- 1PTC Phage Technology Center GmbH, Im Kompetenzzentrum BioSecurity, Bönen, Germany
- 2Division of Food Sciences, School of Biosciences, University of Nottingham, Loughborough, UK
A screen of bacteriophages infecting a panel of Campylobacter jejuni PT14 gene knock-out mutants identified a role for the minor flagellin encoded by the flaB gene, in the defense of the host against CP8unalikevirus bacteriophage CP_F1 infection. Inactivation of the flaB gene resulted in an increase in the susceptibility of PT14 cultures to infection by CP_F1 and an increase in bacteriophage yields. Infection of wild type PT14 with CP_F1 produces turbid plaques in bacterial lawns, from which 78% of the resistant isolates recovered exhibit either attenuation or complete loss of motility. CP_F1 produces clear plaques on the flaB mutant with no regrowth in the lysis zones. Complementation of the mutant restored overgrowth and the development of resistance at the expense of motility. Further analyses revealed an increase in bacteriophage adsorption constant of nearly 2-fold and burst-size 3-fold, relative to the wild type. Motility analysis showed no major reduction in swarming motility in the flaB mutant. Thus, we propose a new role for FlaB in the defense of campylobacters against bacteriophage infection.
Introduction
Campylobacter represents a major zoonotic pathogen, as emphasized in recent reports published by the European Food Safety Authority that document year-on-year recorded caseloads of >210.000, which are estimated to belie an actual annual infection rate of 9 million people (EFSA, 2012, 2014, 2015). Human infection by this gram-negative bacterium leads to gastroenteritis (termed campylobacteriosis) with symptoms including severe abdominal pain, fever and diarrhea (Blaser, 1997; Allos, 2001). The infection is generally self-limiting and can be treated with rehydration therapy. However, in rare cases chronic sequelae can develop, for example reactive arthritis or Guillain-Barré syndrome (GBS), autoimmune disorders that can lead to temporary or in severe cases permanent paralysis (Nachamkin, 2002). The fact that campylobacters are highly prevalent in the intestinal tracts of farm livestock, such as poultry and pigs, renders it a major foodborne pathogen, and therefore represents a significant risk to consumer health (Boes et al., 2005; McCrea et al., 2005).
In order to effectively colonize their host, Campylobacter cells are equipped with one or two polar located flagella that confer high motility. Recent estimates suggest a torque of 3600 pN/nm for C. jejuni flagella, which is more than twice as high as that reported for Salmonella cells (Beeby et al., 2016). These high mobility structures together with CadF and FlpA adhesins help withstand peristaltic forces in the intestines of its colonized host, preventing them from being expelled and to facilitate locomotion in the highly viscous environment of the epithelial cell mucus layer (Monteville et al., 2003; Konkel et al., 2004, 2010). The invasion of intestinal epithelial cells is another key function of the flagella apparatus. A model proposed for Campylobacter pathogenesis suggests attachment to and disruption of epithelial cell barriers, before migrating toward the basal ends of cells, where incorporation takes place (O'Loughlin and Konkel, 2014). For this purpose, the flagella act as a type III secretion apparatus releasing Cia proteins (Campylobacter invasion antigens), that induce the cell invasion process (Konkel et al., 2001). Through these functions flagella are recognized as crucial for colonization and virulence (Bolton, 2015).
Interestingly, these motility structures have also been employed by the natural antagonists of Campylobacter, as they serve as attachment sites for bacteriophage infection. It has been shown that loss of the flagellin structure or motility function will result in resistance to infection from flagellotropic bacteriophages (Coward et al., 2006; Scott et al., 2007; Baldvinsson et al., 2014). Javed et al. (2015) further supported these findings by reporting that glycosylated flagellin serves as target for a phage receptor-binding protein (RBP) from bacteriophage NCTC12673. The main initial binding-target of flagellotropic phages, namely the rotating flagellar filament, is coded for by two tandem genes flaA and flaB, which show 95% sequence identity (Wassenaar et al., 1991). Selective disruption of the major flagellin gene (flaA) or a double deletion mutant lead to loss of the full-length flagellar filament, which results in the loss of motility, reduced colonization efficiency and resistance to flagellotropic bacteriophages (Guerry et al., 1991; Nachamkin et al., 1993; Baldvinsson et al., 2014). Inactivation of flaB does not have a major impact on motility as the FlaB content within the flagellar filament is sparse (Alm et al., 1993). Expression of the alternative flagellin genes are governed by different sigma factors. While flaA is under control of σ28, flaB is σ54 regulated (Nuijten et al., 1990). The functional significance or benefits of this separate gene regulation have yet to be unraveled. There are suggestions that flaB is involved in an intragenomic recombination mechanism aiming to evade immunological responses of the colonized host, and therefore increasing antigenic diversity (Harrington et al., 1997). Whether this could also have an effect on bacteriophage infection has not been tested, since there are no data available in literature concerning links of the minor flagellin gene product (FlaB) to bacteriophage infection.
The aim of the present study was to identify factors that impact on Eucampyvirinae bacteriophage infection that constitute a virulent subfamily of the Myoviridae (Javed et al., 2014), and are candidates for phage therapy of farm animals (Loc Carrillo et al., 2005; El-Shibiny et al., 2009; Kittler et al., 2013; Hammerl et al., 2014) and phage biosanitization applications (Atterbury et al., 2003; Goode et al., 2003; Bigwood et al., 2008) to control campylobacters in the human food chain (Connerton et al., 2011). For this purpose, we have constructed isogenic knock-out mutants targeting virulence and animal host colonization factors in the bacteriophage propagation strain C. jejuni PT14 (Brathwaite et al., 2013). We report for the first time an effect of the flaB gene product in connection to bacteriophage infection. In the absence of functional FlaB we observed an increase in susceptibility to infection by bacteriophage CP_F1 and increased phage propagation after a 24 h incubation period in liquid culture. Further, we observed changes in the adsorption rate and burst size that suggests FlaB has a defensive role against bacteriophage infection.
Materials and Methods
Bacteriophage, Bacterial Strains, and Growth Conditions
Bacteriophage CP_F1 was originally isolated from a pig manure sample and was propagated on C. jejuni PT14 (Brathwaite et al., 2013). All strains, associated plasmids and oligonucleotide PCR primers used in this study are listed in Table 1. The C. jejuni strains were routinely grown on blood agar base No. 2 plates (BA plates; Oxoid) supplemented with 5% horse blood (TCS Biosciences) under microaerobic conditions in either a modular atmosphere controlled cabinet (5% CO2, 5% O2, 2% H2, 88% N2) or in anaerobic jars using gas replacement (7.3% CO2, 5.6% O2, 3.6% H2, 83.5% N2) at 42°C. Escherichia coli strain TOP10 (Invitrogen) was used for cloning and cultured aerobically at 37°C on Luria agar plates or in Luria broth containing appropriate antibiotic as required.
Construction of C. jejuni PT14 Mutant and Complemented Strain
Transformation of C. jejuni
Site-specific knock-out mutations in C. jejuni PT14 were generated by transformation with genomic DNA from established and verified constructs in donor strains (Table 1). As most strains of C. jejuni exhibit efficient natural transformation, the protocol from Wang and Taylor (1990) was employed. The C. jejuni PT14 overnight cultures were collected from BA plates and dissolved in Mueller Hinton (MH) broth (Carl Roth). Cells were adjusted to OD600 0.5 (corresponding to approx. 3 × 109 CFU/ml) and aliquots of 500 μl of adjusted culture were added to 1 ml of MH broth in a 15 ml tube. Following incubation at 42°C under microaerobic conditions for 5 h, 1–5 μg of extracted chromosomal DNA (GenElute Bacterial Genomic DNA Kit, Sigma Aldrich) was added and cells were further incubated overnight. On the following day, cells were concentrated by centrifugation at 13,000 × g for 15 min. Pellets were resuspended in 100 μl of MH broth and spread on BA plates containing the selective agent according to the selective marker in use (34 μg/ml chloramphenicol or 50 μg/ml kanamycin from Sigma Aldrich). Selection plates were then incubated for 24–48 h at 42°C under microaerobic conditions. Genomic insertion of the desired mutant genes were verified via PCR amplification and DNA sequencing.
Trans Complementation of the flaB Mutant Strain
To achieve a trans-complementation of the disrupted flaB gene in strain C. jejuni PT14, a suicide vector based on pCfdxA (Gaskin et al., 2007) was constructed. For this purpose, the wild type flaB gene and the region 100 bp upstream carrying the native promoter were PCR amplified from the genomic DNA of C. jejuni PT14 with primers flab_NgoMIV_fw and flab_BsmBI_rv. After restriction digest the fragment was cloned into the vector adjacent to a chloramphenicol resistance cassette (cat). This vector further harbored regions of pseudogene cj0046 from C. jejuni NCTC11168 flanking the cassette and the gene of interest which enables recombination with the homologous pseudogene A911_00230 of strain C. jejuni PT14. Complementation of the mutant strain C. jejuni PT14 flaB::kan was performed by electroporation, as natural transformation of E. coli derived plasmids was not efficient. Colonies were sub-cultured and analyzed for positive integration by colony PCR using primers cj0046_fw and cj0046_rv that bind in the flanking regions of the A911_00230 pseudogene. Additionally, the amplicon was gel purified and sequenced for additional verification.
Spot Test Assays and Titer Determination
A determination of bacteriophage infection of the C. jejuni PT14 wild type and mutant strains was performed by employing the traditional spot test assay. A fresh overnight culture of the test Campylobacter strain was collected from a BA plate and emulsified in MH broth supplemented with 10 mM MgSO4 (Thermo Fisher) to a cell density of approximately 107 CFU/ml. A volume of 500 μl of emulsified cells was added to 5 ml of molten NZCYM (0.6%, Carl Roth) top agar and poured on top of a NZCYM agar plate. After the top agar had solidified, 5 μl aliquots of the phage suspensions, at the routine test dilution of approximately titer of 107 PFU/ml (Frost et al., 1999), were dispensed on top of the soft agar layer. The plate was then incubated at 42°C under microaerobic conditions for 24–48 h after the spots dried into the agar. In a similar way the phage titer was determined by serial 10-fold dilutions of phage suspensions, which were applied as 10 μl droplets in triplicate to the surface of prepared host bacterial lawns and allowed to dry. After incubation the plaques were counted and titers calculated.
Motility Assays
In order to assess swarming motility, fresh overnight cultures were taken from a BA plate and resuspended in MH broth. After adjustment of cell suspensions to OD600 0.1, 2 μl of suspension was removed with a pipette tip and stabbed into a swarming agar plate (0.4% Mueller Hinton agar). Inoculated plates were then incubated under microaerobic conditions at 42°C for 48 h. Growth zones were measured for each of triplicate samples after 24 and 48 h to assess cell motility.
Analysis of Bacteriophage Infection in Liquid Growth Media
The growth from C. jejuni BA plates incubated overnight at 42°C were collected and emulsified in PBS buffer (Merck Millipore) for the adjustment of inoculum concentrations. Flasks containing 50 ml nutrient broth No. 2 (Oxoid) were inoculated with C. jejuni cells at starting concentrations of approximately 105 or 107 CFU/ml. After inoculation, flasks were sealed with cotton stoppers and placed in anaerobic jars (Oxoid). Microaerobic conditions were introduced using the gas replacement method (John et al., 2011). Cultures were then incubated in a shaking incubator at 42°C and 100 rpm. After 2 h incubation, bacteriophage CP_F1 was added at an MOI of 2. Samples were collected at 2 h intervals for determination of viable cell counts and phage titers using the Miles and Misra method. Phage samples were treated with 2 μl chloroform (Thermofisher) and centrifuged at 13,000 × g for 5 min. The supernatant was removed and used for titration. The C. jejuni PT14 wild type and knock-out strains were tested in triplicate to ensure statistical certainty. Growth controls without phage addition served as references.
Adsorption Assay
Phage adsorption rates were determined as described previously (Siringan et al., 2014) with minor modifications outlined as followed. Suspensions of C. jejuni PT14 wild type, flaB mutant and trans-complemented flaB cells that contained approximately 5 × 107 CFU/ml, were inoculated in nutrient broth No. 2 and incubated at 42°C under microaerobic conditions at 100 rpm for 5 h to obtain cells in the exponential growth state. The actual viable count was determined following serial dilution and incubation as described above. For the experiment, bacteriophage CP_F1 was diluted and added to the suspensions to give a final titer of 105 PFU/ml, then briefly mixed and kept static at 42°C under microaerobic conditions. Sampling was performed every 5 min over a period of 30 min. Samples were immediately centrifuged at 13 000 × g for 5 min and the supernatants removed. The titer of free bacteriophages in the supernatants was determined and used to calculate numbers of bound bacteriophages. Bacteriophage adsorption constants were determined using the formula k = -ln (Pt/P0)/Nt, where Pt = phage titer at time point t (PFU/ml), P0 = initial phage titer (PFU/ml), N = bacterial density (CFU/ml) and t = time (min).
Efficiency of Plating of Phage CP_F1 on Mutant Strains
The efficiency of plating (EOP) of bacteriophage CP_F1 infecting the PT14 mutant strains was determined by enumerating bacteriophages as described above. Subsequently the calculations were made by dividing the bacteriophage titer obtained when applied to the lawns prepared from different mutant strains by the titer obtained when applied to C. jejuni PT14 wild type lawns.
Bacteriophage Growth Parameter Determination
In order to assess the burst size and latent period for bacteriophage CP_F1 infection of C. jejuni PT14 wild type, the flaB mutant and trans-complemented derivatives, single step growth curves were monitored over 4 h. The protocol after Carvalho et al. (2010) was employed for this purpose. Triplicate samples of 10 ml nutrient broth No. 2 were inoculated with 107 CFU/ml cells and grown under microaerobic conditions at 42°C under constant shaking at 100 rpm into early exponential phase (approx. 108 CFU/ml). Bacteriophage CP_F1 was added at an MOI of 0.001. Samples for titer determination were taken every 15 min for 4 h. These were centrifuged at 13,000 × g for 5 min and the supernatants used for titration. Three replicates of each individual experiment were performed and mean values used for presentation of titer development. Non-linear regression was used to determine latent period and burst size of the first burst event.
Whole Genome Sequencing
Genomic DNAs from C. jejuni PT14 non-motile variants were prepared using the GenElute Bacterial Genomic DNA Kit (Sigma Aldrich) following the manufacturer's instructions. DNA sequencing was performed using the Illumina MiSeq platform. The data consisted of 3.1–4.4 million paired-end sequence reads of 250 bp in length. Initial processing of the raw data, mapping of the sequence reads to C. jejuni PT14 (GenBank accession number CP003871) and variant detection were performed using CLC Genomics Workbench version 8.0 (Qiagen, Aarhus, Denmark).
RNA Extraction
Total RNAs were extracted from CP_F1 infected and uninfected control cultures of C. jejuni PT14. For each treatment, three independent early-log phase cultures (eclipse phase of the infected cultures) growing in nutrient broth No. 2 were harvested 50 min after phage addition and the RNA content extracted using TRIzol Max with Max Bacterial Enhancement Reagent (Invitrogen) according to the manufacturer's protocol. Subsequently ethanol-precipitation and purification using the RNeasy Mini kit (Qiagen, Crawley, UK) according to the manufacturer's instructions were performed including DNase treatment using RNase-free DNase and related reagents (Qiagen). Pure RNA samples were collected in 40 μl of RNase free water and analyzed for quantity/purity (Nanodrop ND1000) and quality (Bioanalyser 2100, Agilent Technologies Inc.). Prokaryote Total RNA Nano series II software, Version 2.3 was used for analysis of the RNA quality. All purified RNA samples showed a RNA Integrity Number (RIN) of 10.0.
Real Time qRT-PCR
Total RNAs were converted to cDNA with random hexamer primers using the Superscript II (Fisher Scientific) reverse transcriptase system according to the manufacturer's protocol. Specific primers for qRT PCR were designed with lengths of 18–24 nt and can be found in Supplementary Table S1. An optical 48 well microtiter plate (Applied Biosystems) was used with 20 μl reaction volumes consisting of Power SYBR Green PCR master mix (Life Technologies), 50 nM gene specific primers and 100 ng of the cDNA template. A StepOne real time PCR system (Applied Biosystems) was programmed for an initial set up of 30 s at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 58°C. A melting curve was obtained from 50 to 95°C to control specificities of quantitative PCR reaction for each primer pair. Cycle threshold (CT) values were determined employing the StepOne software version 2.0 (Applied Biosystems). The comparative threshold cycle method was used to calculate change (n-fold) where samples were normalized to the internal control product of the 50 S ribosomal subunit protein L1 gene (rplA), which showed no change in expression levels between phage infected and uninfected cultures of C. jejuni PT14 during acute CP8 infection in previous experiments. Reactions were performed in triplicate. The fold changes were calculated using the 2−ΔΔCt method. Verification of acute phage infection was confirmed by PCR using synthesized cDNA, employing specific primers targeting the gene for the major capsid protein (mcp) of bacteriophage CP_F1 (homolog of gp23 from phage NCTC12673; Kropinski et al., 2011).
Statistical Treatment of Data
Statistical differences between paired control and treatment groups were assessed by using the Student's t-test with significance p < 0.05. Differences between experimental groups were analyzed by analysis of variance. Viable count and phage titer data were log10-transformed for analysis.
Results
Disruption of flaB Yields Clear Lysis Zones for Bacteriophage CP_F1
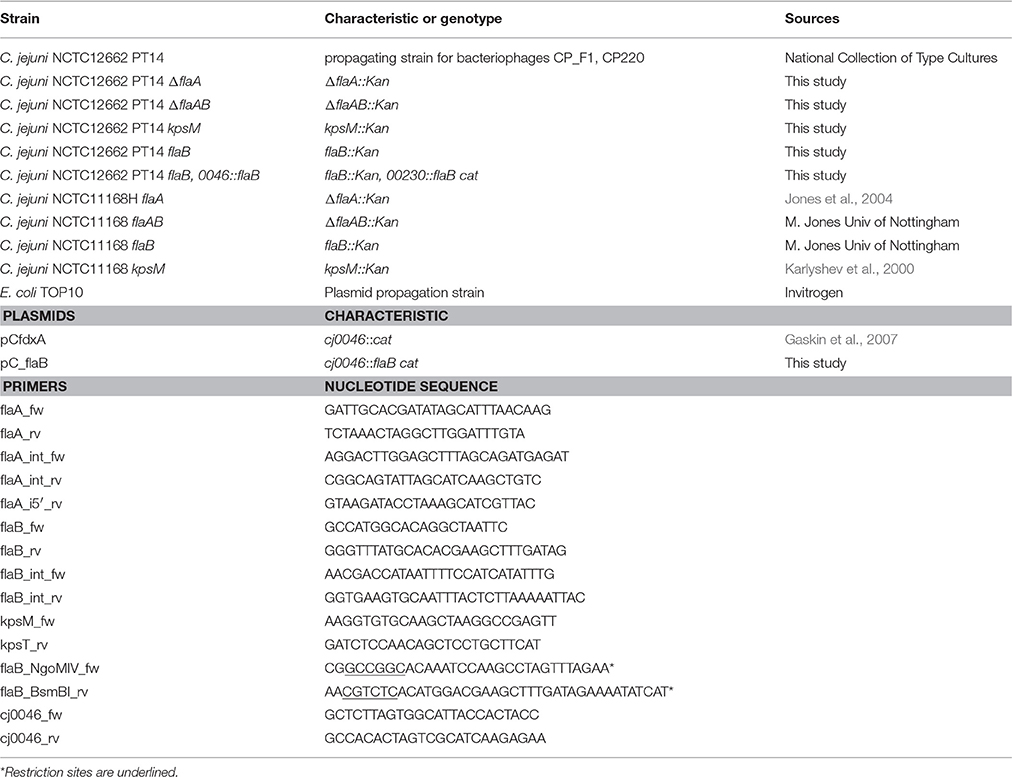
By screening bacteriophage infection of a number of mutant variants of C. jejuni PT14 we have analyzed the effects of flagella related factors on this process. Spot test assays showed a clear dependence on rotating flagella for bacteriophage CP220 (prototype of the Cp220likevirus genus), as no replication and host lysis was observed for flaA or flaAB mutants (Supplementary Figure S1). Similarly, the CP8unalikevirus phage CP_F1 showed decreased infection efficiency on non-motile cells, as infection of flaA and flaAB mutant strains yielded reduced efficiency of plating (EOP) values (flaA 0.08 ± 0.04, n = 3; flaAB 0.12 ± 0.03, n = 3) and highly opaque plaques. Additionally we observed CP_F1 to be strictly dependent on capsular polysaccharide (CPS) as no plaque formation occurred on a kpsM mutant (Figure 1). Interestingly, we found an aberration in infection by bacteriophage CP_F1 of a flaB disruption mutant. CP_F1 was isolated from a pig sample and propagated on strain PT14. It is able to infect and propagate on its host, but yields opaque plaques on soft agar bacterial lawns, which reveal regrowth of the host after infection. We observed similar reactions for phage CP220 (Supplementary Figure S1), suggesting that this reaction is not limited to one class of Campylobacter phage (Javed et al., 2014). However, propagation on a flaB mutant of PT14 produced clear lysis zones that persisted even after prolonged incubation for 24 h (Figure 1). Five individual clones, which all carried the desired disruption of flaB were tested in spot test assays to exclude effects of secondary mutations (de Vries et al., 2015). All showed identical phenotypes toward phage-induced lysis on soft agar lawns and motility (Supplementary Figure S2).
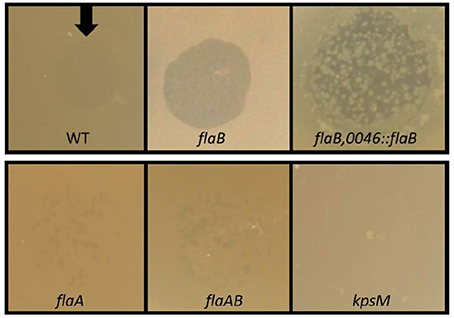
Figure 1. Lysis zones of bacteriophage CP_F1 on bacterial lawns of C. jejuni PT14. Opaque lysis areas were observed upon infection of the wild type strain caused by regrowth of a Campylobacter sub-population less susceptible to phage infection. Disruption of the flaB gene resulted in clear lysis. Complementation of the flaB mutant partially restored opaque lysis. Reduced plating efficiency and opaque plaques were observed in flaA and flaAB mutants. No plaque formation occurred for the kpsM mutant.
DNA sequencing of PCR amplicons of all five clones carrying the desired gene disruption, revealed an integration of the resistance marker in the flaB gene in the orientation of the flaB reading frame, mitigating any polar effects on the adjacent flaA gene. We also detected no mutations in the flaA gene, as further supported by findings from motility tests. Analysis of the motility of the flaB mutant strains revealed no major reduction in swarming motility (Figure 2), which is in accordance with previous observations (Wassenaar et al., 1991; de Vries et al., 2015).
Figure 2. Swarming motility of C. jejuni PT14 wild type (WT), flaB mutant and the complemented strain on low density agar. The diameters of growth zones after 24 and 48 h of incubation are derived from 3 biological replicates and presented as means with standard deviations.
To analyze the regrowth of Campylobacter cells in lysis zones we collected and sub-cultured surviving cells from the surface of turbid spots. Single colony isolates of these were examined for motility, reaction to phage infection by CP_F1 and DNA sequence analysis of the flaA/B region. We found that the majority of clones (78%, n = 100) showed either complete loss of motility or attenuation of their swarming ability (Supplementary Table S2), which was accompanied by resistance to reinfection by phage CP_F1. Additionally, eight clones were observed to have shifted into the carrier state (Siringan et al., 2014), as demonstrated by plaque formation inside of the growth zone on the motility agar plates and detectable phage propagation after serial sub-culturing of these clones (Supplementary Figure S3). PCR amplification and DNA sequence analysis of the flaA gene of the non-motile isolates revealed no alteration in the gene or non-coding sequences. The non-motile isolates were serially sub-cultured and after each passage the motility and phage resistance of the culture were assessed by inoculation of swarming motility agar plates and plaque assay. After two rounds it was observed that cultures arising from a single clone had recovered motility. In one case the process of escape from bacteriophage infection was reversible, which could be attributable to phase variation. Whole genome sequencing of three non-motile clones exhibiting phage resistance revealed all to have suffered a single nucleotide deletion in the N-terminal region of the rpoN gene coding for the RNA polymerase factor σ54 (A911_03265) at nucleotide position 244–250 in a stretch of 7 adenine residues. Further several phase variable genes were shifted to the off state compared to the wild type genome sequences (Table 2). For the flaB mutant, the shift to a phage resistant population could not be observed, which we assume is due to an increased susceptibility of the mutant to phage infection that may also be accompanied by an inability to support the mutation(s) leading to phage resistance.
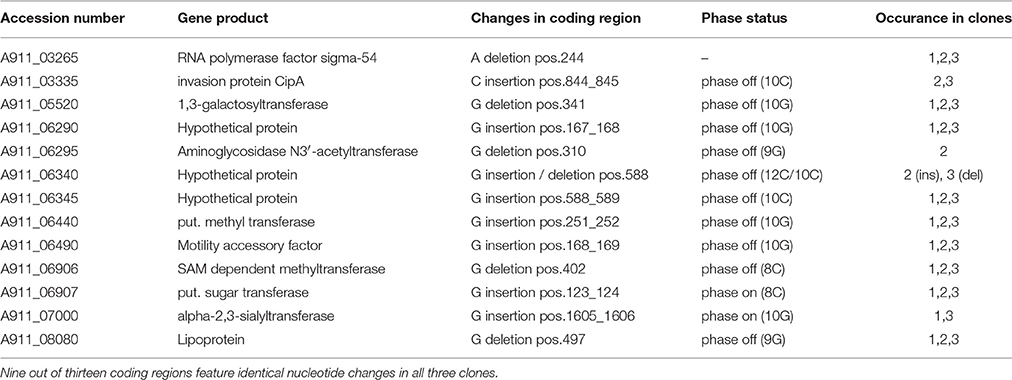
Table 2. Observed single nucleotide changes in the genome sequences of escape mutants recovered after CP_F1 infection relative to the reference sequence of C. jejuni PT14 (CP003871).
Complementation Leads to Partial Restoration of the Wild Type Phenotype
In order to verify that the observed increase in susceptibility toward phage infection was a result of the disruption of the flaB gene, we constructed a plasmid vector for the introduction of an intact copy of flaB into the pseudogene A911_00230 of the mutant strain. This construct was used to transform the flaB mutant strain and the correct insertion verified by DNA sequencing the target region. Trans-complementation of the disrupted flaB gene led to partial restoration of the phenotype with respect to bacteriophage infection. Overgrowth was restored within phage lysis zones but was less turbid than the wild type. The swarming motility of the complemented strain remained comparable to wild type but the survivors of phage infection were compromised in motility (Figure 2).
Increased Susceptibility and Burst Size in Liquid Growth Cultures
In order to analyze the changes in phage susceptibility in greater detail, phage replication experiments were performed in 50 ml liquid medium cultures, using either wild type or the flaB mutant or the flaB trans-complemented strain of C. jejuni PT14 over a period of 24 h (Figure 3). We tested the effects on phage infection at two different host cell densities, above and below the phage proliferation threshold of log10 7 CFU/ml, which represents the density of bacteria required for the productive replication of bacteriophage (Cairns et al., 2009). At higher cell densities CP_F1 infection of wild type, flaB mutant and flaB complement cultures resulted in an initial fall of approximately 1 log10 CFU/ml in the viable count. This event was followed by a recovery in the viable count of wild type cultures (Figure 3A). In contrast the flaB mutant exhibited a drastic reduction in the growth rate (μ) post the phage-induced crash in viable count (Figure 3B). The growth rates post population crash for infected wild type μ = 0.67 ± 0.02/h and infected flaB mutant μ = 0.31 ± 0.05/h cultures (p < 0.01). The flaB complement culture also recovered faster than the mutant (Figure 3C) exhibiting a significantly greater growth rate of μ = 0.44 ± 0.06/h (p < 0.05). Phage proliferation at high initial cell densities showed significant differences between wild type and flaB phage-infected cultures with significantly greater phage yields at 24 h (p < 0.01).
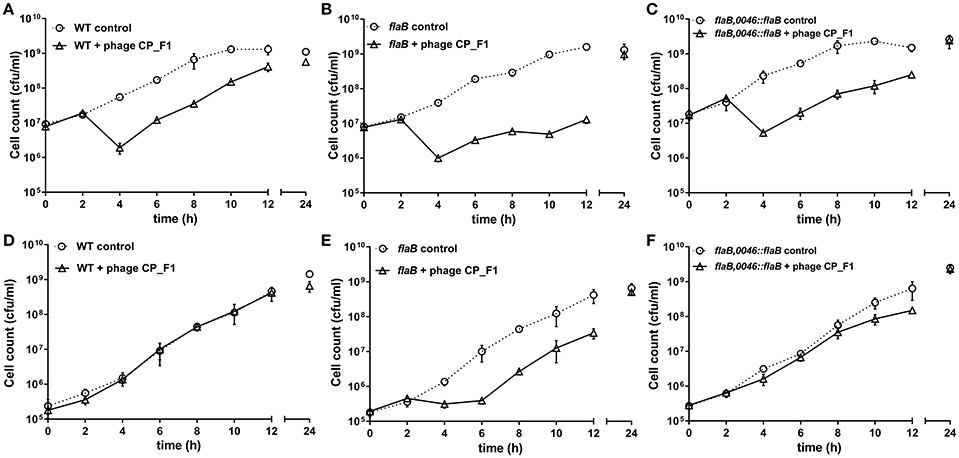
Figure 3. Growth profiles of C. jejuni PT14 wild type strain (A,D), flagellin B mutant (B,E) and trans-complemented flaB cultures (C,F) in nutrient broth No. 2. Phage infection was accomplished by addition of bacteriophage CP_F1 to achieve an MOI 2 whilst uninfected cultures were mock infected. Panels (A–C) show initial infection above the phage proliferation threshold and panels (D–F) below the phage proliferation threshold. Data points indicate mean values for n = 3 biological replicates and the error bars show the standard deviation.
Infection of wild type C. jejuni PT14 at a bacterial density below the phage proliferation threshold with CP_F1 at an MOI of 2 resulted in minimal differences in the viable bacterial counts between infected and control cultures (Figure 3D). In contrast, the viable count for flaB mutant cultures remained static for a period of 4 h, post phage infection but subsequently these cultures entered exponential growth (Figure 3E). The growth rates of the infected cultures post delay were not significantly different to the uninfected control cultures (control: μ = 0.62 ± 0.10/h; infected: μ = 0.74 ± 0.06/h) suggesting under these conditions there was no fitness disadvantage for the emergent phage resistant sub-population. The delay evident in the phage infected flaB mutant was not present in trans-complemented cultures, which behaved similar to wild type with similar growth rates to the uninfected control at early stages of growth (Figure 3F). Analysis of phage propagation showed no increase in phage numbers during the first 12 h of incubation. However, after 24 h an increase in free virions was detected, implying phage replication. There was a significant difference in the phage titer of approximately 1 log10 PFU/ml (p < 0.001) between infected wild type cultures and the infected flagellin B mutant strain after 24 h (Supplementary Figure S4). Further analyses of the phage propagation parameters were examined using a one-step growth experiment. The latent period for CP_F1 was determined as 90 min with two burst events, marked B1 (90–105 min) and B2 (180–210 min) in Figure 4, evident over the 240 min period. The latent period for the CP_F1 infection process was in a comparable range to other studies (Cairns et al., 2009; Carvalho et al., 2010). The number of phage particles liberated per cell showed an increase for CP_F1 infecting the flaB mutant strain relative to the wild type and flaB complement. A burst size of 1.4 ± 0.4 PFU/cell in the wild type and 4.2 ± 1.2 PFU/cell in the mutant strain were calculated for the first burst event. Differences in the phage yields between the phage infected cultures were statistically significant (p < 0.05). Further, the phage yields were magnified after a second burst event. This resulted in a difference of 1 log10 in PFU between mutant culture and the wild type or the flaB complement at 225 min after the initial addition of the phage. However, the significant change in the replication process was not accompanied by a major difference in the relative efficiency of plating of phage CP_F1 between infection of the PT14 wild type and the flagellin B mutant strain (1.59 ± 0.38, n = 3).
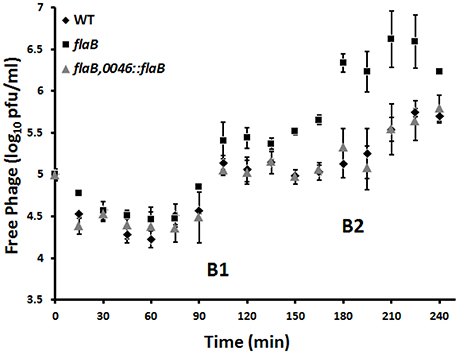
Figure 4. Determination of burst size of Campylobacter bacteriophage CP_F1 infecting C. jejuni PT14 wild type (WT), flagellin B mutant (flaB) and the flaB trans-complement (flaB, 0046::flaB). Cultures were infected with a MOI of 0.001. Two notable rises in phage titer are marked as burst steps B1 and B2. Calculations of burst size, during the first burst event (90–105 min), resulted in a mean value of 1.4 virions (±0.4) per cell for the wild type strain, 1.8 virions (±0.6) per cell for the flaB trans-complement and 4.2 virions (±1.2) for the flagellin B mutant. Data points represent mean values for n = 3 independent biological replicates. Error bars represent standard deviations.
To test if any changes in flagellin gene expression were induced as a response to acute phage infection we performed quantitative RT-PCR analyses. No alterations in the transcript levels of flaA or flaB in wild type PT14 were observed during the eclipse period of phage infection in which phage DNA replication and capsid assembly occurs prior to host lysis. A time point of 50 min post phage addition was selected for transcriptional analysis, since one step growth experiments revealed a latent period of approximately 90 min for CP_F1 infection. Similarly, no change in the expression of flaA was observed in the flaB knock-out strain (Supplementary Figures. S5, S6). These data would suggest that the increase in bacteriophage sensitivity or phage yields of flaB mutants are not linked to a compensatory increase in flaA transcription.
Disruption of flaB Affects Bacteriophage Adsorption
As changes in susceptibility toward phage infection and burst size were observed in the mutant strain, we were interested to examine if a change in adsorption might also be an effect of flaB inactivation. The calculated adsorption constant of CP_F1 binding to the PT14 wild type was in a similar range to that of the Cp8unalikevirus phages CP30 and CP8 (Siringan et al., 2014). However, there were 2-fold and greater increases in the adsorption constants (k) determined for the flaB mutant clones after 30 min incubation (Table 3). Differences in the adsorption constant between the wild type and the mutant were statistically significant for five independent clones based on triplicate experiments (p < 0.01), verifying that the absence of FlaB has an effect on the adsorption process.
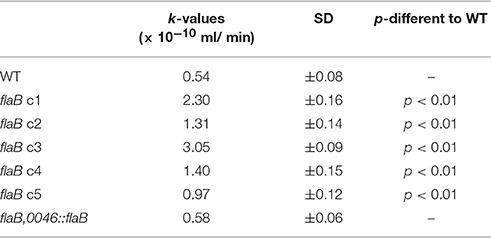
Table 3. Adsorption constants of phage CP_F1 infecting C. jejuni PT14 wild type (WT), five independent clones of flaB mutants and trans-complemented flaB (n = 3).
Discussion
Several reports have demonstrated that Campylobacter bacteriophages are dependent on certain surface structures for the infection of suitable hosts, for example capsular polysaccharide or a functional flagella (Coward et al., 2006; Scott et al., 2007; Sørensen et al., 2011; Baldvinsson et al., 2014). These are targets for bacteriophage adsorption, which have been recognized as being prone to variation in expression at different stages of bacterial cell growth and are often subject to stochastic phase variation. Campylobacters contain homopolymer GC tracts within dispensable reading frames that can expand or contract to place the reading frame in or out of phase and the gene effectively on or off (Bayliss et al., 2012; Anjum et al., 2016). Variable expression of surface-associated structures may contribute an obstacle to efficient infection by bacteriophages and may affect the efficacy of phage based biocontrol approaches (Nuijten et al., 1995; Park et al., 2000; Karlyshev et al., 2002; Connerton et al., 2011; Holst Sørensen et al., 2012). In search of phage target structures that do not exhibit phase variability we tested the effect of several genes, recognized as essential for Campylobacter virulence and colonization, on the infection process of a collection of bacteriophages. The aim was to find novel elements that may be essential for, or have an impact on, bacteriophage infection. During our screening we found a factor that interferes with the infection process of certain bacteriophages. Inactivation of the minor flagellin gene (flaB) showed a number of effects on different parameters of bacteriophage infection. Collectively, these effects increased the efficiency of phage infection and impaired the resilience of the affected host population toward infection. For the first time, we show that the presence of a gene product, classified as a structural protein, confers defensive properties against bacteriophage infection. Further, this effect was not limited to one genetically classified genus of Campylobacter myovirus phages, as two phages CP_F1 and CP220 from two different genera showed similar reactions during infection of a flagellin B mutant strain. CP_F1 is a CP8una-like phage and CP220 is the archetype of the CP220-like phages (Javed et al., 2014). Furthermore, these two phages differ in requirements for effective infection. We found CP220 to be flagellotropic, while CP_F1 strictly relies on capsular polysaccharide (CPS). However, CP_F1 also showed a preference for motile cells as infection of flaA and flaAB mutant strains yielded reduced EOP values.
We hypothesize that flaB is an essential component of an integral defensive mechanism against bacteriophage infection. In our observations, disruption of this gene prevented cells from shifting to resistant, non-motile phenotypes during infection of a bacterial lawn on semi-solid media. This phenomenon may be based on the lysis of a large proportion of cells before mutations or gene regulatory responses can develop that lead to a resistant sub-population. In liquid growth cultures, at cell densities below the phage proliferation threshold, bacteriophage CP_F1 was effectively bacteriostatic for flaB deficient cultures, whereas the phage did not impair the growth of cultures with an intact flaB gene. At cell concentrations that promoted phage proliferation, host bacterial population crashes were observed after phage addition to wild type or mutant cultures. However, the recovery from acute phage infection and the resumption of exponential growth observed for wild type cultures was severely retarded and the growth yields reduced in the flaB mutant cultures compared to wild type. These results clearly show that flaB deficient strains have a greater susceptibility to phage infection. Inevitably an increase in susceptibility will increase the selective pressure to escape phage infection and the generation of less susceptible sub-populations. Whilst not evident upon infection on semi-solid medium, regrowth was observed post infection of the flaB mutants in liquid culture. Spatial differences between liquid and semi-solid media in terms of diffusion, host mobility and distribution might be some of the key factors contributing to the probability of the cells being able to evade infection and develop resistance (Abedon and Yin, 2009). However, it should also be noted that these conditions represent ideal conditions in terms of nutrient availability, suitable oxygen tension and the absence of competitor organisms that would not exist in intestinal or extra-intestinal environments inhabited by campylobacters.
The increase in phage yields observed for the flaB mutant are accompanied by a significant increase in the burst size, which suggests that phage maturation is more efficient in the flaB mutant. Trans-complementation of the flaB mutation results in a reduction in the burst size from 4.2 virions (±1.2) per cell to 1.8 virions (±0.6) per cell, a value similar to that of wild type. The flaB mutant also shows an increase in the adsorption constant, which would theoretically make the mutant more susceptible at lower cell densities. Figure 3E conclusively demonstrates that the flaB mutant culture remains susceptible to phage infection at cell densities below the phage proliferation threshold, such that host growth is arrested as compared to the wild type and flaB trans-complement cultures. Lysis times are predicted to become shorter in phage exhibiting high adsorption rates (Shao and Wang, 2008), and it is noteworthy that the first significant increase in free phage titers were observed for the flaB mutant in Figure 4 at 90 min post-infection. From these observations it is evident that any host bacterium that can evolve a countermeasure against this perfect storm would be at a selective advantage when subject to bacteriophage predation. Campylobacter bacteriophages have been proposed for the biocontrol of campylobacters in the food chain (Connerton et al., 2011), and from a biotechnological standpoint the introduction of a flaB mutant for Campylobacter phage amplification strategies offers the prospect of increasing phage yields from growth cultures, which would render large scale phage production more cost effective.
If the FlaB protein acts either directly or indirectly to reduce the burst size, then the Campylobacter population would benefit by limiting the release of free virus and the rate of propagation. Such a mechanism could account for the rather low burst size of Campylobacter bacteriophages compared to phages infecting other proteobacteria. The peak expression of the flaB σ54 promoter was reported to occur mid exponential phase (Alm et al., 1993), which would coincide the ideal conditions for efficient phage replication when cells are metabolically replete and at a density beyond the phage proliferation threshold. Under these circumstances the deployment of FlaB as a countermeasure would confer the maximal impact on the bacterial population's ability to evade and adapt to bacteriophage infection.
Campylobacter flagella are highly decorated due to O-linked glycosylation of the flagellin monomer (Guerry, 2007; Ewing et al., 2009). A phage receptor binding protein (RBP) has been reported to specifically interact with flagella decorated with acetamidino-modified pseudaminic acid (Javed et al., 2015). As a consequence, changes in the glycosylation pattern of the flagella may introduce or reduce steric effects on the adsorption process. We have measured 1.8–5.6-fold increases in the adsorption rates of flaB mutant clones compared to wild type but how this is manifest is unclear since it has been reported that for C. coli VC167 surface exposure of the FlaB protein could not be detected (Guerry et al., 1991).
However, it is also reported that inactivation of flaB increases colonization efficiency of C. jejuni (Wassenaar et al., 1993). Glycosylation is recognized as an essential colonization factor; therefore it is possible that an altered distribution of glycan molecules may affect a change in colonization efficiency (Howard et al., 2009). There is evidence for diversity in glycan structure and variation in the numbers of residues serving as flagellar glycosylation targets in different Campylobacter strains (Thibault et al., 2001; Guerry, 2007), and variations in the occupancy of the glycosylation sites adding to the complexity of surface exposed region of the flagellin proteins (Meinersmann and Hiett, 2000; Ulasi et al., 2015). With respect to flagellin sequence in strain C. jejuni PT14, FlaA, and B share 95% identity at the protein level. Differences in amino acid composition can be found predominantly within terminal regions of the protein (26 residues). However, a centrally located region of the protein is predicted to be surface exposed, which contains 4 amino acid differences between FlaA and FlaB proteins of C. jejuni PT14, one of which is a serine at position 224 in the FlaB sequence that represents an additional substrate for glycosylation. An analysis of the glycan distributions of FlaA and FlaB may provide further insight as to their potential for interaction with bacteriophage.
Information regarding the function of the minor flagellin is sparse, although several studies have characterized flaB as not essential for motility (Guerry et al., 1991; Wassenaar et al., 1993; de Vries et al., 2015). These observations lead to the question as to why a paralogous structural gene has emerged and has been preserved throughout all known Campylobacter species. It has been reported that intra-genomic recombination between flaA and flaB can occur in Campylobacter cultures (Wassenaar et al., 1995). Later Meinersmann and Hiett (2000) hypothesized that flaB could serve as a driver for antigenic variation, since differences in the amino acid position were found in residues that function as targets for O-linked glycosylation. A similar conclusion was reached by Harrington et al. (1997), who identified intra-genomic recombination between flaA and flaB in strain VC670, and proposed that the variation enabled adaptation to eukaryotic host species, since functional flagella are essential for colonization and a key target of the host immune system. We assume that recombination events between the flagellin genes will also assist in the escape of phage infection by varying the flagellin structure and the O-linked glycosylation attachment sites, a response mechanism that is likely to have evolved before the immune systems of the animal hosts of Campylobacter species. However, analyses of the flaA coding sequences in this study provided no evidence for recombination events between the flaA and flaB genes in the escape mutants of bacteriophage infection. Further, no alterations in the flaA open reading frame or its 5′-untranslated region were detected. Instead whole genome analysis of escape mutants revealed a single adenine deletion in the N-terminal region of the rpoN gene coding for the σ54 factor. In most polar flagellate species σ54 serves as an essential part of flagellar expression. This is also the case for the flagella biogenesis of Campylobacter. A knock-out mutation in the rpoN gene of strain C. jejuni 11168 resulted in complete absence of flagella and flagellin expression (Jagannathan et al., 2001). Motility loss is one strategy by which campylobacters evade phage infection (Scott et al., 2007; Baldvinsson et al., 2014). Moreover, Campylobacter bacteriophage carrier state cultures also escape phage lysis by undergoing growth phase dependent motility attenuation as a response to phage association (Siringan et al., 2014). Whereas, flaA gene transcription is down-regulated in carrier state cultures, which likely accounts for their impaired mobility, flaB transcription is up-regulated, which based on the current data could be an adaption to limit phage infection leading to cell lysis (Brathwaite et al., 2015; Hooton et al., 2016). It is recognized that σ54 is part of a group of transcriptional regulators termed class 1 genes that demonstrate hierarchical regulation of class 2 genes, which are fundamental for the formation of the flagellar secretory apparatus and code for the structural components of the basal body of the flagellum (Hendrixson and DiRita, 2003; Lertsethtakarn et al., 2011). The absence of σ54 therefore has a profound effect on the formation of functional flagella. We further found several phase variable genes in the off state. Amongst them were several genes coding for sugar transferases, which may have an impact on the cell surface polysaccharides exposed and the phage infection process. High rates of phase variation in Campylobacter can facilitate the adaptation toward environmental effects (Gaasbeek et al., 2009; Bayliss et al., 2012). For example, phase variable frame shifts in the key flagellar export regulator FlhA has been reported to cause transcriptional repression of flaA and flaB to vary motility in C. coli (Park et al., 2000). Further, nucleotide deletions in fliW and flgD have also been found in connection with motility loss. This phenomenon was observed upon analysis of second-site mutations in a flaB knock-out mutant strain (de Vries et al., 2015). To exclude that secondary mutation in connection with flaB disruption were responsible for the observed increase in bacteriophage sensitivity; we tested five independent clones, which all carried the selective marker in the desired position. All five independent clones showed identical phenotypes with respect to phage induced lysis and motility. Further, trans-complementation partially restored the wild type phenotypes with respect to cell lysis in spot test assays and continued growth at low cell densities in phage infected broth cultures. These results confirm that the observed changes were introduced through the inactivation of the flaB gene.
In our efforts to understand how host genes affect bacteriophage propagation in C. jejuni we have identified a novel role for the minor flagellin FlaB. Although not essential for motility, the absence of FlaB makes C. jejuni more susceptible to bacteriophage infection. Here we propose that the maintenance of the flaB gene is not only an evolutionary adaptation to drive antigenic diversity in response to immune pressure but an earlier adaptation to evade infection by flagellotropic phage, and is maintained as a general countermeasure against bacteriophage propagation. Given the ubiquitous presence of virulent bacteriophages in the environment, it is perhaps not surprising that this seemingly redundant gene duplication was fixed and remains a landmark feature of many C. jejuni and C. coli strains.
Author Contributions
LL performed the experiments. LL and IC designed the experiments, analyzed the data and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
LL acknowledges support from Ernährung NRW Grant NA2020 Campy-Präv, FRG. This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BB/I024682/1], UK.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01908/full#supplementary-material
References
Abedon, S. T., and Yin, J. (2009). Bacteriophage plaques: theory and analysis. Methods Mol. Biol. 501, 161–174. doi: 10.1007/978-1-60327-164-6_17
Allos, B. M. (2001). Campylobacter jejuni Infections: update on emerging issues and trends. Clin. Infect. Dis. 32, 1201–1206. doi: 10.1086/319760
Alm, R. A., Guerry, P., and Trust, T. J. (1993). Significance of duplicated flagellin genes in Campylobacter. J. Mol. Biol. 230, 359–363. doi: 10.1006/jmbi.1993.1151
Anjum, A., Brathwaite, K. J., Aidley, J., Connerton, P. L., Cummings, N. J., Parkhill, J., et al. (2016). Phase variation of a Type IIG restriction-modification enzyme alters site-specific methylation patterns and gene expression in Campylobacter jejuni strain NCTC11168. Nucleic Acids Res. 44, 4581–4594. doi: 10.1093/nar/gkw019
Atterbury, R. J., Connerton, P. L., Dodd, C. E., Rees, C. E., and Connerton, I. F. (2003). Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69, 6302–6306. doi: 10.1128/AEM.69.10.6302-6306.2003
Baldvinsson, S. B., Sørensen, M. C., Vegge, C. S., Clokie, M. R., and Brøndsted, L. (2014). Campylobacter jejuni motility is required for infection of the flagellotropic bacteriophage F341. Appl. Environ. Microbiol. 80, 7096–7106. doi: 10.1128/AEM.02057-14
Bayliss, C. D., Bidmos, F. A., Anjum, A., Manchev, V. T., Richards, R. L., Grossier, J. P., et al. (2012). Phase variable genes of Campylobacter jejuni exhibit high mutation rates and specific mutational patterns but mutability is not the major determinant of population structure during host colonization. Nucleic Acids Res. 40, 5876–5889. doi: 10.1093/nar/gks246
Beeby, M., Ribardo, D. A., Brennan, C. A., Ruby, E. G., Jensen, G. J., and Hendrixson, D. R. (2016). Diverse high-torque bacterial flagellar motors assemble wider stator rings using a conserved protein scaffold. Proc. Natl. Acad. Sci. U.S.A. 113, E1917–E1926. doi: 10.1073/pnas.1518952113
Bigwood, T., Hudson, J. A., Billington, C., Carey-Smith, G. V., and Heinemann, J. A. (2008). Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiol. 25, 400–406. doi: 10.1016/j.fm.2007.11.003
Blaser, M. J. (1997). Epidemologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176, S103–S105. doi: 10.1086/513780
Boes, J., Nersting, L., Nielsen, E. M., Kranker, S., Enøe, C., Wachmann, H. C., et al. (2005). Prevalence and diversity of Campylobacter jejuni in pig herds on farms with and without cattle or poultry. J. Food Prot. 68, 722–727.
Bolton, D. J. (2015). Campylobacter virulence and survival factors. Food Microbiol. 48, 99–108. doi: 10.1016/j.fm.2014.11.017
Brathwaite, K. J., Siringan, P., Connerton, P. L., and Connerton, I. F. (2015). Host adaption to the bacteriophage carrier state of Campylobacter jejuni. Res. Microbiol. 166, 504–515. doi: 10.1016/j.resmic.2015.05.003
Brathwaite, K. J., Siringan, P., Moreton, J., Wilson, R., and Connerton, I. F. (2013). Complete genome sequence of universal bacteriophage host strain Campylobacter jejuni subsp. jejuni PT14. Genome Announc. 1, e00969–13. doi: 10.1128/genomeA.00969-13
Cairns, B. J., Timms, A. R., Jansen, V. A. A., Connerton, I. F., and Payne, R. J. (2009). Quantitative Models of in vitro bacteriophage–host dynamics and their application to phage therapy. PLoS Pathog. 5:e1000253. doi: 10.1371/journal.ppat.1000253
Carvalho, C. M., Gannon, B. W., Halfhide, D. E., Santos, S. B., Hayes, C. M., Roe, J. M., et al. (2010). The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 10:232. doi: 10.1186/1471-2180-10-232
Connerton, P. L., Timms, A. R., and Connerton, I. F. (2011). Campylobacter bacteriophages and bacteriophage therapy. J. Appl. Microbiol. 111, 255–265. doi: 10.1111/j.1365-2672.2011.05012.x
Coward, C., Grant, A. J., Swift, C., Philp, J., Towler, R., Heydarian, M., et al. (2006). Phase-variable surface structures are required for infection of Campylobacter jejuni by bacteriophages. Appl. Environ. Microbiol. 72, 4638–4647. doi: 10.1128/AEM.00184-06
de Vries, S. P., Gupta, S., Baig, A., L'Heureux, J., Pont, E., Wolanska, D. P., et al. (2015). Motility defects in Campylobacter jejuni defined gene deletion mutants caused by second-site mutations. Microbiology 161, 2316–2327. doi: 10.1099/mic.0.000184
EFSA (2012). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA J. 10:2597. doi: 10.2903/j.efsa.2012.2597
EFSA (2014). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA J. 12:3547. doi: 10.2903/j.efsa.2014.3547
EFSA (2015). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 13:3991. doi: 10.2903/j.efsa.2015.3991
El-Shibiny, A., Scott, A., Timms, A., Metawea, Y., Connerton, P., and Connerton, I. (2009). Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Prot. 72, 733–740.
Ewing, C. P., Andreishcheva, E., and Guerry, P. (2009). Functional characterization of flagellin glycosylation in Campylobacter jejuni 81–176. J. Bacteriol. 191, 7086–7093. doi: 10.1128/JB.00378-09
Frost, J. A., Kramer, J. M., and Gillanders, S. A. (1999). Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol. Infect. 123, 47–55. doi: 10.1017/S095026889900254X
Gaasbeek, E. J., van der Wal, F. J., van Putten, J. P. M., de Boer, P., van der Graaf-van Bloois, L., de Boer, A. G., et al. (2009). Functional characterization of excision repair and RecA-dependent recombinational DNA repair in Campylobacter jejuni. J. Bacteriol. 191, 3785–3793. doi: 10.1128/JB.01817-08
Gaskin, D. J., Van Vliet, A. H., and Pearson, B. M. (2007). The Campylobacter genetic toolbox: development of tractable and generally applicable genetic techniques for Campylobacter jejuni. Zoon. Publ. Health 54:101.
Goode, D., Allen, V. M., and Barrow, P. A. (2003). Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69, 5032–5036. doi: 10.1128/AEM.69.8.5032-5036.2003
Guerry, P. (2007). Campylobacter flagella: not just for motility. Trends Microbiol. 15, 456–461. doi: 10.1016/j.tim.2007.09.006
Guerry, P., Alm, R. A., Power, M. E., Logan, S. M., and Trust, T. J. (1991). Role of two flagellin genes in Campylobacter motility. J. Bacteriol. 173, 4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991
Hammerl, J. A., Jäckel, C., Alter, T., Janzcyk, P., Stingl, K., Knüver, M. T., et al. (2014). Reduction of Campylobacter jejuni in broiler chicken by successive application of group II and group III phages. PLoS ONE 9:e114785. doi: 10.1371/journal.pone.0114785
Harrington, C. S., Thomson-Carter, F. M., and Carter, P. E. (1997). Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J. Clin. Microbiol. 35, 2386–2392.
Hendrixson, D. R., and DiRita, V. J. (2003). Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol. Microbiol. 50, 687–702. doi: 10.1046/j.1365-2958.2003.03731.x
Hooton, S. P., Brathwaite, K. J., and Connerton, I. F. (2016). The bacteriophage carrier state of Campylobacter jejuni features changes in host non-coding RNAs and the acquisition of new host-derived CRISPR spacer sequences. Front. Microbiol. 7:355. doi: 10.3389/fmicb.2016.00355
Holst Sørensen, M. C., van Alphen, L. B., Fodor, C., Crowley, S. M., Christensen, B. B., Szymanski, C. M., et al. (2012). Phase variable expression of capsular polysaccharide modifications allows Campylobacter jejuni to avoid bacteriophage infection in chickens. Front. Cell Infect. Microbiol. 2:11. doi: 10.3389/fcimb.2012.00011
Howard, S. L., Jagannathan, A., Soo, E. C., Hui, J. P. M., Aubry, A. J., Ahmed, I., et al. (2009). Campylobacter jejuni glycosylation island important in cell charge, legionaminic acid biosynthesis, and colonization of chickens. Infect. Immun. 77, 2544–2556. doi: 10.1128/IAI.01425-08
Jagannathan, A., Constantinidou, C., and Penn, C. W. (2001). Roles of rpoN, fliA, and flgR in expression of flagella in Campylobacter jejuni. J. Bacteriol. 183, 2937–2942. doi: 10.1128/JB.183.9.2937-2942.2001
Javed, M. A., Ackermann, H. W., Azeredo, J., Carvalho, C. M., Connerton, I., Evoy, S., et al. (2014). A suggested classification for two groups of Campylobacter myoviruses. Arch. Virol. 159, 181–190. doi: 10.1007/s00705-013-1788-2
Javed, M. A., van Alphen, L. B., Sacher, J., Ding, W., Kelly, J., Nargang, C., et al. (2015). A receptor-binding protein of Campylobacter jejuni bacteriophage NCTC 12673 recognizes flagellin glycosylated with acetamidino-modified pseudaminic acid. Mol. Microbiol. 95, 101–115. doi: 10.1111/mmi.12849
John, A., Connerton, P. L., Cummings, N., and Connerton, I. F. (2011). Profound differences in the transcriptome of Campylobacter jejuni grown in two different, widely used, microaerobic atmospheres. Res. Microbiol. 162, 410–418. doi: 10.1016/j.resmic.2011.02.004
Jones, M. A., Marston, K. L., Woodall, C. A., Maskell, D. J., Linton, D., Karlyshev, A. V., et al. (2004). Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect. Immun. 72, 3769–3776. doi: 10.1128/IAI.72.7.3769-3776.2004
Karlyshev, A. V., Linton, D., Gregson, N. A., Lastovica, A. J., and Wren, B. W. (2000). Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol. Microbiol. 35, 529–541. doi: 10.1046/j.1365-2958.2000.01717.x
Karlyshev, A. V., Linton, D., Gregson, N. A., and Wren, B. W. (2002). A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 148, 473–480. doi: 10.1099/00221287-148-2-473
Kittler, S., Fischer, S., Abdulmawjood, A., Glünder, G., and Klein, G. (2013). Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl. Environ. Microbiol. 79, 7525–7533. doi: 10.1128/AEM.02703-13
Konkel, M. E., Klena, J. D., Rivera-Amill, V., Monteville, M. R., Biswas, D., Raphael, B., et al. (2004). Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 186, 3296–3303. doi: 10.1128/JB.186.11.3296-3303.2004
Konkel, M. E., Larson, C. L., and Flanagan, R. C. (2010). Campylobacter jejuni FlpA binds fibronectin and is required for maximal host cell adherence. J. Bacteriol. 192, 68–76. doi: 10.1128/JB.00969-09
Konkel, M. E., Monteville, M. R., Rivera-Amill, V., and Joens, L. A. (2001). The pathogenesis of Campylobacter jejuni-mediated enteritis. Curr. Issues Intest. Microbiol. 2, 55–71.
Kropinski, A. M., Arutyunov, D., Foss, M., Cunningham, A., Ding, W., Singh, A., et al. (2011). Genome and proteome of Campylobacter jejuni bacteriophage NCTC 12673. Appl. Environ. Microbiol. 77, 8265–8271. doi: 10.1128/AEM.05562-11
Lertsethtakarn, P., Ottemann, K. M., and Hendrixson, D. R. (2011). Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 65, 389–410. doi: 10.1146/annurev-micro-090110-102908
Loc Carrillo, C., Atterbury, R. J., El-Shibiny, A., Connerton, P. L., Dillon, E., Scott, A., et al. (2005). Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71, 6554–6563. doi: 10.1128/AEM.71.11.6554-6563.2005
McCrea, B. A., Tonooka, K. H., Van Worth, C., Boggs, C. L., Atwill, E. R., and Schrader, J. S. (2005). Prevalence of Campylobacter and Salmonella species on farm, after transport, and at processing in specialty market poultry. Poult. Sci. 85, 136–143. doi: 10.1093/ps/85.1.136
Meinersmann, R. J., and Hiett, K. L. (2000). Concerted evolution of duplicate fla genes in Campylobacter. Microbiology 146, 2283–2290. doi: 10.1099/00221287-146-9-2283
Monteville, M. R., Yoon, J. E., and Konkel, M. E. (2003). Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology 149, 153–165. doi: 10.1099/mic.0.25820-0
Nachamkin, I. (2002). Chronic effects of Campylobacter infection. Microbes Infect. 4, 399–403. doi: 10.1016/S1286-4579(02)01553-8
Nachamkin, I., Yang, X. H., and Stern, N. J. (1993). Role of Campylobacter jejuni flagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl. Environ. Microbiol. 59, 1269–1273.
Nuijten, P. J., Márquez-Maga-a, L., and van der Zeijst, B. A. (1995). Analysis of flagellin gene expression in flagellar phase variants of Campylobacter jejuni 81116. Antonie Van Leeuwenhoek 67, 377–383. doi: 10.1007/BF00872938
Nuijten, P. J., van Asten, F. J., Gaastra, W., and van der Zeijst, B. A. (1990). Structural and functional analysis of two Campylobacter jejuni flagellin genes. J. Biol. Chem. 265, 17798–17804.
O'Loughlin, J. L., and Konkel, M. E. (2014). “The role of the flagellum in Campylobacter jejuni colonization and disease,” in Campylobacter Ecology and Evolution, ed S. K. Sheppard (Norfolk: Caister Academic Press), 159–175.
Park, S. F., Purdy, D., and Leach, S. (2000). Localized reversible frameshift mutation in the flhA gene confers phase variability to flagellin gene expression in Campylobacter coli. J. Bacteriol. 182, 207–210. doi: 10.1128/JB.182.1.207-210.2000
Scott, A. E., Timms, A. R., Connerton, P. L., Loc Carrillo, C., Adzfa Radzum, K., and Connerton, I. F. (2007). Genome dynamics of Campylobacter jejuni in response to bacteriophage predation. PLoS Pathog. 3:e119. doi: 10.1371/journal.ppat.0030119
Shao, Y., and Wang, I. N. (2008). Bacteriophage adsorption rate and optimal lysis time. Genetics 180, 471–482. doi: 10.1534/genetics.108.090100
Siringan, P., Connerton, P. L., Cummings, N. J., and Connerton, I. F. (2014). Alternative bacteriophage life cycles: the carrier state of Campylobacter jejuni. Open Biol. 4:130200. doi: 10.1098/rsob.130200
Sørensen, M. C., van Alphen, L. B., Harboe, A., Li, J., Christensen, B. B., Szymanski, C. M., et al. (2011). Bacteriophage F336 recognizes the capsular phosphoramidate modification of Campylobacter jejuni NCTC11168. J. Bacteriol. 193, 6742–6749. doi: 10.1128/JB.05276-11
Thibault, P., Logan, S. M., Kelly, J. F., Brisson, J. R., Ewing, C. P., and Trust, T. J. (2001). Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276, 34862–34870. doi: 10.1074/jbc.M104529200
Ulasi, G. N., Creese, A. J., Hui, S. X., Penn, C. W., and Cooper, H. J. (2015). Comprehensive mapping of O-glycosylation in flagellin from Campylobacter jejuni 11168: a multienzyme differential ion mobility mass spectrometry approach. Proteomics 15, 2733–2745. doi: 10.1002/pmic.201400533
Wang, I., and Taylor, D. E. (1990). Natural Transformation in Campylobacter Species. J. Bacteriol. 172, 949–955. doi: 10.1128/jb.172.2.949-955.1990
Wassenaar, T. M., van der Zeijst, B. A., Ayling, R., and Newell, D. G. (1993). Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 139, 1171–1175. doi: 10.1099/00221287-139-6-1171
Wassenaar, T. M., Bleumink-Pluym, N. M., and van der Zeijst, B. A. (1991). Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 10, 2055–2061.
Keywords: Campylobacter, bacteriophage, flagellin, flaB, phage escape
Citation: Lis L and Connerton IF (2016) The Minor Flagellin of Campylobacter jejuni (FlaB) Confers Defensive Properties against Bacteriophage Infection. Front. Microbiol. 7:1908. doi: 10.3389/fmicb.2016.01908
Received: 16 September 2016; Accepted: 15 November 2016;
Published: 29 November 2016.
Edited by:
Giovanna Suzzi, University of Teramo, ItalyReviewed by:
Hongxia Wang, University of Alabama at Birmingham, USAKerstin Stingl, Federal Institute for Risk Assessment, Germany
Copyright © 2016 Lis and Connerton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian F. Connerton, ian.connerton@nottingham.ac.uk
 Lukas Lis
Lukas Lis Ian F. Connerton
Ian F. Connerton