- 1 Department of Pathology and Laboratory Medicine, University of Texas Health Sciences Center, Houston, TX, USA
- 2 Department of Internal Medicine, Baylor College of Medicine, Houston, TX, USA
- 3 Department of Biochemistry, University of Texas Health Sciences Center, Tyler, TX, USA
- 4 Department of Internal Medicine and Infectious Disease, University of Texas Health Sciences Center, Houston, TX, USA
Mycobacterium tuberculosis (Mtb) replicates within the human macrophages and we investigated the activating effects of retinoic acid (RA) and vitamin D3 (VD) on macrophages in relation to the viability of intracellular Mtb. A combination of these vitamins (RAVD) enhanced the levels of DC-SIGN and mannose receptors on THP-1 macrophages that increased mycobacterial uptake but inhibited the subsequent intracellular growth of Mtb by inducing reactive oxygen species and autophagy. RAVD also enhanced antigen presenting and chemotactic receptors on THPs suggesting an activated phenotype for RAVD activated THPs. RAVD mediated activation was also associated with a marked phenotypic change in Mtb infected THPs that fused with adjacent THPs to form multinucleated giant cells (MNGCs). Typically, MNGCs occurred over 30 days of in vitro culture and contained non-replicating persisting Mtb for more than 60 days in culture. Latent tuberculosis occurs in over a third of mankind and we propose that RAVD mediated induction of persistent Mtb within human macrophages provides a novel model to develop therapeutic approaches and investigate pathogenesis of latency.
Introduction
Tuberculosis (TB), a disease that afflicts mankind, causes 1.3 million deaths per year (WHO, 2006). The etiologic agent, Mycobacterium tuberculosis (Mtb) is unique among bacterial pathogens in being able to survive in vivo for years before reactivation to cause disease. The clinical manifestations of TB represent a complex interaction between the pathogen and the human host immune response. Although Mtb can parasitize, survive and grow within macrophages only a minority of people exposed to infection progress to disease. This indicates that innate mechanisms may contain disease in exposed populations. It is widely believed that protection against the human disease requires the T-helper 1-type (Th1) immune responses (Toossi, 2000).
The host mediated Th1 immune response results in granuloma formation that is sufficient to contain the infection and prevent active disease in most healthy individuals. However, granulomas are unable to completely eradicate the infection (Saunders et al., 1999). Pulmonary TB infection is primarily acquired through inhalation of aerosol droplets containing Mtb. Following Mtb infection of alveolar macrophages, an intense local inflammatory response involving a series of innate immune and Th1 dominant adaptive pathways are triggered. These responses lead to the recruitment of macrophages, lymphocytes, and dendritic cells to the site of infection resulting in the formation of a granuloma, a signature histopathological feature of TB (Ulrichs and Kaufmann, 2006).
The major function of granulomas in TB disease is the containment of Mtb to a localized area, to prevent the spread of disease to other healthy regions of tissue. TB disease occurs upon Mtb growth and disruption of the granuloma structure, leading to cavitation and dissemination. The structure, function, and evolution of granulomas have been studied using various animal models (Bouley et al., 2001; Saunders et al., 2002; Via et al., 2008), within lungs of tuberculous humans (Im et al., 1993; Poey et al., 1997; Long et al., 1998), explant tissue (Kaplan et al., 2003; Ulrichs et al., 2004; Tsai et al., 2006; Ulrichs and Kaufmann, 2006), and in vitro cell cultures (Puissegur et al., 2004). These studies have identified that Mtb infected monocytes differentiate into macrophages, epithelioid cells, and several types of multinucleated giant cells (MNGCs). Interestingly, some of these studies have implicated MNGCs as enhancing bacterial containment and clearance within the granuloma. MNGCs were thought to express increased lytic enzyme activity.
Although the presence of MNGCs was described very early within tuberculosis lesions, their precise formation and contribution is only being analyzed recently. Cultured monocytes and macrophages have been induced to differentiate into MNGCs by exposure to various stimulants, such as cytokines (Weinberg et al., 1984; McInnes and Rennick, 1988; Enelow et al., 1992; Lemaire et al., 1996; DeFife et al., 1997), lectins (Chambers, 1977; Takashima et al., 1993), monoclonal antibodies (Lazarus et al., 1990; Orentas et al., 1992; Tabata et al., 1994), and conditioned media (Sone et al., 1981; Postlethwaite et al., 1982; Kreipe et al., 1988; Abe et al., 1991). However, Mtb is a slow growing organism with a doubling time of about 18 h and the interaction of Mtb and macrophages is a chronic process in vivo lasting years. Paradoxically, the available models are limited by short life span of macrophages. The only long-term study of in vitro MNGC formation utilized freshly isolated PBMCs where the infection was carried through to 28 days.
We discovered that human THP-1 cells (henceforth cited as THPs) could be infected with live Mtb in vitro and treated with physiologically compatible vitamins to induce a differentiation process that resulted in the generation of MNGCs, that in turn, could be maintained for more than 60 days in culture. Due to the difficulty of obtaining alveolar macrophages, a human monocytoid cell line, THP-1, is commonly substituted for in vitro studies of macrophage responses to Mtb infection (Tsuchiya et al., 1980). THPs however need to be activated before they differentiate into a macrophage-like cell line (Theus et al., 2004). Phorbol myristate acetate (PMA) is the most common chemical activator for THPs (Tsuchiya et al., 1982), inducing adherence, upregulation of phagocytic receptors, and an overall macrophage-like state. However, PMA is toxic for humans in vivo, and is not a substance endogenously produced in the host. Unlike PMA, all-trans retinoic acid (RA; Kim et al., 2007) and vitamin D3 (VD; McCarthy et al., 1983) are THP activating agents that are basic components of a healthy diet and have been shown to be linked to immune responses against Mtb infection (Crowle et al., 1987; Crowle and Ross, 1989, 1990; Liu et al., 2007). We therefore examined the effects of PMA and retinoic acid and vitamin D3 (RAVD) on the phenotype and function of THP cells to develop an in vitro model that is closer to human alveolar macrophages. During these studies, we observed differing effects of PMA and RAVD activation of THP cells. PMA activated THPs (PMA-THPs) allowed enhanced growth of Mtb as observed previously. However, RAVD activated THPs (RAVD-THPs) inhibited Mtb and, with the addition of physiologically relevant, increasing doses of RA and VD, sustained bacteriostasis occurred. Furthermore, only in RAVD-THPs, Mtb induced the generation and gradual evolution of various morphotypes of macrophages to represent epithelioid cells, binucleate and MNGCs, culminating in syncytia. Thus, for the first time, it appears possible to study a chronic interaction between a slowly replicating bacterium that also has the unique ability to remain in a persistent state in macrophages.
Materials and Methods
Activation of THPs and Mycobacteria
Human THP-1 monocytoid cell line (TIB#3456, ATCC, MD, USA) was maintained at 37°C and 5% CO2 in HEPES buffered RPMI-1640 (Sigma Aldrich, St Louis, MO, USA) medium with 10% heat inactivated FBS, 50 μg/mL gentamicin and 100 U/mL penicillin (pH 7.2). THPs were genotyped by ATCC to exclude contamination with other cell lines. Cells were passed so that cell counts did not exceed 106/mL. When needed, cells were expanded into 75 cm2 flasks and were activated with RA (1 μM) and VD (1 μM) or PMA (10 ng/mL = 16 nM; all from Sigma Aldrich, St Louis, MO, USA) for 3 days. RAVD concentrations were selected based on the physiological levels that occur in the fluids of humans (Hollis, 2005; Moan et al., 2009). It is noted here that the cholecalciferol version of VD was used in this study. VD is predominantly metabolized to 1,25-dihydroxyvitamin D3 in the liver and kidneys. However, during infection, macrophages and dendritic cells have been shown to metabolize cholecalciferol for use in their antimicrobial processes. Mtb H37Rv or H37Ra were obtained from ATCC repository and gfp-strains of Mtb were prepared as described before (Dhandayuthapani et al., 1995). They were cultured in 7H9 broth with (gfp expressing Mtb H37Rv or Mtb H37Ra strains) or without (Mtb H37Rv and Mtb H37Ra) 25 μg/mL kanamycin for 10 days and highly viable suspension aliquots frozen stored at −80°C.
Surface Receptor Analysis
Cells were activated for 3 days using varying doses of PMA (1, 10, and 50 ng/mL) and RAVD (10, 100, and 1000 nM) and infected with Mtb H37Rv for 24 h. The infected cells were washed once with PBS and stained, on ice for 30 min, with FITC or PE conjugated anti-CD1d, CD14, CD44, CD80, CD86, CD184, CD195, DC-SIGN, and HLA-DR (all from BD Pharmingen, San Jose, CA, USA) antibodies. The cells were post-fixed with 2% paraformaldehyde before analysis using BD Facscan. PMA and RAVD activated and infected THPs were analyzed for mannose receptor expression by incubation with FITC-tagged mannosylated-BSA (FITC-mBSA; Sigma Aldrich, St Louis, MO, USA) for 30 min at 37°C after which the cells were washed and fixed for cytometric analysis (Takahara et al., 2004). In this assay, uptake of FITC-mBSA into THPs was performed incubated at both 4 and 37°C. At 4°C minimal uptake occurs compared to active uptake at 37°C.
Infection of THPs with Mtb and Short-Term Growth Curves
Mtb suspensions were subjected to gentle sonication at 4 W, matched to McFarland standard #1 and spun at 500 rpm for 2 min. The supernatant contained single colony forming unit (CFU) of Mtb without clumps and were used for infection. Activated THP cells were infected with an MOI of 10 with Mtb for 24 h at 37°C and 5% CO2 with gentle mixing to ensure uniform infection. Cells were washed three times with sterile medium to remove non-phagocytosed bacteria, counted and dispensed to 24-well plates (CFU counts, MNGC cultures) or 8-well chamber slides (Mtb uptake, immunostains) at 106 cells/mL medium in replicates. For growth curves between days 1 and 10, cells from 24-well plates were aspirated, pelleted, and lysed in 0.05% SDS within the same well thus accounting for adherent as well as some floating macrophages. When macrophages were adherent both floater cells as well as adherent macrophages were pooled for preparing lysates, which were then plated at 10-fold dilutions on 7H11 agar (Remel, Lenexa, KS) for CFU counts. PMA-THPs generally die between days 7 and 10 due to an excess growth of Mtb while RAVD-THPs control growth of Mtb and cells remain viable. Viability of THPs during Mtb infection was determined by adding 10% by volume of alamar blue vital stain that turns pink when macrophages are fully viable. The dye conversion was quantitated using an Ascent fluorometer; it is also readable using an ELISA reader (Invitrogen, USA).
Growth and Maintenance in Slide Chambers
Cells plated onto 8-well chamber slides (Permanox plastic chamber slides, Nalge Nunc International, Rochester, NY, USA), became adherent after Mtb infection and were fixed either with absolute alcohol for Ziehl–Neelsen stain and scored by microscopy for Mtb uptake into the macrophages or with 2.7% paraformaldehyde for immunostaining with antibodies. THPs in slide chambers were kept alive for several weeks or months with medium replenishment as described below.
Long-Term Cultures of THPs and Induction of Multinucleated Giant Cells
THPs infected with Mtb as above for short-term infection were continued into long-term cultures of MNGCs as follows. Only RAVD-THPs were viable beyond 10 days of Mtb infection. On day 3 post infection, when RAVD-THPs begin to adhere to 24-well plates, cells were aspirated and fresh culture media containing RAVD (1 μM) was added to the culture plates. On day 10, when the cells had differentiated and most adhered to the plastic surface, fresh media with RAVD supplement was added along with freshly activated but uninfected RAVD-THP cells to a final concentration not exceeding 106 cells/mL. Thus, the only time Mtb was added to long-term cultures of THPs was during an initial infection. Thereafter, fresh media supplemented with RAVD was added every 3 days and freshly activated cells every 7 days. Pre-formed giant cells on monolayers fused with freshly added THP cells yielding increasingly larger MNGC. Control cultures of THP-1 cells activated with RAVD and without Mtb infection did not survive beyond 10 days. (a) Growth curve of Mtb: During long-term infection lasting 10–60 days, Mtb infected THPs were adherent but some floaters were present. Thus, during this time interval cells were aspirated, pelleted, and lysed along with adherent cells on monolayers to determine CFU counts. (b) Cell fusion to expand MNGCs: To demonstrate cell fusion, preformed MNGCs adherent to tissue culture wells were added with carboxyfluorescein diacetate (CFSE) stained, RAVD-activated THPs. Cell fusion was visible between MNGCs fusing with CFSE stained single THPs. (c) Cytokine effects: RAVD and cytokines were compared for their ability to induce MNGCs by infecting THPs with Mtb and addition of either RAVD or recombinant human IL-4 and GM-CSF separately or in combination (R&D Systems; 20 ng/ml/106 macrophages each). THPs were re-incubated with fresh IL-4 or GM-CSF, replenished daily. To determine synergistic effects, RAVD activated THPs were also added with the cytokines. (d) Viability analysis: Alamar blue was used as vital stain to determine viability of MNGCs. (e) Cytokines and chemokine secretion from MNGCs: Cytokines and chemokines secreted from MNGCs were quantitated using sandwich ELISA with paired antibodies (R&D Sciences, USA). Long-term cultures for MNGCs were performed over 20 times with reproducible results within a 2-year time frame. (d) Phagosome analysis for proteases: Mtb phagosomes were purified from RAVD-THPs on day 12 using sucrose gradients and analyzed for Cathepsin-D (Cat-D) using western blot (Singh et al., 2006; Katti et al., 2008; Rao et al., 2009).
Immunofluorescent Labeling
Activated THPs infected with Mtb gfp H37Rv were grown in 8-well chamber slides were washed once with PBS, fixed in 2.7% paraformaldehyde and permeabilized for 30 min with a staining buffer containing 1% saponin, 0.1% glycine, and 2% heat-inactivated autologous human serum in PBS. The slides were washed and stained overnight at 4°C with 1/250 dilution of antibodies against p47/67phox, CD63, LAMP-1, Cat-D, and Cathepsin-G (Cat-G). Antibody dilutions were arrived at using preliminary dose-titrations. The slides were washed three times with staining buffer and then a 1:2500 dilution of the appropriate secondary Texas-Red conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was added and incubated at room temperature for 1 h. After further washing, the slides were mounted in Fluorsave (Calbiochem, Gibbstown, NJ, USA) with or without DAPI stain and examined on a Nikon fluorescence microscope equipped with a deconvolution software followed by analysis in a laser confocal microscope. Negative controls consisted of naïve THPs (no activation) and uninfected activated THPs. In addition, isotype controls used were Mtb gfp H37Rv infected cells stained with normal goat or mouse IgG followed by a secondary antibody conjugated with Texas-Red. All uninfected and inactivated THPs presented a uniform low background fluorescence using this procedure. Phagosomes staining for distinct antibodies were scored for colocalization as described before in our previous studies using mouse macrophages (Daniel et al., 2006). For colocalization experiments, bacteria of over 100 macrophages in each chamber of triplicate well slides were counted and averaged for three separate experiments. The colocalization percentage was plotted and Student’s t test was used to determine significance between the groups.
Detection of Reactive Oxygen Species and Nitric Oxide
Activated THPs were treated with 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA, Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s instructions to measure internal reactive oxygen species (ROS) production in pre-and post Mtb infected macrophages. Basal levels of ROS in activated THPs were analyzed for a 3-day period by flow cytometry. Mtb infected cells were incubated for 5 days and tested for intracellular fluorescence as above using fluorometry (Ascent Fluoroscan) instead of flow cytometry because of contamination issues and infectious risk with virulent Mtb. The specificity of ROS mediated conversion of dichlorofluorescein (DCF) was confirmed by using the ROS inhibitor, 10 mM diphenyleneiodonium (DPI; Sigma Aldrich, St Louis, MO, USA). The contribution of inducible nitric oxide synthase (iNOS) to the killing of internalized Mtb was determined by the addition of 10 mM N(G)-monomethyl-L-arginine [L-NMMA; Alexis Biochemicals, San Diego, CA; an inhibitor of nitric oxide (NO) response] to the cultures and the supernatants were titrated using diaminonaphthalene (DAN) fluorescent dye and Ascent Fluoroscan instrument.
siRNA Knock-Down of Beclin-1 to Determine Induction of Autophagy
THP-1 cells were seeded 2 × 106 cells per well onto a 6-well plate and rested for 24 h in media without antibiotics. They were then activated with RAVD as above and 3 days later, the knock-down of beclin-1 was performed as per manufacturer’s instructions (Lonza AG, Walkersville, MD, USA) where the beclin-1 siRNA (Sigma Aldrich, St Louis, MO, USA) was nucleofected into the cells and allowed to rest for 24 h. Infection with Mtb was then performed for 4 h, washed cells were re-incubated and lysates of macrophages were plated for CFU counts of Mtb. Just before infection, western blot analysis with an antibody to beclin-1 (Santa Cruz Biotechnology, USA) was conducted to determine knock-down. The fluid phase autophagosome marker monodansylcadaverine (MDC) was used to label Mtb phagosomes of beclin-1 knockdown and control THPs as described before (Jagannath et al., 2009).
Electron Microscopy
Infected RAVD-THP cells were prepared for examination by transmission electron microscopy. After the indicated incubation periods, the glutaraldehyde-fixed (2% in PBS, pH 7.6) cells were washed in Millonig’s buffer, post-fixed in 50/50 osmium tetroxide/Millonig’s, dehydrated through graded ethanol solutions and embedded in 50/50 LX-112 resin/propylene oxide. Sections of 500 nm were cut from each block using a glass knife on a Leica Ultracut R microtome and stained with 0.5% Toluidine Blue. The blocks were trimmed and thin sections (80 and 100 nm) are cut using a DiATOME diamond knife, one each of the two thicknesses are floated on either 100 or 150 mesh copper grids (Electron Microscopy Sciences) and heat fixed in a 70° oven for at least 1 h. The grids were stained for 15 min using 2% uranyl acetate, rinsed with double distilled water, stained 5 min in Reynolds lead citrate, rinsed and dried in a 70° oven. The specimen grids were imaged in a JEOL 1200 transmission electron microscope at 60 kV with digital images collected using a 1 k × 1 k Gatan BioScan camera, Model 792.
Analysis of Persisting and Non-Replicating Mtb in THPs
RAVD-THPs were activated and infected with Mtb gfp H37Rv as above and maintained as MNGCs in 8-well slide chambers or 24-well TC plates up to 30 days or more. At time intervals (a) THPs of the 8-well slide chambers in triplicate were lysed with 200 μL per chamber of 0.01% SDS and the entire lysate was plated on 7H11 agar plates for CFUs expressed per 106 macrophages, (b) replicate chambers of macrophages were washed at the same time, fixed and examined for Mtb gfp H37Rv through microscopic evaluation, and expressed as number of Mtb per 100 macrophages per chamber in triplicate, and (c) THPs in parallel cultures of 24-well plates were lysed with RNAzol and mRNA obtained was analyzed using real-time PCR as described using the following primers for antigen 85B (Hu et al., 2000; Pai et al., 2000). The primers were: forward primer 5′-TCAGGGGATGGGGCCTAGCC-3′; reverse primer 5′-GCTTGGGGATCTGCTGCGTA-3′; RT primer 5′-GCCGGCGCCTAACGAACTCTGC-3′; Taqman Probe: 85B-TP 5′-FAM-TCGAGTGACCCGGCATGGGAGCGT-BHQ-1-3′. Fold-expression of mRNA from macrophage lysates was compared to mRNA messages from a 7-day viable culture of Mtb that was adjusted to contain 106 CFU/mL diluted from a suspension matched to McFarland #1 turbidity standard (108 CFU/mL).
Results
Phorbol Myristyl Acetate and a Combination of Retinoic Acid and Vitamin D3 Induce Different Surface Receptor Expression in THPs
Macrophages express several types of receptors with different functional consequences. Some mediate uptake of Mtb (CD44, DC-SIGN), while others help cross talk with T cells (HLA-DR, CD1d, CD80, CD86). Chemokine receptors like CD195 and CD184 enable homing of macrophages to infected sites while other receptors like CD14 bind mycobacterial cell wall components altering cytokine signaling. In our studies, RAVD and PMA had differential effects on receptor expression of THPs analyzed using flow cytometry. Receptors were generally up regulated in THPs activated with RAVD compared to PMA and in general there was a dose–response to RAVD. In an experiment repeated three times with similar results, THPs were tested naïve or treated with various concentrations of PMA or RAVD, infected with Mtb, and then stained for CD11c after 24 h. PMA and RAVD-treated THPs enhanced the expression of antigen presenting molecules HLA-DR, CD1d, CD80, and CD86 (Figures 1A–C). CD184 and CD195 were also increased in RAVD vs. PMA activated THPs along with the TLR-4 co-receptor, CD14. Such receptors may make THPs more responsive to chemokines enabling homing, infiltration, and bacterial detection. CD44 has been implicated as a receptor for Mtb but showed no difference between RAVD and PMA activated cells (Leemans et al., 2003). However, DC-SIGN was increased in RAVD-THPs, which is important for capture of Mtb and antigen presentation to T cells (Tailleux et al., 2003). Besides DC-SIGN, RAVD-THPs also showed an enhanced expression of macrophage mannose receptor (MMR). Since antibodies binding to MMR are internalized, we used an active uptake of mBSA tagged with FITC (FITC-mBSA; Sigma Chemical Co., USA) at 37°C as a measure of MMR expression (Figure 1D; Takahara et al., 2004). When uptake of FITC-mBSA was measured at 4°C to rule out adsorption, there was reduced labeling. Uptake of FITC-mBSA was also analyzed among Mtb infected RAVD or PMA THPs and the MMR expression remained stable only in RAVD-THPs (not shown). MMR is a receptor for mannose capped moieties like lipoarabinomannan of Mtb (Kang et al., 2005). Thus, data on DC-SIGN and MMR together indicate that RAVD-THPs yield a more mature phenotype of macrophages that could internalize Mtb more efficiently than PMA-THPs. Using separate studies, we confirmed that RA and VD each had activation effects on THPs, but the maximal expression of receptors was obtained only with their combination (Figure A1 in Appendix).
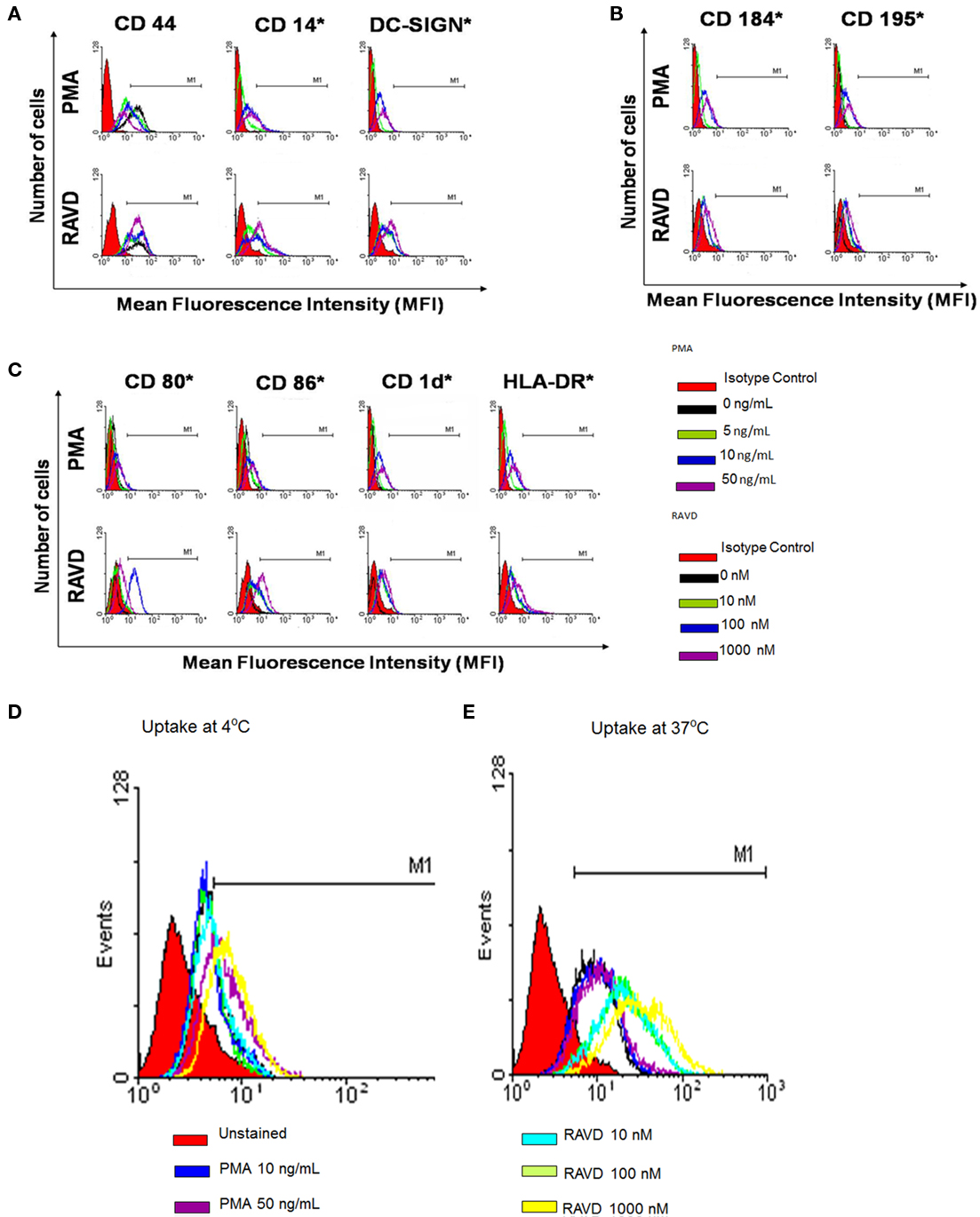
Figure 1. Phorbol myristyl acetate (PMA) and a combination of retinoic acid (RA) and vitamin D3 (VD) induce a differential expression of surface receptors on human THP-1 monocytoid cells. THPs were activated with varying doses (as indicated; C) of PMA or a mix of RA and VD for 72 h and were stained for surface receptors using either fluorescent antibodies (A–C) or fluorescent tagged ligands (D,E) and analyzed using Cellquest software. MFIs from three experiments were calculated to show significance as indicated (*MFI significantly increased in three experiments; t test). (A) THPs were activated and stained for receptors involved in uptake or binding to mycobacteria [DC-SIGN, CD14, and CD44] (B) chemokine receptors, CD184 and CD195; and (C) CD80, CD86, CD1d, and HLA-DR receptors involved in antigen presentation. (D) THPs were activated and stained with mannosylated-BSA-FITC (mBSA-FITC) or BSA-FITC at 37°C for active uptake and at 4°C for absorption control using flow cytometry as above. RAVD enhanced the expression of macrophage mannose receptor compared to PMA activated THPs.
PMA and RAVD Have Differential Effects on the Uptake and Survival of Mycobacterium tuberculosis in THPs
Receptor expression in RAVD-THPs suggested that the optimal doses of RAVD and PMA were respectively 1 μM and 10 ng/mL (16 nM). The optimal activation time was 72 h. All further studies utilized this optimized dose and activation. Activated THPs were then infected with Mtb H37Rv (MOI of 10) and evaluated for uptake and control of Mtb infection. Microscopic analysis of Ziehl–Neelsen stained Mtb (acid fast bacilli; AFB) in fixed PMA and RAVD treated THPs demonstrated a differential uptake of Mtb (Figure 2A). RAVD-THPs were better able to engulf Mtb, where over 75% of the macrophage population had internalized at least one AFB, while only 60% of the PMA-THPs demonstrated internalized AFB. This correlated with the increased expression of mycobacterial up-take receptors (Figure 1). Paradoxically, subsequent to uptake, RAVD-THPs controlled the infection with Mtb better than PMA-THPs (Figure 2B). Mtb grew 10-fold over 7 days within PMA-THP macrophages while RAVD treatment decreased Mtb colony counts (CFUs) by a log10 over 7 days of infection. RA and VD each had modest inhibitory effects against intracellular Mtb but exerted stronger effects when combined (Figure A2 in Appendix).
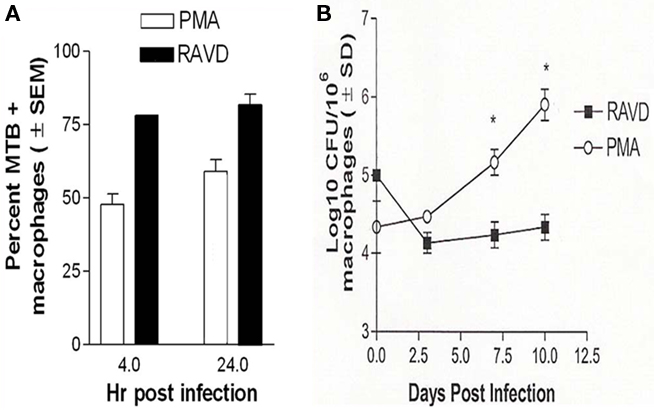
Figure 2. Phorbol myristate acetate and RAVD have differential effects on the survival of Mycobacterium tuberculosis (Mtb) within THP-1 macrophages. (A) THPs were activated with either 10 ng/mL of PMA or 1 μM each of RA and VD for 72 h and phagocytosed with Mtb for 4 and 24 h. Bacteria internalized were visually scored by microscopy using acid fast stain. Mtb uptake by THPs is significantly increased after RAVD treatment (*p < 0.009, t test; three experiments). (B) THPs activated and infected as above were incubated for 10 days and were lysed at intervals and plated for colony (CFU) counts. RAVD treatment inhibited bacterial replication while Mtb showed an increase in colony counts in PMA treated macrophages (*p < 0.009, t test; three experiments).
RAVD Enhance Reactive Oxygen Species and Nitric Oxide Responses in THPs that Lead to Inhibition of Intracellular Mtb
To determine the basis for the anti-TB effects of RAVD, ROS and NO production was evaluated among activated and Mtb infected THPs. When Mtb is internalized by macrophages, it is sequestered within a phagosome that triggers the acquisition of the multimeric components of the NADPH oxidase [phagocyte oxidase (phox)]. In our earlier study, we demonstrated that NADPH oxidase colocalizes with Mtb phagosomes in macrophages and the colocalization, one evidence of targeting of the enzyme to Mtb phagosomes, is detectable using antibody stain against p47/67phox components (Daniel et al., 2006). Accordingly, RAVD or PMA treated THPs were infected with Mtb gfp H37Rv, immunostained and scored microscopically for colocalization (Daniel et al., 2006). PMA-THPs showed a reduced colocalization of the NADPH oxidase proteins with Mtb phagosomes while RAVD-THPs showed an enhanced colocalization (Figures 3A,B).
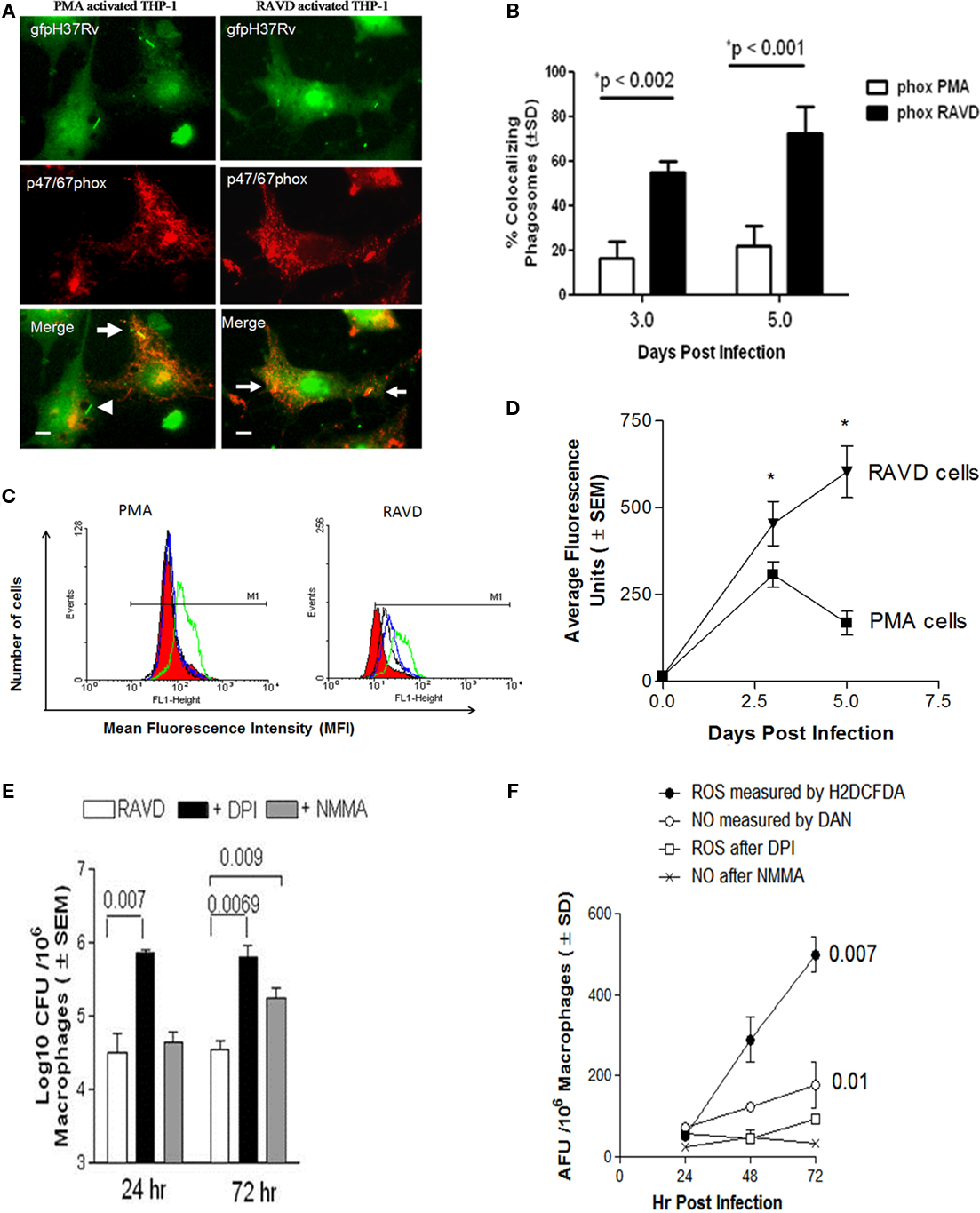
Figure 3. RAVD induces an enhanced reactive oxygen species (ROS) response in THP-1 macrophages that leads to inhibition of intracellular Mtb. (A) THPs were activated with either PMA or RAVD and phagocytosed with gfp-expressing strain of Mtb (gfp H37Rv) for 4 h followed by fixation and staining with antibodies against p47/p67phox components of the phagocyte (NADPH) oxidase. Percent phagosomes colocalizing with gfp H37Rv (arrows) were scored in three experiments and average colocalization shown (B). Arrowhead in (A) shows a non-colocalizing gfp H37Rv (bars = 5 μM). (C) THPs activated with PMA or RAVD were labeled with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), a probe for intracellular ROS, and quantitated using flow cytometry on day 1 (black), day 2 (green), and day 3 (blue). RAVD-THPs maintained ROS production over 3 days, whereas ROS response declined in PMA-THPs after day 2 (one of three similar experiments shown). Red fill shows basal levels of ROS production in naïve (inactivated) THPs. (D) THPs activated with RAVD were infected and measured for intracellular ROS using an Ascent-Fluoroscan that quantitated ROS in average fluorescence units (AFU). Data show that ROS increased in RAVD-THPs over 3 days of activation but decreased by day 3 in PMA-THPs. (E) THPs were activated with RAVD, infected with Mtb and then incubated in the presence or absence of 10 mM of diphenyleneiodonium chloride (DPI) an inhibitor of ROS response or N-monomethyl-L-arginine (L-NMMA) an inhibitor of nitric oxide response. Macrophage lysates were then plated for CFU counts at the end of 24 and 72 h of infection. Inhibition of ROS response with DPI increased the viability of intracellular Mtb compared to inhibition of NO response (p values shown above bars for groups compared, t test; three experiments). (F) Supernatants of macrophage cultures of (E) were tested added with H2DCFDA or diaminonaphthalene (DAN) and read for fluorescence in an Ascent fluoroscan to measure respectively, ROS or NO induced average fluorescence units (AFU).
These studies correlated with the intracellular levels of ROS in PMA and RAVD activated THPs, determined using a ROS specific probe, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). Non-fluorescent H2DCFDA is cleaved by intracellular esterases to yield the DCF, which is then oxidized by ROS to yield a fluorescent hydrophobic product trapped within the cell. Figure 3C shows the flow cytometric analysis of fluorescent ROS positive cells after activation and before infection as analyzed by flow cytometry. PMA-THPs showed an increased shift due to ROS induced fluorescence on day 1 that returned to background levels by day 3. On the other hand, RAVD-THPs showed a shift on day 1 that was sustained until day 3 when the cells were infected with Mtb. The specificity of ROS mediated conversion of DCF was confirmed by using the ROS inhibitor, DPI (not shown). Intracellular ROS was also quantitated following activation and Mtb infection using a fluorometer. RAVD-THPs with internalized Mtb showed increasing ROS levels over 5 days while PMA-THPs showed a spike that declined by day 5 (Figure 3D). Together, these studies indicate that both before and after infection, RAVD-THPs had stronger ROS responses than PMA-THPs.
Finally, ROS specific effects were correlated to bactericidal killing. THPs were activated in the presence or absence of 10 mM each of DPI chloride, an inhibitor of ROS response or L-NMMA, an inhibitor of iNOS that generates NO. After inhibition, cell lysates of infected RAVD-THPs were plated for viable colony counts (CFU) at the end of 24 and 72 h infection. At both time points, inhibition of ROS response with DPI increased the viability of intracellular Mtb compared to inhibition of NO response (Figure 3E). Figure 3F shows that the supernatants of cultures contained detectable ROS and somewhat lesser levels of NO through 3 days of incubation. Differences in NO levels before and after inhibition were not as significant as those for ROS. However, inhibition of iNOS also allowed a greater number of Mtb to survive at 72 h of culture. We speculate that RAVD may promote intracellular changes associated with NO pathway that still remain to be unraveled.
RAVD Induced Autophagy in THPs Leads to Inhibition of Mtb Growth
A recent study demonstrated that VD can induce autophagosome formation to kill internalized Mtb (Yuk et al., 2009). Since autophagy could be another antimicrobial pathway that can control Mtb infection, we investigated the ability of RAVD to induce autophagy. RAVD-THPs were treated or untreated with siRNA vs. beclin-1, to abolish autophagy and then were infected with Mtb. RAVD induced killing of Mtb was inhibited by siRNA vs. beclin-1 (Figure 4A). Mtb phagosomes also colocalized with the autophagosome marker MDC in RAVD-THPs while MDC uptake was absent in siRNA knockdown THPs (Figure 4B) and the THPs needed to be stained with a nuclear DAPI stain to visualize macrophages. These data suggested that the bactericidal activity of RAVD was also attributable in part to the induction of autophagy.
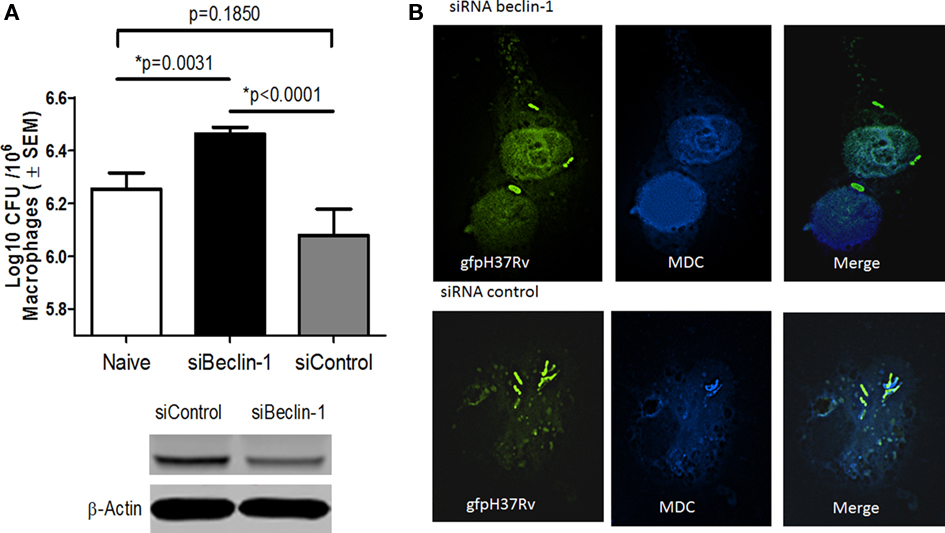
Figure 4. Induction of autophagy in RAVD-THPs leads to enhanced survival of intracellular Mtb. RAVD activated THPs treated or untreated with siRNA vs. beclin-1 to knock-down autophagy, were infected with Mtb gfp H37Rv (MOI of 10). Lysates were then plated for CFU counts at the end of 24 h infection. (A) Inhibition of autophagy with siRNA Beclin-1 increased the viability of intracellular Mtb compared to siRNA control (p values shown above bars for groups compared, t test; three experiments). (B) Monodansylcadaverine (MDC) was used to label autophagosomes in similar type of THPs. Mtb gfp H37Rv colocalizes with MDC in RAVD treated macrophages. MDC uptake was poor in siRNA vs. beclin-1 macrophages so that the nuclei were labeled with DAPI to visualize macrophages and gfp H37Rv.
Activation of THP-1 Macrophages with RAVD Leads Macrophages to Differentiate into Giant Cells
A striking change observed during in vivo TB is the morphological transformation of macrophages into various morphotypes in guinea pigs, rats, rabbits, and humans (Parks and Weiser, 1975; Cain and Kraus, 1982; Kraus, 1982). Interestingly, PMA-THPs with phagocytosed Mtb progressively led to a loss of viability of macrophages after 7 days due to uncontrolled growth of bacteria (Figure 5A). On the other hand, RAVD-THPs were able to control Mtb infection and prevent loss of macrophages. Furthermore, RAVD induced cell fusion to form giant cells. Figure 5B illustrates the growth curve of intracellular Mtb recovered from adherent macrophages between days 1 and 10 and then from adherent MNGCs between days 10 and 35. Notably, not all THPs fused to form giant cells and some remained non-fused but markedly enlarged. Such cells contained single nuclei and a single intracellular Mtb. Figure 5C illustrates the nuclear morphology of DAPI stained MNGCs that contain Mtb gfp H37Rv grown over 35 days of in vitro culture with representative phase contrast images. Finally, when MNGCs fuse with additional macrophages, the zone of fusion is delineated by characteristic inter-digitations observable through electron microscopy (EM; Bainton and Golde, 1978). Figure 5D illustrates an EM image of MNGC fusing with individual THP. In addition, it has been reported that “a disintegrin and metalloproteinase” (ADAM) family of proteins mediate mycobacteria induced cell fusion (Puissegur et al., 2007). We stained RAVD-THPs for several ADAMs and found that RAVD induced only a transient staining for ADAM-9 between THPs undergoing cell fusion early during infection (Figure A3 in Appendix).
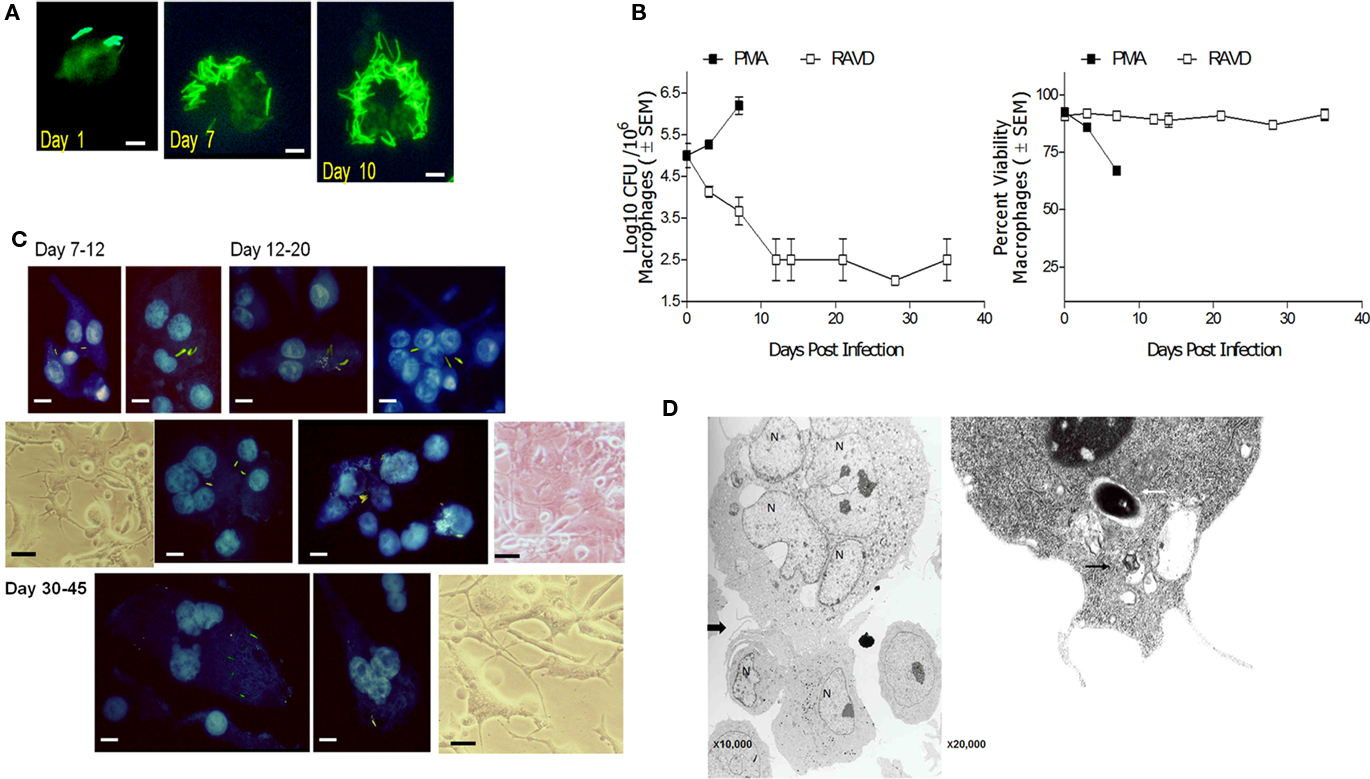
Figure 5. Long-term activation with RAVD allows phenotypic changes in M. tuberculosis infected THP-1 macrophages that lead to multi-nucleate giant cell (MNGC) formation. (A) THPs activated with either PMA or RAVD were infected at an MOI of 1 with gfp H37Rv and incubated for 10 days. By day 7 PMA-THPs show robust intracellular growth of Mtb and by day 10, most of the PMA cells die of bacterial growth (bar = 5 μM). (B) Left: Growth curves of PMA and RAVD-THPs with Mtb show the kinetics of growth and phenotypic change. Right: Alamar blue was added to replicate cultures and supernatants measured for emission at 590 nm in a fluorometer and expressed as AFUs (100 AFU = 100% viability; 106 THPs well; dye conversion in 4 h). (C) Replenishment of RAVD, fresh medium and uninfected THPs every 7 days to the cell culture, prolonged the survival of adherent Mtb infected RAVD-THPs, progressively showing morphology typical of MNGCs. Progressive formation of giant cells is shown by DAPI stained nuclei of THPs with intact gfp H37Rv bacilli and representative phase contrast images (bars = 5 μM). (D) Electron microscopy (left panel) shows that macrophages fuse with adjacent THPs through a zone represented by inter-digitating- septae. A single intact Mtb phagosome (white arrow) with tight membranes and a phagosome in a vacuole (black arrow) are shown in a RAVD-THP in the right panel.
Long-Term Activation of THP-1 Macrophages with RAVD Leads to Long Living MNGCs that Contain Persistent M. tuberculosis
Mycobacterium tuberculosis infected THPs were constantly monitored using alamar blue fluorescent dye that was added to cultures and rapidly converted by viable cells into red-fluorescent products in turn measured using fluorometry (100 AFU emission equals 100% viability for 1 × 105 THPs).
Effect of Cell Fusion Inducing Cytokines IL-4 and GM-CSF vs. RAVD on MNGC Formation from Mtb Infected THPs
Some cytokines like IL-4 and GM-CSF have been known to induce fusion of human peripheral blood derived monocytes or rat macrophages leading to the formation of short lived MNGCs (Sorokin et al., 1992; Purton et al., 1996). To determine their effects on longevity of MNGCs, THPs were activated with IL-4, GM-CSF, their combination or a combination with RAVD followed by infection with Mtb. MNGCs and viability were monitored using microscopy and alamar blue assay in culture for 40 days. Figure 6A1 illustrates that only THPs treated with RAVD induced prolonged survival of THPs while those treated with cytokines died within 20 days due to extensive multiplication of intracellular Mtb (not shown). In the continuation of RAVD treated cultures shown in Figure 6A1, that were maintained up to 60 days without the loss of viability, freshly added uninfected THPs (arrowhead, Figures 6A2–4) fused with preformed adherent MNGC (arrows, Figures 6A3,4) in culture to yield long living MNGCs surviving up to 60 days (Figure 6A5). While cytokines were not required for MNGC formation using RAVD-THPs followed by Mtb infection, interestingly, they secreted both cytokines and chemokines. After an initial burst of cytokines (TNF-α, IL-6, and IL-10), RAVD-THPs showed a decline in the level of cytokines (TNFα, IL-6, and IL-10) after day 21 but switched to higher levels of chemokine (MCP1, MIP1α, and KC) secretion (Figure 6B). These data support a novel protocol that has been optimized to enhance in vitro longevity of MNGCs.
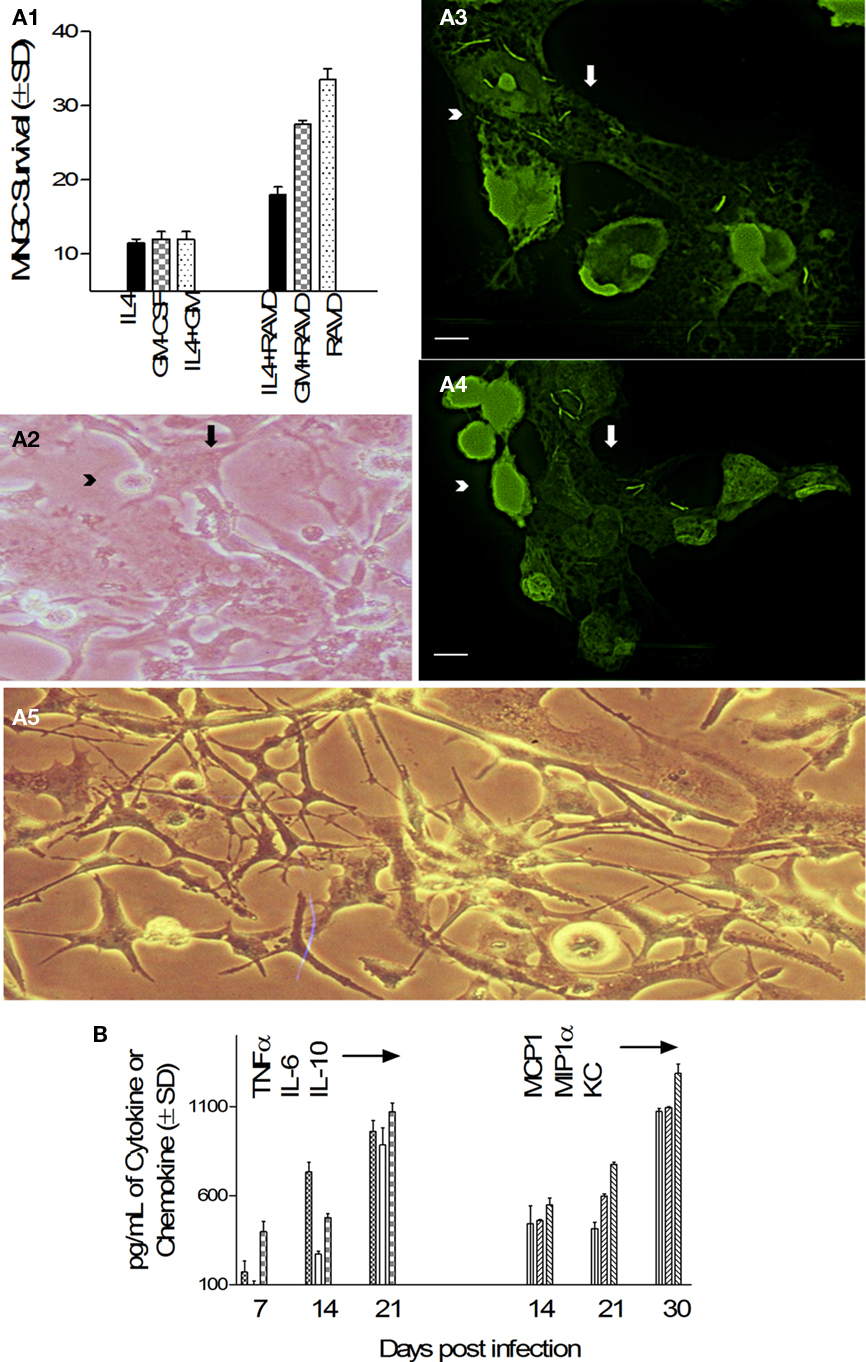
Figure 6. Activation with RAVD but not cell fusion-inducing cytokines leads to long lived MNGCs containing M. tuberculosis. (A1) Survival of Mtb infected THPs measured after treatment with either recombinant human IL-4 or GM-CSF or their combination with RAVD. Fluorescent conversion of the vital dye alamar blue was used to monitor viability of THPs (100% viability = 100 AFUs from 24-well cultures measured at 485/530 nm using Ascent fluoroscan). Only THPs treated with RAVD-THPs prolong survival measured up to 40 days in this experiment. Those treated with IL-4 or GM-CSF die because of excess growth of Mtb (not shown). (A2) RAVD cells of (A1) examined under phase contrast shows a MNGC (arrow) fusing with single THPs (arrowhead) on day 45. (A3,4) Preformed (day 45) adherent MNGCs (pale green; white arrows) that contain multiple gfp H37Rv fuse with freshly added uninfected, carboxyfluorescein- diacetate (CFSE) stained THPs (T; arrowheads) to form expanded MNGCs (bar = 5 μM). (A5) Phase contrast images of MNGCs on day 60 tend to show syncytium formation (bars = 5 μM). (B) Supernatants of RAVD MNGCs of (A1) collected on days of culture shown were measured for cytokine–chemokines using sandwich ELISA. RAVD activated and Mtb infected THPs show an initial burst of cytokines and later burst of chemokines.
Long Living MNGCs Contain Persisting M. tuberculosis in Protease Rich Compartments
To the best of our knowledge, there are no in vitro cell culture models to study long-term persistence of Mtb. Since RAVD-induced MNGCs remained viable even after several weeks and contained Mtb gfp H37Rv, we sought to determine mechanisms that led to their persistence. We speculated that Mtb in RAVD was persistent since replicating bacteria were either killed in acidic compartments of THPs or after the initial bactericidal action from RAVD, Mtb did not multiply subsequently and remained dormant. In addition to oxidant mediated killing of Mtb, macrophages use phagosome–lysosome (P–L) fusion to kill Mtb via lysosomal hydrolases. Generally, Mtb avoids P–L fusion in human macrophages; however, VD has been reported to enhance P–L fusion (Hmama et al., 2004). An evidence of P–L fusion is the accumulation of active proteases that can kill Mtb. Thus, Mtb phagosomes were fractionated from RAVD-THPs on day 12 using sucrose gradients (Singh et al., 2006).
Blots were initially analyzed for Cat-D, a major enzyme proteolytic for Mtb in acidic environments (Singh et al., 2006) whose active forms are demonstrable only by western blot. Cat-D breaks down from a 52-kDa immature inactive form to an enzymatically active 32 kDa form in an acidic pH. Figure 7A illustrates that Mtb phagosomes contained the inactive Cat-D form 24 h after infection while by the end of 72 h, active fragments of Cat-D appeared within the phagosomes. Immunofluorescence was then used to confirm that both Cat-D and another bactericidal protease, Cat-G (Rivera-Marrero et al., 2004) localized to Mtb gfp H37Rv phagosomes (Figures 7B,C). Thus, Mtb gfp H37Rv within RAVD-THPs localized to protease rich compartments after RAVD activation. Finally, the lysosomal localization of Mtb was confirmed by using antibodies to the lysosomal markers, LAMP1 and CD63 (Figures 7D,E). CD63, a definitive marker of lysosomes is present in abundance in activated macrophages and is speculated to play a role in phagocytic and P–L fusion events (Astarie-Dequeker et al., 1999). Likewise, LAMP1 is involved in maintaining lysosome acidity and protecting the lysosomal membranes from autodigestion. RAVD-THP phagosomes containing Mtb showed extensive colocalization with CD63 and LAMP1 (Figures 7D,E). Together, these studies argue that RAVD markedly enhances the localization of Mtb into protease rich degradative lysosomal compartments within THPs that could account for persistence without replication.
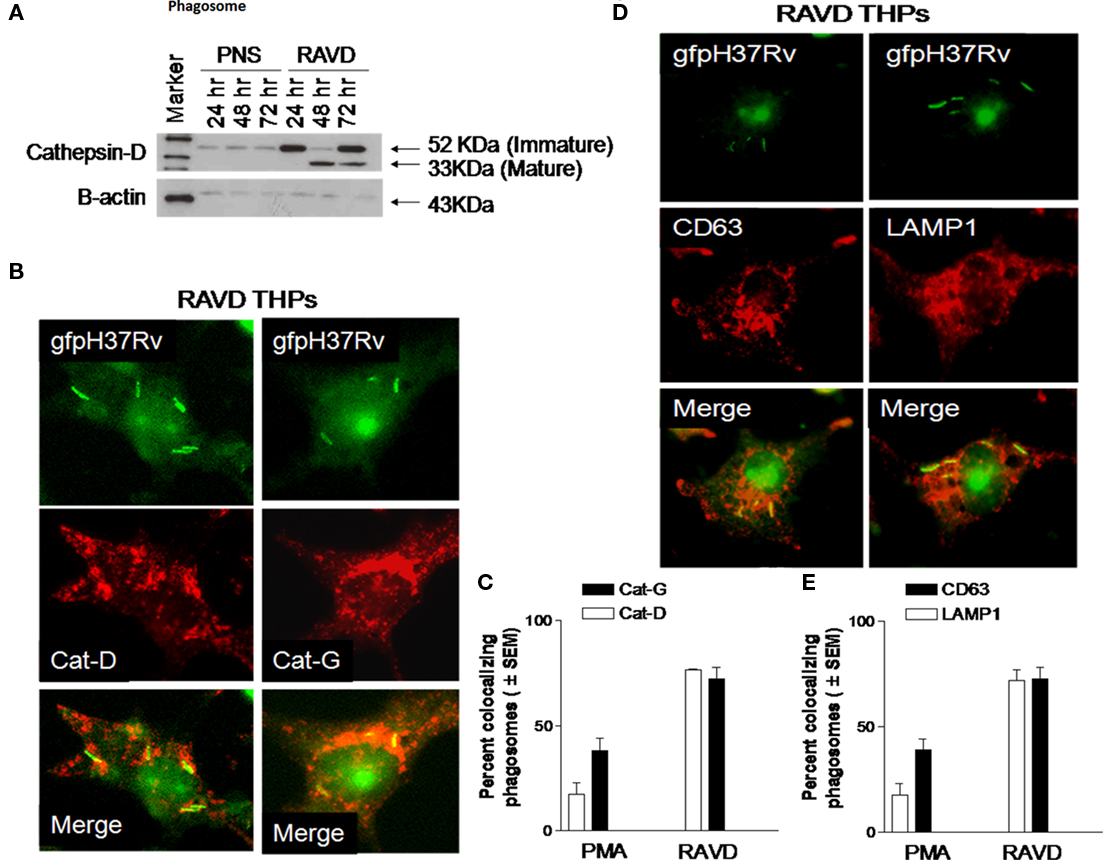
Figure 7. Long-term activation with RAVD leads to increased protease activity and lysosomal localization of Mtb in THP1 macrophages. THPs were activated with RAVD infected with gfp H37Rv and processed as follows. (A) Phagosomes containing gfp H37Rv were purified from day 12 RAVD-THPs using sucrose gradients. Phagosome pellets and post-nuclear supernatants (PNS) were analyzed with Western blot using antibodies against Cathepsin-D (Cat-D). Blot shows the enrichment of Cat-D protease in the phagosomes compared to PNS and the accumulation of enzymatically mature 33 kDa fragment over time. Bottom lane shows loading control β-actin. (B–D) RAVD-THPs on day 12 were stained for Cat-D, Cat-G (B,C), and CD63 and LAMP1 (D,E) using specific antibodies and counterstained with Texas-Red labeled conjugates. (B–D) Show Mtb phagosomes of RAVD-THPs colocalizing with antibodies for proteases and lysosomal markers. Bar graphs of (C–E) show quantitation of colocalizing phagosomes. The average length of Mtb was 5 μM.
Persistent M. tuberculosis in MNGC is Metabolically Active
Irrespective of antibody stains used or the time of examination for THPs, some gfp Mtb remained completely unstained by either protease or lysosomal markers within THPs. Figure 8A illustrates such Mtb bacilli, which are apparently viable and gfp-fluorescent even after 20 days in culture do not stain with LAMP1, a late P–L marker. Preliminary EM studies showed similar mycobacteria that apparently remained in the cytosol of macrophages without a distinct phagosomal membrane (not shown).
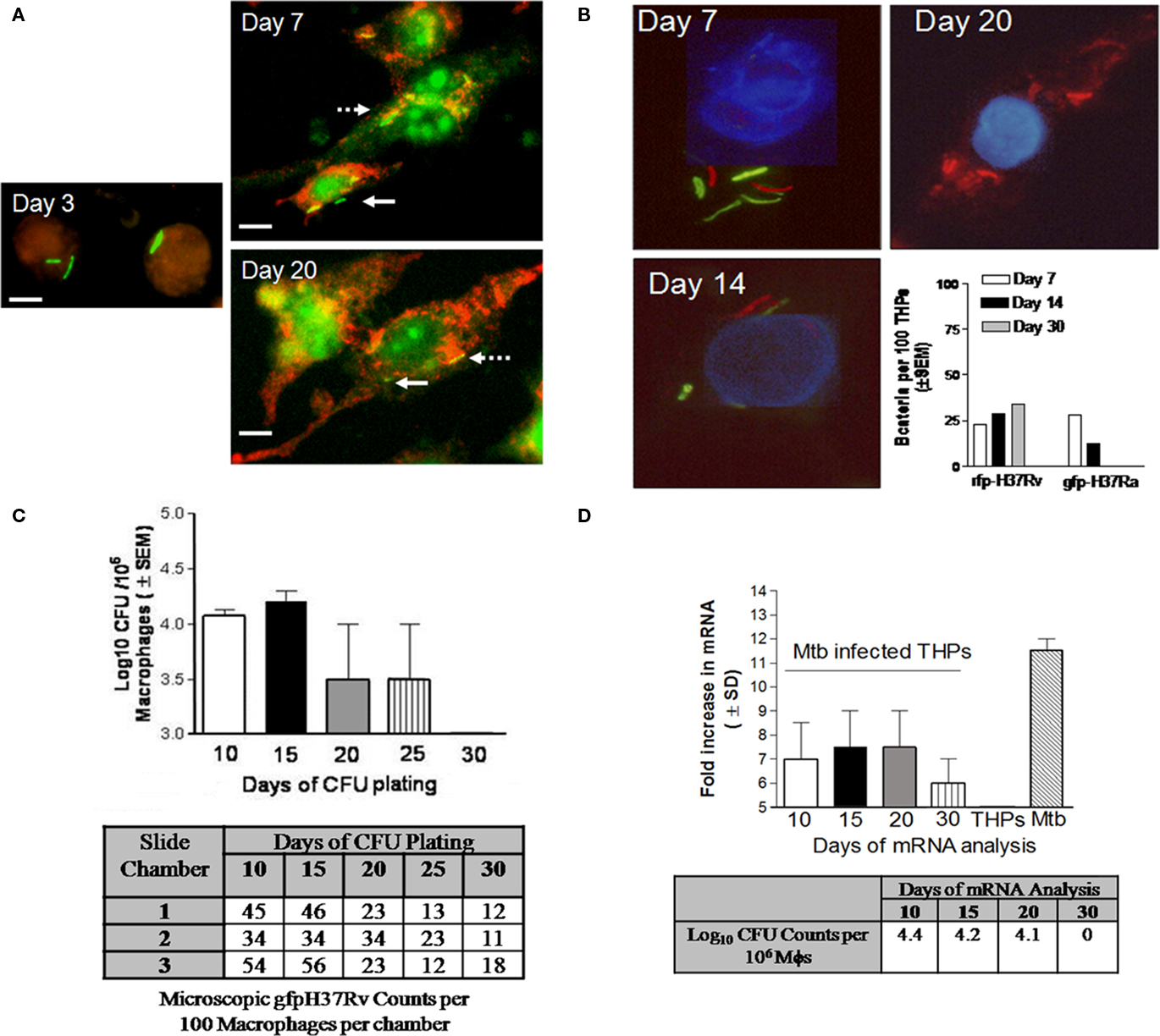
Figure 8. Persistence of M. tuberculosis in RAVD-THPs is a characteristic of virulent H37Rv strain. RAVD-THPs were infected with Mtb strains, maintained in parallel over 30 days in 8-well slide chambers or 24-well plates and analyzed as follows: (A) RAVD-THPs immunostained at intervals showed gfp H37Rv counterstained for LAMP1 from day 7 through day 20 (broken arrow) but some bacilli remain unstained (solid arrow). (B) RAVD-THPs were concurrently infected with gfp H37Ra and rfp H37Rv and bacteria were scored in 100 THPs at each time point as indicated. Only rfp H37Rv survived in RAVD-THPs while gfp H37Ra was eliminated (bar graph). The average length of Mtb was 5 μM. (C)gfp H37Rv were enumerated per 100 THPs through microscopy in triplicate chambers of slide culture and replicate chamber THPs were lysed and plated for CFUs on 7H11 agar as a whole to recover CFUs. RAVD-THPs with microscopically visible, persisting Mtb showed no growth in agar plates by day 30. Data from three separate experiments were averaged and shown. (D) A parallel culture of experiment shown in (C) was set up and RAVD-THPs were analyzed at time intervals for mRNA specific for Antigen 85B of Mtb using RT-PCR and for CFU counts in macrophage lysates on days indicated. On day 30, mRNA messages were detected even when Mtb were not recoverable on agar media from lysates of THPs suggesting non-replicating persistence of Mtb in RAVD-THPs.
We speculated that such Mtb were cytosolic and thus do not stain with membrane markers such as LAMP1. We also proposed that persistence was likely related to the virulence of Mtb. To investigate this aspect, RAVD-THPs were infected concurrently with Mtb gfp H37Ra and red-fluorescent expressing Mtb H37Rv (rfp H37Rv). THPs were then cultured for 30 days and fluorescent bacteria enumerated by counting 100 THPs per triplicate chambers (Figure 8B). Progressively, Mtb gfp H37Ra were eliminated from RAVD-THPs while only Mtb rfp H37Rv was detectable until day 30. This suggested that persistence within macrophages is a feature of only virulent H37Rv strain of Mtb.
Finally, attempts were made to determine whether persistent Mtb were metabolically active. For this purpose, gfp Mtb infected RAVD-THPs were maintained concurrently in 24-well plates as well as 8-well slide chambers over 30 days. First, gfp Mtb infected RAVD-THPs cultured in 8-well slide chambers were fixed at different time intervals and intracellular gfp Mtb were scored per 100 macrophages and correlated to viable counts by plating whole cell lysates of replicate chamber THPs. Figure 8C illustrates that even when viable Mtb were not culturable on agar plates, gfp Mtb were microscopically observable within THPs of slide chambers. In the second experiment, Mtb infected RAVD-THPs were tested over time for the expression of mRNA for antigen-85B, which is a reliable marker for viability of replicating as well as non-replicating, persistent Mtb (Hu et al., 2000; Pai et al., 2000). Figure 8D shows that even when viable Mtb were not recovered from THPs on day 30, mRNA for antigen 85B was expressed indicating that viable Mtb persisted within THPs. We therefore suggest that treatment of THPs with RAVD leads, over time, to a decline in the viability of Mtb and that some of bacteria appear to enter a persistent state.
Discussion
Monocytes and monocytoid cell lines generally express reduced numbers of receptors and are less efficient in phagocytosing pathogens (Schwende et al., 1996). However, differentiated macrophages express abundant receptors, are more phagocytic, and have enhanced intracellular mechanisms of bactericidal activity mediated by phox, iNOS, increased targeting of proteolytic enzymes such as cathepsins and enhanced P–L fusion (Schwende et al., 1996). Monocyte to macrophage differentiation occurs after binding of pathogens to monocytes via their receptors in combination with the release of self-activating cytokines such as TNF-α, G-CSF, and GM-CSF. Differentiation can be induced in vitro by phorbol esters (Tsuchiya et al., 1982), VD (Kim et al., 2007), and RA (McCarthy et al., 1983). However, following infection in vivo macrophages transform into Langhans type of MNGCs that are surrounded by immune cells of granulomas and contain Mtb thought to be non-replicating or dormant. Classic histological studies show that such bacilli reactivate to cause active infection while MNGCs can revert into macrophage phenotype and spread infection in rabbits and guinea pigs (Hektoen, 1898). To the best of our knowledge, an in vitro model of human macrophages that undergo phenotypic changes closely associated with a transition from replicating Mtb into persistent Mtb has not been reported before. We suggest that an RAVD induced MNGC model is more similar to events that occur within humans during TB. We observed that RA and VD were separately able to activate THPs, although maximal differentiation occurred in the synergistic presence of the two vitamins. Subsequently, RAVD exerted more effective bactericidal effects compared to RA or VD alone. These effects were due in part to the induction of NADPH oxidase although there was evidence that modest induction of iNOS also occurred. Significantly, RAVD induced macrophage mediated killing of most Mtb organisms but a few were left to survive within the macrophages that remarkably transformed into MNGCs. Such MNGCs produced copious cytokines and chemokines suggesting that they may be capable of recruiting and activating immune cells should they were in vivo. This is the first report of the induction of long living MNGCs with Mtb infection using activation with physiologically compatible macrophage modulators such as RA and VD. Addition of cytokines such as GM-CSF and IL-4 did not markedly alter the RAVD induced transformation of MNGCs. This suggested that RA and VD mediated biochemical processes separate from those induced by the cytokines.
The induction of MNGCs by RAVD correlated and clarified previously reported observations. VD was found to enhance DC-SIGN levels in THPs earlier and the well documented role of DC-SIGN in mycobacterial recognition was consistent with the increased efficacy of RAVD-THPs during phagocytosis. Likewise, increased expression of mannose receptor (MMR) in RAVD-THPs correlated with the finding that inhibition of MMR prevented MNGC formation (McNally et al., 1996). Clearly, RAVD transformed THPs and we suggest that, by inference, they regulate differentiation of human monocytes to a more efficient macrophage phenotype.
The bactericidal function of RAVD in THPs was partly due to the induction of ROS, since the ROS inhibitor DPI enhanced survival of Mtb. Although other pathways cannot be ruled out, this was similar to the previously reported induction of ROS by RA (N’Diaye et al., 1997). Recently a study showed that VD alone can induce autophagosome formation to kill internalized Mtb (Yuk et al., 2009). Knock-down of beclin-1, a key protein involved in targeting vesicles for autophagy, demonstrated that RAVD can also activate autophagy to keep intracellular Mtb growth in check. It should be noted here that we tested a synergistic effect of RAVD, whereas, the individual effects of RA and VD were measured in cells with slightly different phenotype in earlier studies. RA and VD are ingested by humans together and we further propose that it is the synergistic effect of RA and VD that is likely to be important in vivo.
Our initial studies indicated that inhibition of iNOS with L-NMMA enhanced the survival of intracellular Mtb in RAVD-THPs although, curiously, we did not find elevated levels of NO in the medium. VD has been reported to induce iNOS in murine and human macrophages (Rockett et al., 1998) and it remains to be determined whether it does induce sustained iNOS in THPs.
Among the spectrum of effects of RAVD on THPs, we observed a striking change in the macrophage morphology that led to the formation of MNGCs containing persistent Mtb. Since persistence of Mtb in humans is the leading cause of reactivation TB, these findings need additional elaboration. Neither RA nor VD alone led to long living THP derived MNGCs but their combination was very effective. Since VD induced or suppressed giant cell formation depending upon the cell phenotype and RA only transformed trophoblasts and osteoclasts (Kaji et al., 2000), we propose that their combination is likely more effective in inducing MNGC formation in THPs. In addition, we found that RAVD induced cell fusion through the induction of the matrix metalloproteinase (ADAM-9) that was detectable only as a transient staining between the cell junctions (Figure A3 in Appendix). Finally, RAVD induced MNGCs could be kept in vitro for observation for 60 days yielding the longest living giant cell phenotype in culture.
A more interesting observation was the persistence of Mtb in MNGCs in a non-replicating stage for as long as 60 days in vitro. First, microscopy showed more fluorescent-Mtb per MNGC than recoverable colony counts from lysates of MNGCs. Second, MNGCs positive for gfp Mtb but negative for CFU growth on plate media still expressed mRNA for Antigen-85B, which is a marker for viable as well as persistent Mtb (Hu et al., 2000). This was reminiscent of our previous observation that persistent, non-replicating Mtb in mice treated with antibiotics still expressed abundant mRNA for Antigen 85B (Pai et al., 2000). Third, only Mtb gfp H37Rv persisted in MNGCs after continuous treatment with RAVD, while under similar conditions, Mtb gfp H37Ra organisms were progressively eliminated. These studies together indicate that RAVD eliminate most replicating Mtb through inducing bactericidal functions but a few surviving Mtb are driven into a non-replicating persistence. Thus, we have successfully developed a novel macrophage model to study the long-term persistence of Mtb. To the best of our knowledge an in vitro model to maintain MNGCs for prolonged periods (60 days) or Mtb in a persistent state has not been described. The availability of a cell culture model that reflects the natural niche for Mtb in vivo is important from several directions. It may be possible to study the dynamics of persistence over time and devise methods to eliminate persistent Mtb. Analysis of MNGCs may reveal mechanisms that keep replicating Mtb contained and unable to spread disease. Preliminary studies were performed to neutralize both ROS and NO in MNGCs to determine whether persistent Mtb could be revived with unsuccessful results. Perhaps this could be due to the novel fact that Mtb compartments had RAVD induced active Cat-D and Cat-G which are known to be bactericidal. Thus, we propose that reactivation of Mtb within macrophages is dependent upon multiple, perhaps time-dependent, factors that need to be carefully dissected out. These include the blockade of multiple mechanisms of bactericidal activity and cytokine mediated modulation of MNGCs in conjunction with stimulation of intracellular Mtb through growth stimulatory “quorum sensing factors.” The availability of persistent Mtb within a macrophage niche is an attractive model to address these issues.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by the National Institutes of Health (F31AI061836) (JE) and a pilot grant award to CJ from 5 NIH grant P30 AI36211-15/15 (PI: Janet Butel, CFAR, Baylor College of Medicine, Houston, TX. USA)
References
Abe, E., Ishimi, Y., Jin, C. H., Hong, M. H., Sato, T., and Suda, T. (1991). Granulocyte-macrophage colony-stimulating factor is a major macrophage fusion factor present in conditioned medium of concanavalin A-stimulated spleen cell cultures. J. Immunol. 147, 1810–1815.
Astarie-Dequeker, C., N’Diaye, E. N., Le Cabec, V., Rittig, M. G., Prandi, J., and Maridonneau-Parini, I. (1999). The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun. 67, 469–477.
Bainton, D. R., and Golde, D. W. (1978). Differentiation of macrophages from normal human bone marrow in liquid culture. Electron microscopy and cytochemistry. J. Clin. Invest. 61, 1555–1569.
Bouley, D. M., Ghori, N., Mercer, K. L., Falkow, S., and Ramakrishnan, L. (2001). Dynamic nature of host–pathogen interactions in Mycobacterium marinum granulomas. Infect. Immun. 69, 7820–7831.
Crowle, A. J., and Ross, E. J. (1989). Inhibition by retinoic acid of multiplication of virulent tubercle bacilli in cultured human macrophages. Infect. Immun. 57, 840–844.
Crowle, A. J., and Ross, E. J. (1990). Comparative abilities of various metabolites of vitamin D to protect cultured human macrophages against tubercle bacilli. J. Leukoc. Biol. 47, 545–550.
Crowle, A. J., Ross, E. J., and May, M. H. (1987). Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect. Immun. 55, 2945–2950.
Daniel, D. S., Dai, G., Singh, C. R., Lindsey, D. R., Smith, A. K., Dhandayuthapani, S., Hunter, R. L. Jr., and Jagannath, C. (2006). The reduced bactericidal function of complement C5-deficient murine macrophages is associated with defects in the synthesis and delivery of reactive oxygen radicals to mycobacterial phagosomes. J. Immunol. 177, 4688–4698.
DeFife, K. M., Jenney, C. R., McNally, A. K., Colton, E., and Anderson, J. M. (1997). Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J. Immunol. 158, 3385–3390.
Dhandayuthapani, S., Via, L. E., Thomas, C. A., Horowitz, P. M., Deretic, D., and Deretic, V. (1995). Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol. Microbiol. 17, 901–912.
Enelow, R. I., Sullivan, G. W., Carper, H. T., and Mandell, G. L. (1992). Induction of multinucleated giant cell formation from in vitro culture of human monocytes with interleukin-3 and interferon-gamma: comparison with other stimulating factors. Am. J. Respir. Cell Mol. Biol. 6, 57–62.
Hektoen, L. (1898). The fate of the giant cells in healing tuberculous tissue, as observed in a case of healing tuberculous meningitis. J. Exp. Med. 3, 21–52.
Hmama, Z., Sendide, K., Talal, A., Garcia, R., Dobos, K., and Reiner, N. E. (2004). Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1alpha,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J. Cell. Sci. 117, 2131–2140.
Hollis, B. W. (2005). Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 135, 317–322.
Hu, Y., Mangan, J. A., Dhillon, J., Sole, K. M., Mitchison, D. A., Butcher, P. D., and Coates, A. R. (2000). Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J. Bacteriol. 182, 6358–6365.
Im, J. G., Itoh, H., Shim, Y. S., Lee, J. H., Ahn, J., Han, M. C., and Noma, S. (1993). Pulmonary tuberculosis: CT findings – early active disease and sequential change with antituberculous therapy. Radiology 186, 653–660.
Jagannath, C., Lindsey, D. R., Dhandayuthapani, S., Xu, Y., Hunter, R. L. Jr., and Eissa, N. T. (2009). Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat. Med. 15, 267–276.
Kaji, Y., Ikeda, K., Ikeda, T., Kawakami, K., Sasaki, K., Shindo, M., Hatake, K., Harada, M., Motoyoshi, K., Mori, S., Norimatsu, H., and Takahara, J. (2000). IL-4, but not vitamin D(3), induces monoblastic cell line UG3 to differentiate into multinucleated giant cells on osteoclast lineage. J. Cell. Physiol. 182, 214–221.
Kang, P. B., Azad, A. K., Torrelles, J. B., Kaufman, T. M., Beharka, A., Tibesar, E., DesJardin, L. E., and Schlesinger, L. S. (2005). The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202, 987–999.
Kaplan, G., Post, F. A., Moreira, A. L., Wainwright, H., Kreiswirth, B. N., Tanverdi, M., Mathema, B., Ramaswamy, S. V., Walther, G., Steyn, L. M., Barry, C. E. III, and Bekker, L. G. (2003). Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect. Immun. 71, 7099–7108.
Katti, M. K., Dai, G., Armitige, L. Y., Rivera Marrero, C., Daniel, S., Singh, C. R., Lindsey, D. R., Dhandayuthapani, S., Hunter, R. L., and Jagannath, C. (2008). The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell. Microbiol. 10, 1286–1303.
Kim, K. J., Kim, H. H., Kim, J. H., Choi, Y. H., Kim, Y. H., and Cheong, J. H. (2007). Chemokine stromal cell-derived factor-1 induction by C/EBPbeta activation is associated with all-trans-retinoic acid-induced leukemic cell differentiation. J. Leukoc. Biol. 82, 1332–1339.
Kreipe, H., Radzun, H. J., Rudolph, P., Barth, J., Hansmann, M. L., Heidorn, K., and Parwaresch, M. R. (1988). Multinucleated giant cells generated in vitro. Terminally differentiated macrophages with down-regulated c-fms expression. Am. J. Pathol. 130, 232–243.
Lazarus, D., Yamin, M., McCarthy, K., Schneeberger, E. E., and Kradin, R. (1990). Anti-RMA, a murine monoclonal antibody, activates rat macrophages: II. Induction of DNA synthesis and formation of multinucleated giant cells. Am. J. Respir. Cell Mol. Biol. 3, 103–111.
Leemans, J. C., Florquin, S., Heikens, M., Pals, S. T., van der Neut, R., and Van Der Poll, T. (2003). CD44 is a macrophage binding site for Mycobacterium tuberculosis that mediates macrophage recruitment and protective immunity against tuberculosis. J. Clin. Invest. 111, 681–689.
Lemaire, I., Yang, H., Lauzon, W., and Gendron, N. (1996). M-CSF and GM-CSF promote alveolar macrophage differentiation into multinucleated giant cells with distinct phenotypes. J. Leukoc. Biol. 60, 509–518.
Liu, P. T., Stenger, S., Tang, D. H., and Modlin, R. L. (2007). Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 179, 2060–2063.
Long, R., Maycher, B., Dhar, A., Manfreda, J., Hershfield, E., and Anthonisen, N. (1998). Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest 113, 933–943.
McCarthy, D. M., San Miguel, J. F., Freake, H. C., Green, P. M., Zola, H., Catovsky, D., and Goldman, J. M. (1983). 1,25-Dihydroxyvitamin D3 inhibits proliferation of human promyelocytic leukaemia (HL60) cells and induces monocyte–macrophage differentiation in HL60 and normal human bone marrow cells. Leuk. Res. 7, 51–55.
McInnes, A., and Rennick, D. M. (1988). Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J. Exp. Med. 167, 598–611.
McNally, A. K., DeFife, K. M., and Anderson, J. M. (1996). Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am. J. Pathol. 149, 975–985.
Moan, J., Lagunova, Z., Lindberg, F. A., and Porojnicu, A. C. (2009). Seasonal variation of 1,25-dihydroxyvitamin D and its association with body mass index and age. J. Steroid Biochem. Mol. Biol. 113, 217–221.
N’Diaye, E. N., Vaissiere, C., Gonzalez-Christen, J., Gregoire, C., Le Cabec, V., and Maridonneau-Parini, I. (1997). Expression of NADPH oxidase is induced by all-trans retinoic acid but not by phorbol myristate acetate and 1,25 dihydroxyvitamin D3 in the human promyelocytic cell line NB4. Leukemia 11, 2131–2136.
Orentas, R. J., Reinlib, L., and Hildreth, J. E. (1992). Anti-class II MHC antibody induces multinucleated giant cell formation from peripheral blood monocytes. J. Leukoc. Biol. 51, 199–209.
Pai, S. R., Actor, J. K., Sepulveda, E., Hunter, R. L. Jr., and Jagannath, C. (2000). Identification of viable and non-viable Mycobacterium tuberculosis in mouse organs by directed RT-PCR for antigen 85B mRNA. Microb. Pathog. 28, 335–342.
Parks, D. E., and Weiser, R. S. (1975). The role of phagocytosis and natural lymphokines in the fusion of alveolar macrophages to form Langhans giant cells. J. Reticuloendothel. Soc. 17, 219–228.
Poey, C., Verhaegen, F., Giron, J., Lavayssiere, J., Fajadet, P., and Duparc, B. (1997). High resolution chest CT in tuberculosis: evolutive patterns and signs of activity. J. Comput. Assist. Tomogr. 21, 601–607.
Postlethwaite, A. E., Jackson, B. K., Beachey, E. H., and Kang, A. H. (1982). Formation of multinucleated giant cells from human monocyte precursors. Mediation by a soluble protein from antigen- and mitogen-stimulated lymphocytes. J. Exp. Med. 155, 168–178.
Puissegur, M. P., Botanch, C., Duteyrat, J. L., Delsol, G., Caratero, C., and Altare, F. (2004). An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell. Microbiol. 6, 423–433.
Puissegur, M. P., Lay, G., Gilleron, M., Botella, L., Nigou, J., Marrakchi, H., Mari, B., Duteyrat, J. L., Guerardel, Y., Kremer, L., Barbry, P., Puzo, G., and Altare, F. (2007). Mycobacterial lipomannan induces granuloma macrophage fusion via a TLR2-dependent, ADAM9- and beta1 integrin-mediated pathway. J. Immunol. 178, 3161–3169.
Purton, L. E., Lee, M. Y., and Torok-Storb, B. (1996). Normal human peripheral blood mononuclear cells mobilized with granulocyte colony-stimulating factor have increased osteoclastogenic potential compared to nonmobilized blood. Blood 87, 1802–1808.
Rao, P. K., Singh, C. R., Jagannath, C., and Li, Q. (2009). A systems biology approach to study the phagosomal proteome modulated by mycobacterial infections. Int. J. Clin. Exp. Med. 2, 233–247.
Rivera-Marrero, C. A., Stewart, J., Shafer, W. M., and Roman, J. (2004). The down-regulation of cathepsin G in THP-1 monocytes after infection with Mycobacterium tuberculosis is associated with increased intracellular survival of bacilli. Infect. Immun. 72, 5712–5721.
Rockett, K. A., Brookes, R., Udalova, I., Vidal, V., Hill, A. V., and Kwiatkowski, D. (1998). 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 66, 5314–5321.
Saunders, B. M., Frank, A. A., and Orme, I. M. (1999). Granuloma formation is required to contain bacillus growth and delay mortality in mice chronically infected with Mycobacterium tuberculosis. Immunology 98, 324–328.
Saunders, B. M., Frank, A. A., Orme, I. M., and Cooper, A. M. (2002). CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell. Immunol. 216, 65–72.
Schwende, H., Fitzke, E., Ambs, P., and Dieter, P. (1996). Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 59, 555–561.
Singh, C. R., Moulton, R. A., Armitige, L. Y., Bidani, A., Snuggs, M., Dhandayuthapani, S., Hunter, R. L., and Jagannath, C. (2006). Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J. Immunol. 177, 3250–3259.
Sone, S., Bucana, C., Hoyer, L. C., and Fidler, I. J. (1981). Kinetics and ultrastructural studies of the induction of rat alveolar macrophage fusion by mediators released from mitogen-stimulated lymphocytes. Am. J. Pathol. 103, 234–246.
Sorokin, S. P., McNelly, N. A., and Hoyt, R. F. Jr. (1992). Macrophage development: IV. Effects of blood factors on macrophages from prenatal rat lung cultures. Anat. Rec. 233, 415–428.
Tabata, N., Ito, M., Shimokata, K., Suga, S., Ohgimoto, S., Tsurudome, M., Kawano, M., Matsumura, H., Komada, H., and Nishio, M. (1994). Expression of fusion regulatory proteins (FRPs) on human peripheral blood monocytes. Induction of homotypic cell aggregation and formation of multinucleated giant cells by anti-FRP-1 monoclonal antibodies. J. Immunol. 153, 3256–3266.
Tailleux, L., Schwartz, O., Herrmann, J. L., Pivert, E., Jackson, M., Amara, A., Legres, L., Dreher, D., Nicod, L. P., Gluckman, J. C., Lagrange, P. H., Gicquel, B., and Neyrolles, O. (2003). DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127.
Takahara, K., Yashima, Y., Omatsu, Y., Yoshida, H., Kimura, Y., Kang, Y. S., Steinman, R. M., Park, C. G., and Inaba, K. (2004). Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int. Immunol. 16, 819–829.
Takashima, T., Ohnishi, K., Tsuyuguchi, I., and Kishimoto, S. (1993). Differential regulation of formation of multinucleated giant cells from concanavalin A-stimulated human blood monocytes by IFN-gamma and IL-4. J. Immunol. 150, 3002–3010.
Theus, S. A., Cave, M. D., and Eisenach, K. D. (2004). Activated THP-1 cells: an attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect. Immun. 72, 1169–1173.
Toossi, Z. (2000). The inflammatory response in Mycobacterium tuberculosis infection. Arch. Immunol. Ther. Exp. (Warsz.) 48, 513–519.
Tsai, M. C., Chakravarty, S., Zhu, G., Xu, J., Tanaka, K., Koch, C., Tufariello, J., Flynn, J., and Chan, J. (2006). Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell. Microbiol. 8, 218–232.
Tsuchiya, S., Kobayashi, Y., Goto, Y., Okumura, H., Nakae, S., Konno, T., and Tada, K. (1982). Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 42, 1530–1536.
Tsuchiya, S., Yamabe, M., Yamaguchi, Y., Kobayashi, Y., Konno, T., and Tada, K. (1980). Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26, 171–176.
Ulrichs, T., and Kaufmann, S. H. (2006). New insights into the function of granulomas in human tuberculosis. J. Pathol. 208, 261–269.
Ulrichs, T., Kosmiadi, G. A., Trusov, V., Jorg, S., Pradl, L., Titukhina, M., Mishenko, V., Gushina, N., and Kaufmann, S. H. (2004). Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J. Pathol. 204, 217–228.
Via, L. E., Lin, P. L., Ray, S. M., Carrillo, J., Allen, S. S., Eum, S. Y., Taylor, K., Klein, E., Manjunatha, U., Gonzales, J., Lee, E. G., Park, S. K., Raleigh, J. A., Cho, S. N., McMurray, D. N., Flynn, J. L., and Barry, C. E. III. (2008). Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76, 2333–2340.
Weinberg, J. B., Hobbs, M. M., and Misukonis, M. A. (1984). Recombinant human gamma-interferon induces human monocyte polykaryon formation. Proc. Natl. Acad. Sci. U.S.A. 81, 4554–4557.
Keywords: Mycobacterium, tuberculosis, latent tuberculosis, THP-1, DC-SIGN, mannose receptor
Citation: Estrella JL, Kan-Sutton C, Gong X, Rajagopalan M, Lewis DE, Hunter RL, Eissa NT and Jagannath C (2011) A novel in vitro human macrophage model to study the persistence of Mycobacterium tuberculosis using vitamin D3 and retinoic acid activated THP-1 macrophages. Front. Microbio. 2:67. doi: 10.3389/fmicb.2011.00067
Received: 08 September 2010; Paper pending published: 05 November 2010;
Accepted: 25 March 2011; Published online: 18 April 2011.
Edited by:
Amal Amer, The Ohio State University, USAReviewed by:
Jordi Torrelles, Ohio State University, USALuiz Bermudez, Oregon State University, USA
Copyright: © 2011 Estrella, Kan-Sutton, Gong, Rajagopalan, Lewis, Hunter, Eissa and Jagannath. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Chinnaswamy Jagannath, Department of Pathology and Laboratory Medicine, University of Texas Health Sciences Center, 6400 Fannin Street, MSB 2.200, Houston, TX 77030, USA. e-mail: chinnaswamy.jagannath@uth.tmc.edu
 Jaymie L. Estrella1
Jaymie L. Estrella1