Systemic and functional effects of continuous azithromycin treatment in patients with severe chronic obstructive pulmonary disease and frequent exacerbations
- 1Department of Respiratory Medicine, Hospital Universitari de Bellvitge, Institut d’Investigacio Biomedica de Bellvitge – IDIBELL, Universitat de Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain
- 2Department of Respiratory Medicine, Consorci Sanitari Alt Penedès Garraf, Barcelona, Spain
- 3Department of Respiratory Medicine, Hospital de Sabadell, Institut Universitari Parc Taulí-UAB, Sabadell, Spain
- 4Research Network for Respiratory Diseases (CIBERes), ISCIII, Madrid, Spain
- 5Department of Respiratory Medicine, Hospital Germans Trias i Pujol, Institut d’Investigació en Ciències de la Salut Germans Trias i Pujol – IGTP, Universitat Autònoma de Barcelona, Badalona, Spain
- 6Department of Microbiology, Hospital Universitari de Bellvitge, IDIBELL, Universitat de Barcelona, Barcelona, Spain
Background: Continuous treatment with azithromycin may lead to fewer acute exacerbations of chronic obstructive pulmonary disease (AECOPD), but little is known of its impact on systemic and functional outcomes in real-life settings.
Methods: This was a multicenter prospective observational study of patients with severe COPD who started treatment with azithromycin. Tests were compared at baseline and after 3 and 12 months of treatment. These included lung function tests, a 6-min walking test (6MWT), and enzyme-linked immunosorbent assays of serum and sputum markers, such as interleukins (IL-6, IL-8, IL-13, IL-5), tumor necrosis factor receptor 2 (TNFR2), and inflammatory markers. Incidence rate ratios (IRR) and their 95% confidence intervals (95% CI) are reported.
Results: Of the 478 eligible patients, the 42 who started azithromycin experienced reductions in AECOPDs (IRR, 0.34; 95% CI, 0.26–0.45) and hospitalizations (IRR, 0.39; 95% CI, 0.28–0.49). Treatment was also associated with significant improvement in the partial arterial pressure of oxygen (9.2 mmHg, 95% CI 1.4–16.9) at 12 months. While TNFR2 was reduced significantly in both serum and sputum samples, IL-13 and IL-6 were only significantly reduced in serum samples. Moreover, an elevated serum and sputum IL-8 level significantly predicted good clinical response to treatment.
Conclusion: Continuous azithromycin treatment in a cohort of patients with severe COPD and frequent exacerbations can significantly reduce the number and severity of exacerbations and improve gas exchange. Treatment changes the pattern of microorganism isolates and decreases the inflammatory response. Of note, IL-8 may have utility as a predictor of clinical response to azithromycin treatment.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory and systemic disease that affects the airways. Acute exacerbations of COPD (AECOPD) affect not only the natural history of the disease but also quality of life, the number of hospitalizations, and mortality rates (1). Exacerbations, which are frequently caused by respiratory viral or bacterial infections, are events characterized by increases in respiratory symptoms that worsen in <14 days, may be accompanied by tachypnea and/or tachycardia, and are often associated with increased local and systemic inflammation (2). Various interventions have been shown to prevent AECOPDs, including both non-pharmacological measures (e.g., quitting smoking, respiratory rehabilitation, and vaccines) and pharmacological measures (e.g., bronchodilator and corticosteroid inhalers) (3). Nevertheless, 25–30% of patients still experience AECOPD, indicating that these measures do not prevent all cases (4).
Randomized controlled trials have demonstrated the efficacy of prophylactic azithromycin in reducing AECOPDs, and as such, current clinical guidelines recommend its use in patients with frequent exacerbations despite optimal inhaled therapy (5–7). However, there is limited evidence on the most appropriate time of administration or the most effective dose needed to control exacerbations and minimize side effects. Albert et al. compared the use of 250 mg of azithromycin per day for 1 year irrespective of COPD severity or frequency of AECOPD. They showed both an increased time lag between exacerbations and a decrease in the frequency of exacerbations compared to placebo (5). The COLUMBUS trial also showed that 500 mg of azithromycin three times a week for 1 year significantly reduced (42%) the exacerbation rate compared with placebo in patients with three or more AECOPDs annually (6). Finally, a more recent study found that doses of 250 mg and 500 mg three times a week had comparable effectiveness at preventing exacerbations in patients with severe COPD and frequent exacerbations, and that this could minimize the potential side effects of a long-term antibiotic therapy (7).
Antibiotic prophylaxis in AECOPDs may increase the risk of selection for resistant bacteria, facilitating the spread of antimicrobial resistance. Even though azithromycin is usually well-tolerated drug, undesirable side effects can still result from polypharmacy, advanced age, and comorbidities in real-world settings. Moreover, the therapeutic response can vary depending on the characteristics and baseline statuses of patients. Investigating what factors predict a favorable response, both in the number of exacerbations and their functional effects, is of particular clinical interest.
This study aimed to analyze the systemic and pulmonary functional changes associated with severe COPD in patients with frequent exacerbations after continuous azithromycin treatment, their effect on microbiological isolates in residual exacerbations, and the potential involvement of inflammatory and immunomodulatory mechanisms.
2. Methods
2.1. Study design
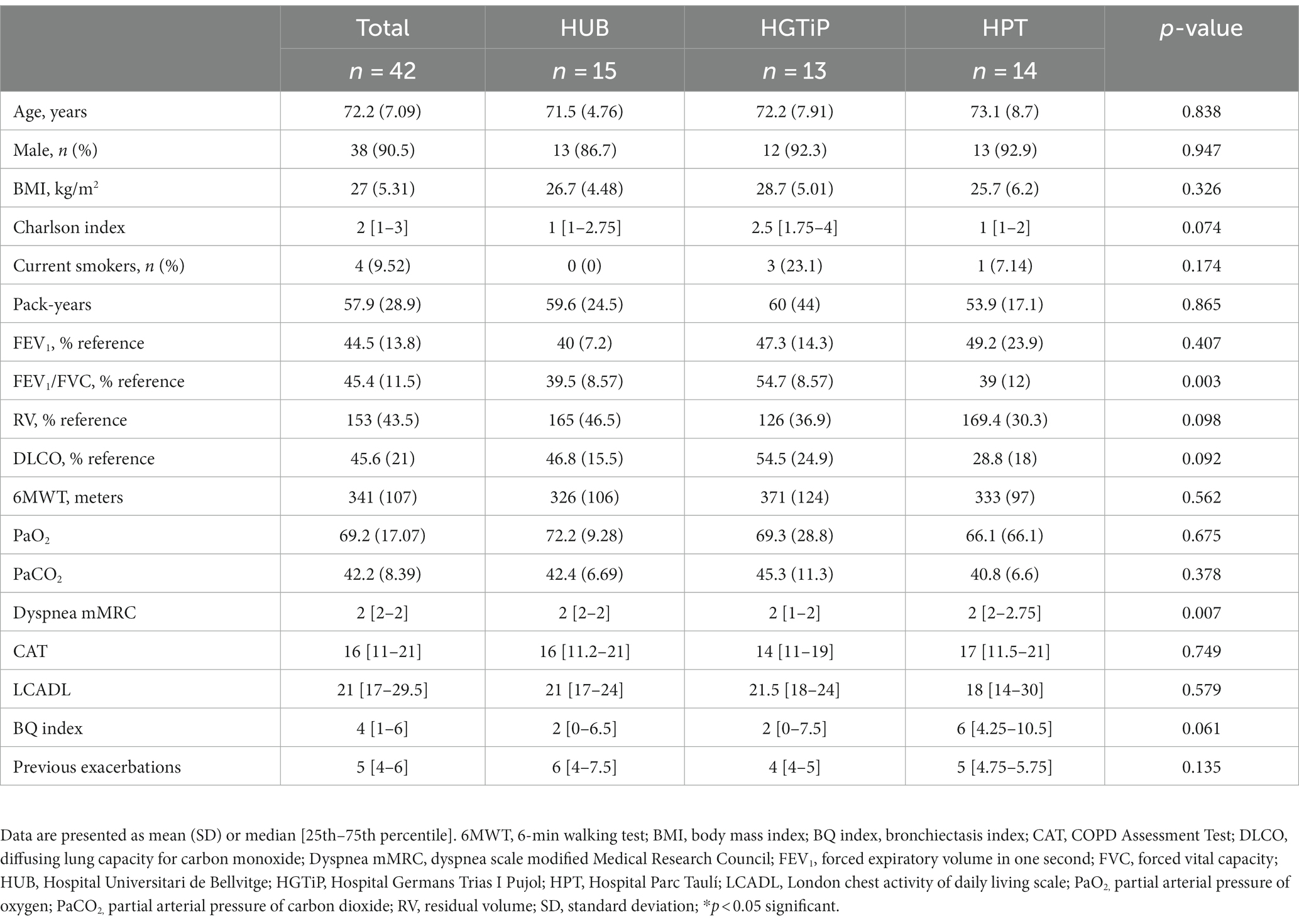
This was a multicenter, observational, and prospective study of patients with severe COPD and frequent exacerbations who regularly attended comprehensive care programs in the respiratory day hospitals of three university hospitals in Barcelona. The participating hospitals were Hospital Universitari de Bellvitge (HUB), Hospital Parc Taulí (HPT), and Hospital Germans Trias i Pujol (HGTiP). All patients who started treatment with azithromycin according to clinical guideline recommendations and practice (8) were included consecutively between January and December, 2017.
2.2. Patient selection
COPD was defined according to the GOLD recommendations: a history of smoking (>10 pack-years) and an FEV1 (forced exhalation volume in 1 second) to FVC (forced vital capacity) ratio < 70% (8). By clinical consensus, and with the objective of homogenizing the selection between the different centers, we included patients at the maximum therapeutic step in the 6 months prior to inclusion if they had frequent exacerbations, defined as ≥4 moderate AECOPDs in the last year (≥3 if two were severe), and had sputum cultures negative for mycobacteria. Patients with contraindications to macrolide therapy (allergy, long QT syndrome), different respiratory pathologies, lack of suitability for macrolide treatment, according to the clinician’s criteria (severe cardiovascular disease, hearing loss, use of co-therapies that prolong the QT interval or drug interactions) and prior use of azithromycin or inhaled colimycin (not concomitant initiation) were excluded.
All patients signed an informed consent form in accordance with the principles outlined in the Declaration of Helsinki, and the local ethics committee approved the study (CEIC, ref. SSP-AZI-2016-01).
2.3. Visits and follow-up
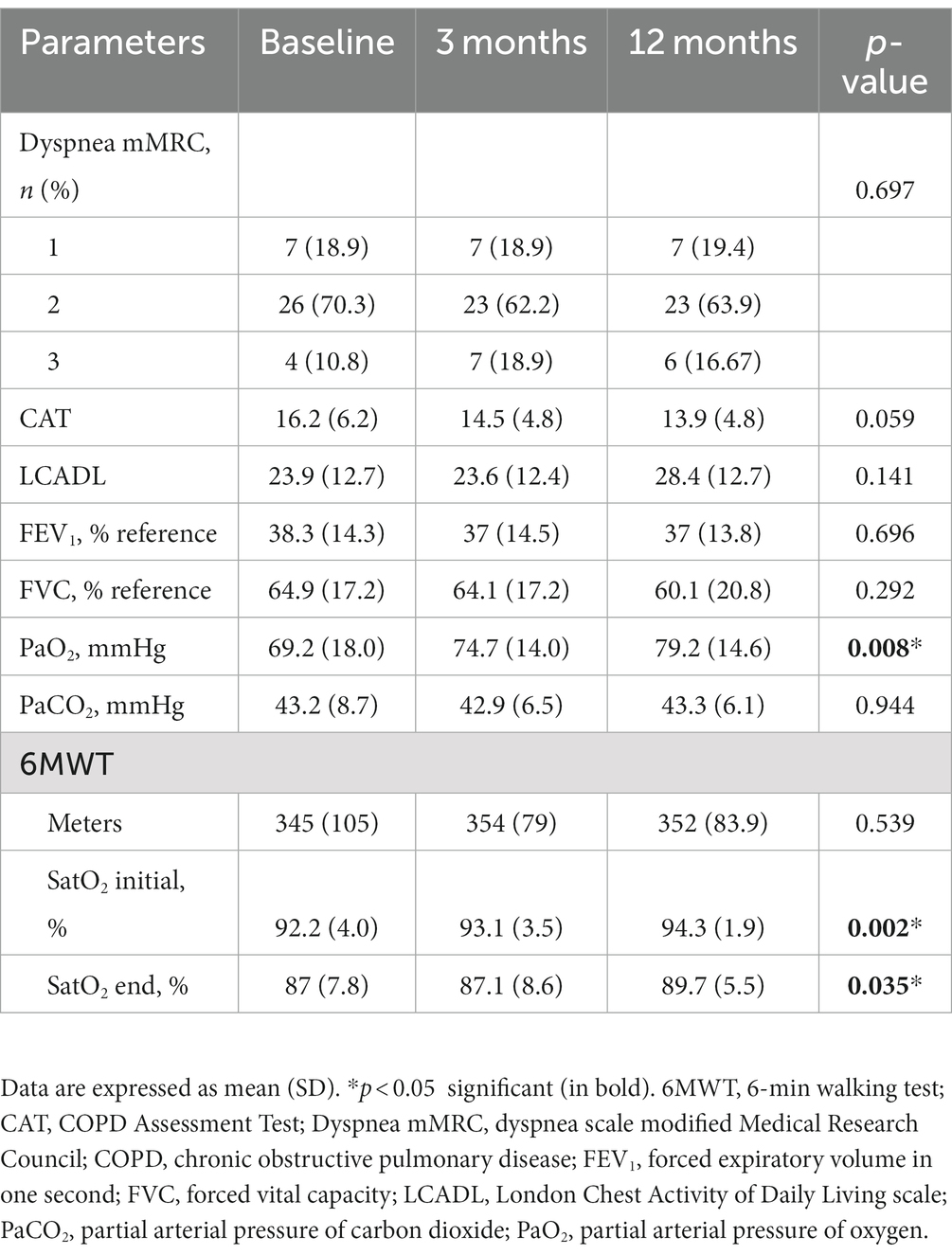
All included patients started treatment with azithromycin 250 mg three times per week for 1 year. Sociodemographic data, anthropometrics, COPD history, and symptom evaluations were collected in all patients. Symptom tests included the COPD Assessment Test (CAT), London Chest Activity of Daily Living Scale (LCADL) (9), and the modified Medical Research Council (mMRC) Dyspnea Scale. In addition, participants underwent a 6-min walking test (6MWT), respiratory functional test, and arterial blood gas analyzes at baseline and after 3 and 12 months of follow-up. Blood and sputum samples were collected at all visits for laboratory determinations. All moderate and severe exacerbations were recorded and treated according to appropriate criteria and guidelines. Exacerbations from the year before starting treatment were also registered. An exacerbation was defined as any event that caused a worsening of respiratory symptoms and that required modification of the patient’s usual treatment.
2.4. Quantification of inflammatory parameters in blood and sputum
Serum samples were obtained from venous blood. After allowing the sample to stand at room temperature for 2 h, it was centrifuged for 15 min at 1000 × g, and the supernatant was taken for analysis.
Sputum was separated from contaminating saliva by macroscopic examination, before being weighed and homogenized with 4 volumes of dithiothritol (Sputasol, Oxoid Ltd., Hants, United Kingdom). After 15 min at room temperature, the same volume of phosphate-buffered saline was added and the whole mixture was filtered and centrifuged at 600 × g for 15 min. The supernatant was stored at −80°C for determinations.
Analysis was performed by enzyme-linked immunosorbent assay (ELISA). Cytokine concentrations (IL-5, IL-6, IL-8, IL-13) were measured in serum samples and supernatant sputum samples using a multiplex immune-bead assay (Milliplex MAP High Sensitivity Human T cell panel Kit; Merck Millipore). TNR2 was analyzed with a Human soluble TNF Receptor 2 ELISA Kit (sTNFRII; RAB0490). All procedures were performed according to the manufacturer’s instructions, centralized in one of the centers.
2.5. Sputum collection and bacterial load detection
Sputum samples were obtained spontaneously at visits and during AECOPD episodes before starting any antimicrobial treatment. Only good quality sputum samples were considered (i.e., 25 leukocytes per low-power field) following the microbiological routine (10). Briefly, samples were homogenized with dithiothreitol (Sputasol, Oxoid Ltd., Hants, United Kingdom), and plated onto blood agar, chocolate agar, and MacConkey agar before overnight incubation at 37°C in a 5% CO2 atmosphere (blood and chocolate agar) or ambient air atmosphere (MacConkey agar). Bacterial colonies were sub-cultured for identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonik GmbH, Bremen, Germany).
2.6. Statistical analyzes
Data are expressed as means ± standard deviation (SD) or medians [25th–75th percentile] for continuous data and as frequencies (percentage) for categorical data. Multiple comparisons were evaluated by chi-squared (categorical), student t (parametric), or Mann–Whitney (nonparametric) tests, applying the Bonferroni method if the Kruskal–Wallis test found significant differences. A negative binomial regression model was used to study the number of exacerbations after treatment, reporting the incidence rate ratio (IRR) and 95% confidence interval (95%CI). Different predictive statistical models were fitted for each analytical or clinical variable of interest, adjusted by age and Charlson index. A value of p of 0.05 was considered statistically significant. IBM SPSS version 22 (IBM Corp., Armonk, NY, United States) was used for all analyzes.
3. Results
3.1. Study subjects
Of the 478 eligible patients with severe COPD in three respiratory day hospitals, we included 42 (8.8%) who started azithromycin (Figure 1; mean age 72.2 ± 7.09 years). Fifty-eight patients were already under treatment with long-term azithromycin therapy and were excluded; therefore, 21% of patients in this cohort were treated. At the time of inclusion, all patients were receiving combined inhaled bronchodilator therapy, long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs), 39 (93%) inhaled corticosteroids (ICS) and nine (21%) long-term oxygen therapy. Participants corresponded to group E of the current GOLD classification (8), having severe airflow obstruction (mean FEV1 44.5% ± 13.8%), an average mMRC dyspnea symptom score of 2, and a median of 5 (4–6) exacerbations during the previous year (Table 1). Patient characteristics were comparable among the three participating hospitals. Only 37 patients finished the study, with patients withdrawn due to cardiac pathology (n = 3; 1 acute myocardial infarction, 2 QT prolongations), diarrhea (n = 1), and personal preference (n = 1).
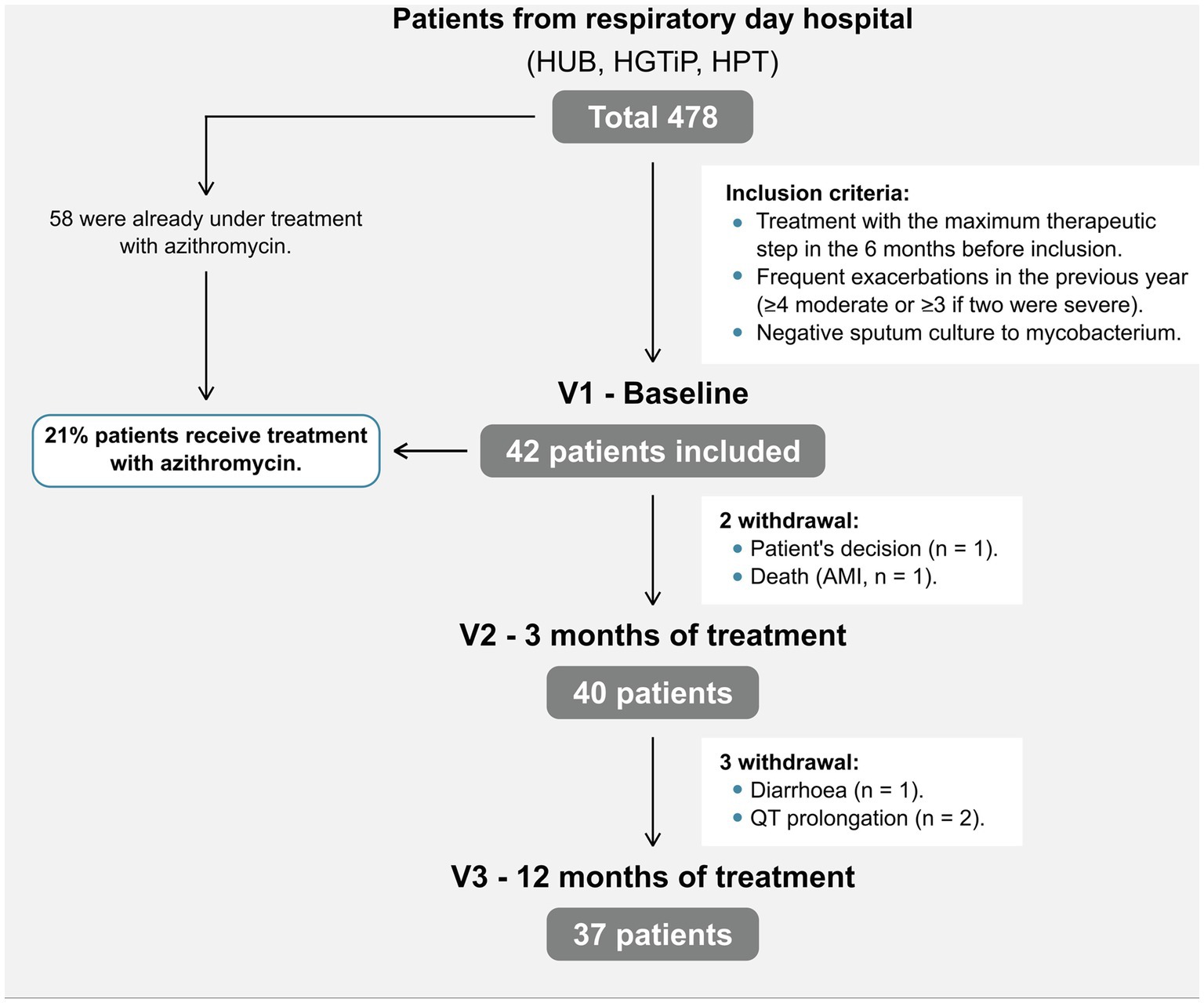
Figure 1. Patient flowchart. AMI, acute myocardial infarction; HUB, Hospital Universitari de Bellvitge; HGTiP, Hospital Germans Trias I Pujol; HPT, Hospital Parc Taulí.
3.2. Clinical and functional effects after continuous azithromycin treatment
A significant improvement in the partial arterial pressure of oxygen (PaO2) was observed after treatment with azithromycin at 3 and 12 months compared with the baseline visit (9.21 mmHg increase at 12 months; 95%CI, 1.45–16.97). This was also associated with a significant improvement in oxygen saturation both at rest and during exercise. However, symptoms (except for a slight improvement in CAT and LCADL scores), the 6MWT distance, and lung function did not differ significantly after 12 months (Table 2).
3.3. Inflammatory changes in serum and sputum
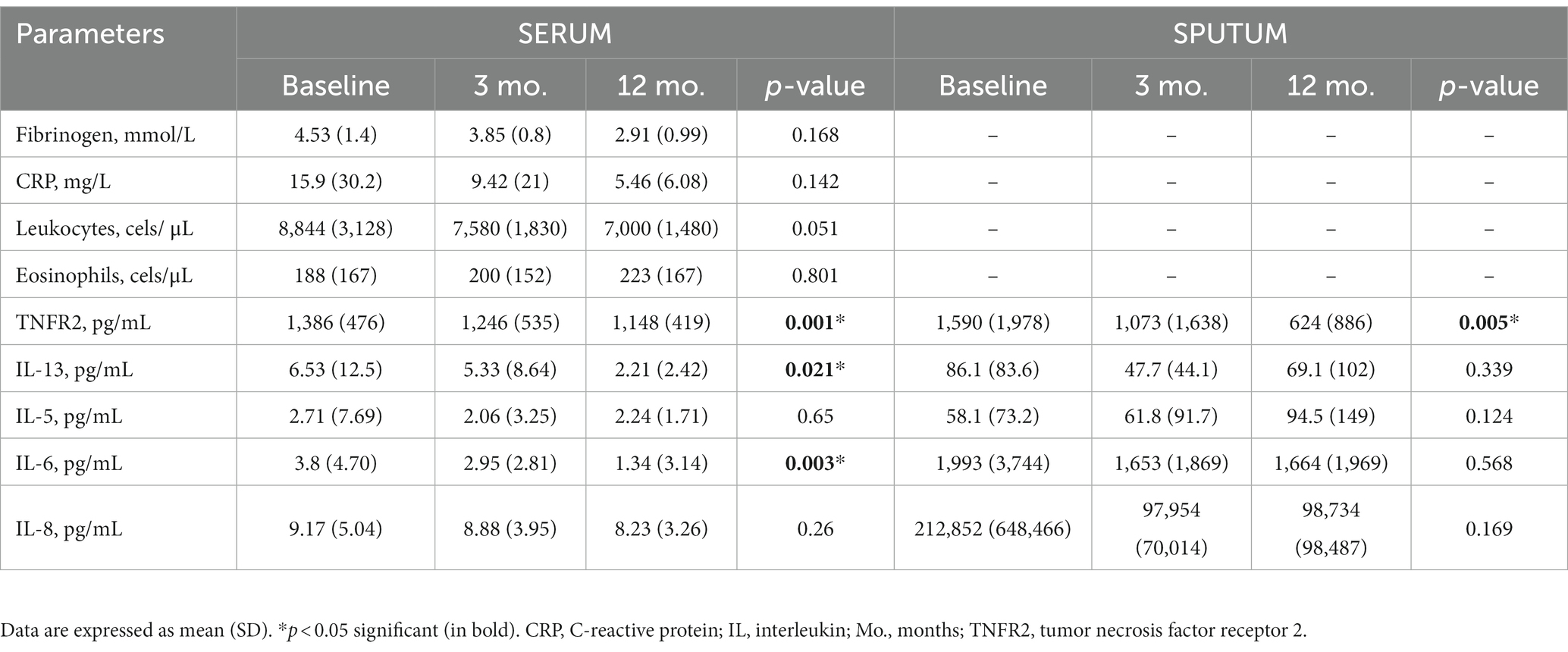
Most serum and sputum inflammatory parameters improved after treatment with azithromycin (Table 3). Of note, treatment significantly lowered serum IL-13, IL-6, and TNFR2 levels, but only lowered sputum TNFR2 levels, with no significant correlation found between serum and sputum levels (data not shown). The decreased inflammatory response was more consistent in serial serum determinations.
3.4. Predictors of clinical response in study participants
After analyzing all serum markers and functional variables, an increase in baseline IL-8 predicted a positive response to azithromycin treatment and a reduction in exacerbations (Supplementary Table S1). Among the sputum markers, a high IL-8 level was also independently associated with good clinical response (Supplementary Table S2).
3.5. Effect of treatment with azithromycin on COPD exacerbations
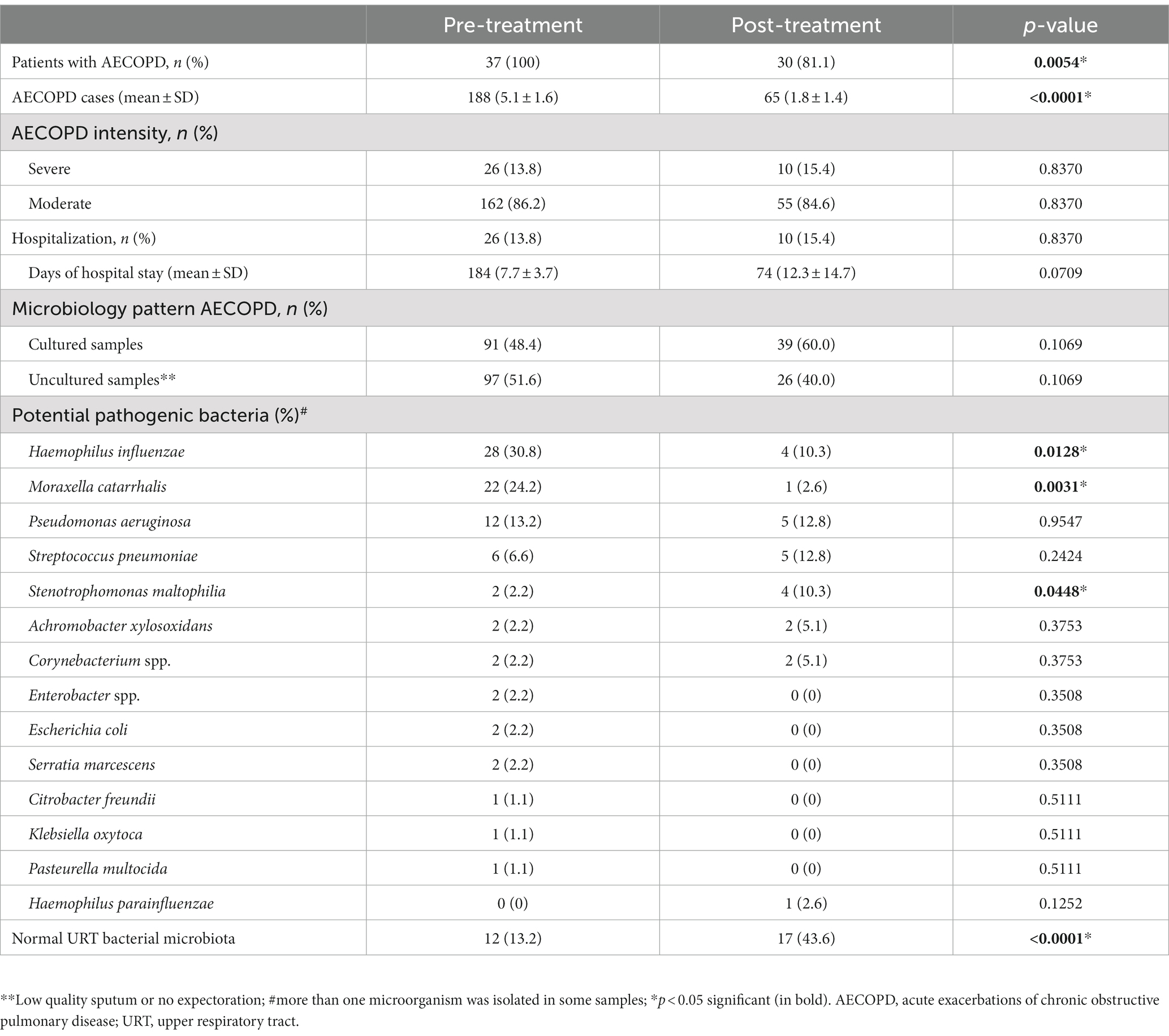
The 37 participants had 188 exacerbations (5.1 ± 1.6 AECOPD/patient) in the year before starting azithromycin compared with only 65 during treatment (1.8 ± 1.4 AECOPD/patient; p < 0.0001; Figure 2). The IRR of AECOPD after treatment was 0.34 (95%CI, 0.26–0.45), indicating that patients had an approximate 66% reduction in acute exacerbations after treatment. Before treatment, 162 (86%) exacerbations were moderate and 26 (14%) were severe and required hospitalization (mean, 184 days in hospital). During treatment, 55 (84.6%) exacerbations were moderate and 10 (15%) were severe and required hospitalization (mean, 74 days; Table 4). Therefore, hospitalizations fell by 61.5% at 12 months (IRR, 0.39; 95%CI, 0.28–0.49). Although fewer patients required hospital admission, their mean hospital stays tended to be longer (7.7 ± 3.7 vs. 12.3 ± 14.7 days).
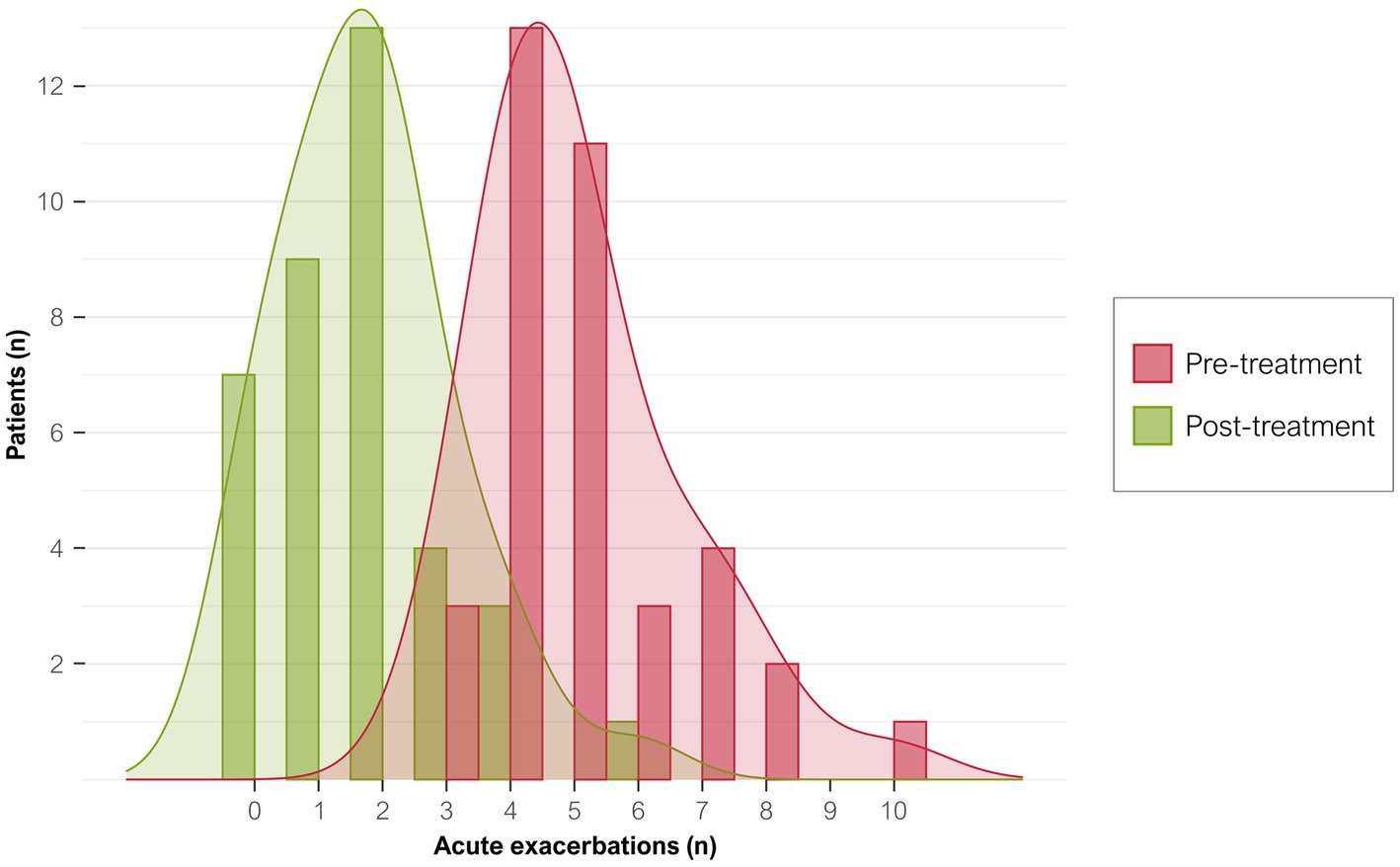
Figure 2. Distribution of exacerbations per patient before and after treatment. Graph shows the number of exacerbations in the year before starting azithromycin treatment (red bars) and after 12 months of treatment (green bars).
3.6. Microbiological effect of continuous azithromycin treatment
A total of 95 microorganisms were obtained during the 188 AECOPD in the year before starting treatment with azithromycin, which decreased to only 41 microorganisms during 65 AECOPD throughout the follow-up period. There was a significant reduction in frequent sputum isolates, such as Haemophilus influenzae (p = 0.0128) and Moraxella catarrhalis (p = 0.0031) (Table 4), and a significant increase in infrequent microorganisms, such as Stenotrophomonas maltophilia (from 2.2 to 10.3%; p = 0.0448). Microorganisms otherwise increased in the upper respiratory tract, and sputum isolates of Pseudomonas aeruginosa and Streptococcus pneumoniae did not change.
Overall, the pattern of microbiological isolates changed with the intensity of AECOPD from before to after azithromycin therapy (Figure 3). In moderate AECOPD, M. catarrhalis decreased from 26.6 to 2.8% (p = 0.0026) and S. maltophilia increased from 0 to 11.1% (p = 0.0026), with a reset observed in the usual upper respiratory tract bacterial microbiota from 11.4 to 47.2% (p < 0.0001). In severe AECOPD, P. aeruginosa isolates increased from 1 to 3 (p = 0.0075) and S. pneumoniae isolates increased from 0 to 2 (p = 0.0078) after azithromycin therapy.
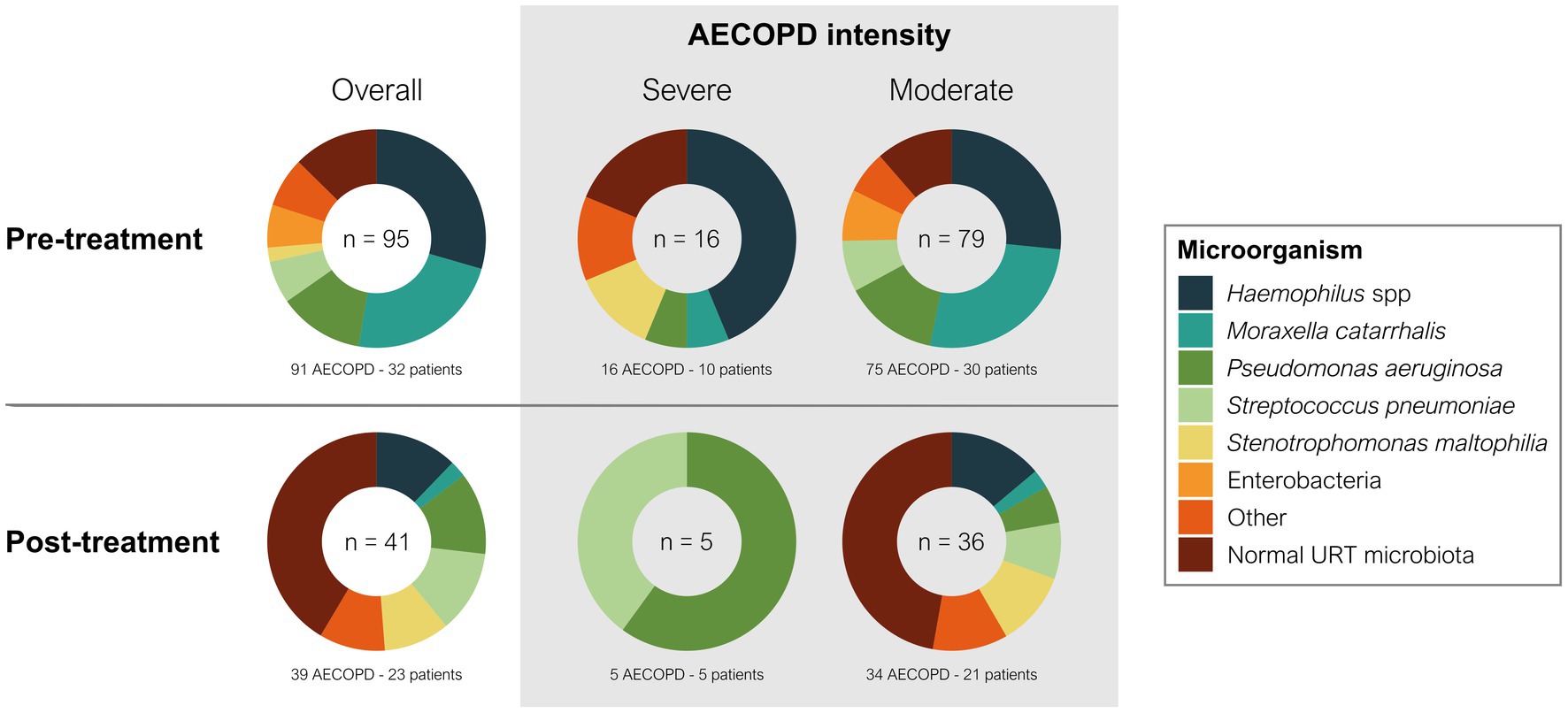
Figure 3. Change of microbiology by AECOPD severity during long-term azithromycin therapy. The number inside each donut chart indicates the number of bacterial isolates. Pre-treatment indicates the year before azithromycin treatment, and post-treatment indicates the first year of azithromycin treatment.
4. Discussion
This multicenter, prospective, observational study analyzed the systemic and functional real-life effects of continuous treatment with azithromycin in patients with severe COPD and frequent exacerbations managed in respiratory day hospitals. In addition to confirming that azithromycin effectively reduced the rate of moderate to severe COPD exacerbations, we provide the first evidence that treatment also improves gas exchange, both at rest and during exercise, after 1 year of treatment. Of the interleukin profiles analyzed in this study, IL-8 and TNFR2 presented as the major inflammatory mediators in the immunomodulatory response to azithromycin in patients with COPD. Baseline elevations of IL-8 in both blood and sputum samples predicted the therapeutic response and may indicate its potential utility as a biomarker. Changes were also observed in the microbial composition of subsequent AECOPD episodes after treatment, with the identification of potentially pathogenic microorganisms with unusual patterns of intrinsic antibiotic resistance that required treatment. This increases our knowledge about how azithromycin treatment affects the inflammatory response and bacteria selection in the airways, together with the implications for future COPD exacerbations.
As expected, continuous azithromycin treatment in patients with severe COPD and frequent exacerbations led to fewer exacerbations (up to 66%) after 1 year of treatment, even using a dosage of 250 mg 3 times a week. From the limited published evidence available (only two clinical trials (5, 6), both carried out in a different profile of COPD patients) does not allow a categorical and unanimous recommendation for the type of patient chosen and the dose to be administered (8). In other chronic inflammatory respiratory diseases, such as bronchiectasis, the dosage of 250 mg 3 times a week for 1 year has been used successfully, minimizing side effects. Moreover, in airways of these patients, this dosage has demonstrated a significant reduction in host neutrophilic inflammatory response (11). The high effectiveness in the control of exacerbations achieved in this study and the few side effects also support the use of this dosage in clinical practice in COPD patients. Previous studies have shown that patients with more severe COPD and higher numbers of exacerbations during the previous year had higher reductions in AECOPD with azithromycin treatment (5–7). These results are consistent with the high effectiveness observed in this study and support the need to identify candidates for preventive therapy accurately and to avoid the use of azithromycin in patients with less severe disease who may not benefit. However, it was notable that neither baseline lung function nor the number of previous exacerbations predicted treatment response in this cohort. Instead, baseline elevations of IL-8 in both blood and sputum samples predicted the therapeutic response, suggesting that this could be a useful biomarker in clinical practice (12, 13).
Another novel finding was the improved arterial oxygenation in patients after 12 months of treatment with azithromycin, both at rest and after exercise, which was associated with effective control of AECOPD. To the best of our knowledge, gas exchange analysis has not been evaluated in studies of azithromycin in patients with COPD. Given that exercise tolerance and lung functional parameters did not improve with azithromycin therapy, this indicates a dissociation between systemic functional data and gas exchange. There are some mechanisms through which azithromycin may improve gas exchange in patients with COPD. The most important is provided by the drug’s anti-inflammatory properties, which reduce the chronic inflammatory response that occurs in the lungs of COPD patients. The decrease in inflammatory markers in blood and sputum after starting prolonged treatment with azithromycin would indicate less airway inflammation and, as a result, an improvement in the pulmonary ventilation-perfusion ratios of treated patients, which presumably contributes to the improvement of gas exchange. In addition, azithromycin can modulate the immune response in the lungs, which may help reduce inflammation; finally, by reducing the frequency and severity of exacerbations, further lung damage can be prevented, leading to more effective lung function.
After analyzing the inflammatory parameters in serum and sputum samples, we observed a numerical improvement in most variables. This was particularly notable in the significant reductions of serum IL-13 and IL-6, as well as serum and sputum TNFR2, after 12 months of treatment. The decreased inflammatory response was more consistent in serial serum determinations than in sputum determinations. Moreover, our predictive models showed that high inflammatory marker and IL-8 levels at baseline predicted a good response to treatment. These immunomodulatory and anti-inflammatory effects of macrolides are well described in the literature (14, 15). Of the interleukin profiles analyzed in this study, IL-8 and TNFR2 presented as the major inflammatory mediators in the immunomodulatory response to azithromycin in patients with COPD. Studies indicate that macrolides take part in proinflammatory cytokine suppression (16–18) and inhibit the transcription and liberation of IL-8, which is important to neutrophil chemotaxis (19). Azithromycin has also been shown to inhibit TNFα-induced production of IL-8 via the JNK signaling pathway and to inhibit MUC5AC production and MMP9. These factors combine to reduce mucus production, which is beneficial for patients with COPD (18). An animal model further showed that TNFα levels were reduced after macrolide treatment, and that this was associated with inhibited neutrophil recruitment in the lungs (12). Other effects of macrolides on cytokines include the modulation of dendritic cells by inhibiting IL-6 and stimulating IL-10 (13). In addition, a potential role of azithromycin in the T helper 2 (Th2) inflammatory pathway has been described, with evidence that it inhibits the expression of different IL- 13-induced genes to reduce mucus expression (i.e., MUC5AC) (20). Overall, our results therefore support previous findings, showing a reduction in cytokine levels associated with neutrophil and eosinophil inflammatory pathways. These help to resolve acute infections and reduce exacerbations in patients with chronic airway diseases.
Despite the significant reduction in exacerbations under continuous azithromycin treatment, some patients still had further AECOPD episodes. Although these patients had fewer hospitalizations, they required more days in hospital to treat severe AECOPD. They also showed changes in the patterns of microbiological isolates in sputum samples after continuous azithromycin treatment, with fewer frequent (H. influenzae and M. catarrhalis) and more potentially pathogenic microorganisms with unusual patterns of intrinsic antibiotic resistance that required treatment (e.g., S. maltophilia or Achromobacter xylosoxidans in 15% of isolates). This has clear implications when selecting empirical antibiotics for residual AECOPDs in patients receiving continuous azithromycin. Viral infections usually act as the trigger of the exacerbation, but no routine study of viral infections was performed in our study. Further research is therefore required.
Although it was not the scope of our study, many antibiotic resistance mechanisms have been described among the common microorganisms after continuous macrolide treatment (21–24). Indeed, the antimicrobial properties of azithromycin seem to affect the respiratory microbiota, and this may change the clinical evolution of COPD. Future studies should therefore look at the impact of limiting the use of some antibiotics in future AECOPD episodes based on the resistance mechanisms that develop after continuous azithromycin therapy.
The main limitation of the present study is the small sample size and absence of a control group. However, we did not want to demonstrate the efficacy of the treatment with azithromycin in a clinical trial. Instead, we wanted to analyze the effectiveness of azithromycin in real-world settings among patients with severe COPD (e.g., older, comorbidities, and frequent exacerbations), who clinical trials might miss, and to study the role of azithromycin on inflammation and bacterial isolates in these cases.
In conclusion, continuous azithromycin treatment for patients with severe COPD, comorbidities, and frequent exacerbations not only significantly reduces exacerbation and severity rates after 1 year of treatment but also improves gas exchange. Changes are also seen in microorganism patterns and inflammatory responses after treatment. Of note, high serum and sputum IL-8 levels at baseline also appear to predict a good clinical response to azithromycin and may indicate its potential utility as a biomarker. If proven, IL-8 as could be used to improve patient selection and reduce microbial resistance in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by local ethics committee (Comité Ética Investigación Clínica, CEIC, ref. SSP-AZI-2016-01). The patients/participants provided their written informed consent to participate in this study.
Author contributions
EC participated in patient’s inclusion, statistical analysis, figure preparation, data interpretation, and manuscript drafting. DH participated in the study design, patient’s inclusion, statistical analysis, data interpretation, and obtaining funding. CM and AM participated in the study design, patient’s inclusion, and data interpretation. AC-S participated in sputum analysis, figure and table preparation, and data interpretation. XP participated patient’s inclusion and data interpretation. MG-N participated in sputum analysis and ELISA experiments. SM participated in sputum analysis, data interpretation, and critical revision of the manuscript. SS contributed to the study concept and design, patient inclusion, funding, study supervision, data interpretation, manuscript drafting, and critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The research was funded by the Instituto de Salud Carlos III (grant PI20/00777 and PI16/00977), co-funded by the European Regional Development Fund (ERDF) “A Way to Build Europe” and grants from Menarini to SS and from SEPAR 088/2016, SEPAR 1116/2020 and FUCAP-2017.
Acknowledgments
The authors thank the nursing staff of the Day hospitals for their dedication to patient care. We also thank to the Institut d’Investigacio Biomedica de Bellvitge for their technical assistance, as well as the CERCA Programme/Generalitat de Catalunya for providing institutional support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1229463/full#supplementary-material
Abbreviations
6MWT, 6-min walking test; AECOPD, acute exacerbation of COPD; AMI, Acute myocardial infarction; COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; ELISA, Enzyme-linked immunosorbent assay; HGTIP, Hospital Germans Trias I Pujol; HPT, Hospital Parc Taulí; HUB, Hospital Universitari de Bellvitge; ICS, inhaled corticosteroids; JNK, c-Jun N-terminal kinase; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MMP9, Matrix metalloproteinase 9; MUC5AC, Mucin 5 AC; URT, Upper respiratory tract.
References
1. Suissa, S, Dell’Aniello, S, and Ernst, P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. (2012) 67:957–63. doi: 10.1136/thoraxjnl-2011-201518
2. Celli, BR, Fabbri, LM, Aaron, SD, Agusti, A, Brook, R, Criner, GJ, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. (2021) 204:1251–8. doi: 10.1164/rccm.202108-1819PP
3. Marchetti, N, Criner, GJ, and Albert, RK. Preventing acute exacerbations and hospital admissions in COPD. Chest. (2013) 143:1444–54. doi: 10.1378/chest.12-1801
4. Ni, W, Shao, X, Cai, X, Wei, C, Cui, J, Wang, R, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One. (2015) 10:e0121257. doi: 10.1371/journal.pone.0121257
5. Albert, RK, Connett, J, Bailey, WC, Casaburi, R, Cooper, JA Jr, Criner, GJ, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. (2011) 365:689–98. doi: 10.1056/nejmoa1104623
6. Uzun, S, Djamin, RS, Kluytmans, JAJW, Mulder, PGH, van't Veer, NE, Ermens, AAM, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. (2014) 2:361–8. doi: 10.1016/S2213-2600(14)70019-0
7. Pomares, X, Montón, C, Huertas, D, Marín, A, Cuevas, E, Casabella, A, et al. Efficacy of low-dose versus high-dose continuous cyclic azithromycin therapy for preventing acute exacerbations of COPD. Respiration. (2021) 100:1070–7. doi: 10.1159/000517781
8. Global Initiative for Chronic Obstructive Lung Disease I. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. (2020). Available at: (www.goldcopd.org).
9. Garrod, R, Bestall, JC, Paul, EA, Wedzicha, JA, and Jones, PW. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London chest activity of daily living scale (LCADL). Respir Med. (2000) 94:589–96. doi: 10.1053/RMED.2000.0786
10. Domenech, A, Puig, C, Martí, S, Santos, S, Fernández, A, Calatayud, L, et al. Infectious etiology of acute exacerbations in severe COPD patients. J Infect. (2013) 67:516–23. doi: 10.1016/J.JINF.2013.09.003
11. Keir, H, Shoemark, A, Dicker, A, Perea, L, Pollock, J, Giam, YH, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med. (2021) 9:873–84. doi: 10.1016/S2213-2600(20)30504-X
12. Tsai, WC, Rodriguez, ML, Young, KS, Deng, JC, Thannickal, VJ, Tateda, K, et al. Azithromycin blocks neutrophil recruitment in pseudomonas endobronchial infection. Am J Respir Crit Care Med. (2004) 170:1331–9. doi: 10.1164/rccm.200402-200OC
13. Sugiyama, K, Shirai, R, Mukae, H, Ishimoto, H, Nagata, T, Sakamoto, N, et al. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin Exp Immunol. (2007) 147:540–6. doi: 10.1111/j.1365-2249.2007.03299.x
14. Friedlander, AL, and Albert, RK. Chronic macrolide therapy in inflammatory airways diseases. Chest. (2010) 138:1202–12. doi: 10.1378/chest.10-0196
15. Martinez, FJ, Curtis, JL, and Albert, R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J COPD. (2008) 3:331–50. doi: 10.2147/copd.s681
16. Uli, O, Erakovi, V, and Parnham, MJ. Anti-inflammatory effects of macrolide antibiotics. Eur J Pharmacol. (2001) 429:209–29. doi: 10.1016/S0014-2999(01)01321-8
17. Rubin, B, and Tamaoki, J. Macrolide antibiotics as biological response modifiers. Curr Opin Investig Drugs. (2000) 1:169–72.
18. Yang, J. Mechanism of azithromycin in airway diseases. J Int Med Res. (2020) 48:030006052093210. doi: 10.1177/0300060520932104
19. Takizawa, H, Desaki, M, Ohtoshi, T, Kawasaki, S, Kohyama, T, Sato, M, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. (1997) 156:266–71. doi: 10.1164/ajrccm.156.1.9612065
20. Mertens, TCJ, Hiemstra, PS, and Taube, C. Azithromycin differentially affects the IL-13-induced expression profile in human bronchial epithelial cells. Pulm Pharmacol Ther. (2016) 39:14–20. doi: 10.1016/J.PUPT.2016.05.005
21. Roberts, MC. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol Lett. (2008) 282:147–59. doi: 10.1111/J.1574-6968.2008.01145.X
22. Serisier, DJ. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir Med. (2013) 1:262–74. doi: 10.1016/S2213-2600(13)70038-9
23. Yamaya, M, Azuma, A, Takizawa, H, Kadota, JI, Tamaoki, J, and Kudoh, S. Macrolide effects on the prevention of COPD exacerbations. Eur Respir J. (2012) 40:485–94. doi: 10.1183/09031936.00208011
24. Carrera-Salinas, A, González-Díaz, A, Ehrlich, RL, Berbel, D, Tubau, F, Pomares, X, et al. Genetic adaptation and acquisition of macrolide resistance in haemophilus spp. during persistent respiratory tract colonization in chronic obstructive pulmonary disease (COPD) patients receiving long-term azithromycin treatment. Microbiol Spectr. (2023) 11:e0386022. doi: 10.1128/SPECTRUM.03860-22
Keywords: inflammatory markers, interleukins, exacerbations, COPD, azithromycin, microorganisms
Citation: Cuevas E, Huertas D, Montón C, Marin A, Carrera-Salinas A, Pomares X, García-Nuñez M, Martí S and Santos S (2023) Systemic and functional effects of continuous azithromycin treatment in patients with severe chronic obstructive pulmonary disease and frequent exacerbations. Front. Med. 10:1229463. doi: 10.3389/fmed.2023.1229463
Edited by:
Amany F. Elbehairy, Alexandria University, EgyptReviewed by:
Zakaria Lahmar, Centre Hospitalier Universitaire de Montpellier, FranceSergey N. Avdeev, I.M. Sechenov First Moscow State Medical University, Russia
Copyright © 2023 Cuevas, Huertas, Montón, Marin, Carrera-Salinas, Pomares, García-Nuñez, Martí and Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Martí, smartinm@bellvitgehospital.cat; Salud Santos, saludsantos@bellvitgehospital.cat
 Ester Cuevas
Ester Cuevas Daniel Huertas
Daniel Huertas Concepción Montón3
Concepción Montón3  Alicia Marin
Alicia Marin Anna Carrera-Salinas
Anna Carrera-Salinas Marian García-Nuñez
Marian García-Nuñez Sara Martí
Sara Martí Salud Santos
Salud Santos