- 1The Clinical Department, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 2The Preventive Department, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 3Center of Research and Innovation of Chinese Traditional Veterinary Medicine, China Agricultural University, Beijing, China
Purpose: To emphasize the importance of tumor-associated macrophages (TAMs) in tumor immunity and to describe the ways in which extracts from Traditional Chinese Medicine (TCM) achieve tumor therapy by modulating macrophages.
Significance: By summarizing these available data, this review focused on TAMs and TCM and can build the foundation for future research on antitumor therapeutics.
Methods: In this review, we summarized the key functions of TAMs in cancer development and overviewed literature on TCM targeting TAMs together with other immune cells aiming to enhance antitumor immunity.
Conclusions: With an indispensable role in antitumor immunity, TAMs contribute to tumor progression, migration, invasion, angiogenesis, lymphangiogenesis, and immunosuppressive microenvironment. In recent years, TCM has gradually gained attention as a potential antitumor adjunctive therapy in preclinical and clinical trials. TCM is also a regulator of cytokine secretion and cell surface molecule expression in balancing the tumor microenvironment (TME), especially macrophage activation and polarization. Therefore, it is believed that TCM could serve as modifiers with immunomodulatory capability.
Introduction
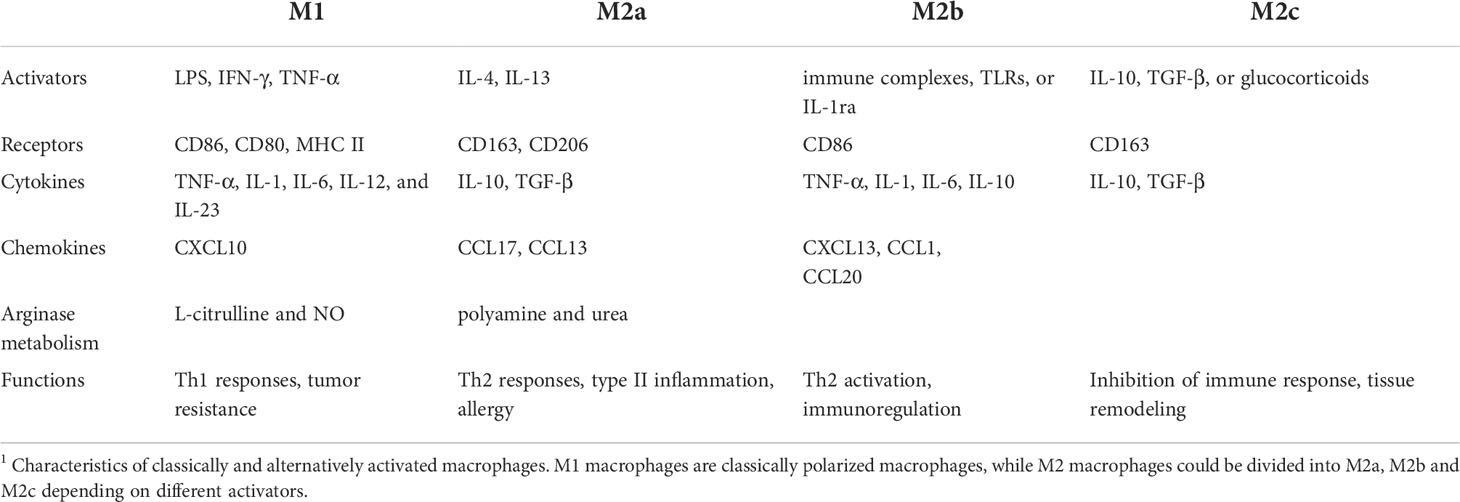
Macrophages are unique components of innate and adaptive immunity to defeat foreign pathogens and tumor cells (1). Tissue-resident macrophages spread through the blood and are usually immobile unless they are induced by stimulations (2). The initial state of tissue macrophages is called M0 macrophages, also known as Mφ macrophages, before being stimulated into the M1(classically activated state) or M2 phenotype (alternatively activated state). The phenotypes can be interchanged in response to various stimuli, (or activation) (3, 4) (Table 1). M1 macrophages are induced by Th1 cytokines, such as lipopolysaccharide (LPS), interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) and glucocorticoid. They highly express major histocompatibility complex (MHC) molecules and produce nitric oxide (NO), reactive oxygen species (ROS), and pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, and IL-12. Above all, M1 macrophages are considered to exert antitumor activity.
Initially, macrophages were simply divided into M1 and M2 two subtypes. With the later research, M2 macrophages could be divided into M2a, M2b, and M2c subtypes according to different activators. M2a macrophages are activated by IL-4 or IL-13, and M2b macrophages are stimulated by immune complexes. M2c macrophages are induced by IL-10 and transforming growth factor (TGF)-β. M2 macrophages secrete anti-inflammatory cytokines and chemokines, including a large amount of IL-10 and little of IL-12 as well as chemokine ligand (CCL)-17, CCL-18, CCL-22, vascular endothelial growth factor (VEGF), TGF-β, and Arginase1 (ARG1). Because of the different cytokines and chemokines they secreted, these three subtypes undertake different functions. M2a macrophages are responsible for Th2 responses, and M2b macrophages could also regulate immune status and therefore lead to the Th2 response. M2c macrophages could suppress immune responses and increase tissue remodeling (6). The dynamic character of phenotype allows macrophages to perform various functions. However, due to the complex internal and external environment, it is difficult to comprehensively summarize the types of macrophages by centralized classification. Therefore, when describing the subtypes of macrophages, it is favorable to choose a series of markers to replace the previous classification methods (3).
Tumor-associated macrophages (TAMs), which are exposed to the tumor microenvironment (TME), undergo M1-like or M2-like activation and then display tumor promoting or suppressing activities (7). When defining the classification of TAMs, we should analyze them in combination with the course of the tumor and the time of cell separation. Choose as many markers as possible, including surface proteins and intracellular proteomics (8), to jointly define the properties of macrophages. TAMs can express VEGF, TGF-β, angiogenesis chemokine CXCL12, and platelet-derived growth factor (PDGF), which promote the formation of partial blood vessels and lymphatic vessels of tumor and even further tumor invasion and migration. During tumor initiation, the infiltrated macrophages display the M1 phenotype, which secretes inflammatory cytokines to defeat tumor cells along with other immune cells (9). However, as cancer advances to the later stages, TAMs convert to the M2 phenotype and create an immunosuppressive microenvironment to support further cancer proliferation, invasion, and metastasis, leading to poor prognoses. However, it is difficult to classify them with specific markers, and a series of markers are typically used for classification (10). The biomarkers of tissue macrophages are complicated because of their distinct locations and functions. F4/80hi cells have been identified as the phenotypic definition of tissue macrophages in mice. Additionally, human macrophages exhibit characteristics that are similar to those of mice macrophages (11).
M1 macrophages express CD68, CD86, CD80, and high MHC class II complex. Scavenger receptor (SR), mannose receptor (MR), low MHC class II complex, and ARG1 are used as M2 phenotype markers (5).
Traditional Chinese Medicine (TCM) has developed for many years and is used as an adjuvant to chemotherapy. Many antitumor natural products come from TCM. However, little is known about its underlying mechanisms and bioactivities because of the complex components and chemical structure and the difficult extraction and purification processes. In addition to the direct cytotoxic effects on tumors, TCM plays various immunomodulatory roles in TME, including angiogenesis inhibition, cell-cycle arrest or apoptosis induction (12), and immune cell regulators, such as activating antigen-presenting cells (APCs) and enhancing NK cell-mediated killing activity. Overall, TCM presents the ability to inhibit tumor progression, angiogenesis, invasion, and metastasis (13).
In this article, we summarized and discussed the characteristics and functions of TAMs in the TME and the mechanisms of TCM targeting TAMs in cancer biological therapy. The evaluations of TCM and TAMs will guide new opportunities in cancer therapeutic strategies.
TAMs and tumor progression
TAMs play indispensable roles in tumor progression, including initiation, promotion, immune suppression, angiogenesis, invasion, and metastasis (14). In the early stages of the tumor, stromal cells secrete colony-stimulating factor (CSF)-1 and other factors to recruit macrophages, which are primarily antitumor M1-like macrophages. However, in the advanced stages, tumor cells secrete other anti-inflammatory cytokines and chemical factors, such as CCL-2 and epidermal growth factor (EGF), leading to the recruitment and conversion of TAMs from the M1 to the M2 phenotype. The flow cytometry results showed that macrophages from advanced stages of hepatic carcinoma were mostly MHC class IIlow TAMs, which were alternatively activated (15). It has also been confirmed that macrophages could be induced from M1 to M2 in a direct or indirect contact co-culture system with tumor cells (16, 17).
Cancer-related inflammation (CRI) refers to the relevance between the instability of the genome and inflammatory mediators, which are mainly composed of TAMs and other white blood cells, representing a hallmark of cancer. The fact that inflammation induces tumor progression through endogenous and exogenous pathways, suggests a relationship between the initiation of cancer and chronic inflammation caused by inflammatory cytokines produced by TAMs (18).
DNA damage could destroy the stability of genome stability. Poor DNA repair, apoptosis disorder, and radiotherapy or chemotherapy can lead to tumor initiation (19). Oxygen-free radicals have also been found to be critical in the initiation and progression of tumors (20). TAMs could produce IL-1 and TNF-α, promoting the formation of oxygen free radicals and further stimulating the macrophage response to other agonists (21). Meanwhile, the accumulation of reactive oxygen species (ROS) encourages macrophages to differentiate into a pro-inflammatory state, and therefore participate in the inflammation-induced tumorigenesis (22).
The density of the infiltrated TAMs and the M2/M1 ratio increases as tumors develop, leading to a poor prognosis (23). For example, the high proportion of CD163+ tumor infiltrated macrophages is related to the poor clinical prognosis in clear cell renal cell carcinoma (RCC) (24), which is supported by another report where the decrease of macrophages partially inhibited the growth of hepatocellular carcinoma (HCC) (15). Clinical datasets show that the overall survival rate of patients with positive expression of M2 macrophages was significantly lower than patients with negative expression. Tian et al. (25) found that among patients with Wilms’ tumor, longer survival time is correlated with a lower density of M2 phenotype macrophages, suggesting that the M2 macrophage index could be a predictor in the pathological examination. CD11c/CD206 signature is associated with macrophage polarization and can be used as an index to predict the prognosis. A CD11chigh/CD206low immune profile leads to a favorable outcome (26). Moreover, TAMs with the M2 phenotype could even affect the efficacy of chemotherapy and radiotherapy through the suppression of T cells (27).
TAMs in invasion and metastases
The protease produced or induced by invasive tumor cells can degrade the extracellular matrix (ECM). Thus, the invasion and migration of tumor cells are significantly enhanced compared to those of normal cells. In tumor stroma, TAMs produce enzymes, such as matrix metalloproteinases (MMPs) and urokinase fibrinolytic enzymes (uPA) to promote matrix degradation, and hence the invasion and metastasis of tumor cells (28).
As one of the MMPs, MMP-9 is a paracrine regulator of tumor progression (29) that degrades ECM, destructs the basement membrane, and spreads cancer through the circulatory system (30). The secretion of MMP-9 and VEGF by M2 phenotype TAMs was notably higher than that by M1 macrophages (31). It has been identified that MMPs are involved in the degrading and remodelling process of ECM (32), and induce epithelial-mesenchymal transition (EMT) by decomposing the adhesion molecules (33). EMT, a process that transits immotile cells to motile mesenchymal cells and therefore weakens the tight junction of tumor cells (34). Within these pathways, the transforming growth factor-β(TGF-β) is the primary regulator, which is also the key factor facilitating the proliferation and differentiation of TAMs (35). As demonstrated in gastric carcinoma (36) and hepatocellular carcinoma (37), EMT is related to the high infiltration of TAMs, which produce higher TGF-β1 than macrophages with other phenotypes.
TAMs in angiogenesis and lymphangiogenesis
Inducing angiogenesis and lymphangiogenesis is one of the major characteristics of tumor cells, the symbol of tumor expansion to distant metastasis. TAMs regulate tumor angiogenesis and lymphangiogenesis in two approaches: paracrine and cell autonomous mode (38). As the tumor proliferates, the supply of oxygen becomes insufficient, generating a hypoxia tumor microenvironment. Macrophages are recruited to the regions between tumor and interstitial cells where vascularization is poor (39). After being stimulated by hypoxia-inducible factor (HIF-1α), TAMs release a set of angiogenic cytokines, such as vascular endothelial growth factors (VEGF)-A (40), TGF-β, CXCL12, PDGF, and MMPs (7), which in turn promote tumor angiogenesis (40). At the same time, macrophages deliver more VEGF-receptors (VEGFRs) under hypoxia to combine with VEGF in the TME, which affects downstream pathways and promotes the transformation of TAMs to M2 phenotype. TAMs could also activate endothelial cells in cervical carcinoma, which highly express VEGF-C and VEGF-D, and stimulate existing lymphatic endothelial cells’ proliferation (41). Furthermore, the existence of macrophage-derived lymphatic endothelial cell progenitors (M-LECP) has proved the autonomous mode. Under the stimulation of inflammatory factors, M-LECP could differentiate into lymphatic endothelial cells (LEC), contributing to pre-existing lymphatic vessels and subsequent lymphogenesis (38).
TAMs could overexpress HIF-1 and HIF-2, further up-regulating CXCL12. CXCL12 was found to be critical in enhancing the GM-CSF/Heparin-binding epidermal growth factor (HB-EGF) paracrine loop of colon cancer metastases in the liver, advancing tumor anti-apoptosis and the recruitment of TAMs (42). CXCL12 has also been identified to promote monocytes to differentiate into CD163+ macrophages and increase the expressions of VEGF and angiogenic chemokine CCL1 (43). To overcome the hypoxia and immunosuppress of the TME, a biomimetic nano-RBC system (V(Hb)) combined with hemoglobin–poly(ϵ-caprolactone) (Hb–PCL) and doxorubicin (V(Hb)@DOX)) was engineered. V(Hb)@DOX could effectively limit the recruitment of CD163+ M2-type macrophages and improve tumor hypoxia by reducing HIF-1α expression. Furthermore, the alleviation of the immunosuppressive TME decreased the secretion of MMP-9 and VEGF-A in tumors, which in turn inhibited tumor growth and metastasis (44).
TAMs and immunosuppressive microenvironment
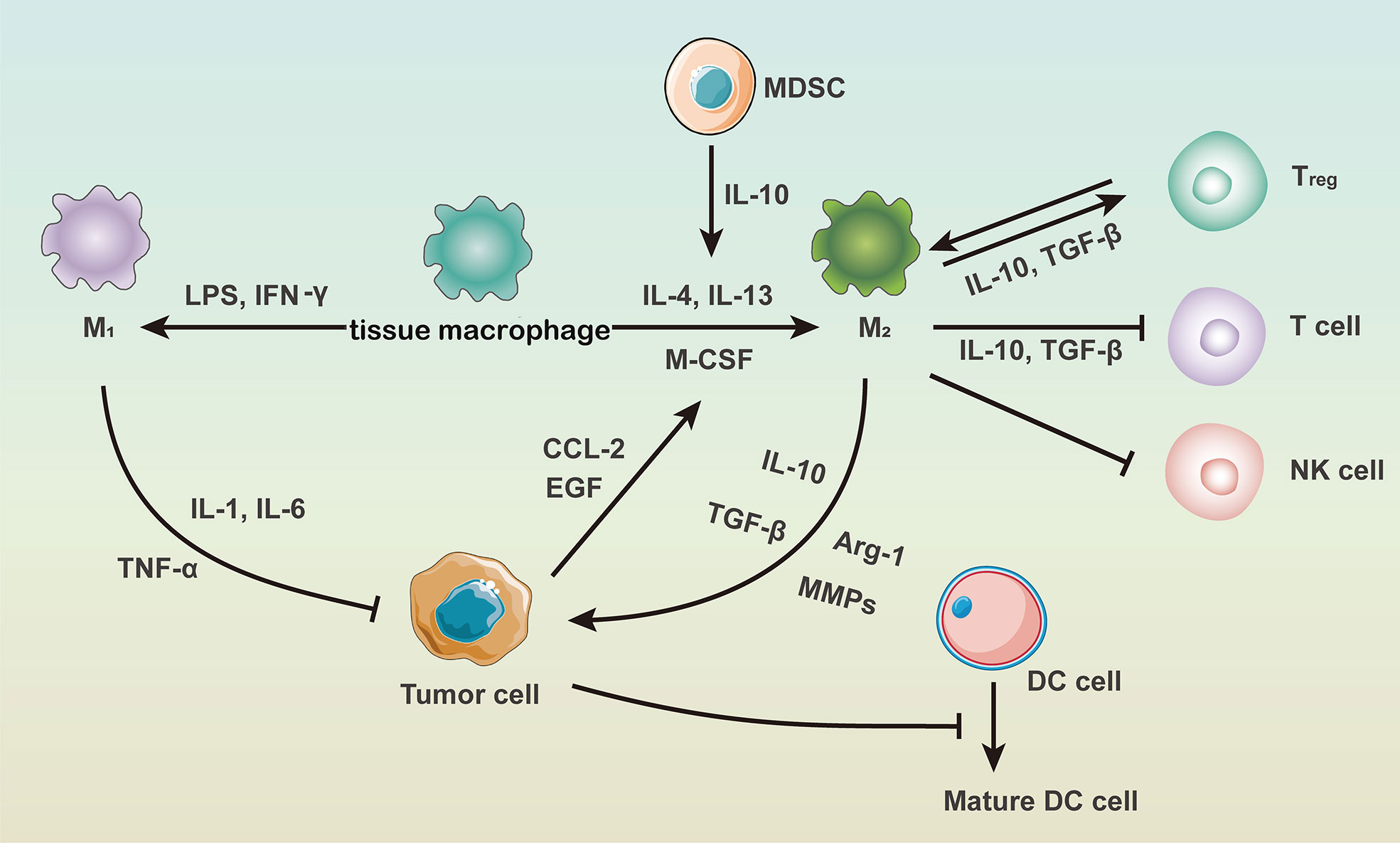
The immunosuppressive tumor microenvironment consists of tumor cells, endothelial cells, fibroblasts, ECM, and immune cells et al. Immune cells therein include macrophages, dendritic cells (DCs), T cells, B cells, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells and regulatory T cells (45). As chronic inflammation is essential in the immunosuppressive microenvironment, immune cells and inflammatory factors highly interacted with each other (46), which are summarized in Figure 1. It has been proposed that IL-10 secreted by MDSCs could down-regulate IL-12 produced by macrophages and thus induce macrophage polarization into the M2 phenotype (47).
TAMs restrain T cell-specific response in various aspects according to the recent findings. Extracellular vesicles (EVs), isolated from M2 phenotype macrophages, crippled CD8+ T cell proliferation, and killing activity, leading to tumor immune evasion in murine hepatocellular carcinoma (48), colon cancer (49) and gastric cancer (50). TAMs depletion with a nanocarrier named BLZ-945SCNs/Pt, which delivers both CSF-1R inhibitor-BLZ-945 and platinum (Pt)-prodrug, can achieve the synergistic antitumor activity of chemoimmunotherapy. The decrease in TAMs significantly reduced the expressions of TAMs-derived VEGF-A and MMP-9 and further less lung metastasis, which demonstrated the therapeutic efficacy of targeting TAMs in tumors (51). Sun (52) reported that Doxorubicin (DOX)-loaded micelles with a hemoglobin crown (Hb-DOXM) have also achieved significant antitumor effects in reprograming the immunosuppressive microenvironment into the immunostimulatory microenvironment by augmenting the release of O2 and DOX and reducing the recruitment of M2-type macrophages in tumors.
For one thing, TAMs act as T cell activators using their surface MHC I or II molecules also by producing cytokines. For another, TAMs could induce T cell inhibition and exhaustion. Through direct and indirect regulations, TAMs are important in each of three steps to activate T cell response: specific binding of T cell receptors and MHC molecules on TAMs, costimulatory molecule signaling pathways, and environmental cytokines derived from TAMs (53). TAMs could secrete IL-10 to induce the expression of inhibitory receptors by T cells, such as programmed death (PD)-1 and cytotoxic T lymphocyte-associated antigen (CTLA)-4. The bindings of receptors and the corresponding ligands (PD-L1 and CD80/CD86) on the surface of TAMs, lead to negative regulations of T cell immune response, including apoptosis, anergy, and exhaustion (54).
Regulatory T cells can also weaken the immune functions of CD4+ and CD8+ T cells (55). Thymus-derived CD4+CD25+Foxp3+ regulatory T cells could increase the percentage of CD206+ and CD163+ macrophages differentiated from monocytes and up-regulate CCL18 and IL-1Ra produced by macrophages (56). Moreover, TAMs and DCs could increase the production of IL-10 and TGF-β, which transform naïve T cells into regulatory T cells (55) and further inhibit the antitumor immunity contributed by NK cells.
In summary, targeting the regulation of immune cell balance and augmenting tumor immunity in the microenvironment has always been the focus of tumor immunotherapy.
TAMs in canine tumors
Similar to human tumors, many studies suggest that TAMs have a relationship with the grading of malignant tumors in veterinary science.
In canine lymphoma, tumor-infiltrating macrophages could be characterized as M1 and M2 according to iNOS, CD204 and CD163. As shown in the immunohistochemical results, the type of macrophages changed from M1 to M2 in the high histological grade. Among the two immunophenotypes of lymphomas, type B and type T, M2 macrophages have a dominant position in T-type lymphoma (57). Unlike human tumors, in many cases, it has been reported that CD204 is a better choice than using CD163 as the marker of the M2 macrophage subtype in canine tumors (58).
In canine mammary gland tumors, another result supported that tumor-infiltrating M2 macrophages have been correlated with the grading of malignant lesions (59). Furthermore, the high density of TAMs in canine mammary tumors has also been considered as a poor prognosis (60). TAMs also have a significant relationship with the expression of VEGF in canine mammary tumors, suggesting that TAMs synergistically promote tumor angiogenesis (61). Above all, TAMs may act as a potential target in the therapy of canine mammary tumors.
TAMs regulated by TCM
Many studies supported that TCM plays an important role in cancer treatment, including promoting immune function, activating immune cells, enhancing the efficacy of antineoplastic, and reducing the side effects of radiotherapy and chemotherapy (62). Some kinds of TCM can directly inhibit the proliferation of tumor cells, while others suppress tumor growth, invasion, and metastasis indirectly by indirectly regulating the immune system (63). Vincristine and paclitaxel are common commercialized chemotherapeutics extracted from TCM in clinical applications. According to National Medical Products Administration, there are more extractions from TCM that are used as adjuvant therapies with radiotherapy and chemotherapy, such as lentinan, krestin et al. Since the 1980s, both China and Japan have approved the use of the mushroom polysaccharide, lentinan, as an adjuvant medicinal medication for the treatment of cancers. Lentinan was mainly used in treating lung (64), gastric, and colorectal cancers as adjuvant therapies and exhibited better efficacy and clinal response rates, as well as improved the quality of life of cancer patients, according to a review that summarized 9474 reported lentinan-associated cancer treatment cases (65). Additionally, polysaccharide-kureha (PSK), also known as Krestin, was authorized for the treatment of various cancers (66). PSK is frequently given orally, either alone or in combination with other drugs. Together with tegafur/uracil (UFT), PSK significantly increased stage II and stage III colorectal cancer patients’ 5-year disease-free survival and reduced the risk of recurrence and lung metastases (67).
The common active components from TCM with macrophage regulatory effects are glycosides, alkaloids, and polysaccharides, which could activate MAPKs, MyD88, and NF-κB related pathways by one or more receptors. The downstream phagocytic activity, ROS, NO, and relevant anti-tumor cytokines of TAMs are further enhanced accounting for the complete antitumor immune regulation (68).
TCM activates antitumor phenotype and inhibits tumor-promoting phenotype of TAMs
TCM regulates macrophages in various ways, including activating anti-tumor macrophages, inhibiting the recruitment and activation of TAMs, transforming the phenotype of TAMs, and indirectly regulating TAMs by altering cytokine secretions in the tumor microenvironment.
It has been proven that acidic polysaccharides from Plantago major leaves could activate J774 macrophages and increase the release of NO and TNF-α (69). The polysaccharide extracts from Plantago depressa have also been shown as an immunomodulatory agent by promoting lymphocyte proliferation and NO production (70). Emodin inhibited the expressions of CCL2 and CSF1, which were involved in the differentiation of macrophages (71). It could also reduce the growth of EO771 and 4T1 breast tumor cells by suppressing macrophage migration and polarization, and inhibiting IRF4 and C/EBPβ signalings (72). Although astragalus polysaccharide (APS) could not inhibit the MCF-7 cell viability directly, it could activate RAW264.7 cells and up-regulate the production of NO and TNF-α, to induce the apoptosis of breast tumor cells (63). Ginseng polysaccharide (GPS) was shown to have similar functions as APS and exerted a cytotoxic effect against mice tumor cells via activating the peritoneal macrophages (PMs) rather than direct cytotoxicity (73).
Furthermore, TCM could cooperate with radiotherapy and chemotherapy, to enhance the curative effect of both and meanwhile alleviate the common side effects. A kind of water-extracted polysaccharides from Fuzi was found to promote the phagocytic activity and the release of NO, IL-6, IL-1, and TNF-α in RAW264.7 cells. Also, it had the ability to reverse the spleen index and thymus index in cyclophosphamide-induced immunosuppressed mice, demonstrating its possible application in antitumor therapy as an immunomodulator (74). Interestingly, not all TCM act as anti-tumor agents. Methanol extracts of Xanthium sibiricum roots (MXS) inhibit the NO, IL-6, IL-1β and TNF-α by suppressing IκBα and STAT3 signaling pathways in LPS-induced RAW264.7 macrophages (75).
Not only the monomers of TCM but also some complex TCM formulas have been proved to have macrophage-regulating functions. Bu-Fei Decoction (BFD), a conventional TCM constituted of six herbs, is often used for tonification and alleviating symptoms of lung cancer. In non-small cell lung cancer (NSCLC), BFD decreased the expressions of IL-10, PD-L1, and CD206 in TAMs induced in vitro by PMA and IL-4. Besides, BFD exhibited a dose-dependent inhibition of the invasion and migration of NSCLC cells via downregulating IL-10 and PD-L1 both in vivo and in vitro (76).
TCM and macrophage polarization
M1-like TAM is the dominant phenotype suppressing tumor growth in the initial immune microenvironment of the tumor, but the M2 phenotype gradually replaces its leading position by recruiting tumor cells as the tumor advances to the later stages (7). In the tumor microenvironment, TAMs can switch between M1 and M2 states depending on the different signal inductions (13). Hence, finding new approaches to change TAMs from M2 to M1 phenotype could assist the antitumor immunity and prevent tumors from immune escape.
Astragalus polysaccharide (PG2) has been indicated as a modifier of macrophage polarization in NSCLC both in vivo and in vitro. PG2 enhanced the M1 polarization and reduced the CD206+ M2 cells in a dose-dependent manner. Also, PG2 could inhibit the tumor enhancement (including proliferation, clonogenicity to form tumorspheres, and invasion via IL-6/STAT3 signaling suppression) from a stem-cell-like phenotype of NSCLC induced by M2 macrophage. Furthermore, PG2 prominently strengthened the tumor-suppressive effect of cisplatin in NSCLC tumor-bearing mice models, but also alleviated dysuria and weight loss caused by cisplatin (77). Macrophages play an important role in baicalin-mediated inhibition of hepatocellular carcinoma (HCC). They were re-programmed towards the M1 phenotype to prevent tumor cells from immune escape, which is characterized as the descending proliferation and invasiveness of HCC cells. This repolarization was related to the autophagy-associated up-regulation of RelB/p52 (78).
A novel polysaccharide WCCP-N-b isolated from Cantharellus cibarius can induce M2-like bone marrow-derived macrophages (BMDMs), mouse peritoneal macrophages, and RAW264.7, to M1 phenotype. After being treated by WCCP-N-b, macrophages affected melanoma cell viability via increasing the production of TNF-α, which was cytotoxic to tumor cells (79). Water extract of ginseng and astragalus (WEGA) is reported to promote macrophages to express M1 markers and down-regulate M2 marker expressions simultaneously. Furthermore, WEGA also promoted immune responses, which were suppressed by cisplatin (62).
Emodin has received much attention due to its inhibiting effect on TAMs and its antitumor activity. It has been found that Emodin could inhibit IRF4, STAT6, and C/EBPβ signaling pathways in vivo to suppress macrophage infiltration and M2 polarization accompanied by T-cell activation, and therefore reduce breast cancer growth (72). Moreover, Emodin significantly inhibited breast cancer lung metastasis by inhibiting M2 polarization in metastatic lungs (80). Emodin inhibited the activation of NF-κB, STAT1, and IRF5 signaling pathways induced by LPS/IFNγ, and the stimulation of STAT6 and IRF4 signaling pathways stimulated by IL4 (81). Taken together, Emodin adopts an inhibitory effect on tumor growth by restoring macrophage homeostasis in the tumor-suppressive immune microenvironment.
TCM and TME regulation
Some TCMs have direct cell killing effects, while most have lower cytotoxicity, but could enhance the bioactivity of immune cells or inhibit immunosuppressive cells for anti-tumor purposes.
Evidence showed that a polysaccharide extracted from the whole plant of Plantago Asiatica L. could recruit immune cells (DCs, macrophages, and T cells) in the murine breast tumor model and accelerate the maturation of DCs, which promoted the proliferation and differentiation of T cells. Plantain polysaccharides had no direct cytotoxicity to breast tumor cells. However, it inhibited tumor growth by promoting the autoimmune response in mice (82). Modified citrus pectin (MCP) has been identified to accelerate the activation of the T-lymphocyte subset, B cells, and NK cells (83). Astragalus polysaccharide (PG2) not only regulated the macrophage phenotype but also promoted the maturation of immature DCs and recruitment of CD8+ T cells for anticancer immune response in NSCLC (77).
Besides, many studies showed a close relationship between immunosuppressive cells and TCM. TCM could suppress the recruitment and metastasis of immunosuppressive cells when tumor-promoting immune cells are dominant in quantity and function. Silibinin, extracted from milk thistle, dwindled tumors in 4T1 tumor-bearing mice by decreasing MDSCs infiltration and M2-like polarization of macrophages. However, in an immunodeficient mouse model, similar efficacy was not observed, suggesting the anti-tumor response of Silibinin was based upon the integrity of the immune system (84). Maitake D (MD)-Fraction, a β-glucan extracted from Grifola frondose, inhibited the growth of mammary carcinoma and colonic adenocarcinoma cells and enhanced immune cell infiltration in the tumor microenvironment, including T cells, B cells, DCs and NK cells. DC maturation, specific T cell responses, and the infiltration and anergy of Tregs and MDSCs were induced by orally administered MD-Fraction, suggesting the significance of converting immunosuppressive elements of the TME in tumor immunotherapy (85).
Not only polysaccharides from TCM could enhance the immune system, but other types of natural products, including terpenes, alkaloids, saponins, and flavonoids, also have the ability. Andrographolide, an isoprenoid extracted from Andrographis paniculate, has been reported to exhibit cytotoxicity to nearly all kinds of cancer cells and mediation of the immune system (86). In another study, Andrographolide released a high level of IL-2 and IFN-γ, promoted cytotoxic T lymphocyte (CTL) production and prolonged the survival time of mice bearing lymphoma (87).
TCM delivery system
Considering the instability and low bioavailability of active components from natural TCM, it is quite challenging to apply them directly in vivo. In recent years, the rapid development of nano-drug delivery systems has made it possible to deliver TCM or employ TCM as drug carriers for cancer treatment. A growing amount of TCM has been delivered to tumor tissues and their stroma through nano-drug delivery, such as liposome, or precision targeted therapy and immune regulation. Astragalus polysaccharide liposome (APSL) has been demonstrated to enhance the phagocytosis of murine peritoneal macrophages and speed up the DC-mediated immune reactions compared to applying AP alone (88). Moreover, cell-membrane-coated nanoparticles showed high efficiency in passing through the biofilm barrier and slowing down the metabolism of the loaded drugs. A novel macrophage-biomimetic drug delivery system carrying Saikosaponin D was reported to inhibit cell migration of MCF-7 and 4T1 cells in vitro and significantly reduced tumor growth and lung and spleen metastasis by promoting dephosphorylation of AKT and Erk in tumor-bearing mice (89).
TCM and canine tumors
The application of TCM in human treatment has gradually increased because of its chemopreventive and chemotherapeutic effects. However, its role in the small animal clinical field should not be underestimated. TCM could also be considered as an approach for clinical therapy to inhibit the growth of canine tumor cells. For example, Paclitaxel has been widely used to treat lung, ovarian, and breast cancer. It was reported to inhibit the migration of canine hemangiosarcoma (HSA) cells with the increase of time and concentration (90). In canine melanoma cells, oral paclitaxel was also tested to decrease the proliferation of tumor cells both in vivo and in vitro by arresting cell cycle (91). Canine mammary tumors are common among female dogs and the risks of malignancy are relatively high. BmKn-2, a peptide extracted from the venom of scorpions, has been proved to have antitumor activity in both human and canine tumor cells. It inhibited canine mammary gland tumor cell proliferation via inducing apoptosis, which was represented by the decrease of Bcl-2 to Bax ratios (92). Besides the direct tumor-killing effect, TCM could contribute to the immunomodulatory effects targeting immune cells and consequently hinder tumor progress. Our team has been focused on the study of TCM in antitumor immunity regulations and proved that although Plantain polysaccharide (PLP) showed no cytotoxicity to canine mammary cells (CIPp), conditioned medium obtained from PLP to DCs had inhibitory effects on CIPp cells. Moreover, it could promote the maturation of DCs and thus facilitate the proliferation of lymphocytes, which exert the main toxicity effect (82). All in all, TCM is of great significance in regulating animals with poor immune status, especially for the tumor patients.
Conclusions
Overall, TAMs could suppress anti-tumor immunity by promoting cancer proliferation, invasion, metastases, and angiogenesis. Besides, TAMs contribute to the immunosuppressive microenvironment to further advance cancer development. TCM has been proved to be an effective method for reprogramming TAMs and other immune cells and turning the immunosuppressive microenvironment into an antitumor one. In this study, we provide support for further studies on antitumor immunity and immunotherapy.
Author contributions
Writing—original draft preparation, JZ and JC. Writing—review and editing, JL and JG. Working concept and design, YJ and YW. Data collation, DZ. Supervision, JL and DL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Nature Science Foundation of China (Grant no. 31972730), the 2115 Talent Development Program of China Agricultural University (Grant no. 00109023), and the Special Fund Project of Fundamental Scientific Research Business Expenses of China Agricultural University (Grant no. 2020TC009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest (2012) 122:787–95. doi: 10.1172/JCI59643
2. Gordon S. Alternative activation of macrophages. Nat Rev Immunol (2003) 3:23–35. doi: 10.1038/nri978
3. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
4. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-Macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol (2006) 177:7303–11. doi: 10.4049/jimmunol.177.10.7303
5. Hao N-B, Lü M-H, Fan Y-H, Cao Y-L, Zhang Z-R, Yang S-M. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol (2012) 2012:1–11. doi: 10.1155/2012/948098
6. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
7. Salmaninejad A, Valilou SF, Soltani A, Ahmadi S, Abarghan YJ, Rosengren RJ, et al. Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell Oncol (2019) 42:591–608. doi: 10.1007/s13402-019-00453-z
8. Li P, Hao Z, Wu J, Ma C, Xu Y, Li J, et al. Comparative proteomic analysis of polarized human THP-1 and mouse RAW264.7 macrophages. Front Immunol (2021) 12:700009. doi: 10.3389/fimmu.2021.700009
9. Najafi M, Hashemi Goradel N, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N, et al. Macrophage polarity in cancer: A review. J Cell Biochem (2019) 120:2756–65. doi: 10.1002/jcb.27646
10. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep (2014) 6:13. doi: 10.12703/P6-13
11. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat Rev Immunol (2014) 14:571–8. doi: 10.1038/nri3712
12. Parekh HS, Liu G, Ming QW. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer (2009) 8:21. doi: 10.1186/1476-4598-8-21
13. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity (2016) 44:450–62. doi: 10.1016/j.immuni.2016.02.015
14. Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol (2020) 11:1731. doi: 10.3389/fimmu.2020.01731
15. Wang B, Li Q, Qin L, Zhao S, Wang J, Chen X. Transition of tumor-associated macrophages from MHC class II(hi) to MHC class II(low) mediates tumor progression in mice. BMC Immunol (2011) 12:43. doi: 10.1186/1471-2172-12-43
16. Solís-Martínez R, Cancino-Marentes M, Hernández-Flores G, Ortiz-Lazareno P, Mandujano-Álvarez G, Cruz-Gálvez C, et al. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol Lett (2018) 196:140–8. doi: 10.1016/j.imlet.2018.02.009
17. Jin X, Su H, Xu L, Wang Y, Su R, Zhang Z, et al. Different co-culture models reveal the pivotal role of TBBPA-promoted M2 macrophage polarization in the deterioration of endometrial cancer. J Hazardous Mater (2021) 413:125337. doi: 10.1016/j.jhazmat.2021.125337
18. Francesco C, Paola A, Antonio S, Cecilia G, Alberto M. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis (2009) 30:1073–81. doi: 10.1093/carcin/bgp127
19. Hoeijmakers JHJ. DNA Damage, aging, and cancer. New Engl J Med (2009) 361:1475–85. doi: 10.1056/NEJMra0804615
20. Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer (1996) 32:30–8. doi: 10.1016/0959-8049(95)00531-5
21. Ward PA, Warren JS, Johnson KJ. Oxygen radicals, inflammation, and tissue injury. Free Radic Biol Med (1988) 5:403–8. doi: 10.1016/0891-5849(88)90114-1
22. Yu W, Tu Y, Long Z, Liu J, Kong D, Peng J, et al. Reactive oxygen species bridge the gap between chronic inflammation and tumor development. Oxid Med Cell Longevity (2022) 2022:1–22. doi: 10.1155/2022/2606928
23. Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res (2014) 7:19. doi: 10.1186/1757-2215-7-19
24. Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, et al. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci (2011) 102:1424–31. doi: 10.1111/j.1349-7006.2011.01945.x
25. Tian K, Wang X, Wu Y, Wu X, Du G, Liu W, et al. Relationship of tumour-associated macrophages with poor prognosis in wilms’ tumour. J Pediatr Urol (2020) 16:376.e1–8. doi: 10.1016/j.jpurol.2020.03.016
26. Xu L, Zhu Y, Chen L, An H, Zhang W, Wang G, et al. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann Surg Oncol (2014) 21:3142–50. doi: 10.1245/s10434-014-3601-1
27. Zhang Y, Liu S, Liu J, Zhang T, Shen Q, Yu Y, et al. Immune Complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through FcγRIIb-dependent PGE2 production. J Immunol (2009) 182:554–62. doi: 10.4049/jimmunol.182.1.554
28. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit Rev Oncol/Hematol (2008) 66:1–9. doi: 10.1016/j.critrevonc.2007.07.004
29. Mira E. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci (2004) 117:1847–57. doi: 10.1242/jcs.01035
30. Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors (Basel) (2018) 18:E3249. doi: 10.3390/s18103249
31. Nie W, Yu T, Sang Y, Gao X. Tumor-promoting effect of IL-23 in mammary cancer mediated by infiltration of M2 macrophages and neutrophils in tumor microenvironment. Biochem Biophys Res Commun (2017) 482:1400–6. doi: 10.1016/j.bbrc.2016.12.048
32. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol (2009) 17:463–516. doi: 10.1146/annurev.cellbio.17.1.463
33. Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis (2008) 25:593–600. doi: 10.1007/s10585-008-9143-9
34. Thiery J-P. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer (2002) 2:442–54. doi: 10.1038/nrc822
35. Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell (2008) 14:818–29. doi: 10.1016/j.devcel.2008.05.009
36. Wang JS, Jia Z, Li JH, Xu L, Wang JZ, Qu HY, et al. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial–mesenchymal transition in gastric cancer. Oncotargets Ther (2016) 9:3975–83. doi: 10.2147/OTT.S103112
37. Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial–mesenchymal transition in hepatocellular carcinoma. Cancer Lett (2014) 352:160–8. doi: 10.1016/j.canlet.2014.05.008
38. Ran S, Montgomery KE. Macrophage-mediated lymphangiogenesis: The emerging role of macrophages as lymphatic endothelial progenitors. Cancers (2012) 4:618–57. doi: 10.3390/cancers4030618
39. Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol (2012) 33:119–26. doi: 10.1016/j.it.2011.12.001
40. Ryota T, Toshihide T, Yohei Y, Akasaki A, Sasaki H. Dual role of macrophage in tumor immunity. Immunotherapy (2018) 10:899–909. doi: 10.2217/imt-2018-0006
41. Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol (2002) 161:947–56. doi: 10.1016/s0002-9440(10)64255-1
42. Rigo A, Gottardi M, Zamò A, Mauri P, Bonifacio M, Krampera M, et al. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol Cancer (2010) 9:273. doi: 10.1186/1476-4598-9-273
43. Sánchez-Martín L, Estecha A, Samaniego R, Sánchez-Ramón S, Vega MÁ., Sánchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood (2011) 117:88–97. doi: 10.1182/blood-2009-12-258186
44. Wang Y, Yu J, Luo Z, Shi Q, Liu G, Wu F, et al. Engineering endogenous tumor-associated macrophage-targeted biomimetic nano-RBC to reprogram tumor immunosuppressive microenvironment for enhanced chemo-immunotherapy. Advanced Mater (2021) 33:2103497. doi: 10.1002/adma.202103497
45. Kim J, Bae J-S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflammation (2016) 2016:6058147. doi: 10.1155/2016/6058147
46. Lin W-W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest (2007) 117:1175–83. doi: 10.1172/JCI31537
47. Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol (2007) 179:977–83. doi: 10.4049/jimmunol.179.2.977
48. Pu J, Xu Z, Nian J, Fang Q, Yang M, Huang Y, et al. M2 macrophage-derived extracellular vesicles facilitate CD8+T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway. Cell Death Discovery (2021) 7:182. doi: 10.1038/s41420-021-00556-3
49. Lan J, Sun L, Xu F, Liu L, Hu F, Song D, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res (2018) 79:146–58. doi: 10.1158/0008-5472.CAN-18-0014
50. Zhang Y, Meng W, Yue P, Li X. M2 macrophage-derived extracellular vesicles promote gastric cancer progression via a microRNA-130b-3p/MLL3/GRHL2 signaling cascade. J Exp Clin Cancer Res (2020) 39:134–54. doi: 10.1186/s13046-020-01626-7
51. Shen S, Li H-J, Chen K-G, Wang Y-C, Yang X-Z, Lian Z-X, et al. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett (2017) 17:3822–9. doi: 10.1021/acs.nanolett.7b01193
52. Sun J-H, Liang X, Cai M, Yan L, Chen Z, Guo L, et al. Protein-crowned micelles for targeted and synergistic tumor-associated macrophage reprogramming to enhance cancer treatment. Nano Lett (2022) 22:4410–20. doi: 10.1021/acs.nanolett.2c00901
53. Guerriero JL. Macrophages: Their untold story in T cell activation and function. Int Rev Cell Mol Biol (2019) 342:73–93. doi: 10.1016/bs.ircmb.2018.07.001
54. Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res (2021) 81:1201–8. doi: 10.1158/0008-5472.CAN-20-2990
55. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol (2006) 6:295–307. doi: 10.1038/nri1806
56. Tiemessen M, Jagger A, Evans H, Van Herwijnen M, John S, Taams L. CD4+ CD25+ Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci United States America (2008) 104:19446–51. doi: 10.1073/pnas.0706832104
57. Vázquez S, Vallejo R, Espinosa J, Arteche N, Vega JA, Pérez V. Immunohistochemical characterization of tumor-associated macrophages in canine lymphomas. Animals (2021) 11:2301. doi: 10.3390/ani11082301
58. Seung B-J, Lim H-Y, Shin J-I, Kim H-W, Cho S-H, Kim S-H, et al. CD204-expressing tumor-associated macrophages are associated with malignant, high-grade, and hormone receptor–negative canine mammary gland tumors. Vet Pathol (2018) 55:417–24. doi: 10.1177/0300985817750457
59. Sfacteria A, Napoli E, Rifici C, Commisso D, Giambrone G, Mazzullo G, et al. Immune cells and immunoglobulin expression in the mammary gland tumors of dog. Animals (2021) 11:1189. doi: 10.3390/ani11051189
60. Raposo T, Gregório H, Pires I, Prada J, Queiroga FL. Prognostic value of tumour-associated macrophages in canine mammary tumours. Vet Comp Oncol (2014) 12:10–9. doi: 10.1111/j.1476-5829.2012.00326.x
61. Raposo TP, Pires I, Carvalho MI, Prada J, Argyle DJ, Queiroga FL. Tumour-associated macrophages are associated with vascular endothelial growth factor expression in canine mammary tumours. Vet Comp Oncol (2015) 13:464–74. doi: 10.1111/vco.12067
62. Chen Y, Bi L, Luo H, Jiang Y, Chen F, Wang Y, et al. Water extract of ginseng and astragalus regulates macrophage polarization and synergistically enhances DDP’s anticancer effect. J Ethnopharmacol (2019) 232:11–20. doi: 10.1016/j.jep.2018.12.003
63. Li W, Song K, Wang S, Zhang C, Zhuang M, Wang Y, et al. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater Sci Eng C Mater Biol Appl (2019) 98:685–95. doi: 10.1016/j.msec.2019.01.025
64. Zhang Y, Zhang M, Jiang Y, Li X, He Y, Zeng P, et al. Lentinan as an immunotherapeutic for treating lung cancer: A review of 12 years clinical studies in China. J Cancer Res Clin Oncol (2018) 144:2177–86. doi: 10.1007/s00432-018-2718-1
65. Zhang M, Zhang Y, Zhang L, Tian Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. In: Progress in molecular biology and translational science. (Progress in Molecular Biology and Translational Science: Elsevier) (2019). p. 297–328. doi: 10.1016/bs.pmbts.2019.02.013
66. Sun C, Rosendahl AH, Wang XD, Wu DQ, Andersson R. Polysaccharide-K (PSK) in cancer - old story, new possibilities? Curr Med Chem (2012) 19:757–62. doi: 10.2174/092986712798992020
67. Alliot C. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer. Br J Cancer (2004) 91:1220–1. doi: 10.1038/sj.bjc.6602100
68. Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol (2006) 6:317–33. doi: 10.1016/j.intimp.2005.10.005
69. Biringanine G, Vray B, Vercruysse V, Vanhaelen-Fastré R, Vanhaelen M, Duez P. Polysaccharides extracted from the leaves of plantago palmata hook.f. induce nitric oxide and tumor necrosis factor-α production by interferon-γ-activated macrophages. Nitric Oxide (2005) 12:1–8. doi: 10.1016/j.niox.2004.10.008
70. Zhao H, Wang Q, Sun Y, Yang B, Wang Z, Chai G, et al. Purification, characterization and immunomodulatory effects of plantago depressa polysaccharides. Carbohydr Polymers (2014) 112:63–72. doi: 10.1016/j.carbpol.2014.05.069
71. Ding J, Guo C, Hu P, Chen J, Liu Q, Wu X, et al. CSF1 is involved in breast cancer progression through inducing monocyte differentiation and homing. Int J Oncol (2016) 49:2064–74. doi: 10.3892/ijo.2016.3680
72. Iwanowycz S, Wang J, Hodge J, Wang Y, Yu F, Fan D. Emodin inhibits breast cancer growth by blocking the tumor-promoting feedforward loop between cancer cells and macrophages. Mol Cancer Ther (2016) 15:1931–42. doi: 10.1158/1535-7163.MCT-15-0987
73. Wang J, Zuo G, Li J, Guan T, Li C, Jiang R, et al. Induction of tumoricidal activity in mouse peritoneal macrophages by ginseng polysaccharide. Int J Biol Macromol (2010) 46:389–95. doi: 10.1016/j.ijbiomac.2010.02.007
74. Yang X, Wu Y, Zhang C, Fu S, Zhang J, Fu C. Extraction, structural characterization, and immunoregulatory effect of a polysaccharide fraction from radix aconiti lateralis preparata (Fuzi). Int J Biol Macromol (2020) 143:314–24. doi: 10.1016/j.ijbiomac.2019.11.208
75. Ju A, Cho Y-C, Cho S. Methanol extracts of xanthium sibiricum roots inhibit inflammatory responses via the inhibition of nuclear factor-κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3) in murine macrophages. J Ethnopharmacol (2015) 174:74–81. doi: 10.1016/j.jep.2015.07.038
76. Pang L, Han S, Jiao Y, Jiang S, He X, Li P. Bu fei decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. Int J Oncol (2017) 51:25–38. doi: 10.3892/ijo.2017.4014
77. Bamodu OA, Kuo K-T, Wang C-H, Huang W-C, Wu ATH, Tsai J-T, et al. Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients (2019) 11:2264. doi: 10.3390/nu11102264
78. Tan HY, Wang N, Man K, Tsao SW, Che CM, Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis (2015) 6:e1942. doi: 10.1038/cddis.2015.271
79. Meng Y, Qu Y, Wu W, Chen L, Cheng H. Galactan isolated from cantharellus cibarius modulates antitumor immune response by converting tumor-associated macrophages toward M1-like phenotype. Carbohydr Polymers (2019) 226:115295. doi: 10.1016/j.carbpol.2019.115295
80. Jia X, Yu F, Wang J, Iwanowycz S, Saaoud F, Wang Y, et al. Emodin suppresses pulmonary metastasis of breast cancer accompanied with decreased macrophage recruitment and M2 polarization in the lungs. Breast Cancer Res Treat (2014) 148:291–302. doi: 10.1007/s10549-014-3164-7
81. Iwanowycz S, Wang J, Altomare D, Hui Y, Fan D. Emodin bidirectionally modulates macrophage polarization and epigenetically regulates macrophage memory. J Biol Chem (2016) 291:11491–503. doi: 10.1074/jbc.m115.702092
82. Gao J, Zhang Y-N, Cui J, Zhang J, Ming Y, Hao Z, et al. A polysaccharide from the whole plant of plantago asiatica l. enhances the antitumor activity of dendritic cell-based immunotherapy against breast cancer. Front Pharmacol (2021) 12:678865. doi: 10.3389/fphar.2021.678865
83. Ramachandran C, Wilk BJ, Hotchkiss A, Chau H, Eliaz I, Melnick SJ. Activation of human T-Helper/Inducer cell, T-cytotoxic cell, b-cell, and natural killer (NK)-cells and induction of natural killer cell activity against K562 chronic myeloid leukemia cells with modified citrus pectin. BMC Complementary Altern Med (2011) 11:1–9. doi: 10.1186/1472-6882-11-59
84. Forghani P, Khorramizadeh MR, Waller EK. Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med (2014) 3:215–24. doi: 10.1002/cam4.186
85. Masuda Y, Inoue H, Ohta H, Miyake A, Nanba H. Oral administration of soluble -glucans extracted from grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int J Cancer (2013) 133:108–19. doi: 10.1002/ijc.27999
86. Islam MT, Alide SE, Uddin SJ, Islam A, Shaw S, Khan IN, et al. Andrographolide, a diterpene lactone from andrographis paniculata and its therapeutic promises in cancer. Cancer Lett (2018) 420:129–45. doi: 10.1016/j.canlet.2018.01.074
87. Sheeja K, Kuttan G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by andrographis paniculata extract and andrographolide. Immunopharmacol Immunotoxicol (2007) 29:81–93. doi: 10.1080/08923970701282726
88. Zhang W, Ma W, Zhang J, Song X, Sun W, Fan Y. The immunoregulatory activities of astragalus polysaccharide liposome on macrophages and dendritic cells. Int J Biol Macromol (2017) 105:852–61. doi: 10.1016/j.ijbiomac.2017.07.108
89. Sun K, Yu W, Ji B, Chen C, Yang H, Du Y, et al. Saikosaponin d loaded macrophage membrane-biomimetic nanoparticles target angiogenic signaling for breast cancer therapy. Appl Mater Today (2020) 18:100505. doi: 10.1016/j.apmt.2019.100505
90. Reckelhoff CR, Lejeune A, Thompson PM, Shiomitsu K. In vitro effects of the chemotherapy agent water-soluble micellar paclitaxel (Paccal vet) on canine hemangiosarcoma cell lines. Vet Comp Oncol (2019) 17:32–41. doi: 10.1111/vco.12442
91. Yang J, Jin B, Kim S, Li Q, Nam A, Ryu M, et al. Antitumour effects of liporaxel (oral paclitaxel) for canine melanoma in a mouse xenograft model. Vet Comp Oncol (2020) 18:152–60. doi: 10.1111/vco.12540
Keywords: tumor-associated macrophages, traditional Chinese medicine, cancer, immunotherapy, tumor microenvironment
Citation: Zhang J, Gao J, Cui J, Wang Y, Jin Y, Zhang D, Lin D and Lin J (2022) Tumor-associated macrophages in tumor progression and the role of traditional Chinese medicine in regulating TAMs to enhance antitumor effects. Front. Immunol. 13:1026898. doi: 10.3389/fimmu.2022.1026898
Received: 26 August 2022; Accepted: 27 September 2022;
Published: 13 October 2022.
Edited by:
Bernd Kaina, Johannes Gutenberg University Mainz, GermanyReviewed by:
Yu-gang Huang, Hubei University of Medicine, ChinaDongfang Zhou, Southern Medical University, China
Jingxian Ding, The Third Hospital of Nanchang, China
Copyright © 2022 Zhang, Gao, Cui, Wang, Jin, Zhang, Lin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Degui Lin, csama@sina.com; Jiahao Lin, jiahao_lin@cau.edu.cn
 Jiatong Zhang
Jiatong Zhang Jiafeng Gao
Jiafeng Gao Jingwen Cui1
Jingwen Cui1 Yongqiang Wang
Yongqiang Wang Di Zhang
Di Zhang Degui Lin
Degui Lin Jiahao Lin
Jiahao Lin