Mortality and Severity in COVID-19 Patients on ACEIs and ARBs—A Systematic Review, Meta-Analysis, and Meta-Regression Analysis
- 1Department of Anesthesiology and Critical Care Medicine, Mayo Clinic, Rochester, MN, United States
- 2Dr. Sampurnanand Medical College and Hospital, Jodhpur, India
- 3Department of Internal Medicine, Islamic International Medical College, Rawalpindi, Pakistan
- 4Department of Gastroenterology and Hepatology, Rutgers Robert Wood Johnson School of Medicine, New Brunswick, NJ, United States
- 5Department of Emergency Medicine, Trinity West Medical Center, Steubenville, OH, United States
- 6Department of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States
- 7Patliputra Medical College and Hospital, Dhanbad, India
- 8GMERS Medical College and Hospital, Vadodara, India
- 9Department of Internal Medicine, Services Institute of Medical Sciences, Lahore, Pakistan
- 10Department of Plastic Surgery, KAHER J. N. Medical College, Belgaum, India
- 11Department of Internal Medicine, North Alabama Medical Center, Florence, AL, United States
- 12Department of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN, United States
Purpose: The primary objective of this systematic review is to assess association of mortality in COVID-19 patients on Angiotensin-converting-enzyme inhibitors (ACEIs) and Angiotensin-II receptor blockers (ARBs). A secondary objective is to assess associations with higher severity of the disease in COVID-19 patients.
Materials and Methods: We searched multiple COVID-19 databases (WHO, CDC, LIT-COVID) for longitudinal studies globally reporting mortality and severity published before January 18th, 2021. Meta-analyses were performed using 53 studies for mortality outcome and 43 for the severity outcome. Mantel-Haenszel odds ratios were generated to describe overall effect size using random effect models. To account for between study results variations, multivariate meta-regression was performed with preselected covariates using maximum likelihood method for both the mortality and severity models.
Result: Our findings showed that the use of ACEIs/ARBs did not significantly influence either mortality (OR = 1.16 95% CI 0.94–1.44, p = 0.15, I2 = 93.2%) or severity (OR = 1.18, 95% CI 0.94–1.48, p = 0.15, I2 = 91.1%) in comparison to not being on ACEIs/ARBs in COVID-19 positive patients. Multivariate meta-regression for the mortality model demonstrated that 36% of between study variations could be explained by differences in age, gender, and proportion of heart diseases in the study samples. Multivariate meta-regression for the severity model demonstrated that 8% of between study variations could be explained by differences in age, proportion of diabetes, heart disease and study country in the study samples.
Conclusion: We found no association of mortality or severity in COVID-19 patients taking ACEIs/ARBs.
Introduction
SARS-CoV-2 originated in Wuhan, China, in December 2019 and has spread to every major country in the world and was subsequently declared a pandemic on March 11, 2020 (1). As of April 29th, 2021, there were 150,088,112 positive patients worldwide; and 3,161,337 of these patients were reported to be deceased because of SARS-CoV-2 (2). The case fatality rate of SARS-CoV-2 in the U.S. is 1.8% as per COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (2). However, the role of different medications and comorbidities has been elicited in recent articles.
The SARS-CoV-2 disease varies from mild to fulminant in reference to several risk variables contributing to a poor prognosis (3–5). While the virus significantly impacts the respiratory tract, other metabolic systems have been involved in numerous case studies and systematic reviews (6–14). Thorough awareness of the risks, pathogenesis, and predisposing factors together with the important aspects in the diagnosis is of paramount importance in order to direct decision-making for acute care and mitigate mortality of COVID-19 (15–18).
Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 uses the receptor angiotensin-converting enzyme (ACE) 2 for entry into target cells, and it was reported that both Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) could increase the mRNA expression of cardiac ACE2 receptors (19, 20). However, controversy about the novel use of Renin-angiotensin system (RAS) blockers has been raised amid this SARS-CoV-2 pandemic. The explanation behind this controversy arises from the very fact that it shares the target receptor site with ACEIs and ARBs, which can cause the upregulation of ACE2 receptors (21). ACE2 is additionally the notable cellular surface receptor and a necessary entry point for SARS-CoV-2 into the target cell (22, 23). As cardiovascular diseases and their therapy affects ACE2 levels, it plays an integral part in consideration of infectivity and outcomes of SARS-CoV-2 (20). It needs to be imperatively determined whether treatment or disease-induced up-regulation of ACE2 impacts the trajectory of SARS-CoV-2 (19). ACEIs/ARBs are often used to treat hypertension, which is the most common comorbidity associated with SARS-CoV-2 (20, 24). As there is no clinical evidence, major international cardiology societies recommend continuing the use of ACEIs and ARBs in SARS-CoV-2 patients (25).
Due to limited literature on the influence of ACE inhibitors and ARBs in COVID-19 patients, we systematically reviewed the relevant medical literature. We performed a meta-analysis and meta-regression to investigate the association of ACEIs and ARBs used in COVID-19 and its effect on the mortality rate and severity of COVID-19.
Materials and Methods
We have presented this review according to the Preferred Systematic Reviews and Meta-Analysis Reporting Items guidelines for documenting analysis (26). We attempted to register this systematic review but opted against it because it was taking an extended amount of time due to the large number of COVID-19-related literature being submitted.
Search Strategy
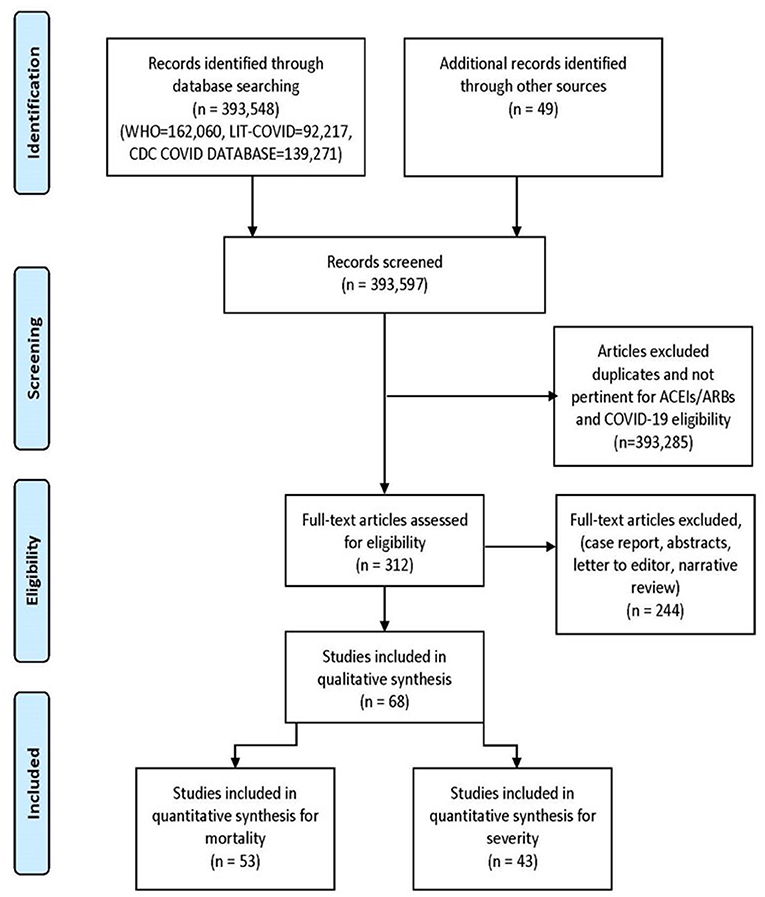
We searched WHO COVID-19 Global research database, Lit-COVID (27), CDC Database of COVID-19 Research including PubMed, Embase, Scopus, Science Web, and Cochrane Central Controlled Trials Registry. The MedRxiv and SSNR preprint servers were also scanned. The searches were performed from December 2019 and revised till January 18th, 2021. The search approach and design can be found in Figure 1. Studies from all around the world were included, there were no language barriers. In an attempt to discover further eligible studies, we manually searched the reference lists of the included studies and the relevant literature. We also scanned the ClinicalTrials.gov registry for completed, as well as in-progress randomized controlled trials (RCTs).
Eligibility Criteria
Observational studies that met all the following criteria were included: (1) study design: case-control, case-crossover, self-controlled case series (SCCS) or cohort study; (2) reported antihypertensive treatment: ACEI/ARB use vs. non-ACEI/ARB use; (3) outcomes: the incidence of COVID-19 mortality or severity; (4) adequate data were used to extract the risk estimates if the adjusted data were not provided in the publication. Studies focusing on patients <18 years of age, focusing on pregnant females, and limited to particular comorbidities and organ dysfunctions were excluded to avoid selection bias. We also excluded case reports, Editorials, correspondences, conference abstracts and commentary articles were excluded in our study. When information was incomplete in the publication, attempts were made to contact the study investigators to obtain missing information.
Study Selection
Three authors (RS, SR, and HK) downloaded all articles from electronic search to EndNote X9 (28), as well as duplicates were eliminated. Titles and abstracts were autonomously evaluated by authors (AT, FA, HK, JM, KM, PG, RS, SA, and SR) to identify and assess key articles. Further, authors (FA, HK, JM, KM, PG, RS, SA, and SR) independently reviewed the entire manuscript and registered justification for the exclusion. Any discrepancies were addressed by arbitration.
Outcome
All-cause mortality in the COVID-19 affected patient was the chief outcome, while severity of disease was the secondary outcome. We defined severity as the need for ICU admission or the need for Mechanical Ventilation. If both severities were given in the article, then we collect the highest amount of data for respective events as the severity for COVID-19.
Data Extraction
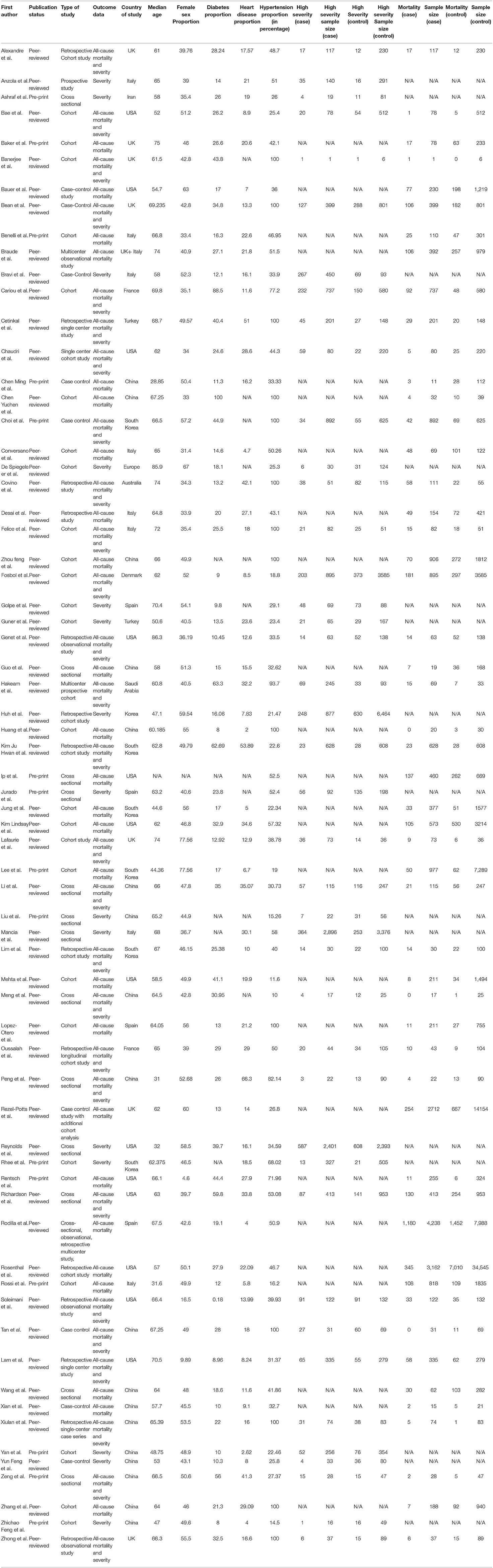
We included all observational studies that satisfied our inclusion criteria. Using a standardized data extraction method, the authors (FA, HK, JM, KM, PG, RS, SA, and SR) extracted information from each survey independently; any conflict was resolved by consensus. The following dataset points were extracted: First author name, cases on ACEI-ARB, total COVID positive patients, country of study, study design, hypertension proportion, diabetes proportion, heart disease proportion, eligibility criteria, Median age, gender (female sex proportion), comorbidities, use of ACEIs or ARBs and primary and secondary outcomes (mortality and severity). Unadjusted and adjusted impact measurements were also extracted where appropriate. The majority of papers differentiated between cases (use of ACEIs and ARBS) and controls (ACEIs/ARBS not used). However, we manually obtained the division in a few publications.
Statistical Analysis
The meta-analysis specifically included longitudinal and cross-sectional studies comparing the effects of COVID-19 in subjects who were on ACEIs/ARBs at the time of infection with those who were not. Meta-analysis was performed first for studies reporting mortality of patients in both groups, followed by that for studies reporting severity of disease assuming independence of results for studies that reported both. Due to anticipated heterogeneity, summary statistics were calculated using a random-effects model. This model accounts for variability between studies as well as within studies. In all cases, meta-analyses were performed using the Mantel-Haenszel method for dichotomous data to estimate pooled odds ratios (OR) and statistical heterogeneity was assessed using Q-value and I2 statistics. The meta-analysis and meta-regression was done with the Comprehensive Meta-Analysis software package (Biostat, Englewood, NJ, USA) (29). We included the region of study in meta-regression model to find out whether Asian studies, which were dated earlier than studies from the rest of the world, contributed disproportionately to the significance of results. This helped rule out location and pipeline biases.
To explore differences between studies that might be expected to influence the effect size, we performed random effects (maximum likelihood method) univariate and multivariate meta-regression analyses. The potential sources of variability defined were median age of study sample, proportion of subjects of female sex, proportion of diabetics and proportion with heart diseases. Covariates were selected for further modeling if they significantly (P < 0.05) modified the association between mortality or severity in the COVID19 infected and treatment with ACEIs/ARBs. Two models were created, one for mortality and the other for severity of disease as outcomes. Subsequently, preselected covariates were included in a manual backward and stepwise multiple meta-regression analysis with P = 0.05 as a cutoff point for removal. P < 0.05 (P < 0.10 for heterogeneity) was considered statistically significant. All meta-analysis and meta-regression tests were 2-tailed.
Risk of Bias Assessment
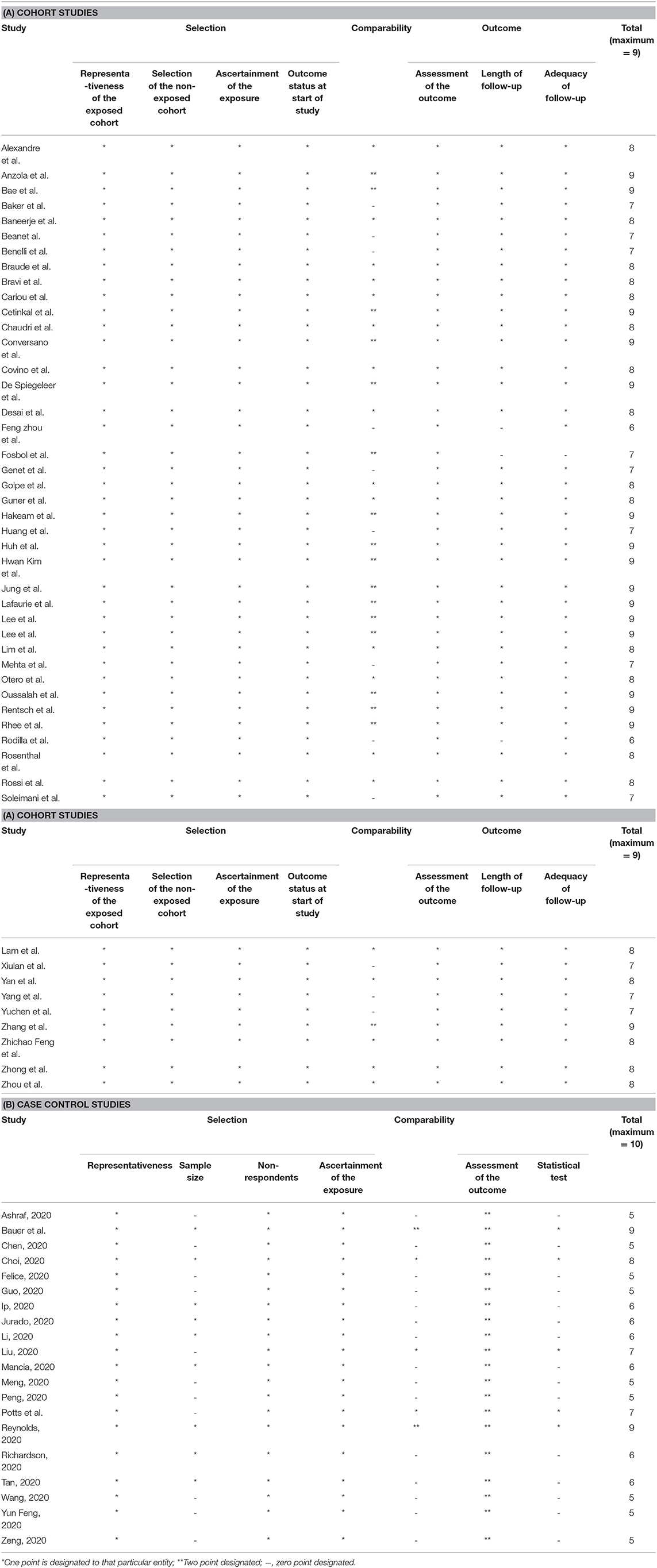
The Newcastle-Ottawa (NOS) scale (30) was used for measuring the risk of bias in cohort and case-control studies (Tables 2A,B). The following classes were rated per study: low bias risk (9 points), moderate bias risk (5–7 points), and high bias risk (0-4 items). For a cross-sectional study, we used the modified version of NOS, assigning the study in the following groups: Low risk of bias (8–10), moderate risk (5–7), high risk of bias (0–4). Three reviewers (RS, SR, and PG) evaluated the likelihood of bias independently, and any conflict was resolved by consensus.
Results
The initial library search identified potentially relevant citations from WHO Global Research Database, CDC COVID-19 Research Articles Downloadable Database, and LitCovid PubMed database comprised of 393,597 articles. Subsequently, 393,285 articles were removed because of unclear evidence and non-relevance to the objective of the manuscript. Out of the remaining 312 articles, a total of 244 articles consisting of case reports, abstracts, letter to editor, and narrative reviews were excluded. Thus, 68 studies (31–98) were included in their entirety as shown in Table 1. The PRISMA flow chart is shown in Figure 1.
Study Characteristics of Included Studies
A total of 53 studies (31–83) were included for meta-analysis for the primary outcome i.e., mortality. In total, these consisted of 112,468 subjects with 16,363 mortality events. Median age for all studies was 64.9 (60.9–67.2) with average 47.3% females (Table 1). Of the comorbidities considered, 25.4% were diabetics, 16.6% had heart diseases overall. Similarly, a total of forty three studies (31, 33, 35, 38–40, 43, 45, 47–49, 51, 55, 57–59, 61–63, 66–68, 71, 75, 76, 78, 79, 81, 84–98) were included for meta-analysis for the secondary outcome i.e., severity of disease. These had a combined sample size of 37,914 with 6,985 patients reaching the endpoint of high disease severity. The median age was 65.2 (60.8–68.7) and 46.2% were females, 25.6% were diabetics, 17.1% had heart diseases overall in this cohort (Table 1).
Meta-Analysis for Mortality Outcome
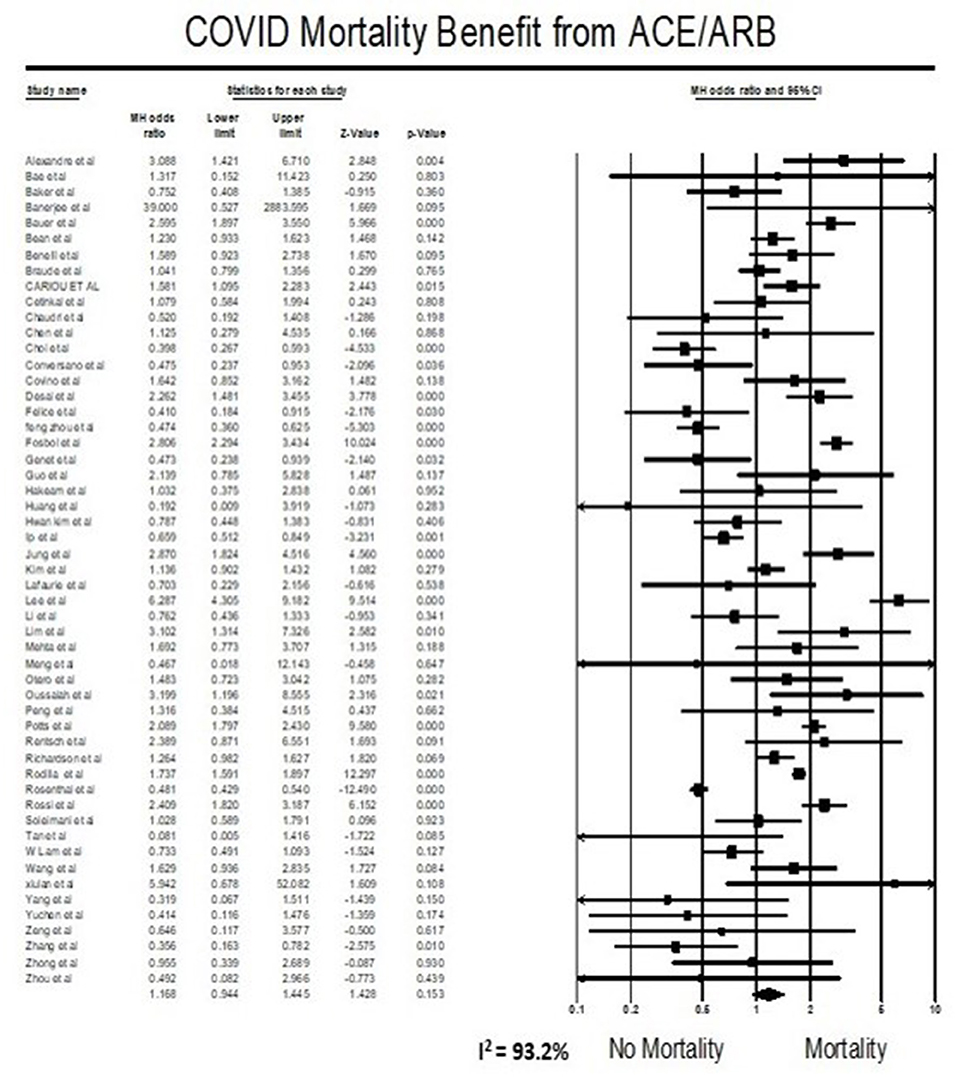
Meta-analysis findings showed that being on ACEIs/ARBs did not have an association with mortality from COVID 19 infections compared to not being on ACEIs/ARBs (OR = 1.17, 95% CI 0.94–1.45, p = 0.15). Heterogeneity was very high with I2 = 93.2% (Figure 2).
Meta-Analysis for Severity Outcome
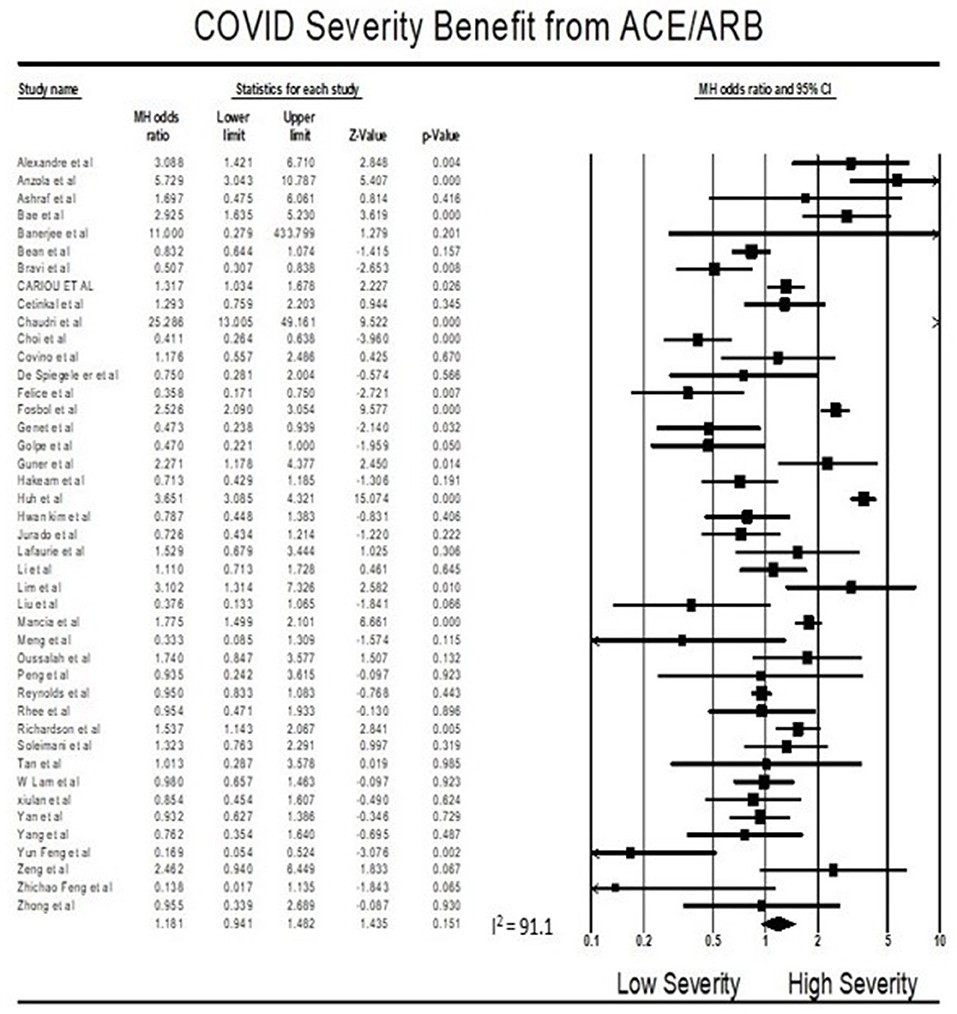
Findings from the meta-analysis showed that being on ACEI/ARBs did not have an association with severity from COVID 19 infections compared to not being on ACEIs/ARBs (OR = 1.18, 95% CI 0.94–1.48, p = 0.15). Heterogeneity was very high with I2 = 91.1% (Figure 3).
Multivariate Meta-Regression Model for Mortality Outcome
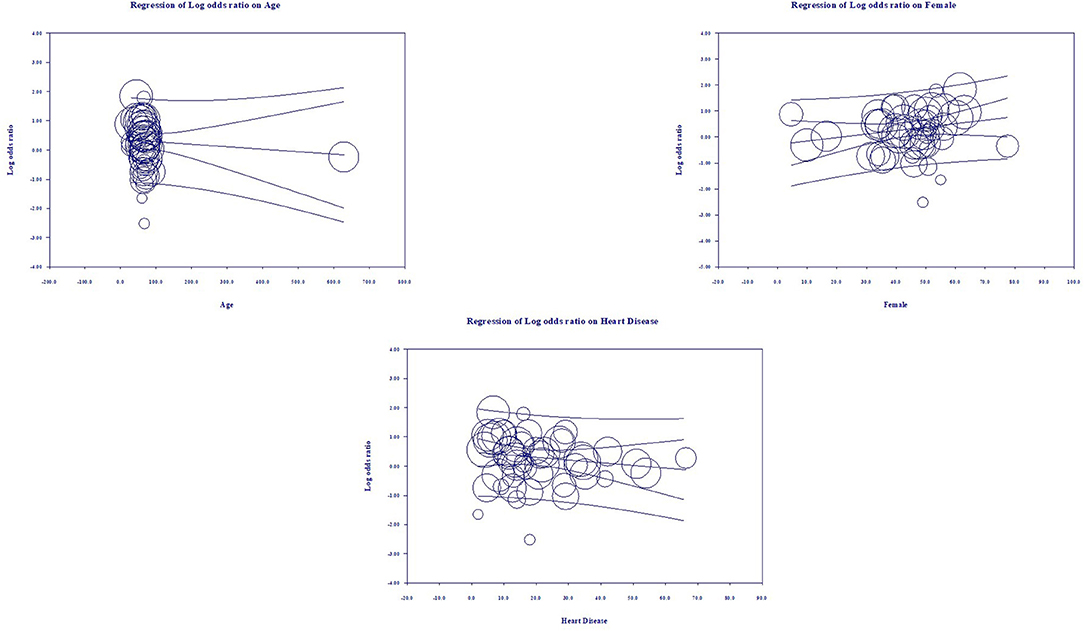
Multivariate meta-regression performed to explain variations in association between mortality and being on ACEIs/ARBs revealed; age, female gender, proportion of heart diseases in included studies covariates to be significant together and explained R2 = 36% of the between study heterogeneity in mortality. Figure 4 shows the resulting equation and individual covariate effect graphs.
Multivariate Meta-Regression Model for Severity Outcome
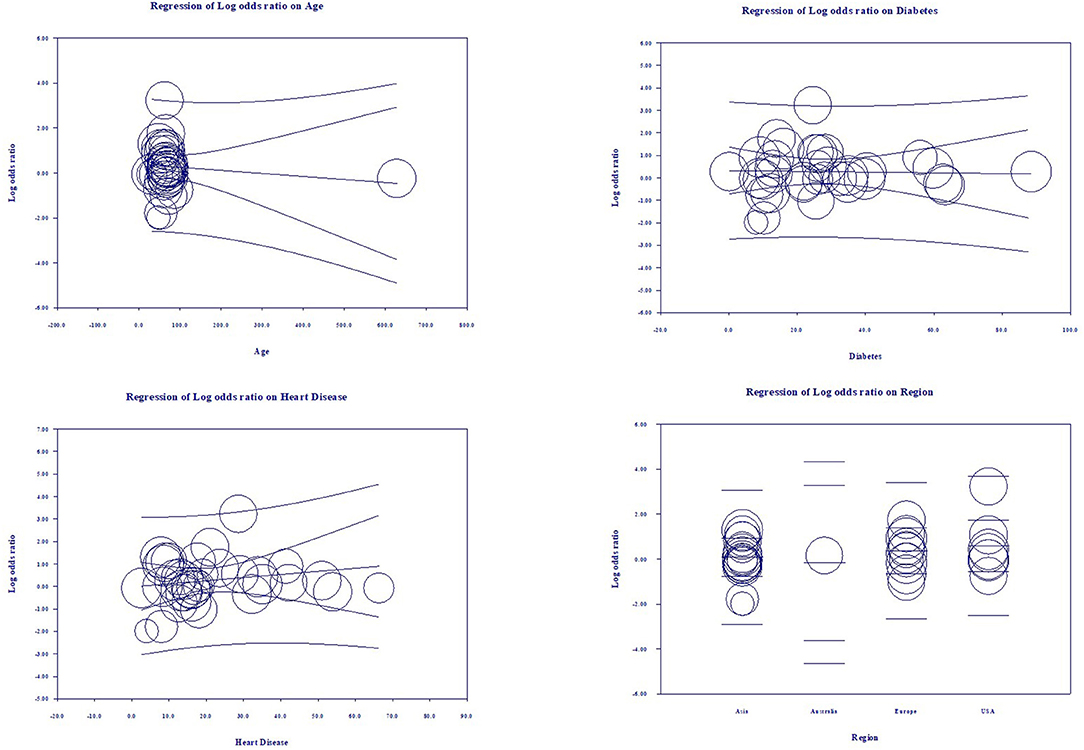
Multivariate meta-regression performed to explain variations in association between severity and being on ACEIs/ARBs revealed age, proportion with diabetes, heart disease, and country of studies covariates to be significant together. These covariates together explained R2 = 8% of the study heterogeneity in severity. Figure 5 shows the resulting equation and individual covariate effect graphs.
Publication Bias
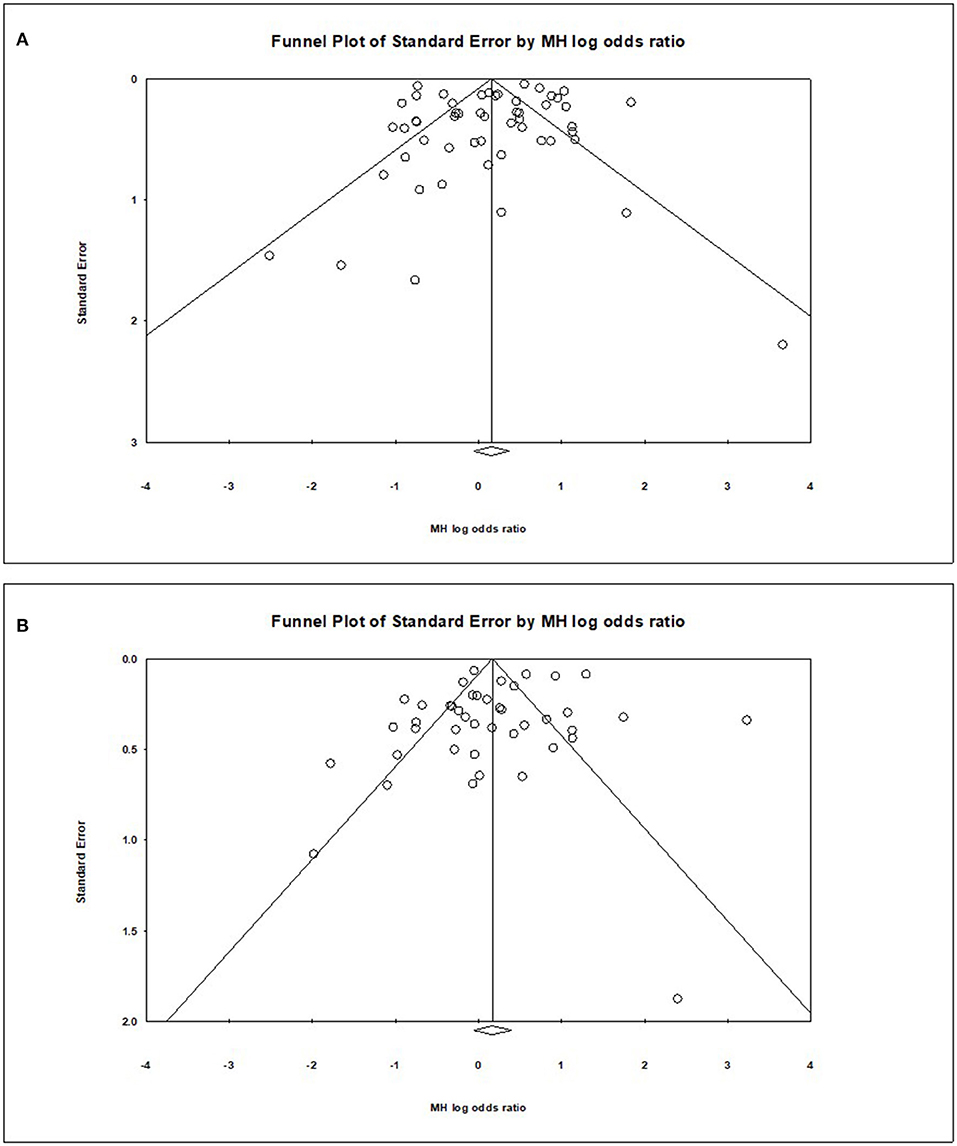
Visual inspection of the standard error plots for the mortality analysis (Figure 6A) suggests symmetry without an underrepresentation of studies of any precision but indicated underrepresentation of studies with smaller effect sizes. Classic fail-safe N analysis computed taking alpha at 0.05 put the number of missing studies at 5. Corroborating inspection findings, in Egger's regression test the null hypothesis of no small study effects was not rejected at P < 0.05 (estimated bias coefficient = −0.28 ± 0.76 SE).
Similarly, visual inspection of the standard error plots for the severity analysis also (Figure 6B) suggest symmetry without an underrepresentation of studies of any precision but indicated underrepresentation of studies with smaller effect sizes. Classic fail-safe N analysis computed taking alpha at 0.05 put the number of missing studies at 8. However, in Egger's regression test the null hypothesis of no small study effects was rejected at P < 0.05 (estimated bias coefficient = −1.14 ± 0.81SE).
Discussion
Based on our meta-analysis consisting of cross-sectional, case-control, and cohort studies, the use of ACEIs and ARBs was neither associated with increased all-cause mortality nor with increased severity of disease progression in COVID-19 patients. Multivariate meta-regression for the mortality model demonstrated that 36% of study variations could be explained by differences in age, female gender, proportion of heart diseases in the study samples. Multivariate meta-regression for the severity model demonstrated that 8% of study variations could be explained by differences in age, proportion of diabetes, heart diseases and country of studies in the study samples. This finding is valuable as association between ACEIs and ARBs use and outcome in COVID-19 patients has been inconclusive so far. To our knowledge, this is the first meta-regression, and the largest meta-analysis to evaluate the role of ACEIs/ARBs as an antihypertensive regimen in hospitalized patients with COVID-19.
The effect of ACEIs/ARBs use on COVID-19 patients has been a controversial topic since the beginning of this pandemic, and some studies even have interposed a risk of taking ACEIs/ARBs using data from previous coronavirus outbreaks and preclinical studies (99). Previously published systematic reviews suggested a lower mortality (25–43%) in patients with hypertension hospitalized for COVID19 (100–102). Furthermore, a large-scale retrospective study demonstrated that in-hospital use of ACEIs/ARBs was associated with a lower risk of 28-day death among hospitalized patients with COVID-19 and coexisting hypertension (adjusted HR 0.32, 95% CI 0.15–0.66) (80). These data suggested that patients with hypertension might obtain benefits from taking ACEIs/ARBs compared with the non-ACEIs/ARBs in the setting of COVID-19 and support the hypothesis that a drug that diminishes angiotensin-2 activity, such as ACEIs and/or ARBs, can reduce the deadliness of inflammation associated injury in COVID-19. Our result trends in contraindicating the above study results but did not reach statistical significance. Our meta-analysis suggests that the use of ACEIs/ARBs neither increase nor decrease mortality in COVID-19 patients (Figure 2). In addition to what is reported in published studies, our systematic review added the most recent studies, and had the largest sample size (53 studies, 112,468 patients).
The main strength of our analysis is the large sample size along with a robust and comprehensive search. The large sample size enables the precision and reliability of risk estimates. Additionally, further meta-regression was performed to adjust for confounding factors. However, despite all the strengths, there are still certain limitations. The major limitation of the meta-regression in the presence of unknown confounders. Multiple previous studies have reported that gender, age, smoking history, and presence of diabetes influence COVID-19 results. Even though these confounders are reported in most of the included studies, further studies focusing on the adjustment of confounders are necessary. We included studies from the medRxiv.org databases and other preprint databases which did not go through peer review at that time. We considered this as a limitation, as peer reviewers could catch more deficiencies in reporting methods and other details. However, it was anticipated that majority of these studies would be peer-reviewed. Third, the use of ACE/ARB has been via medical record review which could be less reliable. Fourth, there is a possibility of publication bias as the definition of COVID-19 severity and outcomes were not uniform among the included studies. Fifth, substantial clinical variability among COVID-19 patients throughout in included studies leads to a high degree of statistical heterogeneity in the analysis of COVID-19 mortality and severity. To overcome this, we did a meta-regression analysis to define heterogeneity in included studies. Sixth, we did not include racial or ethnicity variation as a covariant in meta-regression analysis. However, we included the country of study origin as a covariate in meta-regression to overcome this limitation. Lastly, since most of the patients in the study population were in a hospital, so the results may be subject to selection bias.
Conclusion
Due to the lack of statistical significance in the meta-analysis and observed study variance in the meta-regression analysis, it is not reasonable to conclude that ACEIs and ARBs are either detrimental or beneficial for patients with COVID-19. Larger observational studies (3, 103–105) and clinical trials are warranted to confirm these findings. Providers should continue to manage patient hypertension as per current treatment guidelines (106, 107) and clinical judgement until more robust evidence can say otherwise.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
RS and VB contributed equally in the defining the study outline and manuscript writing. Data review and collection done by AT, FA, HK, JM, KM, PG, RS, SA, and SR. Statistical analysis was done by AB, MS, and VB. Study design and critical review done by IM and RK. RS, VB, AB, and MS are the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This article has been submitted to medRxiv preprint server.
References
1. Shah A, Kashyap R, Tosh P, Sampathkumar P, O'Horo JC. Guide to Understanding the 2019 novel coronavirus. Mayo Clin Proc. (2020) 95:646–52. doi: 10.1016/j.mayocp.2020.02.003
2. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
3. Domecq JP, Lal A, Sheldrick CR, Kumar VK, Boman K, Bolesta S, et al. Outcomes of patients with coronavirus disease 2019 receiving organ support therapies: the international viral infection and respiratory illness universal study registry. Crit Care Med. (2021) 49:437–48. doi: 10.1097/CCM.0000000000004879
4. Bansal V, Singh R, Bhurwal A, Rathore S, Kashyap R. 117: obesity is a risk factor for increased COVID-19 severity: a systemic review and meta-regression. Crit Care Med. (2021). 49:43. doi: 10.1097/01.ccm.0000726356.00193.e7
5. Kirkup C, Pawlowski C, Puranik A, Conrad I, O'Horo JC, Gomaa D, et al. Healthcare disparities among anticoagulation therapies for severe COVID-19 patients in the multi-site VIRUS registry. J Med Virol. (2021) 93:4303–18. doi: 10.1002/jmv.26918
6. Rathore SS, Rojas GA, Sondhi M, Pothuru S, Pydi R, Kancherla N, et al. Myocarditis associated with Covid-19 disease: A systematic review of published case reports and case series. Int J Clin Pract. (2021) 2021:e14470. doi: 10.22541/au.161219538.89676033/v1
7. Sheraton M, Deo N, Kashyap R, Surani S. A review of neurological complications of COVID-19. Cureus. (2020) 12:e8192. doi: 10.7759/cureus.8192
8. Khan H, Sabzposh H, Deshpande S, Kashyap R. Pregnancy during COVID-19 pandemic - Maternal and neonatal outcomes: a concise review. Int J Acad Med. (2020) 6:287–93. doi: 10.4103/IJAM.IJAM_94_20
9. Shah K, Mann S, Singh R, Bangar R, Kulkarni R. Impact of COVID-19 on the mental health of children and adolescents. Cureus. (2020) 12:e10051. doi: 10.7759/cureus.10051
10. Sheraton M, Deo N, Dutt T, Surani S, Hall-Flavin D, Kashyap R. Psychological effects of the COVID 19 pandemic on healthcare workers globally: a systematic review. Psychiatry Res. (2020) 292:113360. doi: 10.1016/j.psychres.2020.113360
11. Singh R, Kashyap R, Hutton A, Sharma M, Surani S. A review of cardiac complications in coronavirus disease 2019. Cureus. (2020) 12:e8034. doi: 10.7759/cureus.8034
12. Bhalala U, Gist K, Tripathi S, Chiotos K, Dapul H, Gharpure V, et al. 145: Pediatric COVID-19: a report from viral infection and respiratory illness universal study (VIRUS). Crit Care Med. (2021). 49:58. doi: 10.1097/01.ccm.0000726468.36252.01
13. Tripathi S, Gist K, Chiotos K, Dapul H, Gharpure V, Bansal V, et al. 61: Risk factors for severe COVID-19 illness in children: analysis of the VIRUS: COVID-19 registry. Crit Care Med. (2021). 49:32. doi: 10.1097/01.ccm.0000726272.88301.cd
14. Menon T, Sharma R, Earthineni G, Iftikhar H, Sondhi M, Shams S, et al. Association of gastrointestinal system with severity and mortality of COVID-19: a systematic review and meta-analysis. Cureus. (2021) 13:e13317. doi: 10.7759/cureus.13317
15. Mehra I, Mahapure K, Armaly P, Madas N, Shah V, Gupta I, et al. 146: controversial role of corticosteroids on mortality in COVID-19: systematic review and meta-analysis. Crit Care Med. (2021). 49:58. doi: 10.1097/01.ccm.0000726472.05866.e3
16. Singh R, Shaik L, Mehra I, Kashyap R, Surani S. Novel and controversial therapies in COVID-19. Open Respirat Med J. (2020) 14:79–86. doi: 10.2174/1874306402014010079
17. Bansal V, Mahapure KS, Bhurwal A, Gupta I, Hassanain S, Makadia J, et al. Mortality benefit of remdesivir in COVID-19: a systematic review and meta-analysis. Front Med. (2020) 7:606429. doi: 10.3389/fmed.2020.606429
18. Jain R, Javeri Y, Nasa P, Kashyap R, Khanna A, Tayar A, et al. Consensus statement for pharmacological management of coronavirus disease 2019 (COVID-19): a pragmatic approach. Asploro J Biomed Clin Case Rep. (2020). 3:241. doi: 10.36502/2020/ASJBCCR.6219
19. Sommerstein R, Kochen MM, Messerli FH, Grani C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. (2020). 9:e016509. doi: 10.1161/JAHA.120.016509
20. Sanchis-Gomar F, Lavie CJ, Perez-Quilis C, Henry BM, Lippi G. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin Proc. (2020) 95:1222–30. doi: 10.1016/j.mayocp.2020.03.026
21. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. (2020) 579:265–9. doi: 10.1038/s41586-020-2008-3
22. Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. (2012) 2012:256294. doi: 10.1155/2012/256294
23. Poland GA, Bass J, Goldstein MR. SARS-CoV-2 infections: an ACE in the hole and systems biology studies-a research agenda. Mayo Clin Proc. (2020) 95:1838–41. doi: 10.1016/j.mayocp.2020.06.044
24. Kanwal A, Agarwala, A, Martin, LW,. COVID-19 Hypertension: What We Know Don't Know. Washington, DC: American College of Cardiology (2020). Available online at: https://www.acc.org/latest-in-cardiology/articles/2020/07/06/08/15/covid-19-and-hypertension (accessesed October 3, 2020).
25. Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. (2020). 5:745–7. doi: 10.1001/jamacardio.2020.1282
26. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
27. Chen Q, Allot A, Lu Z. LitCovid: an open database of COVID-19 literature. Nucleic Acids Res. (2021) 49:D1534–40. doi: 10.1093/nar/gkaa952
28. Hupe M. EndNote X9. J Electron Resour Med Libraries. (2019) 16:117–9. doi: 10.1080/15424065.2019.1691963
29. Borenstein M, Hedges, L, Higgins, J, Rothstein, H,. Comprehensive Meta-Analysis Version 3. Englewood, NJ: Biostat (2013). Available online at: https://www.meta-analysis.com/index.php?cart=BBFA4702757 (accessed November 03, 2021).
30. Wells GA SB, O'Connell, D, Peterson, J, Welch, V, Losos, M, Tugwell, P,. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2009). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed November 03, 2021).
31. Bae DJ Tehrani DM Rabadia SV Frost M Parikh RV Calfon-Press M . Angiotensin converting enzyme inhibitor and angiotensin II receptor blocker use among outpatients diagnosed with COVID-19. Am J Cardiol. (2020) 132:150–7. doi: 10.1016/j.amjcard.2020.07.007
32. Baker KF, Hanrath AT, van der Loeff IS, Tee SA, Capstick R, Marchitelli G, et al. COVID-19 management in a uk nhs foundation trust with a high consequence infectious diseases centre: a retrospective analysis. Med Sci (Basel). (2021) 9:6. doi: 10.3390/medsci9010006
33. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. (2020) 97:1076–82. doi: 10.1016/j.kint.2020.03.018
34. Bauer AZ, Gore R, Sama SR, Rosiello R, Garber L, Sundaresan D, et al. Hypertension, medications, and risk of severe COVID-19: A Massachusetts community-based observational study. J Clin Hypertens. (2021) 23:21–7. doi: 10.1111/jch.14101
35. Bean DM, Kraljevic Z, Searle T, Bendayan R, Kevin O, Pickles A, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail. (2020) 22:967–74. doi: 10.1002/ejhf.1924
36. Gili T, Benelli G, Buscarini E, Canetta C, La Piana G, Merli G, et al. SARS-COV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. PLoS ONE. (2021) 16:e0248498. doi: 10.1371/journal.pone.0248498
37. Braude P, Carter B, Short R, Vilches-Moraga A, Verduri A, Pearce L, et al. The influence of ACE inhibitors and ARBs on hospital length of stay and survival in people with COVID-19. Int J Cardiol Heart Vasc. (2020) 31:100660. doi: 10.1016/j.ijcha.2020.100660
38. Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. (2020) 63:1500–15. doi: 10.1007/s00125-020-05180-x
39. Cetinkal G, Kocas BB, Ser OS, Kilci H, Yildiz SS, Ozcan SN, et al. The association between chronic use of renin-angiotensin-aldosterone system blockers and in-hospital adverse events among COVID-19 patients with hypertension. Sisli Etfal Hastan Tip Bul. (2020) 54:399–404. doi: 10.14744/SEMB.2020.15689
40. Chaudhri I, Koraishy FM, Bolotova O, Yoo J, Marcos LA, Taub E, et al. Outcomes associated with the use of renin-angiotensin-aldosterone system blockade in hospitalized patients with SARS-CoV-2 infection. Kidney360. (2020) 1:801–9. doi: 10.34067/KID.0003792020
41. Chen M, Fan Y, Wu X, Zhang L, Guo T, Deng K, et al. Clinical characteristics and risk factors for fatal outcome in patients with 2019-coronavirus infected disease (COVID-19) in Wuhan, China. SSRN Electron J. (2020). doi: 10.2139/ssrn.3546069
42. Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. (2020) 43:1399–407. doi: 10.2337/dc20-0660
43. Choi HK, Koo H-J, Seok H, Jeon JH, Choi WS, Kim DJ, et al. ARB/ACEI use and severe COVID-19: a nationwide case-control study. medRxiv. (2020). doi: 10.1101/2020.06.12.20129916
44. Conversano A, Melillo F, Napolano A, Fominskiy E, Spessot M, Ciceri F, et al. Renin-angiotensin-aldosterone system inhibitors and outcome in patients with SARS-CoV-2 pneumonia: a case series study. Hypertension. (2020) 76:e10–2. doi: 10.1161/HYPERTENSIONAHA.120.15312
45. Covino M, De Matteis G, Burzo ML, Santoro M, Fuorlo M, Sabia L, et al. Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and prognosis of hypertensive patients hospitalised with COVID-19. Intern Med J. (2020) 50:1483–91. doi: 10.1111/imj.15078
46. Desai A, Voza G, Paiardi S, Teofilo FI, Caltagirone G, Pons MR, et al. The role of anti-hypertensive treatment, comorbidities and early introduction of LMWH in the setting of COVID-19: A retrospective, observational study in Northern Italy. Int J Cardiol. (2021) 324:249–54. doi: 10.1016/j.ijcard.2020.09.062
47. Felice C, Nardin C, Di Tanna GL, Grossi U, Bernardi E, Scaldaferri L, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. (2020) 33:944–8. doi: 10.1093/ajh/hpaa096
48. Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. (2020) 324:168–77. doi: 10.1001/jama.2020.11301
49. Genet B, Vidal JS, Cohen A, Boully C, Beunardeau M, Marine Harle L, et al. COVID-19 in-hospital mortality and use of renin-angiotensin system blockers in geriatrics patients. J Am Med Dir Assoc. (2020) 21:1539–45. doi: 10.1016/j.jamda.2020.09.004
50. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020). 5:811–8. doi: 10.1001/jamacardio.2020.1017
51. Hakeam HA, Alsemari M, Duhailib ZA, Ghonem L, Alharbi SA, Almutairy E, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin II blockers with severity of COVID-19: a multicenter, prospective study. J Cardiovasc Pharmacol Ther. (2021) 26:244–52. doi: 10.1177/1074248420976279
52. Huang Z, Cao J, Yao Y, Jin X, Luo Z, Xue Y, et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. (2020) 8:430. doi: 10.21037/atm.2020.03.229
53. Ip A, Parikh K, Parrillo JE, Mathura S, Hansen E, Sawczuk IS, et al. Hypertension and renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. medRxiv. (2020). doi: 10.1101/2020.04.24.20077388
54. Jung SY, Choi JC, You SH, Kim WY. Association of renin-angiotensin-aldosterone system inhibitors with coronavirus disease 2019 (COVID-19)-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. (2020). 71:2121–8. doi: 10.1093/cid/ciaa624
55. Kim JH, Baek YH, Lee H, Choe YJ, Shin HJ, Shin JY. Clinical outcomes of COVID-19 following the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among patients with hypertension in Korea: a nationwide study. Epidemiol Health. (2021) 43:e2021004. doi: 10.4178/epih.e2021004
56. Kim L, Garg S, O'Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. (2021). 72:e206–14. doi: 10.1093/cid/ciaa1012
57. Lafaurie M, Martin-Blondel G, Delobel P, Charpentier S, Sommet A, Moulis G. Outcome of patients hospitalized for COVID-19 and exposure to angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers in France: results of the ACE-CoV study. Fundam Clin Pharmacol. (2021) 35:194–203. doi: 10.1111/fcp.12613
58. Lahens A, Mullaert J, Gressens S, Gault N, Flamant M, Deconinck L, et al. Association between renin-angiotensin-aldosterone system blockers and outcome in coronavirus disease 2019: analysing in-hospital exposure generates a biased seemingly protective effect of treatment. J Hypertens. (2021) 39:367–75. doi: 10.1097/HJH.0000000000002658
59. Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, et al. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome. J Infect Dis. (2020) 222:1256–64. doi: 10.1093/infdis/jiaa447
60. Lee H-Y, Ahn J, Kang CK, Won S-H, Park J-H, Kang CH, et al. Association of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors on COVID-19-related outcome. SSRN Electron J. (2020). doi: 10.2139/ssrn.3569837
61. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. (2020). 5:825–30. doi: 10.1001/jamacardio.2020.1624
62. Lim JH, Cho JH, Jeon Y, Kim JH, Lee GY, Jeon S, et al. Adverse impact of renin-angiotensin system blockade on the clinical course in hospitalized patients with severe COVID-19: a retrospective cohort study. Sci Rep. (2020) 10:20250. doi: 10.1038/s41598-020-76915-4
63. Liu X, Liu Y, Chen K, Yan S, Bai X, Li J, et al. Efficacy of ACEIs/ARBs vs CCBs on the progression of COVID-19 patients with hypertension in Wuhan: a hospital-based retrospective cohort study. J Med Virol. (2021) 93:854–862. doi: 10.1002/jmv.26315
64. Lopez-Otero D, Lopez-Pais J, Cacho-Antonio CE, Antunez-Muinos PJ, Gonzalez-Ferrero T, Perez-Poza M, et al. Impact of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on COVID-19 in a western population. CARDIOVID registry. Rev Esp Cardiol. (2021) 74:175−82. doi: 10.1016/j.rec.2020.05.018
65. Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020). 5:1020–6. doi: 10.1001/jamacardio.2020.1855
66. Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. (2020) 9:757–60. doi: 10.1080/22221751.2020.1746200
67. Oussalah A, Gleye S, Clerc Urmes I, Laugel E, Callet J, Barbe F, et al. Long-term ACE inhibitor/ARB use is associated with severe renal dysfunction and acute kidney injury in patients with severe COVID-19: results from a referral center cohort in the northeast of France. Clin Infect Dis. (2020) 71:2447–56. doi: 10.1093/cid/ciaa677
68. Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, et al. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV]. Zhonghua Xin Xue Guan Bing Za Zhi. (2020) 48:450–5. doi: 10.3760/cma.j.cn112148-20200220-00105
69. Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv. (2020). doi: 10.1101/2020.04.09.20059964
70. Rezel-Potts E, Douiri A, Chowienczyk PJ, Gulliford MC. Antihypertensive medications and COVID-19 diagnosis and mortality: Population-based case-control analysis in the United Kingdom. Br J Clin Pharmacol. (2021) 87:4598–607. doi: 10.1111/bcp.14873
71. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
72. Rodilla E, Saura A, Jiménez I, Mendizábal A, Pineda-Cantero A, Lorenzo-Hernández E, et al. Association of Hypertension with All-Cause Mortality among Hospitalized Patients with COVID-19. J Clin Med. (2020) 9:3136. doi: 10.3390/jcm9103136
73. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. (2020) 3:e2029058. doi: 10.1001/jamanetworkopen.2020.29058
74. Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS ONE. (2020) 15:e0238281. doi: 10.1371/journal.pone.0238281
75. Soleimani A, Kazemian S, Karbalai Saleh S, Aminorroaya A, Shajari Z, Hadadi A, et al. Effects of angiotensin receptor blockers (ARBs) on in-hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID-19. Am J Hypertens. (2020) 33:1102–11. doi: 10.1093/ajh/hpaa149
76. Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations between angiotensin-converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID-19. Gastroenterology. (2020) 159:1170–2. doi: 10.1053/j.gastro.2020.05.034
77. Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. (2020) 201:1430–4. doi: 10.1164/rccm.202003-0736LE
78. Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, et al. Angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors usage is associated with improved inflammatory status and clinical outcomes in COVID-19 patients with hypertension. medRxiv. (2020). doi: 10.1101/2020.03.31.20038935
79. Zeng Z, Sha T, Zhang Y, Wu F, Hu H, Li H, et al. Hypertension in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. medRxiv. (2020). doi: 10.1101/2020.04.06.20054825
80. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. (2020) 126:1671–81. doi: 10.1161/CIRCRESAHA.120.317242
81. Zhong Y, Zhao L, Wu G, Hu C, Wu C, Xu M, et al. Impact of renin-angiotensin system inhibitors use on mortality in severe COVID-19 patients with hypertension: a retrospective observational study. J Int Med Res. (2020) 48:300060520979151. doi: 10.1177/0300060520979151
82. Zhou F, Liu YM, Xie J, Li H, Lei F, Yang H, et al. Comparative impacts of ACE (angiotensin-converting enzyme) inhibitors versus angiotensin II receptor blockers on the risk of COVID-19 mortality. Hypertension. (2020) 76:e15–7. doi: 10.1161/HYPERTENSIONAHA.120.15622
83. Zhou X, Zhu J, Xu T. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients with hypertension on renin-angiotensin system inhibitors. Clin Exp Hypertens. (2020). 42:656–60. doi: 10.1080/10641963.2020.1764018
84. Ashraf MA, Shokouhi N, Shirali E, Davari-tanha F, Memar O, Kamalipour A, et al. COVID-19 in Iran, A Comprehensive Investigation From Exposure To Treatment Outcomes, PREPRINT (Version 1) (2020). doi: 10.21203/rs.3.rs-26339/v1
85. Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Togni A, et al. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLoS ONE. (2020) 15:e0235248. doi: 10.1371/journal.pone.0235248
86. De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tre G, Belmans L, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. (2020) 21:909–14. doi: 10.1016/j.jamda.2020.06.018
87. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. (2020) 201:1380–8. doi: 10.1164/rccm.202002-0445OC
88. Feng Z, Li J, Yao S, Yu Q, Zhou W, Mao X, et al. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019: a multicenter data analysis. medRxiv. (2020). doi: 10.1101/2020.04.08.20057539
89. Golpe R, Perez-de-Llano LA, Dacal D, Guerrero-Sande H, Pombo-Vide B, Ventura-Valcarcel P, et al. [Risk of severe COVID-19 in hypertensive patients treated with renin-angiotensin-aldosterone system inhibitors]. Med Clin. (2020) 155:488–90. doi: 10.1016/j.medcli.2020.06.013
90. Guner R, Hasanoglu I, Kayaaslan B, Aypak A, Kaya Kalem A, Eser F, et al. COVID-19 experience of the major pandemic response center in the capital: results of the pandemic's first month in Turkey. Turk J Med Sci. (2020) 50:1801–9. doi: 10.3906/sag-2006-164
91. Jurado A, Martin MC, Abad-Molina C, Orduna A, Martinez A, Ocana E, et al. COVID-19: age, Interleukin-6, C-reactive protein, and lymphocytes as key clues from a multicentre retrospective study. Immun Ageing. (2020) 17:22. doi: 10.1186/s12979-020-00194-w
92. Liu Y, Huang F, Xu J, Yang P, Qin Y, Cao M, et al. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. (2020). doi: 10.1101/2020.03.20.20039586
93. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. (2020) 382:2431–40. doi: 10.1056/NEJMoa2006923
94. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. (2020) 382:2441–8. doi: 10.1056/NEJMoa2008975
95. Rhee SY, Lee J, Nam H, Kyoung DS, Shin DW, Kim DJ. Effects of a DPP-4 inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. Diabetes Metab J. (2021) 45:251–9. doi: 10.4093/dmj.2020.0206
96. Yan H, Valdes AM, Vijay A, Wang S, Liang L, Yang S, et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID-19: a large case-control study. Clin Pharmacol Ther. (2020) 108:1185–94. doi: 10.1002/cpt.2047
97. Anzola GP, Bartolaminelli C, Gregorini GA, Coazzoli C, Gatti F, Mora A, et al. Neither ACEIs nor ARBs are associated with respiratory distress or mortality in COVID-19 results of a prospective study on a hospital-based cohort. Intern Emerg Med. (2020) 15:1477–84. doi: 10.1007/s11739-020-02500-2
98. Huh K, Ji W, Kang M, Hong J, Bae GH, Lee R, et al. Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea. Int J Infect Dis. (2021) 104:7–14. doi: 10.1016/j.ijid.2020.12.041
99. Ferrario CM, Ahmad S, Groban L. Mechanisms by which angiotensin-receptor blockers increase ACE2 levels. Nat Rev Cardiol. (2020) 17:378. doi: 10.1038/s41569-020-0387-7
100. Guo X, Zhu Y, Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. (2020) 76:e13–4. doi: 10.1161/HYPERTENSIONAHA.120.15572
101. Ssentongo AE, Ssentongo P, Heilbrunn ES, Lekoubou A, Du P, Liao D, et al. Renin-angiotensin-aldosterone system inhibitors and the risk of mortality in patients with hypertension hospitalised for COVID-19: systematic review and meta-analysis. Open Heart. (2020) 7:e001353. doi: 10.1136/openhrt-2020-001353
102. Lee MMY, Docherty KF, Sattar N, Mehta N, Kalra A, Nowacki AS, et al. Renin-angiotensin system blockers, risk of SARS-CoV-2 infection and outcomes fromCoViD-19: systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2020) doi: 10.1093/ehjcvp/pvaa138
103. Walkey AJ, Kumar VK, Harhay MO, Bolesta S, Bansal V, Gajic O, et al. The viral infection and respiratory illness universal study (VIRUS): an international registry of coronavirus 2019-related critical illness. Crit Care Explor. (2020) 2:e0113. doi: 10.1097/CCE.0000000000000113
104. Turek JR, Bansal V, Tekin A, Sharma M, Bogojevic M, Deo NN, et al. Rapid project management in a time of COVID-19 crisis: lessons learned from a global VIRUS: COVID-19 registry. JMIR Preprints. (2021). doi: 10.2196/preprints.27921. [Epub ahead of print].
105. Walkey AJ, Sheldrick RC, Kashyap R, Kumar VK, Boman K, Bolesta S, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019. Pandemics and beyond: the society of critical care medicine discovery viral infection and respiratory illness universal study registry. Crit Care Med. (2020) 48:e1038–44. doi: 10.1097/CCM.0000000000004572
106. International Society of Hypertension. A Statement From the International Society of Hypertension on COVID-19. (2020). Available online at: https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/ (accessed November 03, 2021).
107. Statement from the American Heart Association Heart Heart Failure Society of America and The American College of Cardiology. Patients Taking ACE-i and ARBs who Contract COVID-19 Should Continue Treatment, Unless Otherwise Advised by their Physician. Heart Failure Society of America. Retreived from: https://hfsa.org/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise (accessesed October 03, 2021).
Keywords: COVID-19, Angiotensin inhibitors, ACEI, ARB, mortality, severity, meta-analysis, meta-regression
Citation: Singh R, Rathore SS, Khan H, Bhurwal A, Sheraton M, Ghosh P, Anand S, Makadia J, Ayesha F, Mahapure KS, Mehra I, Tekin A, Kashyap R and Bansal V (2022) Mortality and Severity in COVID-19 Patients on ACEIs and ARBs—A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Front. Med. 8:703661. doi: 10.3389/fmed.2021.703661
Received: 30 April 2021; Accepted: 08 December 2021;
Published: 10 January 2022.
Edited by:
Mitja Lainscak, University of Ljubljana, SloveniaReviewed by:
Jesus Rico-Feijoo, Hospital Universitario Río Hortega, SpainYi Yang, Zhongda Hospital, Southeast University, China
Copyright © 2022 Singh, Rathore, Khan, Bhurwal, Sheraton, Ghosh, Anand, Makadia, Ayesha, Mahapure, Mehra, Tekin, Kashyap and Bansal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vikas Bansal, bansal.vikas@mayo.edu
†These authors have contributed equally to this work
 Romil Singh
Romil Singh Sawai Singh Rathore
Sawai Singh Rathore Hira Khan
Hira Khan Abhishek Bhurwal
Abhishek Bhurwal Mack Sheraton
Mack Sheraton Prithwish Ghosh6
Prithwish Ghosh6  Sohini Anand
Sohini Anand Janaki Makadia
Janaki Makadia Kiran S. Mahapure
Kiran S. Mahapure Ishita Mehra
Ishita Mehra Aysun Tekin
Aysun Tekin Rahul Kashyap
Rahul Kashyap Vikas Bansal
Vikas Bansal