- 1Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, Zhejiang, China
- 2Department of General, Visceral, and Transplant Surgery, Ludwig-Maximilians-University Munich, Munich, Germany
- 3Department of General, Visceral and Transplantation Surgery, University of Heidelberg, Heidelberg, Germany
- 4Clinical Medical Collage, Southwest Medical University, Luzhou, China
- 5Clinical Molecular Medicine Testing Center, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Cancer vaccines exhibit specificity, effectiveness, and safety as an alternative immunotherapeutic strategy to struggle against malignant diseases, especially with the rapid development of mRNA cancer vaccines in recent years. However, how to maintain long-term immune memory after vaccination, especially T cells memory, to fulfill lasting surveillance against cancers, is still a challenging issue for researchers all over the world. IL-7 is critical for the development, maintenance, and proliferation of T lymphocytes, highlighting its potential role as an adjuvant in the development of cancer vaccines. Here, we summarized the IL-7/IL-7 receptor signaling in the development of T lymphocytes, the biological function of IL-7 in the maintenance and survival of T lymphocytes, the performance of IL-7 in pre-clinical and clinical trials of cancer vaccines, and the rationale to apply IL-7 as an adjuvant in cancer vaccine-based therapeutic strategy.
Introduction
Immunotherapy now is experiencing its “golden age” recently, with the immune checkpoint blocker (ICB) approved by the Food and Drug Administration for the treatment of melanoma and lung cancer, which forever changed the balance in the choice of methods of anticancer therapy (1, 2). The concept of immunotherapy implies antitumor immune response activation or/and immunosuppression inhibition. As one of the critical components of immunotherapy in oncology, cancer vaccines show advantages in specificity, immunogenicity, and low toxicity (3, 4). Unlike ICBs, a passive immunotherapeutic strategy, cancer vaccines possess the capability to induce anti-tumor immune responses actively (5). Moreover, cancer vaccines are time- and labor-saving in manufacturing, when compared to chimeric antigen receptor T cells therapy, making it more feasible for most cancer patients. The current rapid development of the mRNA cancer vaccine brings inspiring results for cancer patients, making this field of research scorching again (6, 7). Although different types of cancer vaccines have been evaluated in clinical trials, few led to satisfying clinical benefits. One of the underlying mechanisms lies in the lack of long-term memory to maintain immune surveillance to prevent the recurrence and metastasis of cancers (3, 8). Increasing studies have revealed that the success of cancer immunotherapeutics, especially cancer vaccines, is critically dependent on the induction of memory T-cell responses against cancers (9, 10). Moreover, the administration of vaccines without immunomodulatory agents is hard to reach the greatest effectiveness to stimulate the antitumor immune response (11). Therefore, searching for adjuvants, which will augment the efficacy of cancer vaccines and contribute to building up a lasting immune memory against cancers, was an attractive issue for researchers.
IL-7, encoded by the IL7 gene, is a 25 kDa secreted soluble protein, which was initially discovered by Hunt et al. in 1987 when they explored the latent role of bone marrow stromal cells in the development of the pre-B cell subset (12, 13). Subsequently, increasing evidence proved that except for thymocytes and stromal non-hematopoietic cells (14), IL-7 is also secreted by lymphoid organs, non-lymphoid tissues, and even cancers (15–20) (Figure 1). The receptor of IL-7 is a heterodimer complex that comprises an IL-7Rα chain (CD127, encoded by the IL7R gene) and a common γ chain (CD132, encoded by the IL2RG gene) shared with receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 (21, 22). It has been reported that the signaling of IL-7/IL-7R participates in the growth and survival of T and B cell precursors critically (21). Recently, accumulating evidence proved that IL-7 is not only the essential factor in every stage of T cell development (23, 24) but also the crucial component for the survival of naïve T cells as well as the generation and maintenance of memory T cells (25, 26). Furthermore, IL-7 was demonstrated to assist T cells to restore homeostasis via the signal transducer and activator of transcription 5 (STAT5)/Suppressor of cytokine signaling (SOCS) pathway (27) and guide T cell homing by inducing the expression of chemokines and integrins (28, 29). In IL-7 knockout mice, a significant depletion of naïve T cells was observed, which can be restored by administrating the exogenous IL-7 (25). Except for T cells, IL-7 was also reported to regulate the immune responses of natural killer cells, dendritic cells, and B cells critically (30–32). As a cytokine therapy for cancer treatment, IL-7 exerts superior activity to induce the expansion of specific T cells against breast carcinoma than IL-2, highlighting its antitumor adjuvant molecular role in oncology (33).
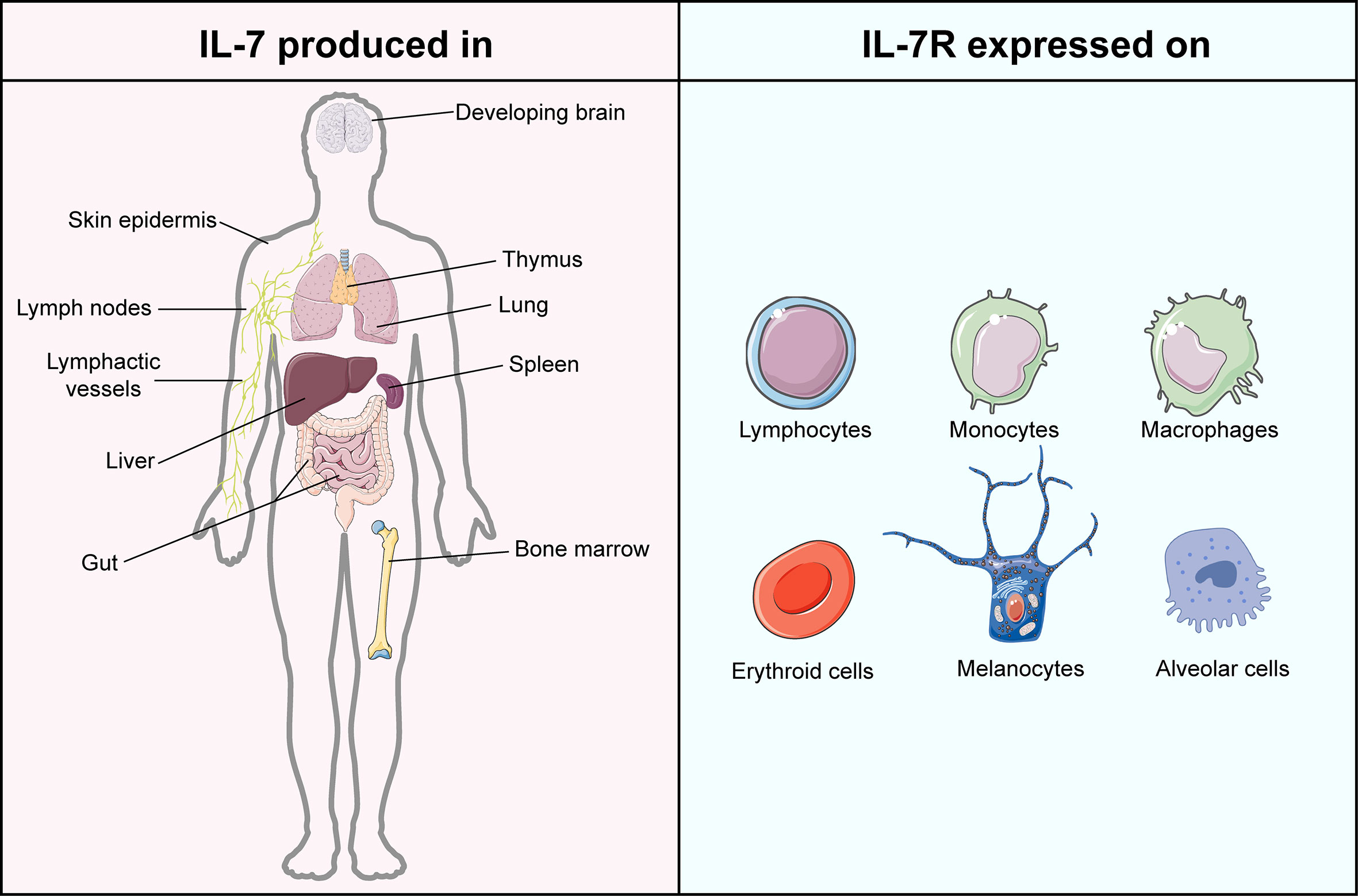
Figure 1 Atlas of IL-7 production and IL-7R expression. IL-7 is mainly produced in but not limited to lymphoid organs, including bone marrow, thymus, spleen, and lymphoid nodes. IL-7 is majorly generated by stromal cells but is also evidenced in epithelial cells, keratinocytes, dendritic cells, and hepatocytes. The expression of IL-7R can be found in lymphocytes, monocytes, macrophages, erythroid cells, melanocytes, and alveolar cells. This figure is created with BioRender.com and smart.servier.com.
Given the pleiotropic and robust biological effects of IL-7, especially its role in the survival, development, proliferation, and even maintenance of memory of T cells, several research teams apply IL-7 as a molecular adjuvant to strengthen the immunogenicity of cancer vaccines, as well as to maintain a long-term memory response against cancers. Therapeutic cancer vaccines aim to stimulate the immune responses in cancer patients, especially T-cell responses, which have been proven to be the predominant safeguard against tumors. Therefore, in this review, we summarized the biological function and mechanism of IL-7 and the signaling pathway of IL-7/IL-7R in T cells, as well as the performance of IL-7 as an adjuvant in combination with cancer vaccines in preclinical and clinical trials. The rationale to utilize IL-7 as an adjuvant in combination with cancer vaccines was also proposed.
The biological activity of IL-7 in T cells
IL-7 in T cell development
Lymphoid progenitors leave the bone marrow and migrate to the thymus for further development into naïve T cells, which were greatly influenced by IL-7 (Figure 2). In IL-7 -/- mice, the development and maturation of γδ T cells were restrained significantly, indicating the crucial role of IL-7 in T cell development (34). However, different stages of T cell development in the thymus exhibit distinct demands on IL-7. Pre-T cell progenitors lacking surface expression of CD4 and CD8 are termed double-negative (DN) cells. Based on the amount of CD44 and CD25, DN cells can be divided into four subgroups (DN1-DN4). Development of T cells from DN1-2 thymocytes showed strong dependence on IL-7 (35), while anti-IL-7 antibody led to the deprivation of T cell maturation by interrupting the expansion of T cells at the DN2 stage (36). Differentiation and proliferation of DN3-4 thymocytes also require the presence of IL-7, while the self-renewal of DN4 cells can be substituted partially by the depletion of Bcl6 in the absence of IL-7 (37). In contrast, IL-7 signaling does not participate in the positive selection of CD4 CD8 double-positive (DP), and the IL-7R is not evidenced on the surface of these cells (38). Intriguingly, cells successfully going through the selection express IL-7R again modulated by developmental T cell antigen receptor (TCR)-dependent signals (39).
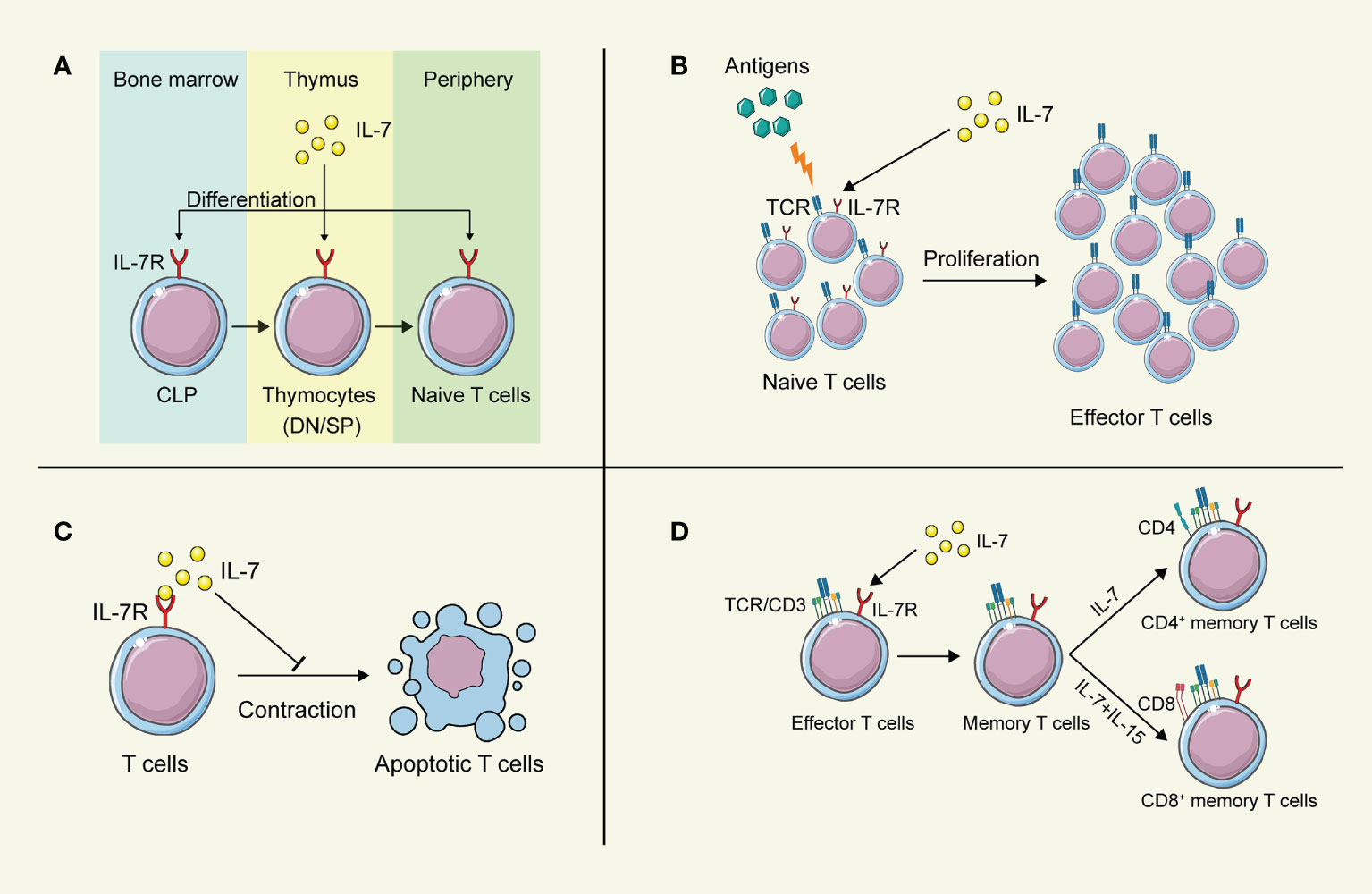
Figure 2 The biological function of IL-7 on T lymphocytes. (A) IL-7 contributes to the development of T cells in the thymus. (B) IL-7 boosts the proliferation of T cells after stimulation by antigens. (C) IL-7 prolongs the survival of T cells. (D) IL-7 promotes the differentiation of memory T cells. This figure is created with BioRender.com and smart.servier.com.
IL-7 in T cell survival
IL-7 was initially discovered as a survival factor for mouse T and B cell precursors. It has been demonstrated that IL-7 activates the transcription factor NFATc1 in DN thymocytes via phosphorylating Tyr371 to prolong the survival of DN thymocytes, while deficiency in NFATc1 blocked thymocyte development at the DN1 (40). Although DP cells are absent in the expression of IL-7R, SP cells regain it to promote their survival and induce the expression of CXCR4 to enhance the recruitment in secondary lymphoid tissues (41, 42). However, increasing evidence indicates that IL-7 fulfills its regulatory role throughout the lymphoid system. Naïve CD4+ T cells consume IL-7 for survival to achieve homeostasis in bone marrow transplant recipients (43). Furthermore, IL-7 also regulates the survival of mature and memory T cells via upregulating the expression of Bcl-2 family proteins, leading to long-term memory (44, 45). Additionally, dendritic epidermal γδ T cells show partial dependence on IL-7 for their survival (46). Interestingly, tumor-bearing mice show a decreased level of IL-7 in the spleen, indicating that insufficient IL-7 might fails to support the survival of activated T cells, attenuating the T cell immune responses against tumors (47).
IL-7 in T cell proliferation
As a highly pleiotropic cytokine, IL-7 provides proliferation signals from hematopoietic stem cells to lymphocytes for their efficient generation (25). At the stage of DN thymocytes, IL-7 appears to promote the proliferation of these cells by upregulating the growth-facilitating genes CD98 in the Stat5-dependent manner (37). After SP thymocytes regain the IL-7R expression, IL-7 still fulfills its capability to stimulate the proliferation of these cells (41). IL-7 facilitates the proliferation of T cells in a concentration-dependent process. A higher concentration of IL-7 stimulates the proliferation of T lymphocytes, while a lower concentration maintains cell survival (48). Administration of IL-7 directly results in an increase in peripheral blood T cells and a broad TCR repertoire diversity (49, 50), while a long-term injection of IL-7 contributes to interrupting the proliferation of T cells, leading to a sharp depletion of naïve T cells (36). Furthermore, the administration of IL-7 in patients suffering from septic syndrome promotes the proliferation of CD4+ and CD8+ T cells in them, indicating the potential clinical application of IL-7 for septic shock (51). Interestingly, IL-7 was found to antagonize the immunosuppressive network, by abrogating the Treg-mediated suppression (52) and reducing the proportion of Treg cells and myeloid-derived suppressor cells (MDSC) (53, 54), even though IL-7 has little effect on the expansion of Treg cells and MDSCs directly (47).
IL-7 in the maintenance of T cell memory
After the clearance of pathogens or cancer cells, most of the effector T cells die, and a small proportion of them turn into memory T cells, maintaining the memory against the same antigens (55, 56). During this differentiation process, the critical role of IL-7 is highlighted. Several studies have revealed that IL-7 improves the long-term immune responses against pathogens by inducing naïve T cells to memory T cells (57, 58). A high expression of IL-7R is evidenced in the memory T cells subset, to maintain their survival by upregulating the anti-apoptotic Bcl-2 family proteins (25, 41). The homeostatic proliferation and maintenance of CD4+ memory T cells heavily depend on the function of IL-7, while it has been demonstrated that these features of CD8+ memory T cells require both IL-7 and IL-15 jointly (59–61). Furthermore, it has been reported that IL-7 is capable to induce more IL-7R+ long-living memory stem T cells, which can self-renew and develop into effector T cells (62). Increased administration of IL-7 can boost the specific memory immune responses against cancer and viral infection (45). The adjuvanticity of IL-7 has been verified to prolong the protective effects of vaccines by inducing the production of memory T and B cells (63–65), highlighting the potential to apply IL-7 as an adjuvant in combination with cancer vaccines to struggle against cancer cells via long-term memory protection.
The signaling pathway of IL-7/IL-7R
The receptor of IL-7, IL-7R, is a transmembrane heterodimer composed of an IL-7Rα chain and a common γ chain. After the binding of IL-7, IL-7Rα dimerizes with the common cytokine γ chain and triggers kinase activation, while IL-7Rα alone fails to prompt the kinase activity and induce signal transduction (25, 66). The signaling of IL-7/IL-7R is mainly transduced by Janus kinase (JAK)- STAT and Phosphoinositide 3-kinase (PI3K)- Ak strain transforming (AKT) pathways in T cells to fulfill the biological functions of IL-7 (Figure 3).
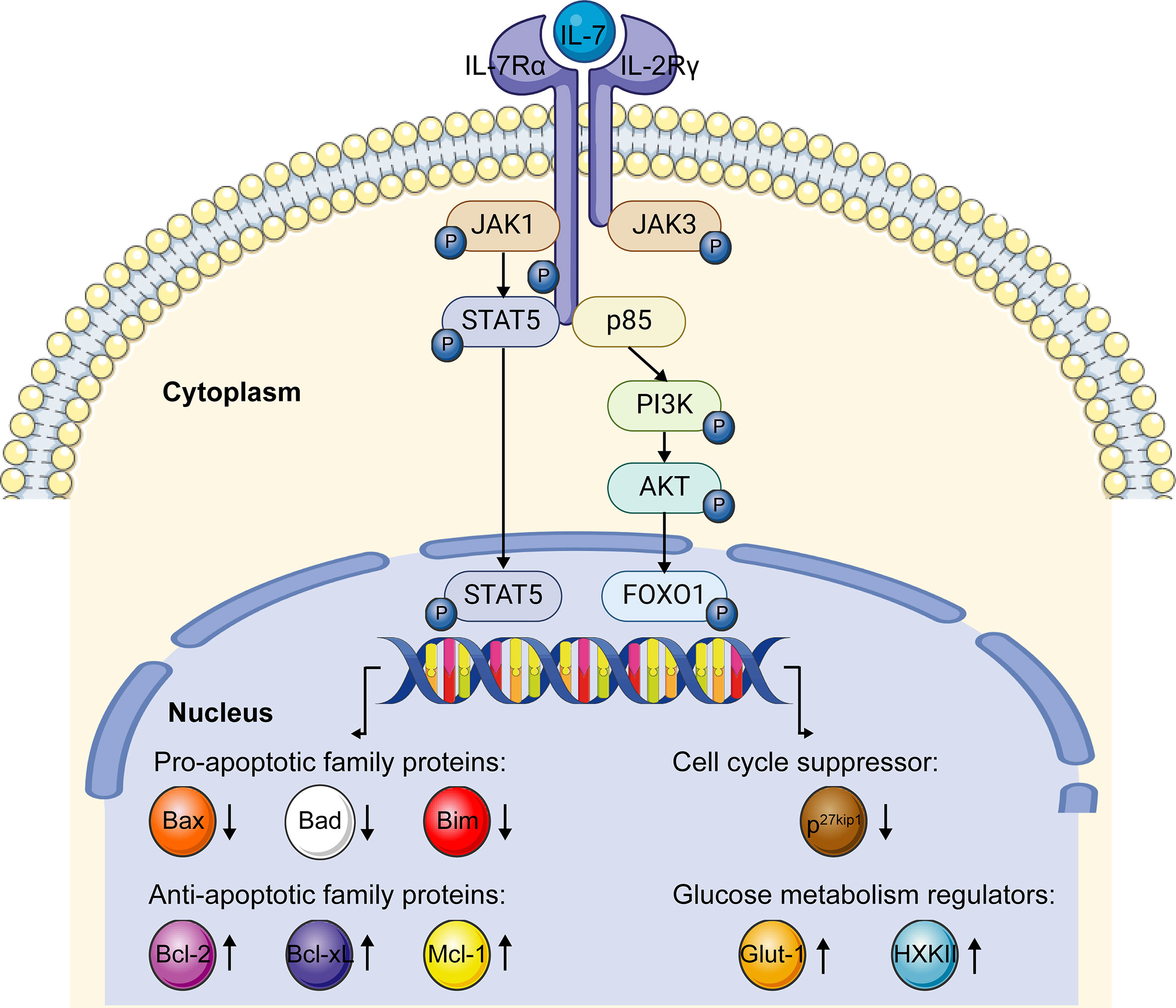
Figure 3 IL-7/IL-7R signaling pathway. When IL-7 interacts with the IL-7R, the α chain and γ chain dimerize. JAK1 and JAK3 were recruited to IL-7Rα and γ chains, separately. After phosphorylation, JAK1 and JAK3 phosphorylate and activate the transcription factor STAT5, which further upregulates anti-apoptotic family genes expression and downregulates pro-apoptotic family genes expression via the JAK-STAT5 pathway. After the interaction of IL-7 and IL-7R, recruited p85 induces the phosphorylation of the cytoplasmic tail of the IL-7Rα chain, which further triggers the phosphorylation of the PI3K-AKT pathway. Activated PI3K-AKT signaling induces glucose metabolism regulator genes expression and impedes the expression of cell cycle suppressor p27kip1 via FOXO1. This figure is created with BioRender.com and smart.servier.com.
When IL-7 combines with IL-7R, JAK1 and JAK3 are recruited to IL-7Rα and γ chain, and then phosphorylated, respectively. Phosphorylated JAK1 and JAK3 create the docking site for STAT5 recruitment and phosphorylation in an IL-7-dependent pattern (67–69). As a transcription factor, phosphorylated STAT5 dimerizes and translocates into the nucleus, mediating the expression of downstream targeted genes associated with the survival and proliferation of T cells (47, 70). It has been reported that IL-7 prolongs the survival of T cells by upregulating the expression of Bcl-2, Bcl-xL, and Mcl-1 proteins via the JAK-STAT signaling pathway (61). Conversely, the inhibitor of JAK activation attenuates IL-7-induced Bcl-2 protein expression (71). Moreover, IL-7 downregulates the expression of the pro-apoptotic genes, like Bad, Bax, and Bim, to prevent apoptosis through the JAK-STAT signaling (72, 73). These studies taken together revealed that the survival of lymphocytes supported by IL-7 is dependent on the JAK-STAT pathway to a large extent.
Another crucial pathway involved in the IL-7/IL-7R signaling is the PI3K-AKT pathway. When IL-7 binds with IL-7R, p85, a regulatory component of PI3K, is recruited and then induces the phosphorylation of tyrosine449 in the IL-7Rα cytoplasmic tail, which triggers the activation of PI3K and then AKT (74). Subsequently, activated AKT participates in the downstream targeted gene regulation by phosphorylating transcription factor the Forkhead box protein 1 (FOXO1) (75). As a targeted molecule of the PI3K-AKT pathway, degraded p27 kinase inhibitor protein 1 (p27kip1) promotes the G1 to S phase transition of T cells in the presence of IL-7, indicating IL-7 can promote the proliferation of T lymphocytes via regulating the expression of p27kip1 (76). Furthermore, the PI3K-AKT signaling mediated by IL-7 was reported to regulate the expression of glycolytic enzymes hexokinase II (HXK II) and glucose transporter-1 (Glut-1), to increase the glucose uptake to support the T cells’ survival and maintain homeostasis (77, 78).
IL-7 as an adjuvant for cancer vaccines in preclinical research
Numerous preclinical trials have evaluated the potential role of IL-7 as an adjuvant to strengthen the effectiveness and long-term responsiveness of cancer vaccines. Here we selectively summarized the performance of IL-7 as an adjuvant in combination with cancer vaccines in preclinical settings (Table 1).
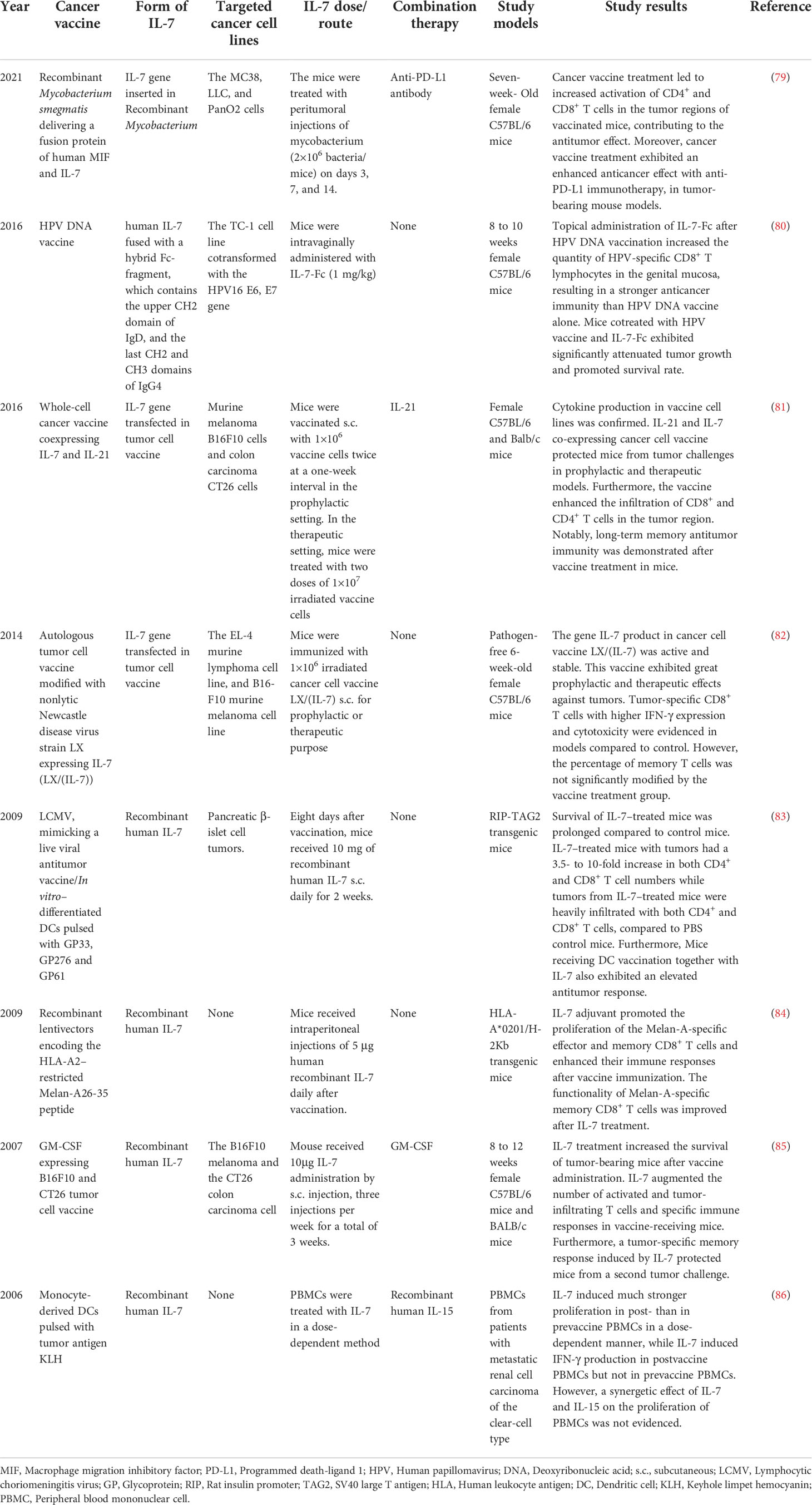
Table 1 Adjuvant effect of IL-7 in cancer vaccine treatment summarized from selective preclinical studies.
Cancer cell vaccines transfected with the IL-7 gene to combat malignant diseases have been extensively explored (81, 82) (Figure 4A). Zhao et al. demonstrated that the production of IL-7 in the cancer cell vaccines modified with the IL-7 gene was active and stable in a soluble form, whereas tumor cells as the control settings exhibited an undetectable level of IL-7. Moreover, 10 μl supernatants of transfected cancer cells were equivalent to 1 ng recombinant human IL-7 protein in promoting T cell proliferation (82). Whole-cell cancer vaccines expressing IL-7 showed robust prophylactic and therapeutic effects against cancers, preventing tumor occurrence or prolonging the survival time in mice models. Furthermore, a higher infiltration of CD4+ and CD8+ T cells in the tumor regions was determined, which was correlated with a better survival outcome in the tumor-bearing mice (81, 82). Except for transfection of the IL-7 gene in tumor cell vaccines, Jeong et al. explored Mycobacterium smegmatis delivering a fusion protein comprising human macrophage migration inhibitory factor (MIF) and IL-7 as cancer vaccines to struggle against tumors (79) (Figure 4B). This bacterial-based cancer vaccine induced antitumor immune responses by recruiting effective T cells while reducing the infiltration of myeloid-derived suppressor cells (MDSCs) in the tumor environment. Intriguingly, an enhanced antitumor effect of the vaccine was observed in combination with programmed death-ligand 1 (PD-L1) immunotherapy.

Figure 4 The adjuvant platform of IL-7 for cancer vaccines in preclinical and clinical trials. (A) Cancer vaccine tumor cells are transfected with the adjuvant IL-7 gene and then produce IL-7 stably. (B) Bacteria are transfected with the adjuvant IL-7 gene and then produce IL-7 stably. (C) Direct administration of human recombinant IL-7 as an adjuvant in combination with cancer vaccines. (D) IL-7 fused with the Fc region of the antibody as an adjuvant. This figure is created with BioRender.com and smart.servier.com.
However, the most common method to apply IL-7 as an adjuvant to enhance the antitumor effect of cancer vaccines in preclinical research was the injection of recombinant human IL-7 directly (83–86) (Figure 4C). Pellegrini and colleagues evidenced that IL-7 treatment in combination with a virus-based vaccine lead to a 3.5-10 fold increase in the number of CD4+ and CD8+ T cells in tumor-bearing mice, as well as higher infiltration levels of T lymphocytes in the tumor milieu compared with the PBS treatment group (83). Adjuvant IL-7 also contributed to the prolonged survival of activated T cells, enhanced effector responses, and increased production of cytokines, which boost vaccine-elicited immunity and survival in tumor-bearing mice. Furthermore, the same adjuvant effects of IL-7 were demonstrated to promote the efficacy of DC-based vaccines. Intriguingly, Pellegrini et al. also found that IL-7 possesses the capability to antagonize the Treg inhibitory network, which might promote antitumor immunity. The effects of recombinant human IL-7 as an adjuvant in augmenting the number of tumor-infiltrating T cells, specific antitumor immune responses, and survival time of tumor-bearing models also have been revealed in other published studies (84–86). Interestingly, Choi and colleagues novelly fused IL-7 with an Fc fragment to enhance the mucosal delivery across the genital epithelial barrier (Figure 4D), aiming at testing the antitumor effects of intravaginal administration of Fc-Fused IL-7 in combination with human papillomavirus (HPV) DNA vaccines against cervical cancer (80). Intravaginal administration of IL-7-Fc induces the recruitment of T cells and various cytokines and chemokines in cervicovaginal tissue, while IL-7 fails to do so. Furthermore, topical administration of IL-7-Fc in combination with the PHV vaccine increased a higher number of HPV-specific CD8+ T lymphocytes and exhibited a greater therapeutic effect against cervical cancer. This research highlighted the possibility to apply Fc-fused IL-7 as an adjuvant to strengthen the cellular immunity induced by vaccines in the genital mucosa to struggle against cancers.
However, the adjuvant capability of IL-7 contributing to cancer vaccines was not only limited to inducing a short-term antitumor effect but also stimulating a long-term T cell memory against cancers. Previous studies have reported that cancer vaccines in combination with IL-7 induced a higher proportion of specific CD8+ and CD4+ memory T cells in mice models bearing tumors and improve the functionality of specific CD8+ memory T cells by enhancing their IFN-γ secretion (81, 84). Furthermore, T cell memory elicited by cancer vaccines and IL-7 protected tumor-free mice model from a second tumor challenge (81, 85). Preclinical research highlighted that adjuvant IL-7, when combined with cancer vaccines, contributes to the generation and maintenance of T cell memory, which is the critical component to building up immune surveillance to restrict the recurrence and even metastasis of cancers. Therefore, the adjuvant value of IL-7 for cancer vaccines is further explored in clinical trials.
The advance of adjuvant IL-7 in combination with cancer vaccines in clinical trials
Preclinical studies have proved that IL-7 could serve as an ideal adjuvant for cancer vaccines to combat cancers. Therefore, based on these preclinical practices, several clinical trials utilizing IL-7 as an adjuvant for cancer vaccines to struggle against malignant diseases were initiated and some encouraging results have been achieved, as summarized in Table 2.
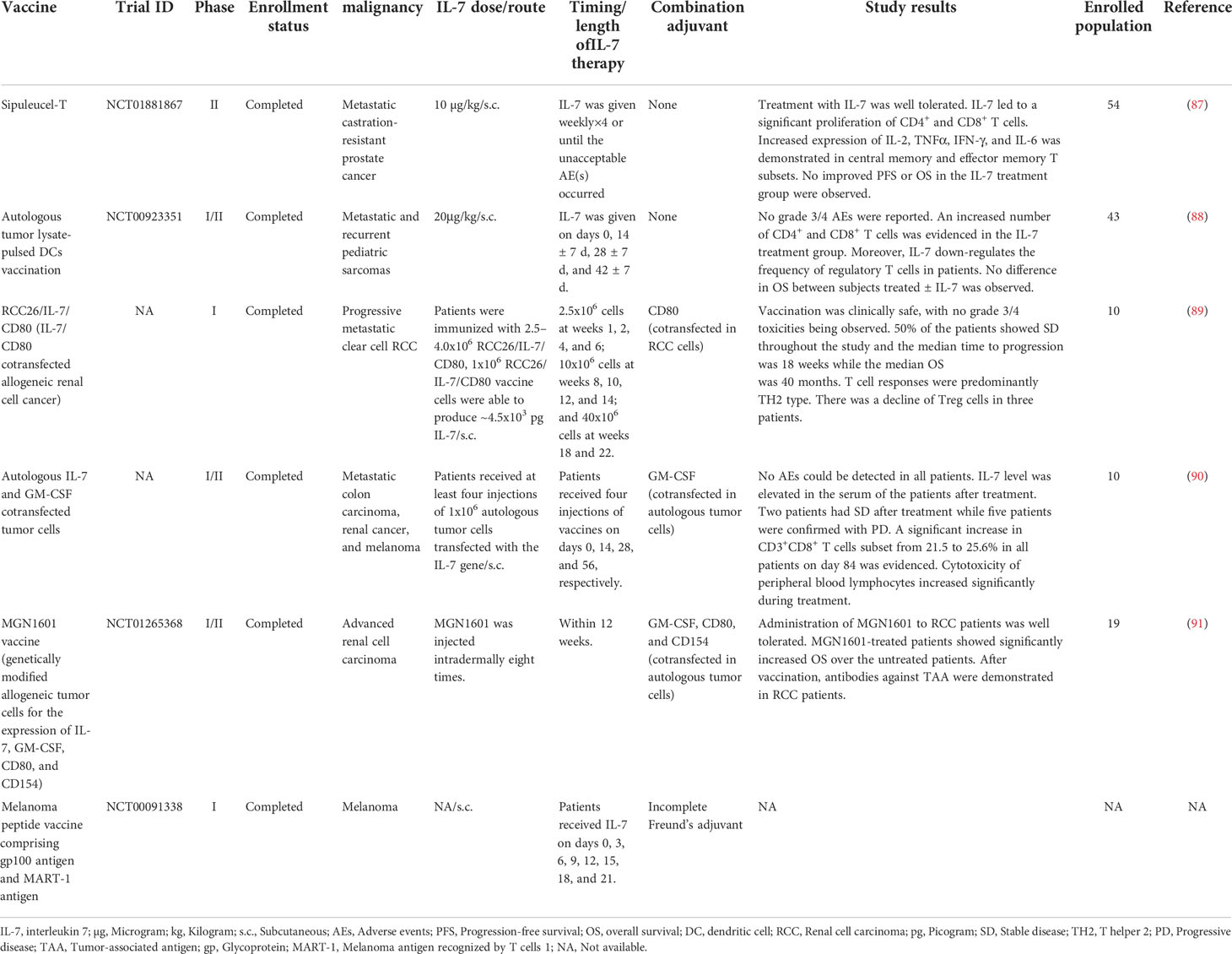
Table 2 Clinical trials of cancer vaccines combined with IL-7 as an adjuvant for the treatment of malignancy.
In the early stage, several clinical trials focus on the development of IL-7 gene-modified tumor cells as a cancer vaccine for patients with advanced malignant diseases (89–91). For instance, in a clinical phase I study launched by Westermann and colleagues, they immunized renal cell cancer (RCC) patients with IL-7 and CD80 genes cotransfected RCC tumor cells cancer vaccine (89). This vaccination has been proven to be feasible and safe with no grade III/IV adverse events (AEs) occurring. Throughout the trial, 5 out of 10 patients developed stable disease (SD) and the median time to progression was 18 weeks, while the median overall survival (OS) was 40 months in all. However, vaccination with IL-7 gene-modified RCC cells induced a TH2-predominant but not TH1-polarized immune response against RCC in most patients. In another phase I/II clinical trial, Wittig and colleagues cotransfected IL-7 and granulocyte-macrophage colony-stimulating factor (GM-CSF) genes in autologous tumor cells as a therapeutic vaccine to immunize patients with progressive metastatic carcinoma (90). This vaccine was clinically tolerable with no AEs being observed. Increased level of IL-7 was evidenced in the serum of the patients after treatment. At the endpoint of the study, 2 out of 10 patients achieved SD while 5 remained in progressive disease. The number of CD3+, CD8+, and CD56+ lymphocytes increased postvaccination. Intriguingly, the cytotoxicity of peripheral blood lymphocytes of patients elevated significantly during treatment, demonstrating that the IL-7-expressing tumor cell vaccine was immunological and capable to stimulate a robust immune response against metastatic cancers.
Recently, some clinical studies attempt to administer recombinant human IL-7 in cancer patients subcutaneously as an adjuvant for cancer vaccines (87, 88). In a phase I/II clinical trial (NCT00923351) (88), recombinant human IL-7 was administered to pediatric sarcoma patients on days 0, 14 ± 7 d, 28 ± 7 d, and 42 ± 7 d after receiving autologous tumor lysate-pulsed DCs vaccination. No grade III/IV AEs were reported during the treatment. Encouragingly, IL-7 recipients showed a higher number of circulating CD4+, CD8+ T cells, and NK cells than the subjects who did not receive IL-7, exhibiting the great potential of IL-7 to induce immunological reconstitution. Moreover, the administration of IL-7 decreased the proportion of regulatory T (Treg) cells in patients. Regrettably, there was no difference in OS between the patients treated ± IL-7. Another phase II clinical study (NCT01881867) (87), initiated by Pachynski et al., reported that subcutaneous administration of IL-7 resulted in the expansion of CD4+, CD8+, and γδ T cells in prostate cancer patients treated with sipuleucel-T, a therapeutic cancer vaccine proved by Food and Drug Administration (92). Furthermore, increased levels of intracellular cytokines were revealed in CD4+,γδ T cells, and NK cells. Notably, increased levels of IL-2, TNF-α, IFN-γ, and IL-6 were demonstrated in the memory subsets, indicating that IL-7 possesses the capability to stimulate the memory immune responses against cancers. Although the IL-7 group does show tails of the curves in both OS and progression-free survival (PFS), this trial failed to evidence a significantly improved OS and PFS in the IL-7 arm.
Taking these clinical data together, adjuvant IL-7 exhibited great potential in immunological reconstitution, including prompting expansion of T cells, inducing cytokine production, maintaining memory response, and arousing resistance to immune suppression, when combined with cancer vaccines to struggle against tumors. Moreover, IL-7 was clinically well-tolerant for cancer patients. Unfortunately, the clinical outcome of the patients who received the IL-7 treatment is still unsatisfying, with no enhanced OS or PFS have been demonstrated. However, the population enrolled in these clinical studies is limited. With more subjects included in trials, the survival benefits of the IL-7 arm might be evidenced statistically. More preclinical and clinical studies are encouraged to explore the potential role of IL-7 as an adjuvant for the development of cancer vaccines.
Proposal of IL-7 as an adjuvant for cancer vaccines in clinical practice
The early-stage clinical trials focus on designing tumor cell cancer vaccines expressing adjuvant molecular IL-7. At least 1x106 IL-7-cotransfected tumor cells were administrated subcutaneously in cancer patients with a minimum of four injections. However, in current clinical trials, direct administration of human recombinant IL-7 subcutaneously as an adjuvant in combination with cancer vaccines is the preferred method. The dose of IL-7 injection varies from 10 µg/kg to 20 µg/kg in cancer patients while at least four doses were administered. Since direct injection of IL-7 as an adjuvant has been proven to be safe and effective, IL-7-cotransfected tumor cell vaccine, which is labor- and time-consuming in manufacturing, seems to be unsuitable and inconvenient in clinical practice. Although direct administration of IL-7 as an adjuvant is more practical, the dose, routine, and time length of IL-7 therapy still need to be optimized according to furthermore clinical trials.
To achieve an effective and long-lasting T cell immune response against cancer to the greatest extent, a combination with other cytokines when utilizing IL-7 as an adjuvant agent for cancer vaccines is recommended. It is crucial to choose suitable chaperone adjuvants rather than a single agent to regulate a full palette of immune responses to combat tumors comprehensively. IL-2 and IL-7 have been well demonstrated to enhance the T cell responses (93), indicating the potential role of IL-2 in combination with IL-7 as adjuvants for cancer vaccines. Another cytokine, IL-15, was also well-defined to boost T cell responses, as well as to maintain long-lasting T cell memory against cancers (94). Intriguingly, antigen-activated T lymphocytes incubated with IL-7 and IL-15 exhibited a more robust capability to induce regression of melanoma and 4T1 mammary carcinoma in comparison to IL-2 alone (33, 95). Moreover, previous studies have indicated that the proliferation and maintenance of CD8+ memory T cells require IL-7 and IL-15 jointly (59–61). However, dose-related adverse events of administration of IL-2 and IL-15, like fever and serious biochemical abnormalities in the liver and kidney should not be ignored (96). Furthermore, IL-2 and IL-15 were evidenced to maintain immunosuppressive Treg cells in the periphery and induce the expression of immunosuppressive receptors, resulting in attenuated immune responses (97). To circumvent the immunosuppression induced by combined cytokines, ICB is suggested.
Although the antitumor role of IL-7 in combination with cancer vaccine has been revealed, there is still a “dark side” that needs to be emphasized. IL-7 was evidence to promote cell viability, cell cycle progression, and growth of T-cell acute lymphoblastic leukemia (T-ALL) in vitro (98). Moreover, in vivo experiments exhibited that the consumption of IL-7 contributes to the development of T-ALL, while IL-7 deficiency reduced the proliferation of leukemia cells (21, 99). Coincidently, IL-7 can also induce the expansion of B-ALL cells (100) and boost the tumorigenesis of B cells in IL-7 transgenic mice (101). These collected facts highly indicated that IL-7 acts as a tumor-promoting factor during the occurrence and progression of hematopoietic malignancy. Therefore, it is unpracticable to apply IL-7, a cytokine that contributes to leukemia development, as an adjuvant for cancer vaccines against hematopoietic malignancy for safety considerations.
Conclusion
Various factors affect the immunogenicity of cancer vaccines, like the selection of antigens (102), the choice of adjuvant (103), and even the delivery vehicles that are applied in the administration strategy (104). To stimulate an effective and long-lasting T cell memory response, cancer vaccines incorporated with adjuvant IL-7 to combat cancers were initiated in numerous experiments. The biological role of IL-7 in the development, survival, proliferation, and memory maintenance of T cells was summarized while the signaling of IL-7/IL-7R was summed up in this manuscript. These facts highlighted the feasibility to apply IL-7 as an adjuvant in combination with cancer vaccines. Preclinical researches bring inspiring results for the rationale to apply IL-7 as an adjuvant for cancer vaccines while further clinical trials enhance this notion. Although boosted T cell expansion and enhanced T cell memory were evidenced in clinical trials when administrating IL-7 as an adjuvant combined with cancer vaccines, the clinical outcomes of cancer patients are still far from satisfactory. Currently, it is still hard to give a conclusion that IL-7 can serve as an ideal adjuvant for cancer vaccines from such a limited number of clinical trials, therefore, clinical trials concerning IL-7 as an adjuvant for cancer vaccines, to fulfill efficacious T cell responses and long-lasting T cell memory against tumors, are urgently encouraged. Here, we also proposed some suggestions on how to utilize IL-7 as an adjuvant incorporated with cancer vaccines, including the form, dose, and chaperone cytokine of IL-7, which might be of value to researchers.
Author contributions
XL and ZX contributed to the study conception design. YZ, KW, and HC performed the literature search and data collection. Figures were drawn by YZ. YZ and KW prepared the draft and the manuscript was revised by XL and ZX. XL provided the funding. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 81871653), the Natural Science Foundation of Chongqing (cstc2021jsyj-yzysbAX0018), Chongqing Science and Health Joint Medical High-end Talent Project (2022GDRC012), Science and Technology Research Program of Chongqing Municipal Education Commission (KJZD-K202100402, KJQN201900449) and CQMU Program for Youth Innovation in Future Medicine (W0073).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sharma P, Allison JP. The future of immune checkpoint therapy. Science (2015) 348(6230):56–61. doi: 10.1126/science.aaa8172
2. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
3. Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer (2021) 21(6):360–78. doi: 10.1038/s41568-021-00346-0
4. Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science (2018) 359(6382):1355–60. doi: 10.1126/science.aar7112
5. Baxevanis CN, Perez SA, Papamichail M. Cancer immunotherapy. Crit Rev Clin Lab Sci (2009) 46(4):167–89. doi: 10.1080/10408360902937809
6. Bidram M, Zhao Y, Shebardina NG, Baldin AV, Bazhin AV, Ganjalikhany MR, et al. Mrna-based cancer vaccines: A therapeutic strategy for the treatment of melanoma patients. Vaccines (Basel) (2021) 9(10):1060. doi: 10.3390/vaccines9101060
7. Sahin U, Karikó K, Türeci Ö. Mrna-based therapeutics–developing a new class of drugs. Nat Rev Drug Discovery (2014) 13(10):759–80. doi: 10.1038/nrd4278
8. Tran T, Blanc C, Granier C, Saldmann A, Tanchot C, Tartour E. Therapeutic cancer vaccine: Building the future from lessons of the past. Semin Immunopathol (2019) 41(1):69–85. doi: 10.1007/s00281-018-0691-z
9. Liu Q, Sun Z, Chen L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell (2020) 11(8):549–64. doi: 10.1007/s13238-020-00707-9
10. Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer (2018) 6(1):87. doi: 10.1186/s40425-018-0399-6
11. Bowen WS, Svrivastava AK, Batra L, Barsoumian H, Shirwan H. Current challenges for cancer vaccine adjuvant development. Expert Rev Vaccines (2018) 17(3):207–15. doi: 10.1080/14760584.2018.1434000
12. Hunt P, Robertson D, Weiss D, Rennick D, Lee F, Witte ON. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell (1987) 48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2
13. Lundström W, Fewkes NM, Mackall CL. Il-7 in human health and disease. Semin Immunol (2012) 24(3):218–24. doi: 10.1016/j.smim.2012.02.005
14. Huang J, Long Z, Jia R, Wang M, Zhu D, Liu M, et al. The broad immunomodulatory effects of il-7 and its application in vaccines. Front Immunol (2021) 12:680442. doi: 10.3389/fimmu.2021.680442
15. Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol (2011) 12(11):1096–104. doi: 10.1038/ni.2112
16. Heufler C, Topar G, Grasseger A, Stanzl U, Koch F, Romani N, et al. Interleukin 7 is produced by murine and human keratinocytes. J Exp Med (1993) 178(3):1109–14. doi: 10.1084/jem.178.3.1109
17. Durum SK, Mazzucchelli RI. Live from the liver: Hepatocyte il-7. Immunity (2009) 30(3):320–1. doi: 10.1016/j.immuni.2009.03.001
18. Maeurer MJ, Walter W, Martin D, Zitvogel L, Elder E, Storkus W, et al. Interleukin-7 (Il-7) in colorectal cancer: Il-7 is produced by tissues from colorectal cancer and promotes preferential expansion of tumour infiltrating lymphocytes. Scand J Immunol (1997) 45(2):182–92. doi: 10.1046/j.1365-3083.1997.d01-384.x
19. Zhang W, Du JY, Yu Q, Jin JO. Interleukin-7 produced by intestinal epithelial cells in response to citrobacter rodentium infection plays a major role in innate immunity against this pathogen. Infect Immun (2015) 83(8):3213–23. doi: 10.1128/iai.00320-15
20. Kim GY, Hong C, Park JH. Seeing is believing: Illuminating the source of in vivo interleukin-7. Immune Netw (2011) 11(1):1–10. doi: 10.4110/in.2011.11.1.1
21. Barata JT, Durum SK, Seddon B. Flip the coin: Il-7 and il-7r in health and disease. Nat Immunol (2019) 20(12):1584–93. doi: 10.1038/s41590-019-0479-x
22. Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. Il-17 receptor-based signaling and implications for disease. Nat Immunol (2019) 20(12):1594–602. doi: 10.1038/s41590-019-0514-y
23. Akashi K, Kondo M, Weissman IL. Role of interleukin-7 in T-cell development from hematopoietic stem cells. Immunol Rev (1998) 165:13–28. doi: 10.1111/j.1600-065x.1998.tb01226.x
24. Hong C, Luckey MA, Park JH. Intrathymic il-7: The where, when, and why of il-7 signaling during T cell development. Semin Immunol (2012) 24(3):151–8. doi: 10.1016/j.smim.2012.02.002
25. Fry TJ, Mackall CL. Interleukin-7: From bench to clinic. Blood (2002) 99(11):3892–904. doi: 10.1182/blood.v99.11.3892
26. Ribeiro D, Melão A, van Boxtel R, Santos CI, Silva A, Silva MC, et al. Stat5 is essential for il-7-Mediated viability, growth, and proliferation of T-cell acute lymphoblastic leukemia cells. Blood Adv (2018) 2(17):2199–213. doi: 10.1182/bloodadvances.2018021063
27. Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, et al. M(6)a mrna methylation controls T cell homeostasis by targeting the il-7/Stat5/Socs pathways. Nature (2017) 548(7667):338–42. doi: 10.1038/nature23450
28. Cimbro R, Vassena L, Arthos J, Cicala C, Kehrl JH, Park C, et al. Il-7 induces expression and activation of integrin α4β7 promoting naive T-cell homing to the intestinal mucosa. Blood (2012) 120(13):2610–9. doi: 10.1182/blood-2012-06-434779
29. Ponte R, Rancez M, Figueiredo-Morgado S, Dutrieux J, Fabre-Mersseman V, Charmeteau-de-Muylder B, et al. Acute simian immunodeficiency virus infection triggers early and transient interleukin-7 production in the gut, leading to enhanced local chemokine expression and intestinal immune cell homing. Front Immunol (2017) 8:588. doi: 10.3389/fimmu.2017.00588
30. Vogt TK, Link A, Perrin J, Finke D, Luther SA. Novel function for interleukin-7 in dendritic cell development. Blood (2009) 113(17):3961–8. doi: 10.1182/blood-2008-08-176321
31. Meazza R, Azzarone B, Orengo AM, Ferrini S. Role of common-gamma chain cytokines in nk cell development and function: Perspectives for immunotherapy. J BioMed Biotechnol (2011) 2011:861920. doi: 10.1155/2011/861920
32. Kulkarni U, Herrmenau C, Win SJ, Bauer M, Kamradt T. Il-7 treatment augments and prolongs sepsis-induced expansion of il-10-Producing b lymphocytes and myeloid-derived suppressor cells. PloS One (2018) 13(2):e0192304. doi: 10.1371/journal.pone.0192304
33. Cha E, Graham L, Manjili MH, Bear HD. Il-7 + il-15 are superior to il-2 for the ex vivo expansion of 4t1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo. Breast Cancer Res Treat (2010) 122(2):359–69. doi: 10.1007/s10549-009-0573-0
34. Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in il-7 -/- mice. J Immunol (1996) 157(6):2366–73.
35. Shitara S, Hara T, Liang B, Wagatsuma K, Zuklys S, Holländer GA, et al. Il-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and tcrγδ+ intraepithelial lymphocytes. J Immunol (2013) 190(12):6173–9. doi: 10.4049/jimmunol.1202573
36. Bhatia SK, Tygrett LT, Grabstein KH, Waldschmidt TJ. The effect of in vivo il-7 deprivation on T cell maturation. J Exp Med (1995) 181(4):1399–409. doi: 10.1084/jem.181.4.1399
37. Boudil A, Matei IR, Shih HY, Bogdanoski G, Yuan JS, Chang SG, et al. Il-7 coordinates proliferation, differentiation and tcra recombination during thymocyte β-selection. Nat Immunol (2015) 16(4):397–405. doi: 10.1038/ni.3122
38. Seddon B, Zamoyska R. Tcr and il-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J Immunol (2002) 169(7):3752–9. doi: 10.4049/jimmunol.169.7.3752
39. Sinclair C, Saini M, Sakaguchi S, Seddon B. The long-term survival potential of mature T lymphocytes is programmed during development in the thymus. Sci Signal (2011) 4(199):ra77. doi: 10.1126/scisignal.2002246
40. Patra AK, Avots A, Zahedi RP, Schüler T, Sickmann A, Bommhardt U, et al. An alternative nfat-activation pathway mediated by il-7 is critical for early thymocyte development. Nat Immunol (2013) 14(2):127–35. doi: 10.1038/ni.2507
41. Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: Intelligent design. Nat Rev Immunol (2007) 7(2):144–54. doi: 10.1038/nri2023
42. Jourdan P, Vendrell JP, Huguet MF, Segondy M, Bousquet J, Pène J, et al. Cytokines and cell surface molecules independently induce Cxcr4 expression on Cd4+ Ccr7+ human memory T cells. J Immunol (2000) 165(2):716–24. doi: 10.4049/jimmunol.165.2.716
43. Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of il-7 in bone marrow transplant recipients: Relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant (1999) 23(8):783–8. doi: 10.1038/sj.bmt.1701655
44. Ponchel F, Cuthbert RJ, Goëb V. Il-7 and lymphopenia. Clin Chim Acta (2011) 412(1-2):7–16. doi: 10.1016/j.cca.2010.09.002
45. Raeber ME, Zurbuchen Y, Impellizzieri D, Boyman O. The role of cytokines in T-cell memory in health and disease. Immunol Rev (2018) 283(1):176–93. doi: 10.1111/imr.12644
46. Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med (2011) 208(3):505–18. doi: 10.1084/jem.20101824
47. Gao J, Zhao L, Wan YY, Zhu B. Mechanism of action of il-7 and its potential applications and limitations in cancer immunotherapy. Int J Mol Sci (2015) 16(5):10267–80. doi: 10.3390/ijms160510267
48. Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. Il-7-Induced proliferation of recent thymic emigrants requires activation of the Pi3k pathway. Blood (2007) 109(3):1034–42. doi: 10.1182/blood-2006-06-027912
49. Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhil-7 in humans increases in vivo tcr repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med (2008) 205(7):1701–14. doi: 10.1084/jem.20071681
50. Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. Il-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA (2001) 98(15):8732–7. doi: 10.1073/pnas.161126098
51. Venet F, Foray AP, Villars-Méchin A, Malcus C, Poitevin-Later F, Lepape A, et al. Il-7 restores lymphocyte functions in septic patients. J Immunol (2012) 189(10):5073–81. doi: 10.4049/jimmunol.1202062
52. Heninger AK, Theil A, Wilhelm C, Petzold C, Huebel N, Kretschmer K, et al. Il-7 abrogates suppressive activity of human Cd4+Cd25+Foxp3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. J Immunol (2012) 189(12):5649–58. doi: 10.4049/jimmunol.1201286
53. Andersson A, Yang SC, Huang M, Zhu L, Kar UK, Batra RK, et al. Il-7 promotes Cxcr3 ligand-dependent T cell antitumor reactivity in lung cancer. J Immunol (2009) 182(11):6951–8. doi: 10.4049/jimmunol.0803340
54. Habibi M, Kmieciak M, Graham L, Morales JK, Bear HD, Manjili MH. Radiofrequency thermal ablation of breast tumors combined with intralesional administration of il-7 and il-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast Cancer Res Treat (2009) 114(3):423–31. doi: 10.1007/s10549-008-0024-3
55. Williams MA, Bevan MJ. Effector and memory ctl differentiation. Annu Rev Immunol (2007) 25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548
56. Gerlach C, Rohr JC, Perié L, van Rooij N, van Heijst JW, Velds A, et al. Heterogeneous differentiation patterns of individual Cd8+ T cells. Science (2013) 340(6132):635–9. doi: 10.1126/science.1235487
57. Colpitts SL, Dalton NM, Scott P. Il-7 receptor expression provides the potential for long-term survival of both Cd62lhigh central memory T cells and Th1 effector cells during leishmania major infection. J Immunol (2009) 182(9):5702–11. doi: 10.4049/jimmunol.0803450
58. Terrazzini N, Mantegani P, Kern F, Fortis C, Mondino A, Caserta S. Interleukin-7 unveils pathogen-specific T cells by enhancing antigen-recall responses. J Infect Dis (2018) 217(12):1997–2007. doi: 10.1093/infdis/jiy096
59. Bradley LM, Haynes L, Swain SL. Il-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol (2005) 26(3):172–6. doi: 10.1016/j.it.2005.01.004
60. Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (Il)-15 and il-7 jointly regulate homeostatic proliferation of memory phenotype Cd8+ cells but are not required for memory phenotype Cd4+ cells. J Exp Med (2002) 195(12):1523–32. doi: 10.1084/jem.20020066
61. Chetoui N, Boisvert M, Gendron S, Aoudjit F. Interleukin-7 promotes the survival of human Cd4+ Effector/Memory T cells by up-regulating bcl-2 proteins and activating the Jak/Stat signalling pathway. Immunology (2010) 130(3):418–26. doi: 10.1111/j.1365-2567.2009.03244.x
62. Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. Il-7 and il-15 instruct the generation of human memory stem T cells from naive precursors. Blood (2013) 121(4):573–84. doi: 10.1182/blood-2012-05-431718
63. Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant il-7 or il-15 overcomes immunodominance and improves survival of the Cd8+ memory cell pool. J Clin Invest (2005) 115(5):1177–87. doi: 10.1172/jci23134
64. Nanjappa SG, Walent JH, Morre M, Suresh M. Effects of il-7 on memory Cd8 T cell homeostasis are influenced by the timing of therapy in mice. J Clin Invest (2008) 118(3):1027–39. doi: 10.1172/jci32020
65. Singh V, Gowthaman U, Jain S, Parihar P, Banskar S, Gupta P, et al. Coadministration of interleukins 7 and 15 with bacille calmette-guérin mounts enduring T cell memory response against mycobacterium tuberculosis. J Infect Dis (2010) 202(3):480–9. doi: 10.1086/653827
66. Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, et al. Cell biology of il-7, a key lymphotrophin. Cytokine Growth Factor Rev (2005) 16(4-5):513–33. doi: 10.1016/j.cytogfr.2005.05.004
67. Leonard WJ, Imada K, Nakajima H, Puel A, Soldaini E, John S. Signaling Via the il-2 and il-7 receptors from the membrane to the nucleus. Cold Spring Harb Symp Quant Biol (1999) 64:417–24. doi: 10.1101/sqb.1999.64.417
68. Mohamed MF, Beck D, Camp HS, Othman AA. Preferential inhibition of Jak1 relative to Jak3 by upadacitinib: Exposure-response analyses of ex vivo data from 2 phase 1 clinical trials and comparison to tofacitinib. J Clin Pharmacol (2020) 60(2):188–97. doi: 10.1002/jcph.1513
69. Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, et al. Impaired Cd8 T cell memory and Cd4 T cell primary responses in il-7r alpha mutant mice. J Exp Med (2007) 204(3):619–31. doi: 10.1084/jem.20061871
70. Zaunders JJ, Lévy Y, Seddiki N. Exploiting differential expression of the il-7 receptor on memory T cells to modulate immune responses. Cytokine Growth Factor Rev (2014) 25(4):391–401. doi: 10.1016/j.cytogfr.2014.07.012
71. Crawley AM, Vranjkovic A, Faller E, McGuinty M, Busca A, Burke SC, et al. Jak/Stat and Pi3k signaling pathways have both common and distinct roles in il-7-Mediated activities in human Cd8+ T cells. J Leukoc Biol (2014) 95(1):117–27. doi: 10.1189/jlb.0313122
72. Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med (2004) 200(9):1189–95. doi: 10.1084/jem.20041328
73. Lu L, Chaudhury P, Osmond DG. Regulation of cell survival during b lymphopoiesis: Apoptosis and bcl-2/Bax content of precursor b cells in bone marrow of mice with altered expression of il-7 and recombinase-activating gene-2. J Immunol (1999) 162(4):1931–40.
74. Venkitaraman AR, Cowling RJ. Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur J Immunol (1994) 24(9):2168–74. doi: 10.1002/eji.1830240935
75. Yang F, Chen E, Yang Y, Han F, Han S, Wu G, et al. The Akt/Foxo/P27(Kip1) axis contributes to the anti-proliferation of pentoxifylline in hypertrophic scars. J Cell Mol Med (2019) 23(9):6164–72. doi: 10.1111/jcmm.14498
76. Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. Il-7 promotes T cell proliferation through destabilization of P27kip1. J Exp Med (2006) 203(3):573–82. doi: 10.1084/jem.20051520
77. Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. Il-7 promotes Glut1 trafficking and glucose uptake Via Stat5-mediated activation of akt to support T-cell survival. Blood (2008) 111(4):2101–11. doi: 10.1182/blood-2007-06-096297
78. Chehtane M, Khaled AR. Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase ii gene. Am J Physiol Cell Physiol (2010) 298(6):C1560–71. doi: 10.1152/ajpcell.00506.2009
79. Jeong H, Lee SY, Seo H, Kim BJ. Recombinant mycobacterium smegmatis delivering a fusion protein of human macrophage migration inhibitory factor (Mif) and il-7 exerts an anticancer effect by inducing an immune response against mif in a tumor-bearing mouse model. J Immunother Cancer (2021) 9(8):e003180. doi: 10.1136/jitc-2021-003180
80. Choi YW, Kang MC, Seo YB, Namkoong H, Park Y, Choi DH, et al. Intravaginal administration of fc-fused Il7 suppresses the cervicovaginal tumor by recruiting hpv DNA vaccine-induced Cd8 T cells. Clin Cancer Res (2016) 22(23):5898–908. doi: 10.1158/1078-0432.CCR-16-0423
81. Gu YZ, Fan CW, Lu R, Shao B, Sang YX, Huang QR, et al. Forced Co-expression of il-21 and il-7 in whole-cell cancer vaccines promotes antitumor immunity. Sci Rep (2016) 6:32351. doi: 10.1038/srep32351
82. Zhao L, Mei Y, Sun Q, Guo L, Wu Y, Yu X, et al. Autologous tumor vaccine modified with recombinant new castle disease virus expressing il-7 promotes antitumor immune response. J Immunol (2014) 193(2):735–45. doi: 10.4049/jimmunol.1400004
83. Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, et al. Adjuvant il-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med (2009) 15(5):528–36. doi: 10.1038/nm.1953
84. Colombetti S, Levy F, Chapatte L. Il-7 adjuvant treatment enhances long-term tumor-Antigen-Specific Cd8+ T-cell responses after immunization with recombinant lentivector. Blood (2009) 113(26):6629–37. doi: 10.1182/blood-2008-05-155309
85. Li B, VanRoey MJ, Jooss K. Recombinant il-7 enhances the potency of gm-Csf-Secreting tumor cell immunotherapy. Clin Immunol (2007) 123(2):155–65. doi: 10.1016/j.clim.2007.01.002
86. Leonhartsberger N, Ramoner R, Putz T, Gander H, Rahm A, Falkensammer C, et al. Antigen-independent immune responses after dendritic cell vaccination. Cancer Immunol Immunother (2007) 56(6):897–903. doi: 10.1007/s00262-006-0245-4
87. Pachynski RK, Morishima C, Szmulewitz R, Harshman L, Appleman L, Monk P, et al. Il-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (Mcrpc). J Immunother Cancer (2021) 9(8):e002903. doi: 10.1136/jitc-2021-002903
88. Merchant MS, Bernstein D, Amoako M, Baird K, Fleisher TA, Morre M, et al. Adjuvant immunotherapy to improve outcome in high-risk pediatric sarcomas. Clin Cancer Res (2016) 22(13):3182–91. doi: 10.1158/1078-0432.CCR-15-2550
89. Westermann J, Florcken A, Willimsky G, van Lessen A, Kopp J, Takvorian A, et al. Allogeneic gene-modified tumor cells (Rcc-26/Il-7/Cd80) as a vaccine in patients with metastatic renal cell cancer: A clinical phase-I study. Gene Ther (2011) 18(4):354–63. doi: 10.1038/gt.2010.143
90. Wittig B, Märten A, Dorbic T, Weineck S, Min H, Niemitz S, et al. Therapeutic vaccination against metastatic carcinoma by expression-modulated and immunomodified autologous tumor cells: A first clinical phase I/Ii trial. Hum Gene Ther (2001) 12(3):267–78. doi: 10.1089/10430340150218404
91. Volz B, Schmidt M, Heinrich K, Kapp K, Schroff M, Wittig B. Design and characterization of the tumor vaccine Mgn1601, allogeneic fourfold gene-modified vaccine cells combined with a tlr-9 agonist. Mol Ther Oncolytics (2016) 3:15023. doi: 10.1038/mto.2015.23
92. Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, et al. Sipuleucel-T. Nat Rev Drug Discov (2010) 9(7):513–4. doi: 10.1038/nrd3220
93. Kim J-H, Lee K-J, Lee S-W. Cancer immunotherapy with T-cell targeting cytokines: Il-2 and il-7. BMB Rep (2021) 54(1):21–30. doi: 10.5483/BMBRep.2021.54.1.257
94. Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. Il-15 in the combination immunotherapy of cancer. Front Immunol (2020) 11:868. doi: 10.3389/fimmu.2020.00868
95. Le HK, Graham L, Miller CH, Kmieciak M, Manjili MH, Bear HD. Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in il-7 + il-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in il-2. Cancer Immunol Immunother (2009) 58(10):1565–76. doi: 10.1007/s00262-009-0666-y
96. Rosenberg SA. Il-2: The first effective immunotherapy for human cancer. J Immunol (2014) 192(12):5451–8. doi: 10.4049/jimmunol.1490019
97. Waldmann TA. The shared and contrasting roles of Il2 and Il15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol Res (2015) 3(3):219–27. doi: 10.1158/2326-6066.Cir-15-0009
98. Oliveira ML, Akkapeddi P, Ribeiro D, Melão A, Barata JT. Il-7r-Mediated signaling in T-cell acute lymphoblastic leukemia: An update. Adv Biol Regul (2019) 71:88–96. doi: 10.1016/j.jbior.2018.09.012
99. Silva A, Laranjeira AB, Martins LR, Cardoso BA, Demengeot J, Yunes JA, et al. Il-7 contributes to the progression of human T-cell acute lymphoblastic leukemias. Cancer Res (2011) 71(14):4780–9. doi: 10.1158/0008-5472.Can-10-3606
100. Digel W, Schmid M, Heil G, Conrad P, Gillis S, Porzsolt F. Human interleukin-7 induces proliferation of neoplastic cells from chronic lymphocytic leukemia and acute leukemias. Blood (1991) 78(3):753–9. doi: 10.1182/blood.V78.3.753.753
101. Rich BE, Campos-Torres J, Tepper RI, Moreadith RW, Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med (1993) 177(2):305–16. doi: 10.1084/jem.177.2.305
102. Zhao Y, Baldin AV, Isayev O, Werner J, Zamyatnin AA Jr., Bazhin AV. Cancer vaccines: Antigen selection strategy. Vaccines (Basel) (2021) 9(2):85. doi: 10.3390/vaccines9020085
103. Paston SJ, Brentville VA, Symonds P, Durrant LG. Cancer vaccines, adjuvants, and delivery systems. Front Immunol (2021) 12:627932. doi: 10.3389/fimmu.2021.627932
Keywords: IL-7, adjuvant, cancer vaccines, T cell immune response, T cell memory
Citation: Zhao Y, Wei K, Chi H, Xia Z and Li X (2022) IL-7: A promising adjuvant ensuring effective T cell responses and memory in combination with cancer vaccines? Front. Immunol. 13:1022808. doi: 10.3389/fimmu.2022.1022808
Received: 19 August 2022; Accepted: 17 October 2022;
Published: 28 October 2022.
Edited by:
Frederique Vegran, U1231 Lipides, Nutrition, Cancer (LNC) (INSERM), FranceReviewed by:
Dan Zhang, State Key Laboratory of Molecular Developmental Biology (CAS), ChinaLuciana D’Apice, National Research Council (CNR), Italy
Yishan Ye, Zhejiang University, China
Copyright © 2022 Zhao, Wei, Chi, Xia and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijia Xia, Zhijia.Xia@med.uni-muenchen.de; Xiaosong Li, lixiaosong@cqmu.edu.cn
†These authors have contributed equally to this work
 Yue Zhao
Yue Zhao Kongyuan Wei
Kongyuan Wei Hao Chi
Hao Chi Zhijia Xia
Zhijia Xia Xiaosong Li
Xiaosong Li