- Department of Biology, Biotechnical Faculty, University of Ljubljana, Ljubljana, Slovenia
Population-wide tolerance and persisters enable susceptible bacterial cells to endure hostile environments, including antimicrobial exposure. The SOS response can play a significant role in the generation of persister cells, population-wide tolerance, and shielding. The SOS pathway is an inducible DNA damage repair system that is also pivotal for bacterial adaptation, pathogenesis, and diversification. In addition to the two key SOS regulators, LexA and RecA, some other stressors and stress responses can control SOS factors. Bacteria are exposed to DNA-damaging agents and other environmental and intracellular factors, including cigarette smoke, that trigger the SOS response at a number of sites within the host. The Escherichia coli TisB/IstR module is as yet the only known SOS-regulated toxin–antitoxin module involved in persister formation. Nevertheless, the SOS response plays a key role in the formation of biofilms that are highly recalcitrant to antimicrobials and can be abundant in persisters. Furthermore, the dynamic biofilm environment generates DNA-damaging factors that trigger the SOS response within the biofilm, fueling bacterial adaptation and diversification. This review highlights the SOS response in relation to antimicrobial recalcitrance to antimicrobials in four clinically significant species, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Mycobacterium tuberculosis.
Introduction
Bacteria are constantly exposed to a changing and stressful environment. The coordinated responses of global regulatory systems enable bacteria to survive and adapt to numerous environmental stresses (Foster, 2007).
Antibiotic resistance is one of the greatest threats to global health. Resistance allows growth in the presence of antibiotics due to genetic alterations that increase the minimal inhibitory concentration (MIC). Nevertheless, other mechanisms such as (i) population-wide tolerance, (ii) persisters, subpopulations characterized by a transient non-growing state and transient tolerance to lethal concentrations of antimicrobials (Wilmaerts et al., 2019), and (iii) shielding enable their survival in the presence of antimicrobial agents. Persisters play a pivotal role in chronic bacterial infections and the evolution of antibiotic resistance (Goormaghtigh and Van Melderen, 2019). Multifactorial and redundant molecular mechanisms are involved in the generation and the survival of persisters and tolerant cells (Molina-Quiroz et al., 2018; Trastoy et al., 2018). The most significant are global stress response, SOS response, oxidant tolerance, toxin–antitoxin system (TA), quorum sensing, energy metabolism, and drug efflux pumps (Dörr et al., 2009; Kwan et al., 2013; Harms et al., 2016; Trastoy et al., 2018). Recent studies have revealed that the SOS response plays significant roles beyond DNA damage repair. The goal of this review is to provide an overview of the SOS response in relation to persisters, tolerance, and shielding, highlighting several significant human pathogens.
The SOS Response
The SOS response is an inducible DNA repair pathway controlled by two key regulators, LexA, a repressor, and RecA, an inducer. Upon DNA damage, RecA is activated (RecA∗) by binding to single-stranded DNA (ssDNA), forming a nucleoprotein filament that stimulates self-cleavage of LexA leading to, in Escherichia coli, de-repression of more than 50 SOS genes. The response is induced by an increase in intracellular ssDNA generated when DNA polymerase stalls at a lesion while helicase continues unwinding DNA. The SOS response is temporally controlled; thus, firstly induced are high-fidelity repair mechanisms, followed by low-fidelity, damage tolerance pathways involving error-prone translesion DNA polymerases PolII (polB), PolIV (dinB), and PolV (umuC, umuD) that are active only after an extensive, persistent DNA damage. These polymerases enable the repair of irrepairable DNA lesions that block DNA replication (Butala et al., 2009; Maslowska et al., 2019).
Conjugative plasmid DNA transfer and transformation also induce the SOS response (Baharoglu et al., 2010; Baharoglu et al., 2012) due to the transfer/uptake of ssDNA.
Exogenous and endogenous triggers induce the SOS response. The exogenous triggers include UV irradiation, drugs, oxidants, and chemical mutagens. The induction of SOS response and mutagenesis by subinhibitory concentrations of antimicrobials has been demonstrated in several bacterial species: Escherichia coli, Vibrio cholerae, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococci species (Baharoglu and Mazel, 2011; Andersson and Hughes, 2014). The endogenous triggers are metabolic intermediates, stalled replication forks, and defects following recombination or chromosome segregation (Pennington and Rosenberg, 2007; McGlynn et al., 2012).
While the SOS response was initially recognized as regulating DNA damage repair, it plays a much broader role. The SOS error-prone polymerases promote an elevated mutation rate, generating genetic diversity and adaptation, including antibiotic resistance. Furthermore, the SOS response controlling the TA systems is significant for biofilms that exhibit increased antimicrobial recalcitrance, horizontal gene transfer (Guerin et al., 2009), intraspecies competition, and phenotypic variation (Maslowska et al., 2019).
Other Bacterial Stress Responses Control SOS Factors
While RecA and LexA are the principal SOS regulators, other stress response pathways such as the general stress responses regulated by alternative sigma factors RpoS and RpoH, the stringent response, reactive oxygen species (ROS), and cAMP are also involved in the control of SOS factors/induction.
RpoS is a regulator of the general stress response as various stresses that inhibit growth provoke RpoS activity (Hengge, 2009). Furthermore, in E. coli, RpoS regulates Pol IV activity (Layton and Foster, 2003) and is a positive regulator of polB (Pol II) (Dapa et al., 2017).
While RpoH (σ32) controls the heat-shock response during exponential growth, its synthesis and activity is also induced by other conditions, leading to unfolded proteins (Morita et al., 2000). In E. coli, RpoH indirectly affects the levels of Pol V and Pol IV via the molecular chaperone GroE. The latter is governed by RpoH and interacts with and protects UmuC (DNA polymerase V) from degradation (Donnelly and Walker, 1992). GroE is also required for the normal and the induced levels of Pol IV, albeit indirectly (Layton and Foster, 2003).
The ROS are produced in metabolic pathways as well as by the immune response upon infection. Environmental agents such as UV, drugs including antimicrobial agents, and heat can provoke and increase the ROS levels that subsequently damage DNA, proteins, and lipids, inducing the SOS response. When more ROS are produced than eliminated, oxidative stress ensues (Zhao and Drlica, 2014).
The stringent response is a conserved global stress response to nutrient and iron starvation that involves the production of (p)ppGpp. Starvation activates the stringent response via induction of the relA and spoT genes to synthesize (p)ppGpp that subsequently affects transcription, translation, and replication. In E. coli, increased levels of (p)ppGpp alters transcription in response to nutrient starvation and other stresses, resulting in reduced growth rate (Ronneau and Hallez, 2019). (p)ppGpp can increase the pause time of transcription elongation complexes and arrest DNA replication forks by targeting DNA primase, exposing ssDNA and inducing the SOS response (Srivatsan and Wang, 2008). In E. coli, the stringent response indirectly induces the expression of recA, ruvA, sbmC, sulA, umuD, and yebG genes, which belong to the SOS regulon (Durfee et al., 2008).
The cyclic AMP receptor protein (CRP) is (primarily) a global transcription regulator of genes involved in carbon catabolite repression (Molina-Quiroz et al., 2018). Nevertheless, cAMP–CRP also regulates the genes involved in virulence, biofilm formation, and SOS response and negatively regulates persistence in uropathogenic E. coli (UPEC) cultures exposed to ampicillin (Molina-Quiroz et al., 2018).
The SOS Response and Biofilms
Biofilms are structured bacterial communities attached to inert or living surfaces that create a protective environment for bacterial cells (Hall-Stoodley et al., 2004). While biofilm formation is an integral part of the prokaryotic life cycle, biofilms also cause biofilm-related diseases that are difficult to eradicate, e.g., urinary tract infections (UTI), chronic infections in cystic fibrosis (CF) patients, colonization of medical devices, and periodontal diseases (Costerton et al., 1999; Arciola et al., 2018). Biofilm formation is a highly regulated process depending upon numerous environmental and genetic factors (López et al., 2010; van Gestel et al., 2015; Qin et al., 2020). Biofilms are induced by antimicrobial stress. In biofilms, bacteria are able to survive a high-dose antibiotic treatment (Costerton et al., 1999). The recalcitrance of biofilms to antimicrobials is multifactorial. The extracellular matrix forms a mechanical barrier that prevents antibiotic diffusion. Furthermore, low oxygen and nutrient concentrations within the mature biofilms create niches with low bacterial metabolic activity. Up to 1% of bacterial cells in biofilms may be persister cells which, due to dormancy, are not affected by antimicrobials (Hall and Mah, 2017). Furthermore, high cell density within biofilms enhances horizontal gene transfer and competition that, together with accumulation of metabolic products, microaerobic areas, and oxidative stress, incite DNA damage and provoke the SOS response. Furthermore, phenotypic variants, e.g., small colony variants (SCVs) from biofilms, are quasi-dormant, slow growing, and very tolerant to host defenses and antimicrobials. SCVs attach strongly to surfaces, display increased production of exopoysaccharides, and may autoaggregate (Soares et al., 2019). They are potentially responsible for difficult-to-treat persistent infections, wherein bacteria persist in the host for prolonged periods of time despite antimicrobial therapy. Thus, the recalcitrance of biofilms to antimicrobials can be due to tolerance, when dispersed biofilm cells exhibit antibiotic sensitivity and low MIC, as well as resistance, characterized by increased MICs and a resistant phenotype of dispersed biofilm bacteria. The SOS response plays a significant role in biofilm formation but, in turn, in the dynamic biofilm environment, SOS-inducing factors that promote mutagenesis and diversification are generated.
SOS-Controlled Persisters and Tolerance in Selected Bacterial Pathogens
Escherichia coli
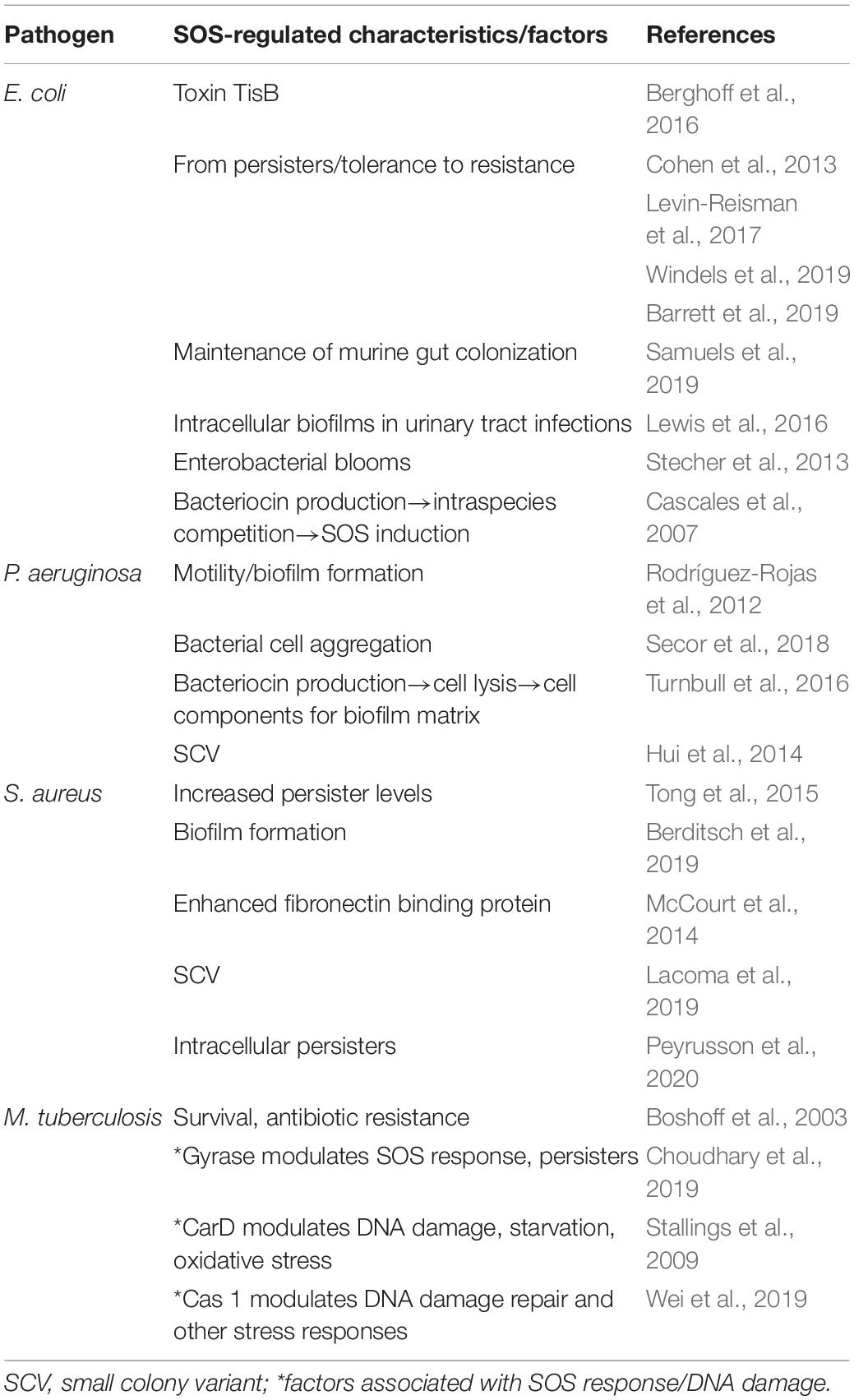
Persisters and antimicrobial tolerance have been most extensively studied in E. coli, a commensal of the gut but also a leading cause of intestinal and extraintestinal infections. One of the best-studied and most clear-cut example of persister cell formation involving the SOS system is activation via the class I toxin–antitoxin TisB/IstR module (Table 1). TisB is a small membrane-acting peptide that decreases the proton motive force and ATP levels, shutting down cell metabolism and inducing dormancy (Dörr et al., 2010). The tisB gene is repressed by LexA, while the IstR-1 antitoxin is constitutively expressed. The regulation of tisB mRNA translation is complex, encompassing inhibition by the RNA IstR-1 antitoxin as well as an inhibitory structure in the 5′UTR of the tisB mRNA. Upon DNA damage and SOS induction, tisB transcription strongly increases and exceeds that of the antitoxin IstR-1 (Berghoff et al., 2016).
Nevertheless, in E. coli, the SOS response is not only involved in persister cell formation but, in persisters, accelerates antibiotic resistance (Cohen et al., 2013; Levin-Reisman et al., 2017; Windels et al., 2019). Thus, from fluoroquinolone (FQ) persisters, the SOS response promotes resistance to unrelated antibiotics following a single FQ exposure (Barrett et al., 2019).
Conditions conducive to SOS induction are encountered by E. coli at various host anatomical sites. The SOS response plays a vital role for maintaining colonization of the murine gut by commensal E. coli with competing commensal organisms, a possible source of genotoxic stress (Samuels et al., 2019).
Urinary tract infections are a worldwide health concern caused mainly by UPEC strains. To provoke UTI, UPEC undergo a complex intracellular cycle (Lewis et al., 2016), forming biofilms within bladder epithelial cells and evading antimicrobial agents. In response, the infected cells produce nitric oxide that attacks DNA, inducing the SOS response. UTI frequently lead to chronic infection, and a persister subpopulation could be responsible for generating relapsing infections (Blango and Mulvey, 2010).
Biofilms are also associated with E. coli intestinal infections (Sharma et al., 2016). DNA damage can be provoked by host factors, e.g., bile salts, and by competing microbes. Furthermore, intestinal inflammation triggered by infection or the gut immune system involving ROS provokes the SOS response and dysbiosis, suppressing anaerobes and inciting Enterobacteriaceae overgrowth with competition for nutrients (Stecher et al., 2013).
Colicins are E. coli LexA-repressed bacteriocins that govern intraspecies competition. Nuclease colicins can, in sensitive E. coli, activate the SOS response via DNA degradation (Cascales et al., 2007). Furthermore, lysis of the producer-releasing colicin, as well as lysis of the sensitive target cell, provides material for bacterial shielding or biofilm matrix. Furthermore, a recent study showed that ampicillin-induced bacterial cell lysis provides a matrix of cell debris that shields viable cells from antimicrobial activity (Podlesek et al., 2016).
Pseudomonas aeruginosa
P. aeruginosa is a ubiquitous Gram-negative bacterium and a major human opportunist. It is a particular threat for immunocompromised patients with CF, burns, or chronic wounds (Salter, 2015). The P. aeruginosa SOS response regulon is comprised of only 15 genes, including recA, lexA, and others involved in DNA repair and induced mutation. Nevertheless, the P. aeruginosa SOS response is complex as, upon induction, the LexA and two other regulons regulated by two LexA-like Ser-Lys dyad repressors, PrtR and PAO906, are also upregulated (Matsui et al., 1993; Michel-Briand and Baysse, 2002; Cirz et al., 2006). In the stressed lung environment, strains evolve via genomic mutations and rearrangements, resulting in intrapopulation phenotypic variation (Darch et al., 2015). One of the most important factors for the survival of P. aeruginosa is the capacity to develop biofilms in various environments, including chronic lung infections in individuals with CF, rendering antibiotics inefficient (Høiby et al., 2010). These biofilms also harbor abundant persisters (1%).
In P. aeruginosa, environmental factors, such as ROS from the inflammatory response, as well as DNA-damaging antibiotics induce biofilm formation via the SOS response (Rodríguez-Rojas et al., 2012). The initial event in biofilm formation is repression of motility, and the results indicate that, in P. aeruginosa, repression is driven by the LexA protein (Chellappa et al., 2013). In Salmonella spp., the activation of the SOS response increases the RecA concentration that subsequently and indirectly impairs swarming motility (Irazoki et al., 2016), while in Clostridium difficile the inactivation of the lexA gene provoked a reduction in swarming motility (Walter et al., 2015).
Aggregation is, in P. aeruginosa, also achieved at chronic infection sites by the depletion mechanism that requires neither biofilm assembly functions nor bacterial viability (Secor et al., 2018). Depletion aggregation markedly and rapidly increases antibiotic tolerance via the SOS response.
Pyocins, chromosomally encoded and SOS-regulated bacteriocins produced by most P. aeruginosa strains, also play a significant role in biofilm formation (Michel-Briand and Baysse, 2002). A recent study revealed that R-type pyocins play an important role in competition among P. aeruginosa strains and could be pivotal for the dominance of epidemic strains in CF patients (Oluyombo et al., 2019). In addition, when pyocins are released via cell lysis, chromosomal DNA and the other released components function as a matrix for biofilm formation (Turnbull et al., 2016).
While persisters are abundant among bacterial isolates from the lungs of chronic CF patients (Mulcahy et al., 2010), SCVs are also commonly isolated from P. aeruginosa infections. Interestingly, the Pf4 bacteriophage plays an important role in P. aeruginosa biofilm formation, stress tolerance, and formation of morphotypic variants such as SCV (Rice et al., 2009). The accumulation of reactive oxygen and nitrogen species and the exposure to sublethal concentrations of antibiotics in the biofilm may result in DNA lesions in Pf4, leading to the formation of superinfection SI phage, which subsequently selects for morphotypic variants, such as SCVs (Hui et al., 2014). These SCVs are usually non-motile and recalcitrant to several different classes of antibiotics (Malone, 2015). In vitro tests have shown that exposure to sublethal concentrations of aminoglycosides selects for SCVs.
Furthermore, in P. aeruginosa, the stringent response enhances antimicrobial tolerance in biofilms by preventing the accumulation of pro-oxidants and upgrading the antioxidant activity, thus reducing oxidative stress (Nguyen et al., 2011; Khakimova et al., 2013).
Staphylococcus aureus
S. aureus is a major human bacterial pathogen that colonizes the nasopharynx and the skin of over one-third of the human population. It can also cause acute as well as persistent infections. Well characterized are biofilm infections on implanted medical devices (Tong et al., 2015). Pretreatment with sublethal concentations of antibiotics substantially increased the levels of persister cells (Johnson and Levin, 2013). Furthermore, in planktonic bacteria, exposure to subinhibitory concentrations of antibiotics induced switching to a biofilm lifestyle (Berditsch et al., 2019). SOS induction has also been shown to enhance the expression of fibronectin-binding protein (Bisognano et al., 2004) that facilitates attachment to host cells and biofilm formation (McCourt et al., 2014). Adhesion is the first step in biofilm formation or host cell invasion.
In S. aureus, only 16 LexA regulated genes have been identified, with one error-prone polymerase, UmuC (Cirz et al., 2007). The SOS response plays a key role in the emergence of antibiotic resistance, as well as the expression and the dissemination of virulence factors encoded on pathogenicity islands, SaPI.
Furthermore, in S. aureus, antibiotic-provoked SOS induction increases the formation of SCV. The mutagenic DNA repair pathway with the AddAB complex (termed RexAB in S. aureus), RecA and PolV, provokes subpopulations of SCVs resistant to H2O2 due to enhanced catalase production (Painter et al., 2015).
Tobacco smoking represents the leading preventable cause of death worldwide. A recent study showed that, in S. aureus, cigarette smoke provoked growth inhibition, increased biofilm formation, increased the invasion of and the persistence within bronchial alveolar epithelial cells, as well as enhanced the mutation frequency, resulting in a significant increase in gentamicin-resistant SCV. SOS DNA mutagenic repair was shown to induce SCV formation (Lacoma et al., 2019).
Recently, the antibiotic treatment of S. aureus was found to elicit intracellular persisters characterized by profound transcriptomic reprogramming with active adaptive responses, including the SOS response (Peyrusson et al., 2020). Furthermore, exposure to a single antibiotic leads to tolerance to several antibiotic classes with unrelated targets. The intracellular survival of S. aureus is considered as a significant factor responsible for recurrent infections (Garzoni and Kelley, 2011).
Mycobacterium tuberculosis
Tuberculosis is one of the leading causes of death worldwide, with a rapid emergence and spread of drug-resistant strains (Choudhary et al., 2019). During infection, the host employs an array of stresses to restrain M. tuberculosis (Mtb) proliferation. Nevertheless, Mtb may persist for decades, indicating the possession of efficient molecular mechanisms to resist host-inflicted damage.
In mycobacteria, two mechanisms control DNA damage repair, a relatively small LexA-regulated and a larger LexA-independent mechanism controlled by a ClpR factor (Smollett et al., 2012). The error prone α subunit of DNA-polymerase III encoded by dnaE2 is required for persistence during infection and for the development of antibiotic resistance (Boshoff et al., 2003).
In contrast to E. coli, which harbors error-prone polymerases, II, IV, and V, Mtb, carries a split cassette, imuA′–imuB/dnaE2. ImuA′ and ImuB are Y-family DNA polymerases essential for induced mutagenesis and damage tolerance in conjunction with the C-family DNA polymerase DnaE2 (Warner et al., 2010). ImuB interacts with ImuA′ and the C-family DNA polymerase, DnaE2, as well as with the beta-clamp. Bacterial genomes are replicated by the DNA polymerase III α subunit (PolIIIα) that classifies into the C-family of DNA polymerases. Two major forms of PolIIIα are recognized, PolC and DnaE (three groups: DnaE1, DnaE2, DnaE3), inferred to have evolved by duplication (Koonin and Bork, 1996). An analysis of approximately 2,000 bacterial genomes showed that they harbor one or more homologs (Timinskas et al., 2014). DnaE2 is part of SOS-inducible mutagenic cassettes identified in many bacterial genomes, including M. tuberculosis. At least some DnaE3s are also error prone and SOS inducible (Boshoff et al., 2003).
Transcriptomic studies showed that various stress response regulons, including the SOS response and different TA genes, are positively regulated in Mtb persisters (Keren et al., 2011; Smollett et al., 2012).
Recently, the suppression of DNA gyrase was found to drastically affect intra- and extracellular Mtb growth. Interestingly, gyrase depletion in Mtb leads to the activation of RecA/LexA-mediated SOS response and drug tolerance via induction of a persister subpopulation (Choudhary et al., 2019).
Furthermore, (p)ppGpp (Prusa et al., 2018) and CarD (Stallings et al., 2009) play significant roles for the successful growth of M. tuberculosis in the host. Lack of CarD leads to killing of M. tuberculosis due to DNA damage, starvation, and oxidative stress (Stallings et al., 2009).
Biofilm growth is also significant for Mtb particularly during infection. Mtb spontaneously grows at the air–liquid interface, forming pellicle biofilms with elevated drug-tolerant persisters compared to planktonic cultures. The intrinsic drug tolerance of biofilm cells was found to determine the frequency of persisters and isonitrile lipopeptide necessary for the architectural development of Mtb biofilms (Richards et al., 2019).
The CRISPR-associated protein 1 (Cas1) is an endonuclease responsible for spacer integration into CRISPR arrays. Interestingly, Cas1 was found to be deleted in many specific drug-resistant strains. In recombinant M. smegmatis, Cas1 increased the sensitivity to multiple anti-tuberculosis drugs by reducing persistence during antimicrobial treatment. Cas1 also impaired the repair of DNA damage and altered the stress response of M. smegmatis (Wei et al., 2019).
Concluding Remarks
Multiple redundant mechanisms are involved in the formation of persister cells, population-wide tolerance, and shielding. DNA damage and other stress pathways trigger the SOS response or control SOS factors in a number of host sites. As yet, only one SOS-regulated TA module has been shown to be involved in persister formation. Nevertheless, the SOS system is a key regulator of biofilm formation that protects bacterial communities against extreme environmental conditions, including antibiotic exposure. Furthermore, in biofilms, persisters and other phenotypic variants can be abundant. The conditions and the mechanisms within a biofilm generate factors that trigger the SOS response, fueling mutagenesis, horizontal gene transfer, competition, and diversification. The discussed four pathogens/opportunists provoke significantly persistent, hard-to-treat infections also due to persister cells and tolerance. Nevertheless, our current understanding of the means and the levels of the SOS response and its links with other global regulatory responses is still lacking. Therapies aimed at preventing persister formation but particularly at suppressing the SOS response, possibly targeting its inducer RecA, could greatly improve patient outcome.
Author Contributions
DŽ provided the general concept. DŽ and ZP wrote the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This study was funded by the grant P1-0198 from the Slovene Research Agency.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andersson, D. I., and Hughes, D. (2014). Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 12, 465–478. doi: 10.1038/nrmicro3270
Arciola, C. R., Campoccia, D., and Montanaro, L. (2018). Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 16, 397–409. doi: 10.1038/s41579-018-0019-y
Baharoglu, Z., Bikard, D., and Mazel, D. (2010). Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation. PLoS Genet. 6:e1001165. doi: 10.1371/journal.pgen.1001165
Baharoglu, Z., Krin, E., and Mazel, D. (2012). Connecting environment and genome plasticity in the characterization of transformation-induced SOS regulation and carbon catabolite control of the Vibrio cholerae integron integrase. J. Bacteriol. 194, 1659–1667. doi: 10.1128/jb.05982-11
Baharoglu, Z., and Mazel, D. (2011). Vibrio cholerae triggers SOS and mutagenesis in response to a wide range of antibiotics: a route towards multiresistance. Antimicrob. Agents Chemother. 55, 2438–2441. doi: 10.1128/AAC.01549-1510
Barrett, T. C., Mok, W. W. K., Murawski, A. M., and Brynildsen, M. P. (2019). Enhanced antibiotic resistance development from fluoroquinolone persisters after a single exposure to antibiotic. Nat. Commun. 10:1177. doi: 10.1038/s41467-019-09058-9054
Berditsch, M., Afonin, S., Reuster, J., Lux, H., Schkolin, K., Babii, O., et al. (2019). Supreme activity of gramicidin S against resistant, persistent and biofilm cells of staphylococci and enterococci. Sci. Rep. 9:17938. doi: 10.1038/s41598-019-54212-z
Berghoff, B. A., Hoekzema, M., Aulbach, L., and Wagner, E. G. (2016). Two regulatory RNA elements affect TisB-dependent depolarization and persister formation. Mol. Microbiol. 103, 1020–1033. doi: 10.1111/mmi.13607
Bisognano, C., Kelley, W. L., Estoppey, T., Francois, P., Schrenzel, J., Li, D., et al. (2004). A recA-LexA-dependent pathway mediates ciprofloxacin-induced fibronectin binding in Staphylococcus aureus. J. Biol. Chem. 279, 9064–9071. doi: 10.1074/jbc.M309836200
Blango, M. G., and Mulvey, M. A. (2010). Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 54, 1855–1863. doi: 10.1128/AAC.00014-10
Boshoff, H. I., Reed, M. B., Barry, C. E. III, and Mizrahi, V. (2003). DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113, 183–193. doi: 10.1016/s0092-8674(03)00270-8
CrossRef Full Text doi: 10.1038/emboj.2009.308 | CrossRef Full Text | Google Scholar
Butala, M., Žgur-Bertok, D., and Busby, S. J. (2009). The bacterial LexA transcriptional repressor. Cell Mol. Life Sci. 66, 82–93. doi: 10.1007/s00018-008-8378-8376
Cascales, E., Buchanan, S. K., Duché, D., Kleanthous, C., Lloubès, R., Postle, K., et al. (2007). Colicin biology. Microbiol. Mol. Biol. Rev. 71, 158–229. doi: 10.1128/MMBR.00036-36
Chellappa, S. T., Maredia, R., Phipps, K., Haskins, W. E., and Weitao, T. (2013). Motility of Pseudomonas aeruginosa contributes to SOS-inducible biofilm formation. Res. Microbiol. 164, 1019–1027. doi: 10.1016/j.resmic.2013.10.001
Choudhary, E., Sharma, R., Kumar, Y., and Agarwal, N. (2019). Conditional silencing by CRISPRi reveals the role of DNA grase in formation of drug-tolerant persister population in Mycobacterium tuberculosis. Front. Cell Infect. Microbiol. 26:70. doi: 10.3389/fcimb.2019.00070
Cirz, R. T., Jones, M. B., Gingles, N. A., Minogue, T. D., Jarrahi, B., Peterson, S. N., et al. (2007). Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J. Bacteriol. 189, 531–539. doi: 10.1128/jb.01464-06
Cirz, R. T., O’Neill, B. M., Hammond, J. A., Head, S. R., and Romesberg, F. E. (2006). Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J. Bacteriol. 188, 7101–7110. doi: 10.1128/jb.00807-06
Cohen, N. R., Lobritz, M. A., and Collins, J. J. (2013). Microbial persistence and the road to drug resistance. Cell Host Microbe 13, 632–642. doi: 10.1016/j.chom.2013.05.009
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
Dapa, T., Fleurier, S., Bredeche, M. F., and Matic, I. (2017). The SOS and RpoS regulons contribute to bacterial cell robustness to genotoxic stress by synergistically regulating DNA polymerase Pol II. Genetics 2017, 1349–1360. doi: 10.1534/genetics.116.199471
Darch, S. E., McNally, A., Harrison, F., Corande, R. J., Barr, H. L., Paszkiewicz, K., et al. (2015). Recombination is a key driver of genomic and phenotypic diversity in a Pseudomonas aeruginosa population during cystic fibrosis infection. Sci. Rep. 5:7649. doi: 10.1038/srep07649
Donnelly, C. E., and Walker, G. C. (1992). Coexpression of UmuD’ with UmuC suppresses the UV mutagenesis deficiency of groE mutants. J. Bacteriol. 174, 3133–3139. doi: 10.1128/jb.174.10.3133-3139.1992
Dörr, T., Lewis, K., and Vulić, M. (2009). SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 5:e1000760. doi: 10.1371/journal.pgen.1000760
Dörr, T., Vulić, M., and Lewis, K. (2010). Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 8:e1000317. doi: 10.1371/journal.pbio.1000317
Durfee, T., Hansen, A. M., Zhi, H., Blattner, F. R., and Jin, D. J. (2008). Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 190, 1084–1096. doi: 10.1128/jb.01092-07
Foster, P. L. (2007). Stress-induced mutagenesis in bacteria. Crit. Rev. Biochem. Mol. Biol. 42, 373–397.
Garzoni, C., and Kelley, W. L. (2011). Return of the Trojan horse: intracellular phenotype switching and immune evasion by Staphylococcus aureus. EMBO Mol. Med. 3, 115–117. doi: 10.1002/emmm.201100123
Goormaghtigh, F., and Van Melderen, L. (2019). Single-cell imaging and characterization of Escherichia coli persister cells to ofloxacin in exponential cultures. Sci Adv. 5:eaav9462. doi: 10.1126/sciadv.aav9462
Guerin, E., Cambray, G., Sanchez-Alberola, N., Campoy, S., Erill, I., Da Re, S., et al. (2009). The SOS response controls integron recombination. Science 324:1034. doi: 10.1126/science.1172914
Hall, C. W., and Mah, T. F. (2017). Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 41, 276–301. doi: 10.1093/femsre/fux010
Hall-Stoodley, L., Costerton, J. W., and Stoodley, P. (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108. doi: 10.1038/nrmicro821
Harms, A., Maisonneuve, E., and Gerdes, K. (2016). Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 16:aaf4268. doi: 10.1126/science.aaf4268
Hengge, R. (2009). Proteolysis of sigmaS (RpoS) and the general stress response in Escherichia coli. Res. Microbiol. 160, 667–676. doi: 10.1016/j.resmic.2009.08.014
Høiby, N., Ciofu, O., and Bjarnsholt, T. (2010). Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 5, 1663–1674.
Hui, J. G., Mai-Prochnow, A., Kjelleberg, S., McDougald, D., and Rice, S. A. (2014). Environmental cues and genes involved in establishment of the superinfective Pf4 phage of Pseudomonas aeruginosa. Front. Microbiol. 5:654. doi: 10.3389/fmicb.2014.00654
Irazoki, O., Mayola, A., Campoy, S., and Barbe, J. (2016). SOS system induction inhibits the assembly of chemoreceptor signaling clusters in Salmonella enterica. PLoS One 11:e0146685. doi: 10.1371/journal.pone.0146685
Johnson, P. J. T., and Levin, B. R. (2013). Pharmacodynamics, population dynamics, and the evolution of persistence in Staphylococcus aureus. PLoS Genet. 9:e1003123. doi: 10.1371/journal.pgen.1003123
Keren, I., Minami, S., Rubin, E., and Lewis, K. (2011). Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio 12:e00100-11. doi: 10.1128/mBio.00100-11
Khakimova, M., Ahlgren, H. G., Harrison, J. J., English, A. M., and Nguyen, D. (2013). The stringent response controls catalases in Pseudomonas aeruginosa and is required for hydrogen peroxide and antibiotic tolerance. J. Bacteriol. 195, 2011–2020. doi: 10.1128/JB.02061-2012
Koonin, E. V., and Bork, P. (1996). Ancient duplication of DNA polymerase inferred from analysis of complete bacterial genomes. Trends Biochem. Sci. 21, 128–129. doi: 10.1016/s0968-0004(96)80165-4
Kwan, B. W., Valenta, J. A., Benedik, M. J., and Wood, T. K. (2013). Arrested protein synthesis increases persister-like cell formation. Antimicrob. Agents. Chemother. 57, 1468–1473. doi: 10.1128/AAC.02135-12
Lacoma, A., Edwards, A. M., Young, B. C., Domínguez, J., Prat, C., and Laabei, M. (2019). Cigarette smoke exposure redirects Staphylococcus aureus to a virulence profile associated with persistent infection. Sci. Rep. 9:10798. doi: 10.1038/s41598-019-47258-47256
Layton, J. C., and Foster, P. L. (2003). Error-prone DNA polymerase IV is controlled by the stress-response sigma factor. RpoS, in Escherichia coli. Mol. Microbiol. 50, 549–561. doi: 10.1046/j.1365-2958.2003.03704.x
Levin-Reisman, I., Ronin, I., Gefen, O., Braniss, I., Shoresh, N., and Balaban, N. Q. (2017). Antibiotic tolerance facilitates the evolution of resistance. Science 355, 826–830. doi: 10.1126/science.aaj2191
Lewis, A. J., Richards, A. C., and Mulvey, M. A. (2016). Invasion of host cells and tissues by uropathogenic bacteria. Microbiol. Spectr. 4:10.1128/microbiolspec.UTI-0026-2016. doi: 10.1128/microbiolspec.UTI-0026-2016
López, D., Vlamakis, H., and Kolter, R. (2010). Biofilms. Cold Spring Harb. Perspect. Biol. 2:a000398. doi: 10.1101/cshperspect.a000398
Malone, J. G. (2015). Role of small colony variants in persistence of Pseudomonas aeruginosa infections in cystic fibrosis lungs. Infect. Drug Resist. 8, 237–247. doi: 10.2147/IDR.S68214
Maslowska, K. H., Makiela-Dzbenska, K., and Fijalkowska, I. J. (2019). The SOS system: a complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 60, 368–384. doi: 10.1002/em.22267
Matsui, H., Sano, Y., Ishihara, H., and Shinomiya, T. (1993). Regulation of pyocin genes in Pseudomonas aeruginosa by positive (ptrN) and negative (ptrR) regulatory genes. J. Bacteriol. 175, 1257–1263. doi: 10.1128/jb.175.5.1257-1263.1993
McCourt, J., O’Halloran, D. P., McCarthy, H., O’Gara, J. P., and Geoghegan, J. A. (2014). Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol. Lett. 353, 157–164. doi: 10.1111/1574-6968.12424
McGlynn, P., Savery, N. J., and Dillingham, M. S. (2012). The conflict between DNA replication and transcription. Mol. Microbiol. 85, 12–20. doi: 10.1111/j.1365-2958.2012.08102.x
Michel-Briand, Y., and Baysse, C. (2002). The pyocins of Pseudomonas aeruginosa. Biochimie. 84, 499–510. doi: 10.1016/s0300-9084(02)01422-1420
Molina-Quiroz, R. C., Silva-Valenzuela, C., Brewster, J., Castro-Nallar, E., Levy, S. B., and Camilli, A. (2018). Cyclic AMP regulates bacterial persistence through repression of the oxidative stress response and SOS-dependent DNA repair in uropathogenic Escherichia coli. mBio 9:e02144-17. doi: 10.1128/mBio.02144-17
Morita, M. T., Kanemori, M., Yanagi, H., and Yura, T. (2000). Dynamic interplay between antagonistic pathways controlling the sigma 32 level in Escherichia coli. Proc. Natl. Acad. Sci. U.S. A. 97, 5860–5865. doi: 10.1073/pnas.080495197
Mulcahy, L. R., Burns, J. L., Lory, S., and Lewis, K. (2010). Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 192, 6191–6199. doi: 10.1128/JB.01651-1659
Nguyen, D., Joshi-Datar, A., Lepine, F., Bauerle, E., Olakanmi, O., Beer, K., et al. (2011). Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334, 982–986. doi: 10.1126/science.1211037
Oluyombo, O., Penfold, C. N., and Diggle, S. P. (2019). Competition in biofilms between cystic fibrosis isolates of Pseudomonas aeruginosa is shaped by R-pyocins. mBio 10:01828-18. doi: 10.1128/mBio.01828-18
Painter, K. L., Strange, E., Parkhill, J., Bamford, K. B., Armstrong-James, D., and Edwards, A. M. (2015). Staphylococcus aureus adapts to oxidative stress by producing H2O2-resistant small-colony variants via the SOS response. Infect. Immun. 83, 1830–1844. doi: 10.1128/IAI.03016-3014
Pennington, J. M., and Rosenberg, S. M. (2007). Spontaneous DNA breakage in single living Escherichia coli cells. Nat. Genet. 39, 797–802. doi: 10.1038/ng2051
Peyrusson, F., Varet, H. T., Nguyen, T. K., Legendre, R., Sismeiro, O., Coppée, J. Y., et al. (2020). Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat. Commun. 11:2200. doi: 10.1038/s41467-020-15966-7
Podlesek, Z., Butala, M., Šakanović, A., and Žgur-Bertok, D. (2016). Antibiotic induced bacterial lysis provides a reservoir of persisters. Antonie Van Leeuwenhoek 109, 523–528. doi: 10.1007/s10482-016-0657-x
Prusa, J., Zhu, D. X., and Stallings, C. L. (2018). The stringent response and Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 1:fty054. doi: 10.1093/femspd/fty054
Qin, B., Fei, C., Bridges, A. A., Mashruwala, A. A., Stone, H. A., Wingreen, N. S., et al. (2020). Cell position fates and collective fountain flow in bacterial biofilms revealed by light-sheet microscopy. Science 11:eabb8501. doi: 10.1126/science.abb8501
Rice, S. A., Tan, C. H., Mikkelsen, P. J., Kung, V., Woo, J., Tay, M., et al. (2009). The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J. 3, 271–282. doi: 10.1038/ismej.2008.109
Richards, J. P., Cai, W., Zill, N. A., Zhang, W., and Ojha, A. K. (2019). Adaptation of Mycobacterium tuberculosis to biofilm growth is genetically linked to drug tolerance. Antimicrob. Agents Chemother 63:e01213-19. doi: 10.1128/AAC.01213-19
Rodríguez-Rojas, A., Oliver, A., and Blázquez, J. (2012). Intrinsic and environmental mutagenesis drive diversification and persistence of Pseudomonas aeruginosa in chronic lung infections. J. Infect. Dis. 205, 121–127. doi: 10.1093/infdis/jir690
Ronneau, S., and Hallez, R. (2019). Make and break the alarmone: regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol. Rev. 43, 389–400. doi: 10.1093/femsre/fuz009
Salter, S. J. (2015). Keeping an eye on P. aeruginosa. Nat. Rev. Microbiol. 13:69. doi: 10.1038/nrmicro3422
Samuels, A. N., Roggiani, M., Zhu, J., Goulian, M., and Kohli, R. M. (2019). The SOS response mediates sustained colonization of the mammalian gut. Infect. Immun. 87:e00711-18. doi: 10.1128/IAI.00711-118
Secor, P. R., Michaels, L. A., Ratjen, A., Jennings, L. K., and Singh, P. K. (2018). Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 115, 10780–10785. doi: 10.1073/pnas.1806005115
Sharma, G., Sharma, S., Sharma, P., Chandola, D., Dang, S., Gupta, S., et al. (2016). Escherichia coli biofilm: development and therapeutic strategies. J. Appl. Microbiol. 121, 309–319. doi: 10.1111/jam.13078
Smollett, K., Smith, K. M., Kahramanoglou, C., Arnvig, K. B., Buxton, R. S., and Davis, E. O. (2012). Global analysis of the regulon of the transcriptional repressor LexA, a key component of SOS response in Mycobacterium tuberculosis. J. Biol. Chem. 287, 22004–22014. doi: 10.1074/jbc.M112.357715
Soares, A., Caron, F., and Etienne, M. (2019). Commentary: tolerance and resistance of Pseudomonas aeruginosa Biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front. Microbiol. 10:2164. doi: 10.3389/fmicb.2019.02164
Srivatsan, A., and Wang, J. D. (2008). Control of bacterial transcription, translation and replication by (p)ppGpp. Curr. Opin. Microbiol. 11, 100–105. doi: 10.1016/j.mib.2008.02.001
Stallings, C. L., Stephanou, N. C., Chu, L., Hochschild, A., Nickels, B. E., and Glickman, M. S. (2009). CarD is an essential regulator of rRNA transcription required for Mycobacterium tuberculosis persistence. Cell 138, 146–159. doi: 10.1016/j.cell.2009.04.041
Stecher, B., Maier, L., and Hardt, W. D. (2013). ‘Blooming’ in the gut: how dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 11, 277–284. doi: 10.1038/nrmicro2989
Timinskas, K., Balvočiûtë, M., Timinskas, A., and Venclovas, Č (2014). Comprehensive analysis of DNA polymerase III α subunits and their homologs in bacterial genomes. Nucleic Acids Res. 42, 1393–1413. doi: 10.1093/nar/gkt900
Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L., and Fowler, V. G. Jr. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi: 10.1128/CMR.00134-114
Trastoy, R., Manso, T., Fernández-García, L., Blasco, L., Ambroa, A., Pérez Del Molino, M. L., et al. (2018). Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin. Microbiol. Rev. 31:e00023-18. doi: 10.1128/CMR.00023-18
Turnbull, L., Toyofuku, M., Hynen, A. L., Kurosawa, M., Pessi, G., Petty, N. K., et al. (2016). Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 7:11220. doi: 10.1038/ncomms11220
van Gestel, J., Vlamakis, H., and Kolter, R. (2015). Division of labor in biofilms: the ecology of cell differentiation. Microbiol. Spectr. 3:MB-0002-2014. doi: 10.1128/microbiolspec.MB-0002-2014
Walter, B. M., Cartman, S. T., Minton, N. P., Butala, M., and Rupnik, M. (2015). The SOS response master regulator LexA is associated with sporulation, motility and biofilm formation in Clostridium difficile. PLoS One 18:e0144763. doi: 10.1371/journal.pone.0144763
Warner, D. F., Ndwandwe, D. E., Abrahams, G. L., Kana, B. D., Machowski, E. E., Venclovas, C., et al. (2010). Essential roles for imuA’- and imuB-encoded accessory factors in DnaE2-dependent mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 107, 13093–13098. doi: 10.1073/pnas.1002614107
Wei, J., Lu, N., Li, Z., Wu, X., Jiang, T., Xu, L., et al. (2019). The Mycobacterium tuberculosis CRISPR-Associated Cas1 involves persistence and tolerance to anti-tubercular drugs.Biomed. Res. Int. 2019:7861695. doi: 10.1155/2019/7861695
Wilmaerts, D., Windels, E. M., Verstraeten, N., and Michiels, J. (2019). General mechanisms leading to persister formation and awakening. Trends Genet. 35, 401–411. doi: 10.1016/j.tig.2019.03.007
Windels, E. M., Michiels, J. E., Fauvart, M., Wenseleers, T., Van den Bergh, B., and Michiels, J. (2019). Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. ISME J. 13, 1239–1251. doi: 10.1038/s41396-019-0344-349
Keywords: SOS response, persisters, tolerance, biofilms, bacterial pathogens/opportunists
Citation: Podlesek Z and Žgur Bertok D (2020) The DNA Damage Inducible SOS Response Is a Key Player in the Generation of Bacterial Persister Cells and Population Wide Tolerance. Front. Microbiol. 11:1785. doi: 10.3389/fmicb.2020.01785
Received: 11 May 2020; Accepted: 08 July 2020;
Published: 04 August 2020.
Edited by:
Maria Tomas, A Coruña University Hospital Complex (CHUAC), SpainReviewed by:
Juan Carlos Alonso, National Center for Biotechnology (CNB), SpainClaudia N. H. Marques, Binghamton University, United States
Copyright © 2020 Podlesek and Žgur Bertok. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darja Žgur Bertok, darja.zgur.bertok@bf.uni-lj.si
 Zdravko Podlesek
Zdravko Podlesek Darja Žgur Bertok
Darja Žgur Bertok