (4) salt tolerance, (5) phytohormone production, and (6) substrate stabilization, all of which are desirable properties of green roof ecosystems. As few studies exist that directly examine these relationships on green roofs, we explore the existing ecological literature on these topics to unravel the mechanisms that could support more complex green roof ecosystem and lead to new insight into the design, performance, and broader applications in green infrastructure.
Introduction
Microorganisms, including fungi and bacteria, comprise the majority of biodiversity on earth, and therefore, have a significant impact on environmental health through the governance of ecosystem functions relating to bio-geochemical processes (Morin and McGrady-Steed, 2004). In particular, soil microbial communities have been found to directly and indirectly promote aboveground biodiversity by enhancing nutrient pools and regulating plant species dominance (Torsvik and Øvreås, 2002; Van Der Heijden et al., 2008). A wide diversity of microorganisms live and reproduce in plant tissues and in the regions around plant roots. The best studied of them are mycorrhizal fungi, which are known to associate with >82% of all land plants (Linderman, 1988; Brundrett, 2002). Most herbaceous plant species used in horticulture normally associate with one or more species of arbuscular mycorrhizal fungi (AMF). AMF hyphae link the internal tissues of roots with large exterior networks, enabling plants to access enhanced water and nutrient supplies from soil in exchange for simplified hexose and other nutrients (Parke, 1991). Equally as influential are the bacteria that occupy the soil, the area of substrate that interacts with the roots (rhizosphere), and those that live directly inside plant tissues—forming important interactions that are only beginning to be understood. All of these organisms contribute to the plant microbiome and are all largely derived from the physical substrate that plants grow in with some additions over time from the atmosphere and dispersing biota. In nutrient poor ecosystems, soil microorganisms are important regulators of plant productivity because they provide plants with essential nutrients such as nitrogen (Van der Heijden et al., 2008) and phosphorus (Mehnaz and Lazarovits, 2006; Tomasi et al., 2008). Therefore, substrates without microbes are generally nutrient poor and lack the resources optimal for plant growth (Nannipieri et al., 2003).
Plant-associated fungi are diverse and have been shown to underpin plant diversity and productivity in natural ecosystems (Van der Heijden et al., 1998; Zak et al., 2003). One gram of soil may possess up to 200 m fungal hyphae and tens of thousands of species of bacteria (Leake et al., 2004; Roesch et al., 2007). Research has revealed that soil microbial communities vary with plant species and development stage, largely because of the specific and changing nature of plant exudates (Broeckling et al., 2008; Houlden et al., 2008; Prober et al., 2015; Bili et al., 2016). Despite the importance of these biological relationships in natural systems, rarely are microbial communities considered in designed plantings, including applications of urban greening, such as green roofs. McGuire et al. (2015) were perhaps the first to explicitly point out the importance of broad functional groups of bacteria and fungi to green roof ecosystems. In this paper we highlight some of the more specific plant-microbial relationships that are key to green roof development.
Green roofs are comprised of plants and substrate on top of conventional building roofs and are created to provide key ecosystem services in an urban setting (Figure 1). For instance, they are used to cool buildings, improve stormwater management, and create green space in cities where traditionally there would be none (e.g., on rooftops) (Oberndorfer et al., 2007). Extensive green roofs (e.g., substrates < 15 cm depth) are difficult growing environments for plants because they experience a high degree of stress resulting from harsh microclimatic conditions including sun and wind exposure as well as limited growing space (Dunnett and Kingsbury, 2008). They also experience drought conditions frequently as water accumulated following a rain event not lost to runoff quickly dries due to the high rates of evapotranspiration in these exposed environments (Bengtsson et al., 2005). Green roof plant communities are also subject to low nutrient availability (Rowe, 2011). Other site-specific factors and construction details can impact survival, including shading, solar reflectance by windows, or excessive wind scour from nearby or adjacent buildings (Buckland-Nicks et al., 2016).

Figure 1. A green roof comprised of shallow substrate and drought tolerant vegetation at the Berliner Wasserbetriebe in Berlin, Germany (Photo credit: Kelly Ksiazek-Mikenas).
There is growing interest in the biological properties of green roof substrates and their contribution to green roof ecosystem service delivery. Newly installed green roofs have depauperate microbial communities because substrates are sterilized prior to installation or are derived mostly from dry mineral materials (John et al., 2014). This is common practice so that any persistent spontaneous weed seedlings, or other presumed potentially harmful biological agents are removed (McGuire et al., 2013). In many installations compost or commercial inoculants may be added, but there is very little research on the microbial content of the various media formations at the outset and over time. McGuire et al. (2013) surveyed the fungal communities of several green roofs in New York comprised of the same substrate and plant communities. All the fungal communities were less diverse than those found in New York park soils. John et al. (2014) planted bait plants into the growing media used on their experimental roofs to look for fungal colonization. Fresh media (as purchased), unplanted media could only “sparsely” colonize the bait plants. Significant mycorrhizal content was only introduced by green roof plantings themselves—and these had originated in fields. Ondoño et al. (2014) compared compost:brick formulations to compost:soil media and showed the former had far less microbial activity and fewer nutrients than the latter. As more and more research shows, plants derive so many benefits from their microbial associates that they operate as multi-organismal holobionts rather than as isolated individuals (Bordenstein and Theis, 2015), As holobionts they are far better equipped to deal with various environmental stressors (Vandenkoornhuyse et al., 2015). In a sterile substrate, plants will be not be able to form these critical relationships. Green roofs can be blank slates to study the underlying mechanism of plant-microbial relationships that improve growing conditions on green roof ecosystems, such as water and nutrient availability. Without a robust microbial community in the substrate, green roofs might be more susceptible to homogenization due to competition from plants capable of surviving in less microbially active substrates.
In this paper, we discuss six hypotheses that collectively suggest there are positive direct and indirect impacts of microbial activity on green roof plant survival and performance. With the rise of ever improving methods of next generation sequencing, it is possible to determine the diversity and abundance and even some functions of microorganisms within communities (Joynt et al., 2006; Caporaso et al., 2012; Schmidt et al., 2013; Lallias et al., 2015). This encourages us to study the ways in which microbial diversity and abundance can be integrated practically into green roof design and construction. The addition of microbial communities into green roof ecosystems could improve plant (1) drought tolerance, (2) protection from pathogens, (3) access to limiting nutrients, (4) salt tolerance, (5) productivity, and (6) stabilization of the green roof substrate (John et al., 2014; Molineux et al., 2014; Ondoño et al., 2014) (Figure 2). There are a considerable number of research studies on these topics from natural and experimental systems but our knowledge is very limited in how these processes occur on green roofs (John et al., 2017). As a result, we include recommendations for research into practical components of microbial additions into green roof ecosystems including the importance of host plant specificity, the role of diversity and the mode of inoculation.
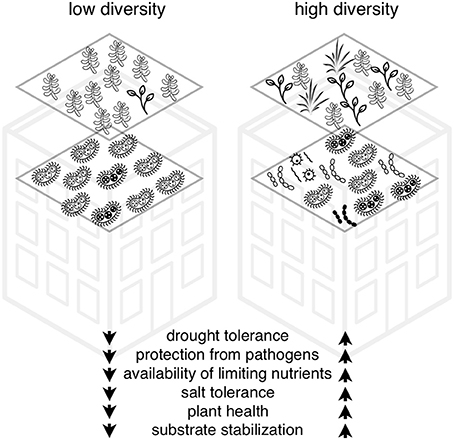
Figure 2. Framework for hypotheses for the role of microbial community diversity on green roof plant survival, diversity, and ecosystem service delivery.
Hypotheses
Hypothesis 1: The Existence of Microbes Increases Green Roof Plant Drought Tolerance
Green roofs experience severe drought conditions which can greatly impact plant survival. This is largely because weight restrictions on green roof substrates dictate that they not be too thick (Berndtsson, 2010) and also because of the inherent lack of shade on roof top systems. On green roofs, water uptake by plant roots declines in efficiency when substrate temperatures increase above 25°C (Sutton et al., 2012). In a study that examined the survival of native perennial wildflowers on extensive green roofs in Michigan, 100% of the plants tested perished due to drought stress (Monterusso et al., 2005). Therefore, drought tolerances dictate planting choices in many cases (Kokkinou et al., 2016; Johannessen et al., 2017; Szota et al., 2017). AMF, an important type of mycorrhiza linking plant and soil nutrients, can alleviate this stress (Smith et al., 2010). These fungi enable plants to have enhanced water and nutrient acquisition with the substrate in exchange for simplified hexose and other nutrients (Parke, 1991), thereby reducing drought stress on the plant communities through a variety of mechanisms (Rillig, 2004; Ruiz-Lozano et al., 2012). AMF hyphae access more substrate by improving organic substrate aggregation through physical binding and conditions for plant root systems (Auge, 2004). AMF penetrate into the soil in natural systems—both vertically and horizontally—in search of water which is then made accessible to the plant roots, permitting plants to form dense patches and survive periods of inundation and drought. Utilizing water more effectively, AMF hyphae should increase the water holding capacity of the substrate and therefore the green roof substrate for the next rain event. In one study, Allen et al. (1980) found that Bouteloua gracilis (Willd. ex Kunth) Lag. ex Griffiths transpiration rates increased 100% and showed no signs of stress during periods of drought when in symbiosis with AMF vs. without. Grasses in the genus Bouteloua are regularly used on green roofs but often planted into sterilized green roof substrate without any consideration of their potential relationships with AMF (Sutton et al., 2012).
In addition to fungi, both rhizospheric and endophytic bacteria can also alleviate drought stress in host plants. Improved drought resilience via inoculation with specific bacteria has been documented in several species, including the common bean (Figueiredo et al., 2008), mung bean (Sarma and Saikia, 2014), maize (Sandhya et al., 2010; Naveed et al., 2014), switchgrass (Wang et al., 2016), broom grass (Brachypodium distachyon) (Gagné-Bourque et al., 2015), and timothy grass (Gagné-Bourque et al., 2016). Our understanding of the mechanisms involved in bacterially-induced drought resilience are not as advanced as for AMF, but the production of compounds that affect osmosis in plants, protection from oxidative stress and changes in plant gene expression have been shown to play important roles (Naveed et al., 2014; Gagné-Bourque et al., 2016). These findings have received no attention in the design of green roofs.
Hypothesis 2: Microbial Associations Increases Plant Protection from Pathogens
Plant health, and consequently, green roof performance, is strongly affected by the interplay between beneficial and pathogenic microorganisms. Plant communities on green roofs may be more susceptible to pathogens present in the urban environment, because of persistent stressful conditions or the high density and low diversity of plantings typical of green roof systems The suppression of pathogenic disease and reduced infection rates in plants has been linked to increased microbial activity (Garbeva et al., 2004) and AMF relations (Bodker et al., 1998). Wehner et al. (2010) examined the role of AMF diversity in substrates on plant pathogenic protection and found few studies illustrating an impact; however, green roofs as “designed experiments” could provide model systems to manipulate these relationships to determine functional roles and additional ecosystem service delivery.
Rhizospheric and endophytic bacteria are frequently phyto-protective and lessen levels of attack from pathogenic bacteria and fungi (Van Loon et al., 1998). Some protective bacteria act through the induction of a general plant systemic immunity, and others via the direct production of antibiotic chemicals. For example, Actinobacteria are well-known for antibiotic production and among most commonly encountered members of endophytic bacterial communities (Rosenblueth and Martínez-Romero, 2006) The production of secondary metabolites produced by plant-associated Actinobacteria are currently understudied (Brader et al., 2014). Some microbes can also lessen the amount of volatile organic compounds emitted that are used by herbivores and parasites to orient toward host plants (e.g., the green roof vegetation) (D'Aleessandro et al., 2014).
More work is needed to investigate these relationships as the augmentation of bacteria populations overall might inadvertently increase the likelihood of a negative association being formed (e.g., accidentally adding microbes that have negative plant associations). Very little is known about the presence or abundance of bacterial species on green roofs vertically isolated from ground level (Molineux et al., 2015). Therefore, a lack of maintenance (e.g., inoculation) could mean that bacterial communities could take longer to develop if left to accumulate naturally over time.
Hypothesis 3: Microbial Diversity Increases the Availability of Limiting Nutrients
Green roofs are limited in both macro- and micro-nutrients required for the growth of most plant species (Ampim et al., 2010). This can generally result in reduced plant growth and the need to manually fertilize or re-plant, adding unknown maintenance-associated costs to a constructed ecosystem that must appease client expectations for how it should appear and function (Rowe, 2011).
For green roofs in temperate climates, newly mixed substrates during installation contribute the phosphorus required for plant growth (Whittinghill and Rowe, 2012). However, phosphorus needs to be converted to a usable form, it becomes readily available because plants, fungi and bacteria alike produce numerous types of phosphatase enzymes that can release phosphate from the substrate (Whitelaw, 1999). Hyphal networks created by mycorrhizal fungi allow them extend further into the soil, where phosphates can be taken up and translocated to the root tissues (Bolan, 1991). One potential issue is that an imbalance of solubilized phosphate and plant uptake may occur, resulting in excess phosphate running off of the green roof after significant storm events. This is known to occur with green roofs receiving compost or fertilizers during set up (Berndtsson, 2010); however, phosphorus leaching is rare in natural ecosystems.
Nitrogen is often limiting on green roofs and microbial communities are responsible for improving its availability. Johnson et al. (2016) showed that plant richness on green roofs increases nitrogen sequestration and reduced runoff. This increase could be mediated by an increase in AMF abundance and/or diversity. Bacteria are also critical for nitrogen availability. For example, rhizobial nodule-forming bacteria of legumes (Proteobacteria such as Azorhizobium, Bradyrhizobium, Rhizobium, Ensifer, Mesorhizobium) reduce atmospheric nitrogen to ammonia, allowing plants to thrive in systems limited by sources of nitrogen and to enrich their surroundings (De Meyer et al., 2016; Lemaire et al., 2016). In addition, several other types of nitrogen fixing bacteria exist in association with different plant groups. Actinorhizal bacteria such as Frankia species form nodules with a wide variety of non-leguminous trees and shrubs and few herbaceous plants (e.g., Datisca sp.) (Swensen, 1996). Non-nodulating diazotrophs have also been found to be active within the tissues of certain grasses and responsible for nitrogen fixation (Santi et al., 2013). For example, Burkholderia sp. are common non-nodulating nitrogen fixing endophytes (Estrada-De Los Santos et al., 2001) and if inoculated into green roof substrates or transported in horticultural plant stock could make green roof systems more habitable for a wider range of plant species.
Availability of organic matter on green roofs can also have a significant impact on plant growth but many green roofs are designed with lightweight minerals and inorganic aggregates (Hill et al., 2016). The decomposition of plant biomass on green roofs through microbial mineralization can be a significant source of organic matter for plants (Buffam and Mitchell, 2015) and could curb reliance on supplemental fertilizers (Ampim et al., 2010). Both above and below ground plant litter is decomposed by a great number of bacteria and fungi and the type, volume, and chemical composition of plant litter and humus can significantly influence the microbial community (Lindahl et al., 2007; Fulthorpe et al., 2008). Numerous saprophytic fungi are responsible for carbon mineralization by decomposing freshly fallen litter, and AMF also contribute to nutrient cycling from organic matter (Cheng et al., 2012; Paterson et al., 2016). Distributed at the underlying soil horizon, mycorrhizal fungi mobilize nitrogen of decomposed litter and humus and deliver them to their host plants. However, excess biomass from grasses and forbs is often removed during maintenance on green roofs to minimize risk of fire (Monterusso et al., 2005). Manipulating litter levels and composition on green roofs to examine the impact on decomposition resulting from microbial communities and surveying the respective communities for the most effective species could lead to improved maintenance strategies and ecosystem service delivery.
Hypothesis 4: Microbial Associations Can Increase Salt Tolerance of Green Roof Plants
Many non-halophilic plant species subjected to saline (NaCl) conditions experience stress and reduced growth rates (Marsalek, 2003; Kaushal et al., 2005). Salt loads on green roofs are of concern in coastal areas, but also potentially in temperate urban regions where the atmospheric resuspension of deicing salt on roads is a real phenomenon (Nicholson and Branson, 1990). Numerous bacteria have been demonstrated to improve plant growth under salt stress when used as soil inoculants (Yang et al., 2009; Egamberdieva and Legtenberg, 2014). For instance, Ashraf et al. (2006) detail the impact of six exopolysaccharide-producing bacteria on wheat growth after inoculated to naturally saline root zone soils. The authors found that the inoculation improved root growth, protection from sodium ions, and nutrient acquisition, all of which would improve plant health and subsequent green roof ecosystem service delivery.
Endophytic bacteria can also reduce the impacts of excess salts in substrates on plant growth (Mayak et al., 2004). Many plants cannot tolerate salt levels higher that 30 mM, but one study showed that the endophyte Bachybacterium paraconglomeratum improved growth of the annual herb, Chlorophytum borivilianum experiencing 150 mM salt stress conditions (Barnawal et al., 2016). Akhtar et al. (2015) found that inoculants of Burkholderia phytofirmans PsJN and Enterobacter FD17 improved maize growth in salt conditions (25 mM NaCl) relative to non-inoculated stressed controls, exhibiting higher photosynthetic rates, stomatal conductance, and reduced sodium uptake. Nabti et al. (2007) reported restoration of wheat growth under stress from 150 to 200 mM NaCl after seed inoculation with a halophilic strain of Azospirillum brasilense isolated from saline soil. Ali et al. (2014) investigated the impact of Pseudomonas fluorescens and P. migulae endophytes on the ability of tomatoes to withstand up to 185 mM NaCl. Both of these strains allowed growth under these extremely stressful conditions, at least partially through the suppression of ethylene. Needless to say, there are many directions for research into microbial species and communities to improve stressful conditions for plants experiencing salt contamination on green roofs and other forms of green infrastructure, such as roadside water retention ponds. The need for this research is expected to increase with increasing sea level rise and a dependence on de-salinized water for irrigation and other non-potable uses in green infrastructure.
Hypothesis 5: Microbial Diversity Will Increase Overall Plant Health
The impact that microbial associates have on plant growth and resistance to various stress is now just being realized, as more endophytic organisms are being studied. Both fungal and bacterial endophytes and inhabitants of the rhizosphere are capable of a producing a variety of phytohormones—auxins, gibberillins, cytokinins, absisic acid, to name a few (Allen et al., 1980; Ali et al., 2017). These plant growth stimulators affect productivity and other processes (Costacurta and Vanderleyden, 1995; Yang et al., 2009).
The difference that microbial associates can make to plant growth is not trivial. Tomato plants inoculated with Sphingomonas sp. LK11 were compared to controls grown in sterilized horticulture media supplemented with water or nutrient broth (Khan et al., 2014). LK11 was originally isolated from Tephrosia appolinea—a dryland legume studied for its wide range of secondary metabolites. Tomato plants inoculated with LK11 exhibited 41, 37, and 14.5% increases in shoot length, dry weights, and chlorophyll contents relative to nutrient broth controls. The effect was attributed to the production of physiologically active gibberillins and indole acetic acids, both of which stimulate plant growth by the bacteria. In another example, Paecilomyces formosus, isolated from cucumber plants, produces giberillins and IAA among other compounds. It was tested for the growth stimulation of japonica rice under normal and heat stress conditions. Waqas et al. (2015) report increases in dry weight of 35 and 47% respectively, and attribute these effects to phytohormones and nutrient effect. For many food plants grown on green roofs where agricultural yield is important function of the plants selected (Whittinghill et al., 2013), microbial diversity and abundance will be important for crop protection from pests and environmental stress.
Particular relevant to plant recovery from various stress is the role that many bacteria play in changing ethylene production levels. Plants produce ethylene throughout their lives to regulate key developmental processes, and ethylene production integrates inputs from environmental stresses and hormones alike (Ecker, 1995). Many microbes can capture 1-aminocyclopropane-1-carbozylate (ACC) and convert it into alpha-ketogutyrate and ammonia, in doing so they interfere with the plants own stress response. This prevents the growth inhibition that would normally follow from a variety of both biotic and abiotic impediments (Glick, 1995; Glick et al., 1998; Santoyo et al., 2016). Phytohormones are also used by plants to reduce damage from insect feeding (Shikano et al., 2017).
Hypothesis 6: Microbial Activity Increases Substrate Stabilization
Newly planted green roofs are susceptible to high winds that can cause substrate to blow off a roof, negatively impacting plant health by increasing water evaporation, exposing roots, and decreasing substrate depth. Moreover, substrate stabilization is important on sloped roofs where gravity leads to substrate loss over time, exposing plants at higher points on the roof. In natural systems, microbial communities play a significant role in stabilizing soil communities (Lützow et al., 2006). By including them in substrate mixes, practitioners could reduce substrate loss due to the unique microclimate and technical factors experienced on green roofs that impact substrate stabilization. The geomorphological processes on green roofs which result in weathering, erosion and other changes to the substrate which impacts the vegetation community can be reduced with substrates that are colonized with AMF as mycorrhizal networks increase plant root binding which will secure them in place (Jastrow and Miller, 1998; Six et al., 2002). Often green roofs utilize a stabilization mat of mesh biodegradable plastic or other products (Hill et al., 2015), and mycorrhizae in substrate mixes might be used in place reducing the necessity for additional materials and associated costs. Even bacteria can contribute to substrate stabilization. For example, Cyanobacteria such as Nostoc can be added as a soil amendment—they contributes to structure by acting like cement, bonding soil particles that could be susceptible to erosion (Maqubela et al., 2009). This can include substrate formation and the decomposition of organic matter required for vegetation. Overall, through their production of extracellular polymeric substances, bacteria contribute to the formation of substrate aggregates, binding particles to roots, organic matter and to each other (Forster, 1990).
Research Challenges for Green Roof Implementation
If we take as given that microbial associates of plants are desirable components of green roof systems, then we must consider the best way of seeing that these are introduced and maintained. To this end there are some knowledge gaps that require filling.
Are Green Roof Plant-Microbial Relationships Specific or is There a “One Size Fits-All” Solution? the Question of Host Plant Specificity
The degree to which most plant species have their own specific microbial associates is not well-studied. Although it is generally accepted that many mycorrhizal fungi are generalists (Opik et al., 2009) and can contribute to the health of many plant species, little is known about the host specificity of rhizospheric and endophytic bacteria. If applied to green roofs, a subset of “generalist” mycorrhizal and bacterial inocula may be sufficient and desirable in terms of benefits to plants and cost but there are few data to support this idea. The tendency for industry to formulate a “one-size fits all” mixture of the same strains of well-known microbes underscores the underlying assumption that the benefits of these microbial communities are not host specific. In contrast, differential plant responses to stress-alleviating bacterial inoculants reveal that plant-microbial relationships are not universal (Hardoim et al., 2008). Rumble and Gange (2017) added mycorrhizae, Bacillus species and trichoderma to 8-year old Sedum planted roofs—with no impact on roof colonization by mycorrhizae. Similarly John et al. (2014) found that a commercial growing media results in differential levels of mycorrhizal colonization of three roof plants, and none at all in Sedum. host-specificity research should be a priority—especially for well-studied green roof plant species groups, such as Sedum Following wider investigations of other common green roof plant species as well as local plants in regional green roof markets, knowledge of host-specificity will increase the range of plant species that are successful on green roofs, diversifying their applications on different buildings in cities.
Is There a Value to Overall Microbial Diversity as Opposed to the Provision of a Simple Set of Green Roof Plant Beneficial Organisms?
Green roof plant communities may also benefit from the addition and maintenance of microbial diversity—not just a small number of generalist or even specialist species. Provision of optimal microbial diversity is the opposite approach from that suggested above. In the absence of knowledge of specific plant-microbial needs, one provides as close to natural levels of diversity as possible. Cultivating diversity in the microbial community might be one approach to ensuring resilience in the plant community to harsh green roof conditions, thereby sustaining ecosystem service delivery. Empirically, diversity has been linked to benefits such as disease suppression and buffering impacts of environmental stress in natural plant communities (Garbeva et al., 2004; Wehner et al., 2011). More specific studies have linked plant diversity to improved green roof ecosystem service delivery (Lundholm et al., 2010; Philippot et al., 2013; Lundholm, 2015). New sequencing techniques that allow us to accurately measure microbial diversity and link this information to plant diversity and ecosystem services in green roofs and other forms of green infrastructure present an exciting new research direction in the field of applied urban ecology. More work is needed to link microbial diversity to key ecosystem services that can be a point of reference for practitioners and ecologists interfacing with designers that create green roof plant and substrate communities.
Are Some Modes of Inoculation Better than Others? Should Microbes be Introduced into the Growing Medium, or is Introduction in Planting Root System (Nursery Stock) Sufficient?
The mode and effectiveness of inoculation is important to ensure added microbes form ongoing associations with the plants and that practitioners can minimize the frequency of re-application and associated costs (Gianinazzi and Vosátka, 2004). These are important to consider on green roofs and the modes of inoculation can vary pre- and post- green roof installation (Figure 2). Green roof media typically include some organic matter or animal manure and many native seed companies will inoculate seeds prior to application to stimulate growth. Do these initial communities persist? The microbial community compositions may change over time due to wind and rain events, and through other biological vectors including animals that visit the green roof, and the growth of the plants themselves. The dynamics of these communities have not been established. More work is needed to elucidate the most effective ways of inoculating green roof systems with microbes that lead to plant health and subsequent ecosystem service delivery.
Very few studies have actually looked at the effect of microbial inoculants on green roof ecosystem service delivery. In those that have the results vary based on the nature and age of the roof installations and of course the nature of the inocula A study carried out on a green roof in London, UK looked at the effects of added AMF, compost “tea” (a source of bacteria) or both on artificial media (Molineux et al., 2014). Inocula enhanced microbial biomass levels, plant heights, and plant root biomass. However, these benefits diminished in a year following inoculation (Molineux et al., 2017).
Are Some Growing Media More Conducive to the Maintenance of Microbial Communities than Others?
Comparative studies on soil microbial communities from different systems using next generation sequencing methods have established that soil characteristics strongly influence the microbial community compositions, with pH being the most influential factor (Fierer and Jackson, 2006). Soil texture also strongly affects microbial community compositions, largely through the effects of particle size on nutrients, habitat space, moisture, and redox levels (Kaiser et al., 2016). Molineux et al. (2017) showed, the effectiveness and persistence of microbial inocula on green roofs differed according to whether or not the substrate was concrete or brick based. There is considerable need to study the impact that media type has on the microbes it will support.
Conclusions
Research and applications in microbial ecology and diversity present new and novel opportunities for integration into green infrastructure and specifically green roof design to improve plant survival and delivery of ecosystem services. Few studies have examined the nature of microbial communities on green roofs, the influence of modes of inoculation, or of substrate types on their establishment. Even fewer have experimentally manipulated these green roof microbial communities to examine impacts of those changes to green roof ecosystem service delivery. In completing this paper, we call for research on these interdisciplinary themes, and in particular on how microbial communities can be cultivated in green roof ecosystems to realize the improvements in plant productivity, nutrient use, and stress resistance. Microbial additions to green roofs presents a potentially fruitful avenue for basic research in ecology, as well as improvements to green roof design that will yield economic and environmental benefits.
Author Contributions
RF and JM concieved the idea for the review. RF led the writing of the manuscript and JM, PJ, and S-LY all contributed to writing. JM created the figures, and JM is acting as corresponding author.
Funding
This work was completed without the need to acknowledge any funding sources.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank two reviewers for constructive comments on the manuscript.
References
Akhtar, S. S., Andersen, M. N., Naveed, M., Zahir, Z. A., and Liu, F. (2015). Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 42, 770–781. doi: 10.1071/FP15054
Ali, S., Charles, T. C., and Glick, B. R. (2014). Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 80, 160–167. doi: 10.1016/j.plaphy.2014.04.003
Ali, S., Charles, T. C., and Glick, B. R. (2017). “Endophytic phytohormones and their role in plant growth promotion,” in Functional Importance of the Plant Microbiome, ed S.L. Doty (Cham: Springer), 89–105.
Allen, M. F., Moore, T. S. Jr., and Christensen, M. (1980). Phytohormone changes in Bouteloua gracilis infected by vesicular–arbuscular mycorrhizae: I. Cytokinin increases in the host plant. Can. J. Bot. 58, 371–374. doi: 10.1139/b80-038
Ampim, P. A., Sloan, J. J., Cabrera, R. I., Harp, D. A., and Jaber, F. H. (2010). Green roof growing substrates: types, ingredients, composition and properties. J. Environ. Hortic. 28, 244–252.
Ashraf, M., Hasnain, S., and Berge, O. (2006). Effect of exo-polysaccharides producing bacterial inoculation on growth of roots of wheat (Triticum aestivum L.) plants grown in a salt-affected soil. Int. J. Environ. Sci. Technol. 3, 43–51. doi: 10.1007/BF03325906
Auge, R. M. (2004). Arbuscular mycorrhizae and plant/water relations. Can. J. Soil Sci. 84, 37–381. doi: 10.4141/S04-002
Barnawal, D., Bharti, N., Triparthi, A., Pandey, S. S., Chanotiya, C. S., and Kalra, A. (2016). ADD-Deaminase-producing endophyte Brachybacterium paraconglomeratum strain SMR20 ameliorates Chlorophytum salinity stress via altering phytohormone generation. J. Plant Growth Regul. 35, 553–564. doi: 10.1007/s00344-015-9560-3
Bengtsson, L., Grahn, L., and Olsson, J. (2005). Hydrological function of a thin extensive green roof in southern Sweden. Hydrol. Res. 36, 259–268.
Berndtsson, J. C. (2010). Green roof performance towards management of runoff water quantity and quality: a review. Ecol. Eng. 36, 351–360. doi: 10.1016/j.ecoleng.2009.12.014
Bili, M., Cortesero, A. M., Mougel, C., Gauthier, J. P., Ermel, G., Simon, J. C., et al. (2016). Bacterial community diversity harboured by interacting species. PLoS ONE 11:e0155392. doi: 10.1371/journal.pone.0155392
Bodker, L., Kjoller, R., and Rosendahl, S. (1998). Effect of phosphate and the arbuscular mycorrhizal fungus Glomus intraradices on disease severity of root rot of peas (Pisum sativum) caused by Aphanomyces euteiches. Mycorrhiza 8, 169–174. doi: 10.1007/s005720050230
Bolan, N. S. (1991). A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134, 189–207.
Bordenstein, S. R., and Theis, K. R. (2015). Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biol. 13:e1002226. doi: 10.1371/journal.pbio.1002226
Brader, G., Compant, S., Mitter, B., Trognitz, F., and Sessitsch, A. (2014). Metabolic potential of endophytic bacteria. Curr. Opin. Microbiol. 27, 30–37. doi: 10.1016/j.copbio.2013.09.012
Broeckling, C. D., Broz, A. K., Bergelson, J., Manter, D. K., and Vivanco, J. M. (2008). Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 74, 738–744. doi: 10.1128/AEM.02188-07
Brundrett, M. C. (2002). Coevolution of roots and mycorrhizas of land plants. New Phytol. 154, 275–304. doi: 10.1046/j.1469-8137.2002.00397.x
Buckland-Nicks, M., Heim, A., and Lundholm, J. (2016). Spatial environmental heterogeneity affects plant growth and thermal performance on a green roof. Sci. Tot. Environ. 553, 20–31. doi: 10.1016/j.scitotenv.2016.02.063
Buffam, I., and Mitchell, M. E. (2015). “Nutrient cycling in green roof ecosystems,” in Green Roof Ecosystems, ed R. K. Sutton (New York, NY: Springer International Publishing), 107–137.
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624. doi: 10.1038/ismej.2012.8
Cheng, L., Booker, F. L., Tu, C., Burkey, K. O., Zhou, L., Shew, H. D., et al. (2012). Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337, 1084–1087. doi: 10.1126/science.1224304
Costacurta, A., and Vanderleyden, J. (1995). Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 21, 1–18. doi: 10.3109/10408419509113531
D'Aleessandro, M., Erb, M., Ton, J., Brandenburg, A., Karlen, D., Zopfi, J., et al. (2014). Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 37, 813–826. doi: 10.1111/pce.12220
De Meyer, S. E., Briscoe, L., Martínez-Hidalgo, P., Agapakis, C. M., de-los Santos, P. E., Seshadri, R., et al. (2016). Symbiotic Burkholderia species show diverse arrangements of nif/fix and nod genes and lack typical high-affinity cytochrome cbb3 oxidase genes. Mol. Plant Microbe Interact. 29, 609–619. doi: 10.1094/MPMI-05-16-0091-R
Dunnett, N., and Kingsbury, N. (2008). Planting Green Roofs and Living Walls. Portland, OR: Timber Press
Ecker, J. R. (1995). The ethylene signal transduction pathway in plants. Science 26, 667–674. doi: 10.1126/science.7732375
Egamberdieva, D., and Legtenberg, B. (2014). “Use of plant growth promoting rhizobacteria to alleviate salinity stress,” in Use of Microbes for the Alleviation of Salt Stresses, Vol. 1, ed M. Miransari (New York, NY: Springer Science), 73–96.
Estrada-De Los Santos, P., Bustillos-Cristales, R., and Caballero-Mellado, J. (2001). Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl. Environ. Microbiol. 67, 2790–2798. doi: 10.1128/AEM.67.6.2790-2798.2001
Fierer, N., and Jackson, R. B. (2006). The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. U.S.A. 103, 626–631. doi: 10.1073/pnas.0507535103
Figueiredo, M. V., Burity, H. A., Martínez, C. R., and Chanway, C. P. (2008). Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 40, 182–188. doi: 10.1016/j.apsoil.2008.04.005
Forster, S. M. (1990). The role of microorganisms in aggregate formation and soil stabilization: types of aggregation. Arid Land Res. Manage. 4, 85–98. doi: 10.1080/15324989009381236
Fulthorpe, R. R., Roesch, L., Cassella, G., and Triplett, E. (2008). Randomly sampled soils have few bacterial species in common. ISME J. 2, 901–910. doi: 10.1038/ismej.2008.55
Gagné-Bourque, F., Bertrand, A., Claessens, A., Aliferis, K. A., and Jabaji, S. (2016). Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front. Plant Sci. 7:584. doi: 10.3389/fpls.2016.00584
Gagné-Bourque, F., Mayer, B. F., Charron, J. B., Vali, H., Bertrand, A., and Jabaji, S. (2015). Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS ONE 10:e0130456. doi: 10.1371/journal.pone.0130456
Garbeva, P., Van Veen, J. A., and Van Elsas, J. D. (2004). Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Ann. Rev. Phytopathol. 42, 243–270. doi: 10.1146/annurev.phyto.42.012604.135455
Gianinazzi, S., and Vosátka, M. (2004). Inoculum of arbuscular mycorrhizal fungi for production systems: science meets business. Can. J. Bot. 82, 1264–1271. doi: 10.1139/b04-072
Glick, B. R. (1995). The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 41, 109–117. doi: 10.1139/m95-015
Glick, B. R., Penrose, D. M., and Li, J. (1998). A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 190, 63–68. doi: 10.1006/jtbi.1997.0532
Hardoim, P. R., van Overbeek, L. S., and van Elsas, J. D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 16, 463–471. doi: 10.1016/j.tim.2008.07.008
Hill, J., Drake, J., and Sleep, B. (2016). Comparisons of extensive green roof media in Southern Ontario. Ecol. Eng. 94, 418–426. doi: 10.1016/j.ecoleng.2016.05.045
Hill, J., Kuszkowski, H., Sleep, B., and Drake, J. (2015). “Gone with the wind,” in Environmental Connection Conference Proceedings (Portland, OR).
Houlden, A., Timms-Wilson, T. M., Day, M. J., and Bailey, M. J. (2008). Influence of plant developmental stage on microbial community structure and activity in the rhizosphere of three field crops. FEMS Microbiol. Ecol. 65, 193–201. doi: 10.1111/j.1574-6941.2008.00535.x
Jastrow, J. D., and Miller, R. M. (1998). “Soil aggregate stabilization and carbon sequestration: feedbacks through organomineral associations,” in Soil Processes and the Carbon Cycle, eds R. Lal, J. M. Kimble, R. F. Follett, and B. A. Stewart (Boca Raton, FL: CRC Press), 207–223.
Johannessen, B. G., Hanslin, H. M., and Muthanna, T. M. (2017). Green roof performance potential in cold and wet regions. Ecol. Eng. 106, 436–447. doi: 10.1016/j.ecoleng.2017.06.011
John, J., Kernaghan, G., and Lundholm, J. (2017). The potential for mycorrhizae to improve green roof function. Urb. Ecosyst. 20, 113–127. doi: 10.1007/s11252-016-0573-x
John, J., Lundholm, J., and Kernaghan, G. (2014). Colonization of green roof plants by mycorrhizal and root endophytic fungi. Ecol. Eng. 71, 651–659. doi: 10.1016/j.ecoleng.2014.08.012
Johnson, C., Schweinhart, S., and Buffam, I. (2016). Plant species richness enhances nitrogen retention in green roof plots. Ecol. Appl. 26, 2130–2144. doi: 10.1890/15-1850.1
Joynt, J., Bischoff, M., Turco, R., Konopka, A., and Nakatsu, C. H. (2006). Microbial community analysis of soils contaminated with lead, chromium and petroleum hydrocarbons. Microb. Ecol. 51, 209–219. doi: 10.1007/s00248-005-0205-0
Kaiser, K., Wemheuer, B., Korolkow, V., Wemheuer, F., Nacke, H., Schöning, I., et al. (2016). Driving forces of soil bacterial community structure, diversity, and function in temperate grasslands and forests. Sci. Rep. 6:33696. doi: 10.1038/srep33696
Kaushal, S. S., Groffman, P. M., Likens, G. E., Belt, K. T., Stack, W. P., Kelly, V. R., et al. (2005). Increased salinization of fresh water in the northeastern United States. Proc. Natl. Acad. Sci. U.S.A. 102, 13517–13520. doi: 10.1073/pnas.0506414102
Khan, A. L., Waqas, M., Kang, S. M., Al-Harrasi, A., Hussain, J., Al-Rawahi, A., et al. (2014). Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 52, 689–695. doi: 10.1007/s12275-014-4002-7
Kokkinou, I., Ntoulas, N., Nektarios, P. A., and Varela, D. (2016). Response of native aromatic and medicinal plant species to water stress on adaptive green roof systems. Hortscience 51, 608–614.
Lallias, D., Hiddink, J. G., Fonseca, V. G., Gaspar, J. M., Sung, W., Neill, S. P., et al. (2015). Environmental metabarcoding reveals heterogeneous drivers of microbial eukaryote diversity in contrasting estuarine ecosystems. ISME J. 9, 1208–1221. doi: 10.1038/ismej.2014.213
Leake, J. R., Johnson, D., Donnelly, D. P., Muckle, G. E., Boddy, L., and Read, D. J. (2004). Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can. J. Bot. 82, 1016–1045. doi: 10.1139/b04-060
Lemaire, B., Chimphango, S., Stirton, C., Rafundeen, S., Honnay, O., Smets, E., et al. (2016). Biogeographical patterns of legume-nodulating Burkholderia: from African Fynbos to continental scales. Appl. Environ. Microbiol. 82, 5099–5115. doi: 10.1128/AEM.00591-16
Lindahl, B. D., Ihrmark, K., Boberg, J., Trumbore, S. E., Högberg, P., Stenlid, J., et al. (2007). Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 173, 611–620. doi: 10.1111/j.1469-8137.2006.01936.x
Linderman, R. G. (1988). Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizosphere effect. Phytopathology 78, 366–371.
Lundholm, J. T. (2015). Green roof plant species diversity improves ecosystem multifunctionality. J. Appl. Ecol. 52, 726–734. doi: 10.1111/1365-2664.12425
Lundholm, J., MacIvor, J. S., MacDougall, Z., and Ranalli, M. (2010). Plant species and functional group combinations affect green roof ecosystem functions. PLoS ONE 5:e9677. doi: 10.1371/journal.pone.0009677
Lützow, M. V., Kögel-Knabner, I., Ekschmitt, K., Matzner, E., Guggenberger, G., Marschner, B., et al. (2006). Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions–a review. Eur. J. Soil Sci. 57, 426–445. doi: 10.1111/j.1365-2389.2006.00809.x
Maqubela, M. P., Mnkeni, P. N. S., Issa, O. M., Pardo, M. T., and D'Acqui, L. P. (2009). Nostoc cyanobacterial inoculation in South African agricultural soils enhances soil structure, fertility, and maize growth. Plant Soil 315, 79–92.
Marsalek, J. (2003). Road salts in urban stormwater: an emerging issue in stormwater management in cold climates. Water Sci. Technol. 48, 61–70.
Mayak, S., Tirosh, T., and Glick, B. R. (2004). Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Phys. Biochem. 42, 565–572. doi: 10.1016/j.plaphy.2004.05.009
McGuire, K. L., Payne, S. G., Orazi, G., and Palmer, M. I. (2015). “Bacteria and fungi in green roof ecosystems,” in Green Roof Ecosystems, ed R. K. Sutton (New York, NY: Springer International Publishing), 175–191.
McGuire, K. L., Payne, S. G., Palmer, M. I., Gillikin, C. M., Keefe, D., Kim, S. J., et al. (2013). Digging the New York City skyline: Soil fungal communities in green roofs and city parks. PLoS ONE 8:e58020. doi: 10.1371/journal.pone.0058020
Mehnaz, S., and Lazarovits, G. (2006). Inoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb. Ecol. 51, 326–335. doi: 10.1007/s00248-006-9039-7
Molineux, C. J., Connop, S. P., and Gange, A. C. (2014). Manipulating soil microbial communities in extensive green roof substrates. Sci. Tot. Environ. 493, 632–638. doi: 10.1016/j.scitotenv.2014.06.045
Molineux, C. J., Gange, A. C., and Newport, D. J. (2017). Using soil microbial inoculations to enhance substrate performance on extensive green roofs. Sci. Tot. Environ. 580, 846–856. doi: 10.1016/j.scitotenv.2016.12.031
Molineux, C. J., Gange, A. C., Connop, S. P., and Newport, D. J. (2015). Are microbial communities in green roof substrates comparable to those in post-industrial sites? A preliminary study. Urb. Ecosyst. 18, 1245–1260. doi: 10.1007/s11252-015-0450-z
Monterusso, M. A., Rowe, D. B., and Rugh, C. L. (2005). Establishment and persistence of Sedum spp. and native taxa for green roof applications. Hortscience 40, 391–396.
Morin, P. J., and McGrady-Steed, J. (2004). Biodiversity and ecosystem functioning in aquatic microbial systems: a new analysis of temporal variation and species richness-predictability relations. Oikos 104, 458–466. doi: 10.1111/j.0030-1299.2004.13256.x
Nabti, E., Sahnoune, M., Adjrad, S., Van Dommelen, A., Ghoul, M., Schmid, M., et al. (2007). A halophilic and osmotolerant Azospirillum brasilense strain from Algerian soil restores wheat growth under saline conditions. Eng. Life Sci. 7, 354–360. doi: 10.1002/elsc.200720201
Nannipieri, P., Ascher, J., Ceccherini, M., Landi, L., Pietramellara, G., and Renella, G. (2003). Microbial diversity and soil functions. Eur. J. Soil Sci. 54, 655–670. doi: 10.1046/j.1351-0754.2003.0556.x
Naveed, M., Mitter, B., Reichenauer, T. G., Wieczorek, K., and Sessitsch, A. (2014). Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 97, 30–39. doi: 10.1016/j.envexpbot.2013.09.014
Nicholson, K. W., and Branson, J. R. (1990). Factors affecting resuspension by road traffic. Sci. Tot. Environ. 93, 349–358. doi: 10.1016/0048-9697(90)90126-F
Oberndorfer, E., Lundholm, J., Bass, B., Coffman, R. R., Doshi, H., and Dunnett, N. (2007). Green roofs as urban ecosystems: ecological structures, functions, and services. Bioscience 57, 823–833. doi: 10.1641/B571005
Ondoño, S., Bastida, F., and Moreno, J. L. (2014). Microbiological and biochemical properties of artificial substrates: a preliminary study of its application as Technosols or as a basis in Green Roof Systems. Ecol. Eng. 70, 189–199. doi: 10.1016/j.ecoleng.2014.05.003
Opik, M., Metsis, M., Daniell, T. J., Zobel, M., and Moora, M. (2009). Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 184, 424–437. doi: 10.1111/j.1469-8137.2009.02920.x
Parke, J. L. (1991). “Root colonization by indigenous and introduced microorganisms,” in The Rhizosphere and Plant Growth, eds D. L. Keister and P. B. Cregan (Dordrecht: Springer), 33–42.
Paterson, E., Sim, A., Davidson, J., and Daniell, T. J. (2016). Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralisation. Plant Soil 408, 243–254. doi: 10.1007/s11104-016-2928-8
Philippot, L., Spor, A., Hénault, C., Bru, D., Bizouard, F., Jones, C. M., et al. (2013). Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 7, 1609–1619. doi: 10.1038/ismej.2013.34
Prober, S. M., Leff, J. W., Bates, S. T., Borer, E. T., Firn, J., Harpole, W. S., et al. (2015). Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 18, 85–95. doi: 10.1111/ele.12381
Rillig, M. C. (2004). Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol. Lett. 7, 740–754. doi: 10.1111/j.1461-0248.2004.00620.x
Roesch, L. F., Fulthorpe, R. R., Riva, A., Casella, G., Hadwin, A. K., Kent, A. D., et al. (2007). Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 1, 283–290. doi: 10.1038/ismej.2007.53
Rosenblueth, M., and Martínez-Romero, E. (2006). Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 19, 827–837. doi: 10.1094/MPMI-19-0827
Rowe, D. B. (2011). Green roofs as a means of pollution abatement. Environ. Pollut. 159, 2100–2110. doi: 10.1016/j.envpol.2010.10.029
Ruiz-Lozano, J. M., Porcel, R., Barzana, G., Azcon, R., and Arcoca, R. (2012). “Contribution of arbuscular mycorrhizal symbiosis to plant drought tolerance: State of the art,” in Plant Responses to Drought Stress, ed R. Acoa (Berlin; Heidelberg: Springer), 335–362.
Rumble, H., and Gange, A. C. (2017). Microbial inoculants as a soil remediation tool for extensive green roofs. Ecol. Eng. 102, 188–198. doi: 10.1016/j.ecoleng.2017.01.025
Sandhya, V., Ali, S. Z., Grover, M., Reddy, G., and Venkateswarlu, B. (2010). Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 62, 21–30. doi: 10.1007/s10725-010-9479-4
Santi, C., Bogusz, D., and Franche, C. (2013). Biological nitrogen fixation in non-legume plants. Ann. Bot. 111, 743–767. doi: 10.1093/aob/mct048
Santoyo, G., Moreno-Hagelsieb, G., del Carmen Orozco-Mosqueda, M., and Glick, B. R. (2016). Plant growth-promoting bacterial endophytes. Microbiol. Res. 183, 92–99. doi: 10.1016/j.micres.2015.11.008
Sarma, R. K., and Saikia, R. (2014). Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 377, 111–126. doi: 10.1007/s11104-013-1981-9
Schmidt, P. A., Bálint, M., Greshake, B., Bandow, C., Römbke, J., and Schmitt, I. (2013). Illumina metabarcoding of a soil fungal community. Soil Biol. Biochem. 65, 128–132. doi: 10.1016/j.soilbio.2013.05.014
Shikano, I., Rosa, C., Tan, C. W., and Felton, G. W. (2017). Tritrophic Interactions: microbe-mediated plant effects on insect herbivores. Annu. Rev. Phytopathol. 55, 313–331. doi: 10.1146/annurev-phyto-080516-035319
Six, J., Conant, R. T., Paul, E. A., and Paustian, K. (2002). Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241, 155–176.
Smith, S. E., Facelli, E., Pope, S., and Smith, F. A. (2010). Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326, 3–20. doi: 10.1007/s11104-009-9981-5
Sutton, R. K., Harrington, J. A., Skabelund, L., MacDonagh, P., Coffman, R. R., and Koch, G. (2012). Prairie-based green roofs: literature, templates, and analogs. J. Green Build. 7, 143–172. doi: 10.3992/jgb.7.1.143
Swensen, S. M. (1996). The evolution of actinorhizal symbioses: evidence for multiple origins of the symbiotic association. Am. J. Bot. 83, 1503–1512. doi: 10.2307/2446104
Szota, C., Farrell, C., Williams, N. S., Arndt, S. K., and Fletcher, T. D. (2017). Drought-avoiding plants with low water use can achieve high rainfall retention without jeopardising survival on green roofs. Sci. Tot. Environ. 603, 340–351. doi: 10.1016/j.scitotenv.2017.06.061
Tomasi, N., Weisskopf, L., Renella, G., Landi, L., Pinton, R., Varanini, Z., et al. (2008). Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol Biochem. 40, 1971–1974. doi: 10.1016/j.soilbio.2008.02.017
Torsvik, V., and Øvreås, L. (2002). Microbial diversity and function in soil: from genes to ecosystems. Curr. Opin. Microbiol. 5, 240–245. doi: 10.1016/S1369-5274(02)00324-7
Van Der Heijden, M. G., Bardgett, R. D., and Van Straalen, N. M. (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310. doi: 10.1111/j.1461-0248.2007.01139.x
Van der Heijden, M. G., Klironomos, J. N., Ursic, M., Moutoglis, P., Streitwolf-Engel, R., Boller, T., et al. (1998). Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396, 69–72. doi: 10.1038/23932
Van Loon, L. C., Bakker, P. A. H. M., and Pieterse, C. M. J. (1998). Systemic resistance induced by rhizosphere bacteria. Ann. Rev. Phytopathol. 36, 453–483. doi: 10.1146/annurev.phyto.36.1.453
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., and Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206. doi: 10.1111/nph.13312
Wang, B., Seiler, J. R., and Mei, C. (2016). A microbial endophyte enhanced growth of switchgrass under two drought cycles improving leaf level physiology and leaf development. Environ. Exp. Bot. 122, 100–108. doi: 10.1016/j.envexpbot.2015.09.004
Waqas, M., Khan, A. L., Shahzad, R., Ullah, I., Khan, A. R., et al. (2015). Mutualistic fungal endophytes produce phytohormones and organic acides that promote Japonica rice plant growth under prolonged heat stress. Biomed. Biotechnol. 16, 1011–1018. doi: 10.1631/jzus.B1500081
Wehner, J., Antunes, P. M., Powell, J. R., Caruso, T., and Rillig, M. C. (2011). Indigenous arbuscular mycorrhizal fungal assemblages protect grassland host plants from pathogens. PLoS ONE 6:e27381. doi: 10.1371/journal.pone.0027381
Wehner, J., Antunes, P. M., Powell, J. R., Mazukatow, J., and Rillig, M. C. (2010). Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia 53, 197–201. doi: 10.1016/j.pedobi.2009.10.002
Whitelaw, M. A. (1999). Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv. Agron 69, 99–151. doi: 10.1016/S0065-2113(08)60948-7
Whittinghill, L. J., and Rowe, D. B. (2012). The role of green roof technology in urban agriculture. Renew. Agric. Food Syst. 27, 314–322. doi: 10.1017/S174217051100038X
Whittinghill, L. J., Rowe, D. B., and Cregg, B. M. (2013). Evaluation of vegetable production on extensive green roofs. Agroecol. Sustainable Food Syst. 37, 465–484. doi: 10.1080/21683565.2012.756847
Yang, J., Kloepper, J. W., and Ryu, C. M. (2009). Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14, 1–4. doi: 10.1016/j.tplants.2008.10.004
Keywords: mycorrhizal fungi, endophytic bacteria, mycorrhizosphere, green infrastructure, biodiversity, plant-fungal interactions, plant-bacteria interactions
Citation: Fulthorpe R, MacIvor JS, Jia P and Yasui S-LE (2018) The Green Roof Microbiome: Improving Plant Survival for Ecosystem Service Delivery. Front. Ecol. Evol. 6:5. doi: 10.3389/fevo.2018.00005
Received: 06 September 2017; Accepted: 10 January 2018;
Published: 02 February 2018.
Edited by:
Galina Churkina, Yale University, United StatesReviewed by:
Anna L. Johnson, University of Maryland, Baltimore County, United StatesKrista L. McGuire, University of Oregon, United States
Kelly Ksiazek-Mikenas, Northwestern University, United States
Copyright © 2018 Fulthorpe, MacIvor, Jia and Yasui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Scott MacIvor, scott.macivor@utoronto.ca
 Roberta Fulthorpe
Roberta Fulthorpe J. Scott MacIvor
J. Scott MacIvor Pu Jia
Pu Jia Simone-Louise E. Yasui
Simone-Louise E. Yasui